The present study assessed the impact of >25 lymph node retrieval on the survival outcome of patients with advanced gastric cancer after curative‐intent gastrectomy. The clinicopathological parameters and overall survival were analyzed according to the number of lymph nodes examined. Retrieving more than 25 lymph nodes during curative‐intent gastrectomy substantially improved survival and survival stratification of advanced gastric cancer without compromising patient safety.
Keywords: Lymph node retrieval, Gastric cancer, Gastrectomy, Long‐term survival
Abstract
Background.
The present study assessed the impact of the retrieval of >25 lymph nodes (LNs) on the survival outcome of patients with advanced gastric cancer after curative‐intent gastrectomy.
Patients and Methods.
A total of 5,386 patients who had undergone curative gastrectomy for gastric cancer from 1994 to 2011 were enrolled. The clinicopathological parameters and overall survival (OS) were analyzed according to the number of LNs examined (≤15, n = 916; 16–25, n = 1,458; and >25, n = 3,012).
Results.
The percentage of patients with >25 LNs retrieved increased from 1994 to 2011. Patients in the LN >25 group were more likely to have undergone total gastrectomy and to have a larger tumor size, poorer tumor differentiation, and advanced T and N stages. Hospital mortality among the LN ≤15, LN 16–25, and LN >25 groups was 6.1%, 2.7%, and 1.7%, respectively (p < .0001). The LN >25 group consistently exhibited the most favorable OS, in particular, with stage II disease (p = .011) when OS was stratified according to tumor stage. Similarly, the LN >25 group had significantly better OS in all nodal stages (from N1 to N3b). The discrimination power of the lymph node ratio (LNR) for the LN ≤15, LN 16–25, and LN >25 groups was 483, 766, and 1,560, respectively. Multivariate analysis demonstrated that the LNR was the most important prognostic factor in the LN >25 group.
Conclusion.
Retrieving more than 25 lymph nodes during curative‐intent gastrectomy substantially improved survival and survival stratification of advanced gastric cancer without compromising patient safety.
Implications for Practice.
D2 lymph node (LN) dissection is currently the standard of surgical management of gastric cancer, which is rarely audited by a third party. The present study, one of the largest surgical series worldwide, has shown that the traditionally recognized retrieval of ≥16 LNs during curative‐intent gastrectomy might not be adequate in regions in which locally advanced gastric cancers predominate. The presented data show that retrieval of >25 LNs, which more greatly mimics D2 dissection, improves long‐term outcomes and survival stratification without compromising patient safety.
Introduction
Gastric cancer is the fourth most commonly diagnosed cancer and the second leading cause of cancer‐related deaths worldwide, despite improvement in the techniques of radical resection and multidisciplinary management [1], [2]. The debate on the extent of lymphadenectomy that began in the 1980s has resulted in at least five randomized clinical trials with mixed outcomes [3], [4], [5], [6], [7]. However, a trend toward improved survival was shown among patients undergoing D2 lymphadenectomy without splenectomy or pancreatectomy [8]. D2 gastrectomy in the management of gastric cancer is the current reference standard in Eastern Asian countries and some parts of Europe. However, adherence to D2 dissection has rarely been audited by a third party.
In 2010, both the 7th edition of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) TNM system [9] and the 14th Japanese gastric cancer classification [10] unanimously proposed that the absolute number of positive lymph nodes, rather than the anatomical locations, represents nodal staging of gastric cancer. Both the AJCC classification [9] and National Comprehensive Cancer Network (NCCN) guidelines [11] recommend examination of 16 or more regional lymph nodes (LN ≥16) to determine the N status. However, several investigators have suggested that the number of lymph nodes assessed should vary according to the tumor stage and should be fewer than 15 for early gastric cancer and greater than 15 for advanced gastric cancer [12], [13], [14]. Furthermore, studies have demonstrated a strong association between the number of lymph nodes analyzed and improved survival [15], [16]. As a result, many investigators have proposed their ideal cutoff values for the number of lymph nodes dissected to accurately stage the disease and achieve local control [17]. Smith et al. [16], Schwarz and Smith [18], and Chen et al. [19] argued that the goal of LN ≥16 dissection appeared insufficient in the context of locally advanced gastric cancer and recommended that the minimum goal should be set at 25 (LN >25). The aim of the present study was to assess the impact of LN >25 retrieval on the survival outcome and survival stratification for patients with gastric cancer undergoing curative‐intent gastrectomy.
Patients and Methods
The present retrospective study was based on an analysis of 5,386 patients with gastric cancer who had undergone curative gastrectomy from 1994 to 2011 at Chang Gung Memorial Hospital in Linkou, and Veteran General Hospital in Taipei. The clinicopathological data on patient demographics, tumor location, types of operation, pathological findings, and tumor stage were obtained from the prospectively collected electronic medical records designed for registration of gastric cancer [6], [20]. The type of resection was mainly determined by the anatomic location of the tumor. The standard procedure for a distal third tumor was radical subtotal gastrectomy with reconstruction by Billroth I, Billroth II, or Roux‐en‐Y gastrojejunostomy according to surgeon preference. For a middle or upper third tumor, radical total gastrectomy with Roux‐en‐Y esophagojejunostomy was performed. For selected early‐stage upper third tumors, radical proximal gastrectomy was performed with esophagogastrostomy reconstruction. The extent of lymphadenectomy was performed according to the preoperative imaging findings, intraoperative assessment of macroscopic lymph node involvement, surgeon preference, and the patient's general condition. The surgical specimens and lymph nodes were examined by pathologists specializing in gastric cancer using the updated edition of the UICC/AJCC TNM staging system, which was then converted to the seventh edition at the time of our analysis [9]. For the purposes of the present study, the patients were divided into three groups according to the number of lymph nodes retrieved: patients with ≤ 15 harvested lymph nodes (LN ≤15), patients with 16–25 harvested lymph nodes (LN 16–25), and patients with >25 harvested lymph nodes (LN >25). The lymph node ratio (LNR) was defined as the ratio between metastatic and examined lymph nodes. Clinical outcome was evaluated from the date of surgery to the date of the last follow‐up visit or December 31, 2012. Perioperative deaths were excluded from the survival analysis.
Statistical Analysis
Numerical data are expressed as the mean ± SD. Clinical records were compared using Fisher's exact test, Pearson's chi‐square test, or the analysis of variance test, as appropriate. Linear regression analysis was used to estimate the relationship between the proportion of N3 disease and the number of lymph nodes retrieved. The Kaplan‐Meier method was used to assess survival outcomes, and differences were analyzed using the log‐rank test. The optimal cutoff values were chosen for the LNR according to the survival tree using R software, version 3.1.3 (March 9, 2015; R Core Team, available at http://www.r-project.org). The potentially relevant factors obtained from the univariate analysis were assessed in the multivariate model using Cox's regression. A p value of <.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows, version 13 (SPSS, Inc., Chicago, IL, http://www.ibm.com).
Results
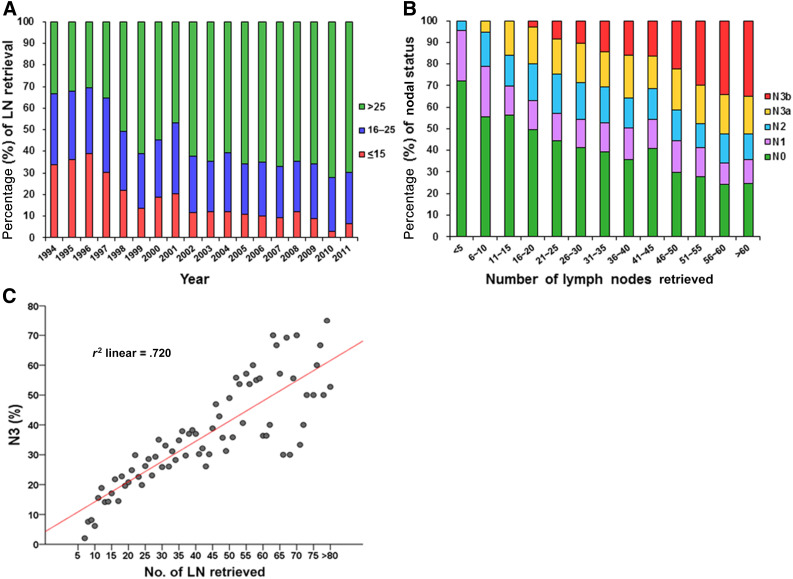
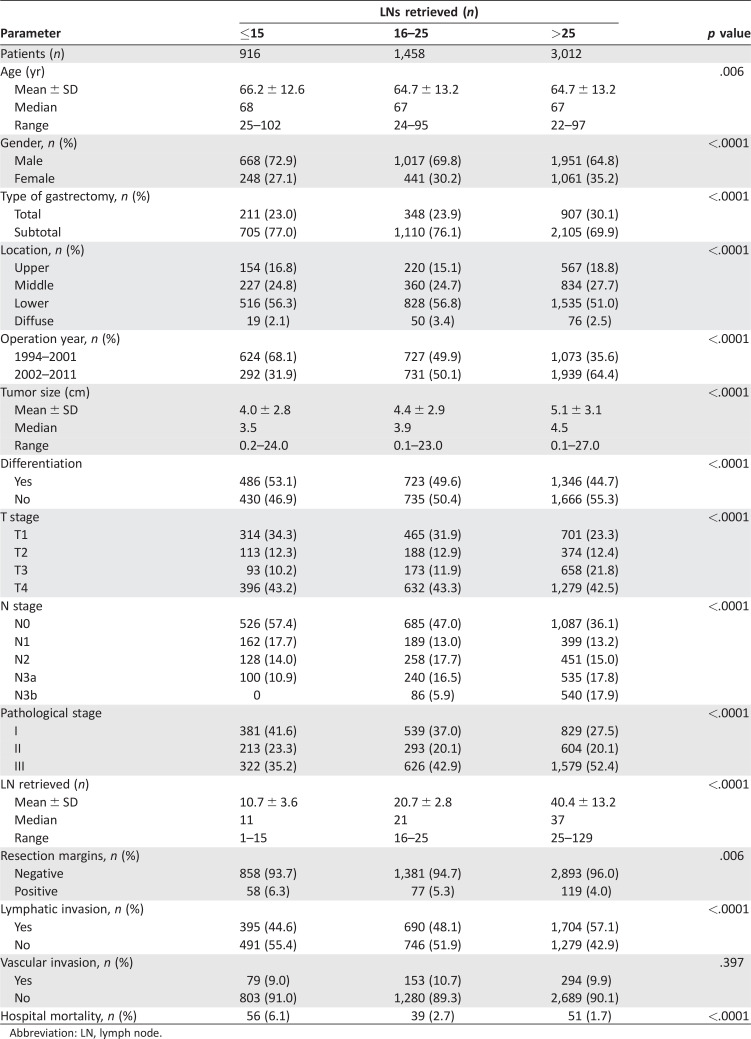
The proportion of LN ≤15, LN 16–25 and LN >25 by year is shown in Figure 1A. A trend toward a greater percentage of LN >25 was seen over the years. The AJCC nodal stage correlated with the number of lymph nodes retrieved (Fig. 1B). Specifically, N3 disease was strongly associated with the number of retrieved lymph nodes (r2 = .72; Fig. 1C). The patient demographics and clinicopathological data of the three groups are listed in Table 1. The percentage of LN >25 was 65.4% from 2002 to 2011 compared with 43.4% from 1994 to 2001 (p < .0001). The LN >25 group was associated with a higher proportion of total gastrectomy, larger tumor size, poorer tumor differentiation, and more advanced T and N stages compared with the LN ≤15 and LN 16–25 groups. Hospital mortality was significantly higher in the LN ≤15 and LN 16–25 groups than in the LN >25 group (6.1%, 2.7%, and 1.7%, respectively; p < .0001).
Figure 1.
The proportion of LN groups by year, AJCC nodal stage, and linear regression analysis is shown. (A): The proportion of LN ≤15, LN 16–25, and LN >25 per year from 1994 to 2011, highlighting an increase in the percentage of LN >25. (B): The correlation between nodal stage and the number of lymph nodes retrieved. (C): Linear regression analysis of percentage of nodal stage N3 versus number of lymph nodes retrieved in a cohort of 5,386 patients with gastric cancer.
Abbreviations: AJCC, American Joint Committee on Cancer; LN ≤15, ≤15 harvested lymph nodes; LN 16–25, 16–24 harvested lymph nodes; LN >25, ≥25 harvested lymph nodes.
Table 1. Clinicopathological data for three groups of resectable gastric cancer patients stratified by number of lymph nodes retrieved.
Abbreviation: LN, lymph node.
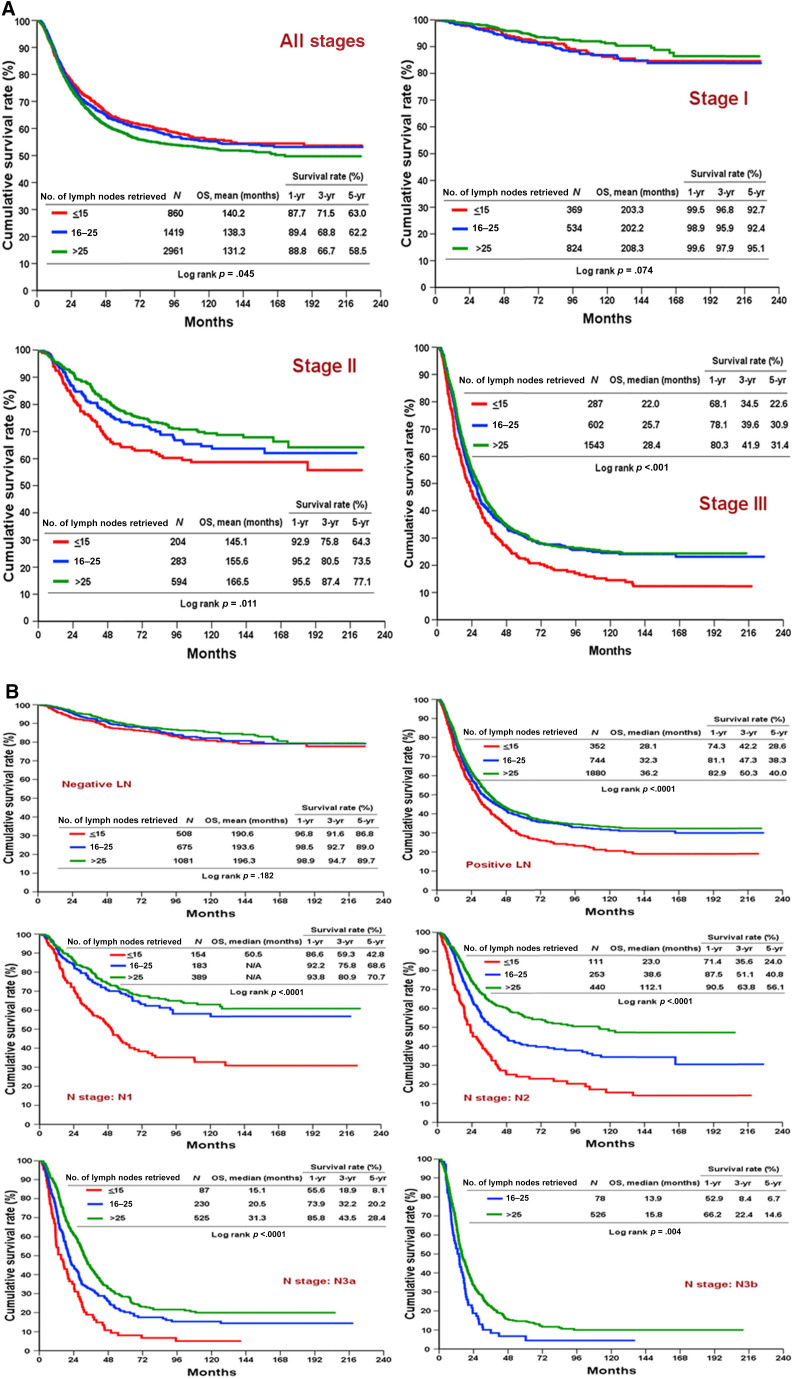
The mean overall survival (OS) of the LN >25 group was 131 months compared with 140 months and 138 months in the LN ≤15 and LN 16–25 groups, respectively. The apparent poorer survival outcome was a reflection of the higher proportion (52%) of patients with stage III disease in the LN >25 group compared with the LN ≤15 group (35%) and LN 16–25 group (42%). When OS was stratified according to the pathological stage, the LN >25 group consistently exhibited the best OS in stage I, II, and III disease, although the difference did not reach statistical significance between the LN 16–25 and LN >25 groups for stage II and III. The difference in OS between the LN ≤15 and LN 16n–25 groups in stage II disease also failed to reach statistical significance (Fig. 2A). With respect to nodal stage (from N1 to N3b), the LN >25 group had the most favorable OS, followed by the LN 16–25 and LN ≤15 groups (Fig. 2B).
Figure 2.
Comparisons of OS stratified by overall stage and nodal stage. (A): Comparisons of OS for LN ≤15, LN 16–25, and LN >25 groups stratified by all stages and stage I, II, and III. (B): Comparisons of OS for LN ≤15, LN 16–25, and LN >25 groups stratified by nodal stage.
Abbreviations: LN ≤15, ≤ 15 harvested lymph nodes; LN 16–25, 16–24 harvested lymph nodes; LN >25, ≥25 harvested lymph nodes; OS, overall survival.
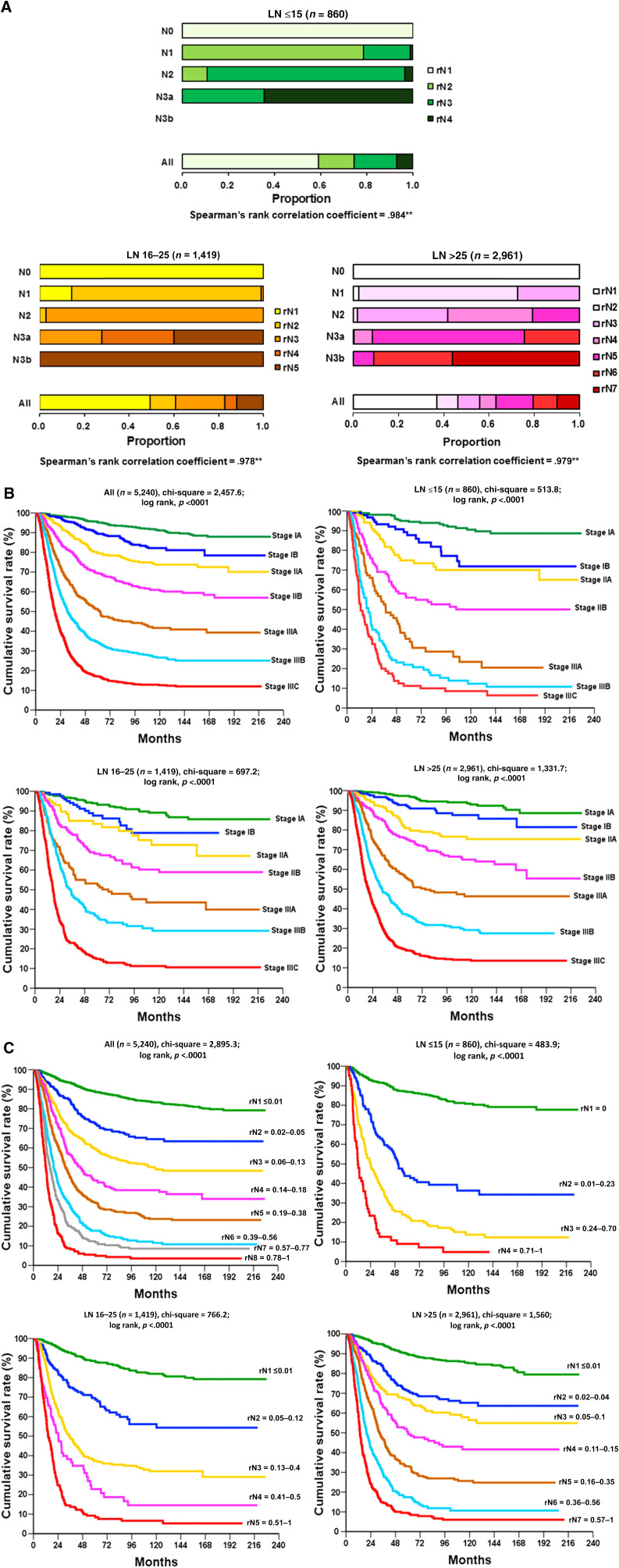
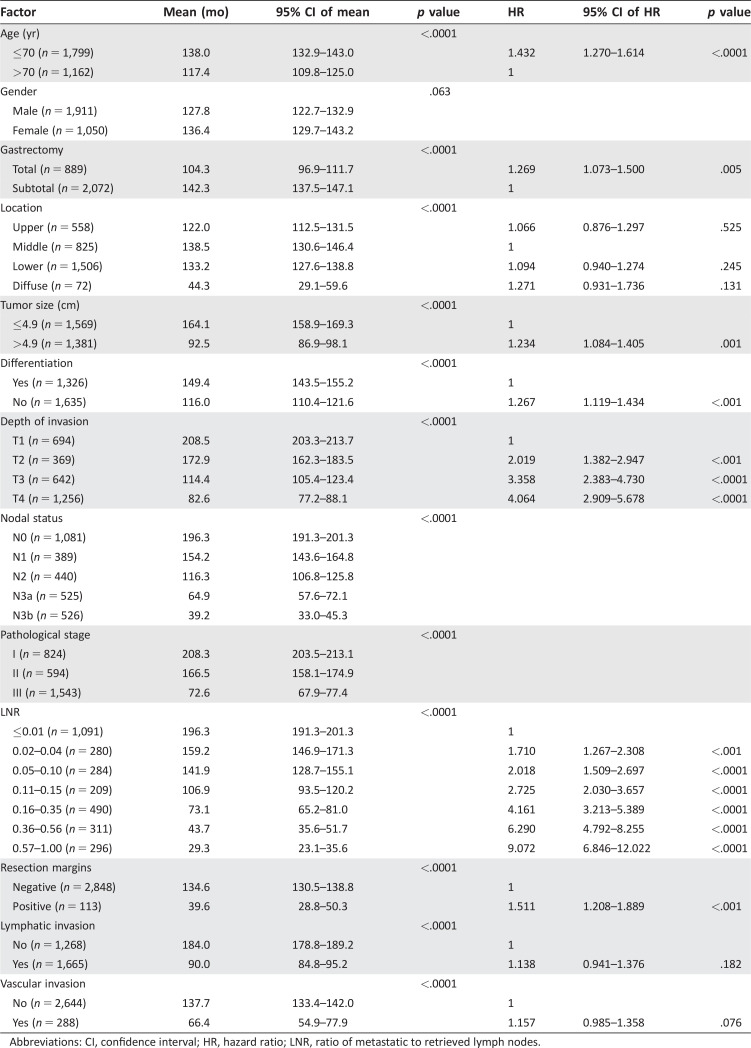
The optimal LNR cutoff values and tiers for each group (LN ≤15, LN 16–25, and LN >25) were determined using a survival tree (Fig. 3A). The LNR tiers were 4, 5, and 7 for LN ≤15, LN 16–25, and LN >25, respectively. When OS discrimination was determined using the seventh edition AJCC system, the discrimination power for LN ≤15, LN 16–25, and LN >25 was 513, 697, and 1,331, respectively (Fig. 3B). When OS discrimination was determined from the LNR alone, the discrimination power for LN ≤15, LN 16–25, and LN >25 was 483, 766, and 1,560, respectively (Fig. 3C). The LNR conferred better survival discrimination than the nodal staging of the AJCC system when lymph node retrieval was more than 25. In the multivariate analysis of prognostic factors in the LN >25 group, age, type of gastrectomy, tumor size, tumor differentiation, LNR, and resection margins were predictive of survival, but nodal stage and pathological stage failed to reach statistical significance (Table 2). The LNR was the most significant prognostic factor, with a hazard ratio of 9 in the most advanced tier.
Figure 3.
The optimal lymph node ratio (LNR) cutoff values and tiers for each group and overall survival discrimination. (A): Graphic comparisons of LNR staging versus seventh‐edition American Joint Committee on Cancer (AJCC) nodal staging. The optimal cutoff values and tiers for each group (LN ≤15, LN 16–25, LN >25) chosen for the LNR were determined using a survival tree (detailed in the Patients and Methods section). The “All” bar shows the proportion of each tier of LNR across its own categories. (B): Kaplan‐Meier overall survival curves stratified by AJCC staging system (seventh edition). LN >25 exhibited better survival stratification compared with that of LN ≤15 and LN 16–25. (C): Kaplan‐Meier overall survival curves stratified by AJCC nodal stage (left) and LNR staging system. The LNR tiers were 4,5, and 7 for LN ≤15, LN 16–25, and LN >25, respectively. The discrimination power for LN ≤15, LN 16–25, and LN >25 was 483, 766, and 1,560, respectively, when overall survival was stratified according to LNR. **, Correlation insignificant at the .01 level (two‐tailed).
Abbreviations: LN ≤15, ≤15 harvested lymph nodes; LN 16–25, 16–24 harvested lymph nodes; LN >25, ≥25 harvested lymph nodes.
Table 2. Univariate and multivariate analysis of prognostic factors in resectable gastric cancer patients with lymph node retrieval >25.
Abbreviations: CI, confidence interval; HR, hazard ratio; LNR, ratio of metastatic to retrieved lymph nodes.
Discussion
In the present study, we reported the data from a cohort of 5,386 patients with resected gastric cancer who had been treated at the two leading medical centers in Taiwan during an 18‐year period, representing one of the largest surgical series worldwide. A total of 83% of the cohort had a minimal number of 16 lymph nodes retrieved in compliance with the AJCC [9] and NCCN [11] guidelines. Wagner et al. [21] investigated the relationship between the number of regional lymph nodes and the level of lymphadenectomy in cadavers with gastric cancer. An average of 27 nodes was found with D2 dissection and an average of 43 nodes could be retrieved with D3 dissection. Chen et al. [19] reported that the mean number of lymph nodes retrieved by D1, D1+, and D2 lymphadenectomy was 17, 21, and 28, respectively. Using 25 lymph nodes as the cutoff value, survival benefits were observed in patients with N2 and N3 gastric cancer in the LN >25 group compared with those in the LN <25 group. Our data showed that the median number of lymph nodes retrieved in the LN ≤15, LN 16–25, and LN >25 groups was 11, 21, and 37, respectively. Lymph node retrieval of more than 25 should be the minimal requirement for D2 dissection and the benchmark for surgical radicality and pathological examination.
It is noteworthy that 83% of our cohort had a minimal number of 16 retrieved lymph nodes. This is comparable to that reported by Biondi et al. [15] and far superior to the 23% reported by Smith et al. [16] in 2005 and 42% reported by Morgan et al. [22] in 2015, both of which were based on the Surveillance, Epidemiology, and End Results database. Biondi et al. [15] demonstrated that retrieval of 16 or more lymph nodes was associated with better survival (hazard ratio, 0.59; p = .002). This survival benefit was also observed by Smith et al. [16]. Our data showed that LN >25 was paradoxically associated with the poorest OS (p = .045) compared with that of the LN ≤15 and LN 16–25 groups. This apparent contradiction was due to two distinctions between the series. First, the stage of disease at diagnosis was different between the series reported by Biondi et al. [15] and the present study. In the former, only 25% of cases were classified as locally advanced gastric cancer (stage III), whereas 47% of cases were categorized as stage III in our study. Second, the percentage of stage III disease among the LN <16 and LN ≥16 groups was reported by Biondi et al. [15] to be similar (23% and 24%, respectively). However, in our series, the percentage of stage III disease among the LN ≤15, LN 16–25, and LN >25 groups was 35%, 42%, and 52%, respectively (p < .0001). When the OS of the three groups was stratified according to tumor and nodal stage, LN >25 consistently exhibited the best OS. The survival advantage of LN >25 compared with LN 16–25 in patients with N2 and N3 disease is similar to that observed by Chen et al. [19]. Thus, we would recommend that, in regions where population screening for gastric cancer is not available and advanced gastric cancer predominates at the initial presentation, D2 gastrectomy with LN >25 is more appropriate to avoid the stage migration phenomenon and provide better locoregional disease control.
The lymph node ratio was originally developed to overcome inadequate lymph node retrieval and avoid incorrect tumor staging. Several studies have shown that the LNR is an independent prognostic factor that can potentially better stratify for patient survival than the AJCC nodal status [23], [24], [25], [26], [27]. In the U.S. Intergroup 0116 trial [28], 54% of patients underwent less than D1 lymphadenectomy, 36% underwent D1 lymphadenectomy, and only 10% underwent D2 lymphadenectomy. The number of lymph nodes retrieved and assessed relies on the attitude of the surgeon on radical‐intent dissection and the commitment of the pathologist [29]. Thus, the use of the LNR might obviate the confounding effect of these variables and provide a more reliable indication for adjuvant therapy. It would also permit direct comparisons among different institutions reporting on gastric cancer survival outcomes after gastrectomy. Our analysis showed 4, 5, and 7 tiers of LNR for LN ≤15, LN 16–25, and LN >25, respectively, that predicted the survival outcome. The discrimination power for LN ≤15, LN 16–25, and LN >25 was 483, 766, and 1,560, respectively, when OS was stratified by the LNR. This shows that the LNR has excellent survival discrimination with stepwise increments of lymph node retrieval. Our multivariate analysis, which revealed the LNR as the most important prognostic factor in the setting of LN >25, further confirmed that a higher cutoff value for lymph node retrieval not only substantially enhances long‐term survival per se, but also helps in the delicate survival stratification that would be vital in individualizing gastric cancer treatment strategies.
It is important to note that the pursuit of higher lymph node retrieval in the present study did not come at the expense of patient safety. This was reflected by the comparable hospital mortality rates between the LN 16–25 and LN >25 groups (2.7% vs. 1.7%). In our experience, most patients in the LN ≤15 group underwent less extensive lymph node dissection because of early disease status, medical comorbidities, morbid obesity, age (oldest old), or frailty. In contrast, patients in the LN >25 group were generally medically fit to undergo standard D2 gastrectomy.
Our study had several limitations. Owing to the retrospective nature of the study design, the extent of lymph node dissection could have been influenced by patient factors or surgeon preference. Selection bias in this setting could have considerably confounded the OS. The high percentage of LN >25 and the low hospital mortality rate achieved in the present study might not be easily reproducible in other hospitals because the two participating hospitals are highvolume medical centers that perform more than 200 radical gastrectomies annually at each institution [6], [18]. Finally, the number of lymph nodes retrieved might have been influenced by patient, disease, surgeon, and pathologist factors.
Conclusion
The traditionally recognized LN ≥16 retrieval after curative‐ intent gastrectomy might not be adequate in regions where locally advanced gastric cancer predominates. Our data show that LN >25 improves the long‐term outcome and survival stratification without compromising patient safety.
Acknowledgments
We thank Shu‐Fang Huang for updating the database and performing the data analysis. This study was partly supported by the Chang Gung Medical Research Program, Taiwan, Grant CMRPG 380161 and Department of Health, Taiwan, Grants DOH99‐TD‐C‐111‐006 and PMRPG390071.
Contributed equally.
Author Contributions
Conception/Design: Cheng‐Tang Chiu, Ta‐Sen Yeh
Provision of study material or patients: Jun‐Te Hsu, Chun‐Yi Tsai, Keng‐Hao Liu, Chun‐Nan Yeh, Tse‐Ching Chen, Ren‐Chin Wu, Ta‐Sen Yeh
Collection and/or assembly of data: Frank Wang, Jun‐Te Hsu, Chun‐Yi Tsai, Keng‐Hao Liu, Chun‐Nan Yeh, Tse‐Ching Chen, Ta‐Sen Yeh
Data analysis and interpretation: Yu‐Yin Liu, Wen‐Liang Fang, Frank Wang, Jun‐Te Hsu, Chun‐Yi Tsai, Keng‐Hao Liu, Chun‐Nan Yeh, Tse‐Ching Chen, Ren‐Chin Wu, Cheng‐Tang Chiu, Ta‐Sen Yeh
Manuscript writing: Yu‐Yin Liu, Wen‐Liang Fang, Ta‐Sen Yeh
Final approval of manuscript: Ta‐Sen Yeh
Disclosures
The authors indicated no financial relationships.
References
- 1. Jemal A, Siegel R, Xu J et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 2. Dikken JL, Jansen EP, Cats A et al. Impact of the extent of surgery and postoperative chemoradio‐therapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430–2436. [DOI] [PubMed] [Google Scholar]
- 3. Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988;75:110–112. [DOI] [PubMed] [Google Scholar]
- 4. Cuschieri A, Weeden S, Fielding J et al. Patient survival after D1 and D2 resections for gastric cancer: Long‐term results of the MRC randomized surgical trial. Br J Cancer 1999;79:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartgrink HH, van de Velde CJ, Putter H et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004; 22:2069–2077. [DOI] [PubMed] [Google Scholar]
- 6. Wu CW, Hsiung CA, Lo SS et al. Nodal dissection for patients with gastric cancer: A randomised controlled trial. Lancet Oncol 2006;7:309–315. [DOI] [PubMed] [Google Scholar]
- 7. Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643–649. [DOI] [PubMed] [Google Scholar]
- 8. Seevaratnam R, Bocicariu A, Cardoso R et al. A meta‐analysis of D1 versus D2 lymph node dissection. Gastric Cancer 2012;15(suppl 1):S60–S69. [DOI] [PubMed] [Google Scholar]
- 9. Edge SB, Byrd DR, Compton CC. Cancer Staging Manual. 7th ed. New York: Springer, 2010. [Google Scholar]
- 10.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guideline 2010(ver.3). Gastric Cancer 2011;14:113–123. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: gastric cancer version 2. 2013. Available at http://www.nccn.org/professionals/physician-gls/pdf/gastric.pdf. Accessed February 9, 2014.
- 12. Karpeh MS, Leon L, Klimstra D et al. Lymph node staging in gastric cancer: Is location more important than number? An analysis of 1,038 patients. Ann Surg 2000;232:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siewert JR, Bottcher K, Stein HJ et al. Relevant prognostic factors in gastric cancer: Ten‐year results of the German Gastric Cancer Study. Ann Surg 1998; 228:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu KJ, Loewen M, Atten MJ et al. The survival of stage III gastric cancer patients is affected by the number of lymph nodes removed. Surgery 2003;134:639–646. [DOI] [PubMed] [Google Scholar]
- 15. Biondi A, D'Ugo D, Cananzi FC et al. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer? Eur J Surg Oncol 2015;41:779–786. [DOI] [PubMed] [Google Scholar]
- 16. Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US‐ population database. J Clin Oncol 2005;23:7114–7124. [DOI] [PubMed] [Google Scholar]
- 17. Seevaratnam R, Bocicariu A, Cardoso R et al. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer 2012;15(suppl 1):S70‐S88. [DOI] [PubMed] [Google Scholar]
- 18. Schwarz RE, Smith DD. Clinical impact of lympha‐ denectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317–328. [DOI] [PubMed] [Google Scholar]
- 19. Chen HN, Chen XZ, Zhang WH et al. Necessity of harvesting at least 25 lymph nodes in patients with stage N2‐N3 resectable gastric cancer: A 10‐year, single‐institution cohort study. Medicine (Baltimore) 2015;94:e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh CN, Wang SY, Hsu JT et al. N3 subclassification incorporated into the final pathologic staging of gastric cancer: A modified system based on current AJCC staging. Medicine (Baltimore) 2015;94:e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner PK, Ramaswamy A, Rüschoff J et al. Lymph node counts in the upper abdomen: Anatomical basis for lymphadenectomy in gastric cancer. Br J Surg 1991;78:825–827. [DOI] [PubMed] [Google Scholar]
- 22. Morgan JW, Ji L, Friedman G et al. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg 2015;150:37–43. [DOI] [PubMed] [Google Scholar]
- 23. Smith DD, Nelson RA, Schwarz RE. A comparison of five competing lymph node staging schemes in a cohort of resectable gastric cancer patients. Ann Surg Oncol 2014;21:875–882. [DOI] [PubMed] [Google Scholar]
- 24. Wong J, Rahman S, Saeed N et al. Prognostic impact of lymph node retrieval and ratio in gastric cancer: A U.S. single center experience. J Gastrointest Surg 2013;17:2059–2066. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Dang P, Raut CP et al. Comparison of a LNR‐based staging system with the 7th AJCC system for gastric cancer: Analysis of 18043 patients from the SEER database. Ann Surg 2012;255:478–485. [DOI] [PubMed] [Google Scholar]
- 26. Marchet A, Mocellin S, Ambrosi A et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: Result from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu XJ, Miao RL, Li ZY et al. Prognostic value of metastatic lymph node ratio as an additional tool to the TNM stage system in gastric cancer. Eur J Surg Oncol 2015;41:927–933. [DOI] [PubMed] [Google Scholar]
- 28. Macdonald JS, Smalley SR, Benedetti J et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345: 725–730. [DOI] [PubMed] [Google Scholar]
- 29. Parkash V, Bifulco C, Feinn R et al. To count and how to count, that is the question: Interobserver and intraobserver variability among pathologists in lymph node counting. Am J Clin Pathol 2010;134: 42–49. [DOI] [PubMed] [Google Scholar]