Abstract
Currently, no targeted therapies are available for metastatic triplenegative breast cancer (mTNBC). We evaluated the safety, efficacy, and biomarkers of response to cabozantinib, a multikinase inhibitor, in patients with mTNBC. We conducted a single arm phase II and biomarker study that enrolled patients with measurable mTNBC. Patients received cabozantinib (60 mg daily) on a 3‐week cycle and were restaged after 6 weeks and then every 9 weeks. The primary endpoint was objective response rate. Predefined secondary endpoints included progression‐free survival (PFS), toxicity, and tissue and blood circulating cell and protein biomarkers. Of 35 patients who initiated protocol therapy, 3 (9% [95% confidence interval (CI): 2, 26]) achieved a partial response (PR). Nine patients achieved stable disease (SD) for at least 15 weeks, and thus the clinical benefit rate (PR+SD) was 34% [95% CI: 19, 52]. Median PFS was 2.0 months [95% CI: 1.3, 3.3]. The most common toxicities were fatigue, diarrhea, mucositis, and palmar‐plantar erythrodysesthesia. There were no grade 4 toxicities, but 12 patients (34%) required dose reduction. Two patients had TNBCs with MET amplification. During cabozantinib therapy, there were significant and durable increases in plasma placental growth factor, vascular endothelial growth factor (VEGF), VEGF‐D, stromal cell‐derived factor 1a, and carbonic anhydrase IX, and circulating CD3 + cells and CD8 + T lymphocytes, and decreases in plasma soluble VEGF receptor 2 and CD14+ monocytes (all p < .05). Higher baseline concentrations of soluble MET (sMET) associated with longer PFS (p = .03). In conclusion, cabozantinib showed encouraging safety and efficacy signals but did not meet the primary endpoint in pretreated mTNBC. Exploratory analyses of circulating biomarkers showed that cabozantinib induces systemic changes consistent with activation of the immune system and antiangiogenic activity, and that sMET should be further evaluated a potential biomarker of response.
Implications for Practice
Triple‐negative breast cancer (TNBC)—a disease with a dearth of effective therapies—often overexpress MET, which is associated with poor clinical outcomes. However, clinical studies of agents targeting MET and VEGF pathways—alone or in combination—have shown disappointing results. This study of cabozantinib (a dual VEGFR2/MET) in metastatic TNBC, while not meeting its prespecified endpoint, showed that treatment is associated with circulating biomarker changes, and is active in a subset of patients. Furthermore, this study demonstrates that cabozantinib therapy induces a systemic increase in cytotoxic lymphocyte populations and a decrease in immunosuppressive myeloid populations. This supports the testing of combinations of cabozantinib with immunotherapy in future studies in breast cancer patients.
Keywords: Triple‐negative breast cancer, Cabozantinib, Soluble MET, Lymphocytes, Vascular endothelial growth factor receptor
Short abstract
This study evaluates the safety, efficacy, and biomarkers of response to cabozantinib, a multikinase inhibitor, in patients with metastatic triple‐negative breast cancer (mTNBC). Cabozantinib showed encouraging safety and efficacy signals but did not meet the prespecified primary endpoint in pretreated mTNBC. Exploratory analyses of circulating biomarkers showed that cabozantinib induces systemic changes consistent with activation of the immune system and antiangiogenic activity, and soluble MET should be further evaluated as a potential biomarker of response.
Background
Breast cancer is the second highest cause of cancer mortality among American women 1. Triple‐negative breast cancer (TNBC) represents approximately 15% of all breast cancers but is associated with high‐grade disease, early visceral metastases, and death 2, 3, 4, 5.
Currently, there are no targeted therapies for this subtype. MET is a receptor tyrosine kinase that promotes cell proliferation, invasion, and survival when activated by its ligand, hepatocyte growth factor (HGF) 6. MET and HGF overexpression are associated with tumor hypoxia, increased invasiveness and metastasis, and reduced survival in metastatic breast cancer 7, 8, 9, 10, 11, 12. Furthermore, MET expression is disproportionately elevated in TNBC and associated with poorer prognosis 13, 14. MET copy number was found to be elevated in 14% of TNBC, as opposed to 8% of hormone receptorpositive (HR+) breast cancer and 7% of human epidermal growth receptor 2‐positive (HER2+) breast cancer 15. Preclinical studies suggest that MET expression drives differentiation of tumors into the TNBC subtype. Mice harboring an activating mutant MET knock‐in or mutant MET transgene under mouse mammary tumor virus promoter developed TNBCs, suggesting that inhibition of MET signaling may be a promising therapeutic approach 16, 17.
Cabozantinib (XL184, Exelixis, South San Francisco, CA, http://www.exelixis.com) is a small molecule inhibitor of multiple tyrosine kinases, including MET and vascular endothelial growth factor receptor 2 (VEGFR2), a mediator of tumor angiogenesis 18. Cabozantinib has demonstrated efficacy in advanced renal cell carcinoma and metastatic medullary thyroid cancers 19, 20, 21. In this phase II study, we assessed the efficacy and safety and examined potential biomarkers of cabozantinib in metastatic TNBC (mTNBC) patients.
Patients and Methods
Patient Eligibility
Patients 18 years of age or older with measurable mTNBC were eligible. Triple‐negative status was defined as estrogen receptor‐negative (ER—) (<10% staining by immunohis‐ tochemistry [IHC]), progesterone receptor‐negative (PR—) (<10% staining by IHC), and HER2‐negative (0 or 1+ by IHC or fluorescence in situ hybridization [FISH] < 2.0). Patients had measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and may have received 0 to 3 prior chemotherapeutic regimens for mTNBC. They were required to be off any myelosuppressive agent for 21 days before initiation of cabozantinib and must have discontinued all biologic therapy and radiation therapy at least 14 days before initiation of study treatment. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and were required to have availability of formalin‐fixed, paraffin‐embedded (FFPE) tumor tissue. Key exclusion criteria included the following: receipt of another investigational agent within 14 days of the first dose of the study drug; prior receipt of a MET inhibitor other than tivantinib (ARQ‐197); known brain metastases that were untreated, symptomatic, or required therapy to control symptoms; and corrected QT >470 milliseconds. Research was approved by local human research protections programs and institutional review boards, and studies were conducted in accordance with the Declaration of Helsinki.
Study Design and Treatment
This was a single‐arm, two‐stage phase II study assessing the efficacy of cabozantinib monotherapy in patients with mTNBC. The study sponsor, Exelixis, provided cabozantinib. Treatment consisted of oral dosing of cabozantinib at 60 mg daily over a 21‐day cycle. Patients underwent radiographic restaging at 6 weeks and every 9 weeks thereafter. Patients with complete or partial RECIST responses continued to receive study treatment, whereas those with progressive disease were taken off study. Dose reductions for toxicity occurred if patients experienced grade 3 or 4 neutropenia or thrombocytopenia, or nonhematologic adverse events. From the starting dose of 60 mg daily, doses were reduced as needed to 40 and 20 mg daily. For the purposes of determining the effect of cabozantinib treatment on pain and analgesic medication use, pain was assessed by a participant‐reported questionnaire, and daily analgesic medication usage was recorded. These were completed at baseline and during week 3, 6, and every 6 weeks thereafter until the date of the participant's last follow‐up visit. The primary endpoint was the activity of cabozantinib, as defined by objective response rate (ORR) in patients with mTNBC. Predefined secondary endpoints included progression‐free survival (PFS), toxicity, and pain. Correlative studies included analysis of MET and phospho‐MET expression in archival tumortissue, and molecular and cellular biomarkers of cabozantinib.
Fluorescence In Situ Hybridization Assessment of MET Amplification in Tissue
A MET FISH probe labeled with SpectrumRed and a CEP7 reference probe labeled with SpectrumGreen were purchased from Abbott Molecular (Des Plaines, IL, https://www.abbottmolecular.com). FISH was performed following standard protocols. Briefly, 5 μm tissue slides were baked overnight at 60°C, deparaffinized, treated in 1% sodium borohydride for 4 hours and heated in pressure cooker for 20 minutes in citrate buffer (pH 6). After treatment with 150 μg/mL solution of Proteinase K, slides were fixed in 1% neutral‐buffered formalin and denatured in 70% for mamide for 4 minutes at 72°C. Probes were denatured for 5 minutes at 80°C and incubated for 30 minutes at 37°C for preannealing. Hybridization was carried out overnight at 37°C; posthybridization slide washes were carried out for 20 minutes in 50% for mamide/2 × standard saline citrate (SSC) at 45°C, followed by 5 minutes wash in 1 × SSC at 45°C. FISH signal evaluation and acquisition were performed manually by using filter sets and software developed by Applied Spectral Imaging (Carlsbad, CA, http://www.spectral-imaging.com). Several fields with at least 50 tumor cells total were captured, and ratio of MET to CEP7 signal numbers was calculated. An assessment of ploidy was made by visual screening of all tumor area; cells with the maximum number of signals were recorded. MET amplification was defined as a MET/CEP7 ratio ≥2. Samples with a MET/CEP7 ratio between 1.5 and 2 were defined as having relative MET gain. Samples with a MET/CEP7 ratio of 1, but with more than two copies of each probe, were deemed to have polysomy of chromosome 7.
Assessment of MET Amplification in Circulating Tumor Cells
Circulating tumor cells (CTCs) were enriched from 7.5 mL of a patient's whole blood at the Circulating Tumor Cell Core Facility (Brigham and Women's Hospital, Boston, MA, http://www.brighamandwomens.org) by using the Circulating Tumor Cell Profile Kit (Veridex/Janssen Diagnostics, Raritan, NJ, http://www.janssen.com). Processed samples were received as cells suspended in 900 μL of buffer. Equal volume of PBS was added before tubes were spun down at 200g for 8 minutes. Supernatant was carefully removed, leaving approximately 60 μL of buffer. Cell pellets were gently resuspended, and the suspension was applied on the labeled slide and allowed to dry in the vacuum dessicator at room temperature. Slides were placed in methanol at −20°C for aging and storage.
For FISH, dried slides were treated in 2 × SSC at 37°C for 30 minutes, followed by 10 minutes of treatment with 0.002% pepsin solution in 0.01 M HCl at 37°C and 15 minutes of fixation in 1% formalin at room temperature. Slides were dehydrated in the series of ethanols, dried, and codenatured with MET/CEP7 FISH probe (Kreatech/Leica Microsystems Inc., Buffalo Grove, IL, http://www.leica-microsystems.com) on an 80°C plate for 2 minutes. Hybridization was carried out at 37°C overnight, followed by a 0.4 × SSC/0.3% Igepal wash at 72°C for 3 minutes and a 2 × SSC/0.1% Igepal wash at room temperature for 1 minute. Slides were dehydrated in the series of ethanols and dried before application of Vectashield mounting medium with 4′,6‐diamidino‐2‐phenylindole (Vector Laboratories Inc., Burlingame, CA, http://vectorlabs.com). FISH signal evaluation and acquisition were performed manually by using filter sets and software developed by Applied Spectral Imaging.
Circulating Biomarker Assays
Potential biomarkers of cabozantinib activity were identified by measuring plasma proteins at baseline, on day 8 of therapy, on day 1 of each cycle of therapy, and, if available, at the time of progression. Eight milliliters of blood was collected in purple‐ top (plasma EDTA) vacutainers and shipped on wet ice to a Clinical Laboratory Improvement Amendments‐certified core in the Steele Laboratories (Massachusetts General Hospital), where whole blood was separated by centrifugation into cellular fraction and plasma. The fraction of stem/progenitor cell, lymphocyte, and myeloid populations of total circulating mononuclear cells were counted by flow cytometry using a LSR‐II cytometer and FACSDiva software in fresh blood samples using the following markers: CD3, CD4, CD8, CD14, CD25, CD34, CD45, CD56, CD127, and CD133 (Becton Dickinson, Franklin Lakes, NJ, http://www.bd.com).
Plasma was prepared in the standard fashion and stored at −78°C until collection and analysis of all samples. The biomarkers measured included VEGF, placental growth factor (PlGF), VEGF‐C, VEGF‐D, soluble VEGFR1 (sVEGFR1), basic fibroblast growth factor (bFGF), and sTie‐2 (using a 7‐plex Growth Factor array) and granulocyte‐macrophage colony stimulating factor (GM‐CSF), interferon γ (IFN‐γ), tumor necrosis factor α (TNF‐α), and interleukin‐1β (IL‐1β), IL‐2, IL‐6, IL‐8, IL‐10, and IL‐12 heterodimer p70 (using a 9‐plex Inflammatory Factor array; both Meso Scale Discovery, Gaithersburg, MD, https://www.mesoscale.com); and HGF, sMET, carbonic anhydrase IX (CAIX), stromal cell‐derived factor 1α (SDF1α), and sVEGFR2 by single analyte enzyme‐linked immunosorbent assay (R&D Systems, Minneapolis, MN, https://www.rndsystems.com).
Statistical Analysis
This study used Simon optimal two‐stage design to control type I error at 10% and have at least 90% power to detect the acceptable response rate. By study design, 13 participants were to be enrolled in the first stage. If there was at least 1 response, accrual was to continue to the second stage, where an additional 22 patients were to be enrolled. If there were at least 4 responses among the 35 total patients, the regimen was to be considered worthy of further study. With a true response rate of 5%, the chance that the regimen would be declared worthy of further study was 10%, and with a true response rate of 20%, the chance that the regimen would be declared worthy of further study was 90%.
Objective response was evaluated by using RECIST1.1. Per protocol, patients who do not achieve a confirmed complete response (CR) or confirmed partial response (PR) were considered nonresponders. Objective response rate was reported with 95% confidence interval (CI) for the two stage designs 22. PFS and 95% CI were described using Kaplan‐Meier methods. PFS was defined as the duration of time from study entry to time of objective disease progression, or time of death from any cause, whichever came first. For patients who were taken off of protocol treatment for any reason other than progression, the date of PFS was censored at the date of last staging study (either on or off protocol therapy) on which the patient was documented not to have progressed, or the date of initiation of alternative anticancer therapy, whichever came first. Clinical benefit rate was included as an exploratory analysis. Clinical benefit included confirmed CR, PR, and stable disease (SD) of 15 weeks or longer. If patients had unconfirmed PR followed by SD, they were considered to receive clinical benefit.
Descriptive statistics were used to summarize biomarker values at protocol‐specific time points. The Wilcoxon ranked sum test evaluated the difference of baseline biomarker values between patients who did or did not experience clinical benefit. The Wilcoxon signed rank test assessed biomarker change from day 1 to 8. Mixed effects linear models assessed the change in biomarker values at days 1, 8, 22, 43, and 64; values beyond day 64 were not analyzed because of the small number of patients still on protocol. In the mixed effects linear model, the fixed effects were times of assessment, and patients were entered as a random effect. Logarithmic transformation was used to achieve normality, when applicable. Baseline biomarkers were stratified by using the median values for the entire cohort. The log‐rank test compared PFS among patients with low or high baseline sMET. All tests were conducted with two‐sided α = 0.05. The Benjamini‐Hochberg procedure was used to adjust p values to control the false discovery rate from evaluating multiple circulating biomarkers 23.
Results
Patient Characteristics
Thirty‐five mTNBC patients were enrolled between January 2013 and June 2014—after signing an informed consent form—and were included in the analyses. Median age was 50 years (range 31–78); patients had received 0 (n = 6; 17%), 1 (n = 18; 51%), 2 (n = 4; 11%), or 3 (n = 7; 20%) lines of chemotherapy for mTNBC (Table 1). The median number of metastatic sites was 3 (range 1–6). The most common sites of metastatic disease were regional lymph nodes (n = 26; 74%), lung (n = 18; 51%), breast or chest wall (n = 16; 46%), bone (n = 13; 37%), and liver (n = 12; 34%).
Table 1.
Baseline patient and tumor characteristics
| Characteristics | Value |
|---|---|
| Median age, yr (range) | 50 (31–78) |
| Female sex, n (%) | 35 (100) |
| Race, n (%) | |
| White | 32 (91) |
| African American | 3 (9) |
| Triple negative primary tumor, n (%) | 25 (71) |
| Triple negative metastatic tumor, n (%) | 33 (94) |
| Prior lines of chemotherapy for resectable disease preceding metastasesa, n (%) | |
| 0 | 19 (54) |
| 1 | 6 (17) |
| 2 | 1 (3) |
| Not applicable | 9 (26) |
| Prior lines of chemotherapy for metastatic or unresectable diseaseb, n (%) | |
| 0 | 6 (17) |
| 1 | 18 (51) |
| 2 | 4 (11) |
| 3 | 7 (20) |
| ECOG performance status | |
| 0 | 26 (74) |
| 1 | 8 (23) |
| 2 | 1 (3) |
| Median metastatic sites (range) | 3 (1–6) |
| Sites of metastatic disease, n (%) | |
| Lung | 18 (51) |
| Pleural effusion | 2 (6) |
| Liver | 12 (34) |
| Bone | 13 (37) |
| Breast or chest wall | 16 (46) |
| Lymph nodes | 26 (74) |
| Others | 15 (43) |
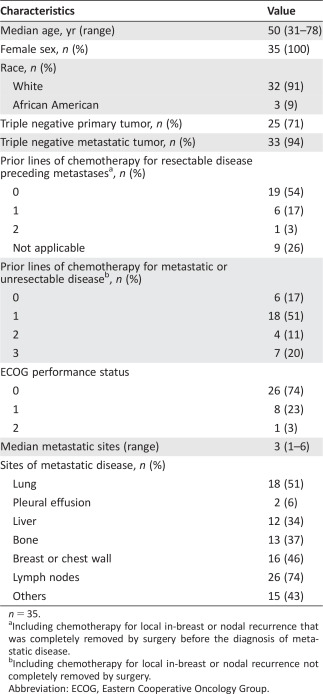
n = 35.
Including chemotherapy for local in‐breast or nodal recurrence that was completely removed by surgery before the diagnosis of metastatic disease.
Including chemotherapy for local in‐breast or nodal recurrence not completely removed by surgery.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Efficacy
Patients received a median of 3 cycles (9 weeks) of therapy (range 1–17). One patient achieved a PR within the first 13 patients, so the study was continued to the second stage. A total of 3 patients achieved PR (ORR, 9% [95% CI: 2, 26]; Table 2 and Fig. 1A). Thus, the study did not reach the level of clinical activity to define success under the Simon 2‐stage design. Of these patients, one received 17 cycles of protocol therapy and was on treatment for 11.7 months, and another received 8 cycles of protocol therapy and was on treatment for 6.5 months. Twenty of 35 patients (57%) had SD as their best response, and 9 of 35 (26%) patients had SD for >15 weeks. The clinical benefit rate at 15 weeks was 34% [95% CI: 19%, 52%], and the median PFS was 2.0 months [1.3, 3.3] (Fig. 1B).
Table 2.
Best overall response by RECIST 1.1
| Best overall response | n (%) |
|---|---|
| PR | 3 (9) |
| SD | 20 (57) |
| ≥15 weeks | 9 (26) |
| <15 weeks | 11 (31) |
| PDa | 11 (31) |
| Not evaluated due to toxicity | 1 (3) |
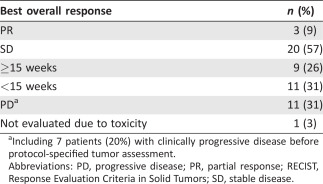
Including 7 patients (20%) with clinically progressive disease before protocol‐specified tumor assessment.
Abbreviations: PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 1.
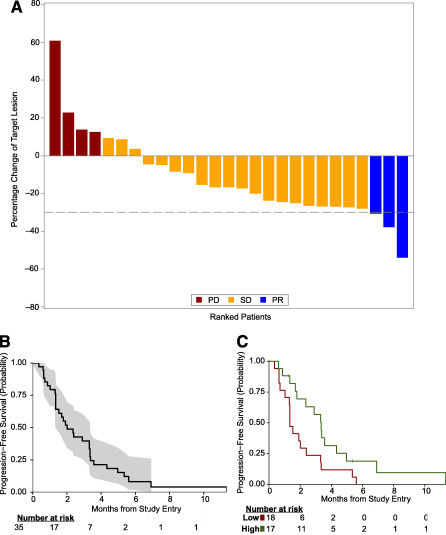
Efficacy data and biomarker associations for cabozantinib in metastatic triple‐negative breast cancer (mTNBC) patients. (A): Waterfall plot of objective responses by Response Evaluation Criteria in Solid Tumors 1.1. (B, C): Kaplan‐Meier survival distributions in mTNBC patients: progression‐free survival (PFS) (B) and PFS stratified by baseline circulating soluble MET level (C). *, p < .05.
Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease.
Twenty‐one of 24 patients who reported pain upon entering the study completed at least one pain survey at week 1 or 4. Eleven (52%) of them reported a decrease in pain since baseline, and 10 of these had discontinued using pain medications.
Toxicity
The most common toxicities (all grades that were possibly related to protocol therapy) were fatigue (77%), diarrhea (40%), oral mucositis (37%), and palmar‐plantar erythrody sesthesia (PPE; 37%; Table 3). There were 15 grade 3 adverse events, including elevated aspartate aminotransferase (n = 2), elevated lipase (n = 3), or hypertension (n = 2). There were no grade 4 toxicities. Twelve patients (34%) required dose reduction, 4 due to PPE and 8 due to other toxicities. All but one patient omitted at least one dose while on protocol therapy, 26 due to toxicity and 8 due to other reasons (supplemental online Table 1). Overall, 32 patients (91%) went off treatment due to progressive disease and 3 (9%) due to toxicity.
Table 3.
Summary of adverse events with at least 20% incidence (all grades) or any grade 3 or 4 event that was deemed related (definite, probable, or possible) to protocol therapy
| Maximum grade | ||||
|---|---|---|---|---|
| Adverse event | Total (% of 35) | Mild | Moderate | Severe |
| Fatigue | 27 (77) | 18 | 9 | 0 |
| Diarrhea | 14 (40) | 8 | 6 | 0 |
| Oral mucositis | 13 (37) | 11 | 2 | 0 |
| PPE | 13 (37) | 3 | 9 | 1 |
| Anorexia | 12 (34) | 10 | 2 | 0 |
| Elevated aspartate aminotransferase | 12 (34) | 7 | 3 | 2 |
| Hypertension | 12 (34) | 6 | 4 | 2 |
| Nausea | 10 (29) | 10 | 0 | 0 |
| Elevated alanine aminotransferase | 7 (20) | 6 | 0 | 1 |
| Dysgeusia | 7 (20) | 5 | 2 | 0 |
| Elevated lipase | 3 (9) | 0 | 0 | 3 |
| Prolonged activated partial thromboplastin time | 1 (3) | 0 | 0 | 1 |
| Bone pain | 1 (3) | 0 | 0 | 1 |
| Hypophosphatemia | 1 (3) | 0 | 0 | 1 |
| Infection | 1 (3) | 0 | 0 | 1 |
| Thromboembolic event | 1 (3) | 0 | 0 | 1 |
| Wound dehiscence | 1 (3) | 0 | 0 | 1 |
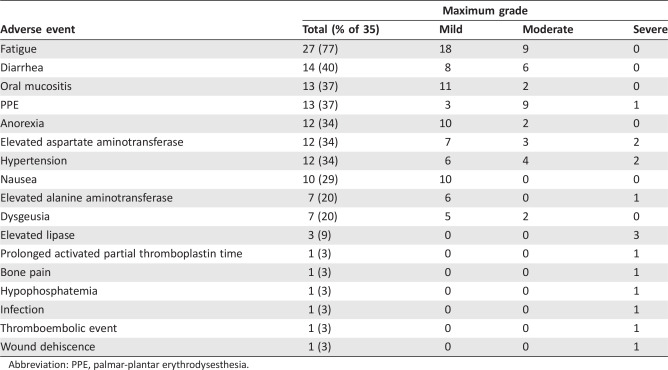
Abbreviation: PPE, palmar‐plantar erythrodysesthesia.
MET Amplification and Expression
Archival tissue analysis showed MET amplification in 2 of 35 patients (MET/CEP7 2.14 and 2.16), and relative MET amplification (MET/CEP7 1.7) in 1 patient. These 3 patients were also the only ones to show relative MET gain in CTCs.
Plasma Biomarkers
Cabozantinib treatment was associated with an increase in plasma PlGF, VEGF, and VEGF‐D from baseline to day 22, which was maintained at day 64 (p < .001). Plasma CAIX also increased and sVEGFR2 decreased at days 43 and 64 (p < .001). Plasma HGF initially decreased at day 8, and then increased at day 64 (p = .02), whereas plasma SDF1α transiently increased at day 22 (p = .002) (Table 4). Plasma sVEGFRl, sMET, sTIE‐2, or bFGF did not significantly change over time (Table 4). The kinetics of VEGF‐C, GM‐CSF, IL‐lβ, IL‐2, IFN‐γ, IL‐6, IL‐8, IL‐10, TNF‐α, and IL‐12/p70 were not analyzed because of the large number of undetectable measurements.
Table 4.
Change in plasma biomarkers
| Day 1 | Day 8 | Day 22 | Day 43 | Day 64 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | p valuea |
| HGF | 35 | 1,319 (1,093–1,816) | 33 | 1,078 (964–1,485) | 29 | 1,132 (986–1,716) | 22 | 1,191 (1,051–1,324) | 17 | 1,260 (1,039–1,470) | .01 |
| sMET | 35 | 795 (678–1,054) | 33 | 890 (761–987) | 29 | 902 (738–1,005) | 22 | 903 (740–1,074) | 17 | 923 (822–1,104) | .45 |
| CAIX | 34 | 111 (58–205) | 31 | 132 (77–290) | 26 | 182 (116–281) | 21 | 215 (143–355) | 17 | 264 (153–413) | <.001 |
| SDF1α | 35 | 2,017 (1,742–2,258) | 33 | 2,232 (1,765–2,326) | 29 | 2,264 (2,056–2,443) | 22 | 2,130 (1,940–2,355) | 17 | 2,215 (2,022–2,373) | .002 |
| VEGFR2 | 35 | 8,872 (8,305–10,545) | 33 | 8,479 (7,271–9,729) | 29 | 6,726 (5,818–7,360) | 22 | 5,812 (4,951–6,781) | 17 | 5,966 (5,578–6,705) | <.001 |
| bFGF | 35 | 39 (19–56) | 33 | 41 (29–53) | 29 | 28 (17–46) | 22 | 33 (24–49) | 17 | 21 (15–34) | .15 |
| PlGF | 35 | 53 (44–69) | 33 | 89 (79–140) | 29 | 119 (94–184) | 22 | 124 (82–162) | 17 | 119 (105–150) | <.001 |
| sFLT‐1 | 35 | 124 (82–310) | 33 | 90 (63–180) | 29 | 90 (69–232) | 22 | 124 (69–245) | 17 | 87 (72–168) | .39 |
| TIE2 | 35 | 4,648 (3,932–5,627) | 33 | 5,038 (4,303–5,724) | 29 | 4,845 (4,368–5,638) | 22 | 4,636 (4,384–5,576) | 17 | 5,256 (4,546–5,472) | .06 |
| VEGF | 35 | 98 (71–143) | 32 | 188 (124–316) | 26 | 206 (167–410) | 21 | 206 (125–342) | 17 | 195 (177–221) | <.001 |
| VEGF‐D | 35 | 1,062 (748–1,257) | 33 | 1,419 (1,102–1,806) | 29 | 1,582 (1,365–2,018) | 22 | 1,437 (1,035–1,870) | 17 | 1,429(1,121–2,063) | <.001 |
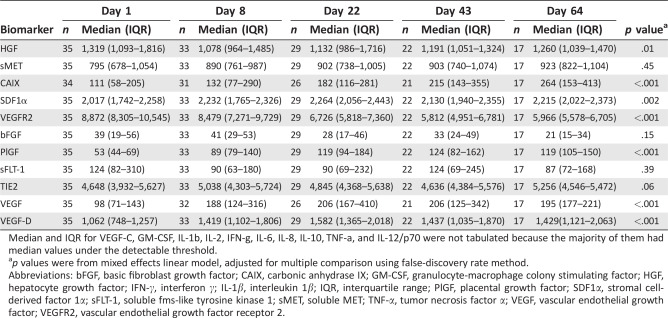
Median and IQR for VEGF‐C, GM‐CSF, IL‐1b, IL‐2, IFN‐g, IL‐6, IL‐8, IL‐10, TNF‐a, and IL‐12/p70 were not tabulated because the majority of them had median values under the detectable threshold.
p values were from mixed effects linear model, adjusted for multiple comparison using false‐discovery rate method.
Abbreviations: bFGF, basic fibroblast growth factor; CAIX, carbonic anhydrase IX; GM‐CSF, granulocyte‐macrophage colony stimulating factor; HGF, hepatocyte growth factor; IFN‐γ, interferon γ; IL‐1β, interleukin 1β; IQR, interquartile range; PlGF, placental growth factor; SDF1α, stromal cell‐derived factor 1α; sFLT‐1, soluble fms‐like tyrosine kinase 1; sMET, soluble MET; TNF‐α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
Of all biomarkers analyzed at baseline, only high baseline sMET (≥795 ng/mL median value) was associated with prolonged PFS (median PFS 3.3 months, lower 95% confidence limit 2.4), compared with low sMET ( <795 ng/mL, median PFS 1.3 [1.3, 3.3] months, p = .03) (Fig. 1C). There was a nonsignificant trend toward greater baseline sMET in patients with clinical benefit (1,008 pg/mL [interquartile range (IQR): 858, 1089] compared with those who did not (759 pg/mL [IQR: 663, 921]) (unadjusted p = .06). The changes in plasma VEGF‐C at day 22 correlated with clinical benefit (p = .03), but only samples from 19 of 35 patients were available at this time‐point.
Cell Biomarkers
After cabozantinib treatment, we detected a significant increase in the fraction of circulating CD3 + cells and CD3 + CD4‐CD8 + T lymphocytes at days 22 and 64 (p = .04 and p = .01, respectively), and a decrease in percentage of CD14+ monocytes at days 22 and 64 (p = .01) (Table 5). There was a nonsignificant trend toward increase in CD3+CD4+CD8− T (p = .08) and CD3‐CD56 + NK lymphocytes (p = .07), but changes in the fractions of CD133 + progenitor/stem cells, CD4+CD25 + regulatory T cells, CD4+CD127 + memory T cells, or CD3+CD56 + NKT cells (Table 5). None of the cell biomarkers associated with outcome measures.
Table 5.
Change in circulating cell subpopulations evaluated by flow cytometry
| Day 1 | Day 8 | Day 22 | Day 43 | Day 64 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker % of WBC | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | p valuea |
| CD34+CD133 + (progenitor/stem cells) | 34 | 0.17 (0.07–0.37) | 32 | 0.11 (0.05–‐0.27) | 28 | 0.12 (0.05–0.32) | 19 | 0.18 (0.08–0.31) | 16 | 0.11 (0.06–0.29) | .83 |
| CD14+ (monocytes) | 33 | 42.20 (34.67–45.98) | 32 | 31.44 (23.83–41.12) | 28 | 19.03 (15.25–27.46) | 20 | 24.79 (16.37–31.30) | 15 | 23.48 (17.40–32.42) | .01 |
| CD117 (KIT)+ | 33 | 0.47 (0.30–0.74) | 32 | 0.45 (0.27–0.85) | 28 | 0.49 (0.28–1.09) | 20 | 0.47 (0.35–0.62) | 15 | 0.53 (0.29–0.84) | .72 |
| CD3+ (lymphocytes) | 34 | 24.73 (13.45–29.95) | 31 | 24.96 (17.72–32.54) | 28 | 33.56 (23.46–45.58) | 21 | 31.79 (24.86–42.88) | 16 | 30.99 (19.49–43.93) | .04 |
| CD3+CD4−CD8 + (CTLs) | 34 | 7.63 (4.38–11.41) | 31 | 8.36 (5.79–13.05) | 28 | 11.99 (7.71–16.88) | 21 | 11.44 (6.76–16.82) | 16 | 11.32 (7.28–14.33) | .01 |
| CD3+CD8−CD4+ | 34 | 0.15 (0.09–0.19) | 31 | 0.17 (0.09–0.19) | 28 | 0.18 (0.14–0.26) | 21 | 0.19 (0.12–0.26) | 16 | 0.19 (0.12–0.30) | .08 |
| CD3+CD8−CD4+CD25+ | 34 | 0.66 (0.26–1.53) | 31 | 0.79 (0.22–1.34) | 28 | 0.59 (0.35–2.24) | 21 | 0.73 (0.15–1.06) | 16 | 0.61 (0.36–1.61) | .98 |
| CD3+CD8−CD4+CD25+CD127− (Tregs) | 34 | 0.62 (0.26–1.30) | 31 | 0.68 (0.20–1.01) | 28 | 0.55 (0.32–1.95) | 21 | 0.71 (0.15–1.03) | 16 | 0.61 (0.35–1.49) | .83 |
| CD3+CD8−CD4+CD25− | 34 | 11.41 (7.57–14.62) | 31 | 14.18 (7.06–17.74) | 28 | 16.41 (12.41–23.24) | 21 | 17.71 (11.45–23.02) | 16 | 15.29 (9.67–25.14) | .06 |
| CD4+CD25−CD127+ (memory T cells) | 34 | 0.32 (0.05–0.98) | 31 | 0.46 (0.13–1.08) | 28 | 0.32 (0.08–0.87) | 21 | 0.45 (0.16–1.06) | 16 | 0.40 (0.10–1.29) | .72 |
| CD3−CD56+ (NK cells) | 34 | 5.84 (4.86–9.32) | 31 | 7.27 (4.58–9.44) | 28 | 8.51 (4.75–13.49) | 21 | 6.50 (5.71–9.80) | 16 | 8.13 (6.39–15.98) | .07 |
| CD3+CD56+ (NKT cells) | 34 | 0.77 (0.35–1.83) | 31 | 0.86 (0.49–3.06) | 28 | 0.94 (0.49–3.17) | 21 | 1.02 (0.52–2.13) | 16 | 0.98 (0.72–1.94) | .48 |
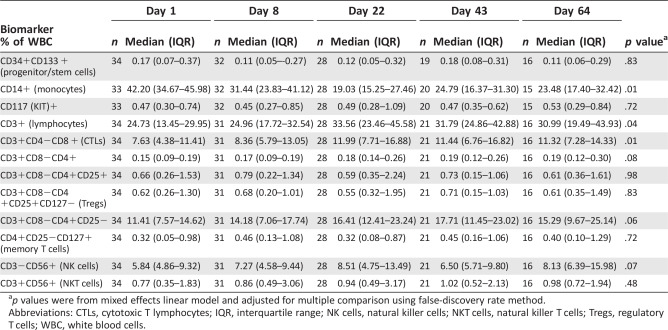
p values were from mixed effects linear model and adjusted for multiple comparison using false‐discovery rate method.
Abbreviations: CTLs, cytotoxic T lymphocytes; IQR, interquartile range; NK cells, natural killer cells; NKT cells, natural killer T cells; Tregs, regulatory T cells; WBC, white blood cells.
Discussion
Cabozantinib monotherapy did not meet the prespecified efficacy endpoint (ORR was 9%) but showed a clinical benefit rate of 34% at 15 weeks and a median PFS of 2.0 months in pretreated mTNBC patients. Treatment was well tolerated, and most common grade 3 toxicities were fatigue, diarrhea, oral mucositis, and PPE. Patients often reported decreases in pain, with some able to discontinue analgesics, consistent with previous results showing improvements in pain and reduction in narcotic use after cabozantinib 24.
MET remains an attractive target in TNBC, as shown in recent preclinical studies 25. Two patients enrolled in this study (6%) had tumors with MET amplification (consistent between archival tumor specimen and CTC evaluations), one of whom discontinued therapy due to toxicity. Thus, no potential correlation could be established between MET amplification and response. However, high baseline plasma concentrations of sMET were associated with longer PFS, indicating that cancers producing increased sMET may be more likely to respond to MET inhibition. Larger randomized studies should validate the association of sMET with outcomes (OS, PFS, or pain) and to establish whether sMET is a prognostic or predictive in TNBC. The concentration of plasma HGF, the MET ligand, was lower in patients with clinical benefit versus those without, but this association did not reach statistical significance. Further larger studies examining the association of MET amplification in the tumor and circulating HGF with response to MET inhibition in TNBC are warranted.
Cabozantinib treatment was associated with changes in biomarker concentrations that are consistent with antivascular effects and increases in tissue hypoxia—increases in plasma CAIX, PlGF, VEGF, VEGF‐D, and SDF1a Moreover, cabozantinib significantly decreased plasma concentrations of sVEGFR2, a potential “pharmacodynamic” biomarker for anti‐VEGFR2 TKIs 26. None of these systemic changes were associated with clinical outcomes. An increase in plasma VEGF‐C associated with lack of clinical benefit and is worthy of further investigation 26, 27.
Flow‐cytometric analyses showed a persistent increase in the fraction of circulating CD3 + T cells after cabozantinib therapy, largely driven by the increased CD4‐/CD8+ cytotoxic T lymphocyte (CTL) population. Moreover, there was a persistent decrease in the CD14+ monocytes, a mixed population that encompasses immunosuppressive and proangiogenic myeloid cells. These findings may reflect an activation of systemic antitumor immunity after treatment with cabozantinib, as observed in preclinical models 28, 29, but did not associate with outcome. These findings are provocative given recent interest in combining cabozantinib with immune checkpoint inhibitors 30 (NCT02496208).
The mechanism of action and of clinical benefit of VEGFR and MET inhibitors, when used alone or in combination, remains unclear. Several VEGF and MET inhibitors have been previously shown to be ineffective in metastatic breast cancer 31, 32. The mechanism of benefit to VEGF blockade may be related to vascular normalization rather than antivascular effects and inducing hypoxia in the tumors 33. HGF and MET are hypoxia‐inducible proteins, and increased MET expression after VEGFR2 inhibition has been associated with evasive treatment resistance 34, 35. Unfortunately, antibody blockade of both VEGF (bevacizumab) and MET (onartuzumab) with paclitaxel demonstrated no clinical benefit in patients with mTNBC who had not previously received paclitaxel for metastatic disease 36. Our circulating biomarker data indicate that cabozantinib might have potent antivascular effects in mTNBC. To overcome these limitations, our hypothesisgenerating results indicate that (a) sMET should be further studied as a potential biomarker of response and (b) the systemic changes in antitumor immunity may be leveraged by rational combinations with immunotherapies.
Our study has several limitations, related to the single‐arm design and small number of patients. Clinically, the median PFS was modest, largely driven by the early PD in the patients without benefit. Future studies (such as NCT01441947 and NCT02260531) are warranted and should validate our biomarker data and characterize the tumors in the patients who benefit from therapy.
Conclusion
Our phase II study of cabozantinib showed an ORR of 9%, preliminary activity and favorable safety in mTNBC patients. Exploratory analyses showed that circulating sMET levels may be potentially a response biomarker for cabozantinib and that this agent may have an intriguing immunomodulatory activity. These hypotheses should be tested in larger studies in mTNBC and other malignancies.
Author Contributions
Conception/Design: Sara M. Tolaney, Eric P. Winer, Dan G. Duda
Provision of study material or patients: Sara M. Tolaney, Michaela J. Higgins, Steven J. Isakoff, Jane E. Brock, Eric P. Winer, Dan G. Duda
Collection and/or assembly of data: Sara M. Tolaney, David R. Ziehr, Hao Guo, Mei R. Ng, Michaela J. Higgins, Steven J. Isakoff, Jane E. Brock, Elena V. Ivanova, Cloud P. Paweletz, Michelle K. Demeo, Nikhil H. Ramaiya, Beth A. Overmoyer, Dan G. Duda
Data analysis and interpretation: Sara M. Tolaney, David R. Ziehr, Hao Guo, Mei R. Ng, William T. Barry, Elena V. Ivanova, Cloud P. Paweletz, Michelle K. Demeo, Nikhil H. Ramaiya, Beth A. Overmoyer, Rakesh K. Jain, Eric P. Winer, Dan G. Duda
Manuscript writing: Sara M. Tolaney, David R. Ziehr, Hao Guo, Eric P. Winer, Dan G. Duda
Final approval of manuscript: Sara M. Tolaney, David R. Ziehr, Hao Guo, Mei R. Ng, William T. Barry, Michaela J. Higgins, Steven J. Isakoff, Jane E. Brock, Elena V. Ivanova, Cloud P. Paweletz, Michelle K. Demeo, Nikhil H. Ramaiya, Beth A. Overmoyer, Rakesh K. Jain, Eric P. Winer, Dan G. Duda
Disclosures
Sara M. Tolaney: Exelixis, Genentech, Pfizer, Novartis, Eli Lilly, Merck (RF); William T. Barry: Pfizer (RF); Beth A. Overmoyer: Incyte, Genentech, Eisai (RF); Rakesh K. Jain: Ophthotech, SynDevRx, XTuit (C/A, OI), SPARC (C/A), Medimmune, Roche (RF), Enlight (OI), XTuit,Tekla Healthcare Investors, Tekla Life Science Investors, Tekla Healthcare Opportunities Fund (Other); Dan G. Duda: Merrimack, Bayer, Leap Therapeutics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/ inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supporting Information
Acknowledgments
We thank Anna Khachatryan and Cristina Koppel for technical assistance. The clinical trial was supported by funding from Exelixis, Rosa's Pursuit of Hope (Sara M. Tolaney); the TripAdvisor Charitable Foundation (Sara M. Tolaney); Department of Defense Breast Cancer Research Program Innovator Award W81XWH‐10‐ 1‐0016 (to Rakesh K. Jain); and National Cancer Institute (NCI) Grant R01CA159258 (to Dan G. Duda) and NCI Proton Beam/ Federal Share Program grants (to Rakesh K. Jain and Dan G. Duda). Mei R. Ng was supported by a U.S. Department of Defense Breast Cancer Research Program Postdoctoral Fellowship (W81XWH‐14‐1‐0034). Clinical trial information: NCT01738438.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Sara M. Tolaney, Email: stolaney@partners.org.
Dan G. Duda, Email: gduda@partners.org.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien KM, Cole SR, Tse CK et al. Intrinsic breast tumor subtypes, race, and long‐term survival in the Carolina Breast Cancer Study. Clin Cancer Res 2010; 16:6100–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iqbal J, Ginsburg O, Rochon PA et al. Differences in breast cancer stage at diagnosis and cancer‐specific survival by race and ethnicity in the United States. JAMA 2015;313:165–173. [DOI] [PubMed] [Google Scholar]
- 4. Dent R, Trudeau M, Pritchard KI et al. Triple‐negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 2007;13: 4429–4434. [DOI] [PubMed] [Google Scholar]
- 5. Millikan RC, Newman B, Tse CK et al. Epidemiology of basal‐like breast cancer [published correction appears in Breast Cancer Res Treat 2008;109:141]. Breast Cancer Res Treat 2008;109:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gherardi E, Birchmeier W, Birchmeier C et al. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 2012;12:89–103. [DOI] [PubMed] [Google Scholar]
- 7. Beviglia L, Matsumoto K, Lin CS et al. Expression of the c‐Met/HGF receptor in human breast carcinoma: Correlation with tumor progression. Int J Cancer 1997; 74:301–309. [DOI] [PubMed] [Google Scholar]
- 8. Jin L, Fuchs A, Schnitt SJ et al. Expression of scatter factor and c‐met receptor in benign and malignant breast tissue. Cancer 1997;79:749–760. [DOI] [PubMed] [Google Scholar]
- 9. Lengyel E, Prechtel D, Resau JH et al. C‐Met overexpression in node‐positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer 2005;113:678–682. [DOI] [PubMed] [Google Scholar]
- 10. Raghav KP, Wang W, Liu S et al. cMET and phospho‐cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res 2012;18: 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuck AB, Park M, Sterns EE et al. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol 1996;148: 225–232. [PMC free article] [PubMed] [Google Scholar]
- 12. Yamashita J, Ogawa M, Yamashita S et al. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res 1994;54:1630–1633. [PubMed] [Google Scholar]
- 13. Ho‐Yen CM, Green AR, Rakha EA et al. C‐Met in invasive breast cancer: Is there a relationship with the basal‐like subtype? Cancer 2014;120:163–171. [DOI] [PubMed] [Google Scholar]
- 14. Zagouri F, Bago‐Horvath Z, Rössler F et al. High MET expression is an adverse prognostic factor in patients with triple‐negative breast cancer. Br J Cancer 2013;108:1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez‐Angulo AM, Chen H, Karuturi MS et al. Frequency of mesenchymal‐epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide‐3‐kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancer. Cancer 2013;119:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graveel CR, DeGroot JD, Su Y et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc Natl Acad Sci USA 2009;106:12909–12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponzo MG, Lesurf R, Petkiewicz S et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc Natl Acad Sci USA 2009; 106:12903–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yakes FM, Chen J, Tan J et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–2308. [DOI] [PubMed] [Google Scholar]
- 19. Choueiri TK, Escudier B, Powles T et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elisei R, Schlumberger MJ, Müller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith DC, Smith MR, Sweeney C et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J Clin Oncol 2013;31:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkinson EN, Brown BW. Confidence limits for probability of response in multistage phase II clinical trials. Biometrics 1985;41:741–744. [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300. [Google Scholar]
- 24. Basch E, Autio KA, Smith MR et al. Effects of cabozantinib on pain and narcotic use in patients with castration‐resistant prostate cancer: Results from a phase 2 nonrandomized expansion cohort. Eur Urol 2015;67:310–318. [DOI] [PubMed] [Google Scholar]
- 25. Sameni M, Tovar EA, Essenburg CJ et al. Cabozantinib (XL184) inhibits growth and invasion of preclinical TNBC models. Clin Cancer Res 2016;22: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jain RK, Duda DG, Willett CG et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol 2009;6:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leibowitz‐Amit R, Pintilie M, Khoja L et al. Changes in plasma biomarkers following treatment with cabozantinib in metastatic castration‐resistant prostate cancer: A post hoc analysis of an extension cohort of a phase II trial. J Transl Med 2016;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwilas AR, Ardiani A, Donahue RN et al. Dual effects of a targeted small‐molecule inhibitor (cabozantinib) on immune‐mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med 2014;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwilas AR, Donahue RN, Tsang KY et al. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron 2015;2:e677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Escudier B. Subgroup analyses of meteor, a randomized phase 3 trial of cabozantinib versus everolimus in patients (pts) with advanced renal cell carcinoma (RCC). J Clin Oncol 2016;34(suppl 2):499a. [Google Scholar]
- 31. Tolaney S, Guo H, Barry W et al. A phase II study of tivantinib (ARQ‐197) for metastatic triple‐negative breast cancer. J Clin Oncol 2014;32(suppl 5):1106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miles DW, Diéras V, Cortés J et al. First‐line bevacizumab in combination with chemotherapy for HER2‐negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann Oncol 2013;24:2773–2780. [DOI] [PubMed] [Google Scholar]
- 33. Tolaney SM, Boucher Y, Duda DG et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci USA 2015; 112:14325–14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu KV, Chang JP, Parachoniak CA et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012;22:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 2012; 12:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diéras V, Campone M, Yardley DA et al. Randomized, phase II, placebo‐controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple‐negative breast cancer. Ann Oncol 2015;26: 1904–1910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supporting Information


