Abstract
Erectile dysfunction (ED) will visit every man at some time in his life. The age at when that knock on the door is heard is totally dependent on one’s genetics as well as other extrinsic factors. Unlike guests who come for a visit and then leave, once ED shows up it tends to hang around forever. To add insult to injury, the longer ED hangs around, the worse it will get. It is estimated that by the time a man is in his 40’s, he has about a 40% chance of having some form of ED and this prevalence increases about 10% per decade thereafter. This suggests that the aging related process that leads to ED begins early in life. It turns out that the most common cause of ED, regardless of the patient’s age, is due to a problem with the vascular system of the penis. However, this specific aging related vascular problem is not caused by arterial disease but due to a dysfunction and/or loss of the corporal smooth muscle cells (SMC), the main constituent of the corporal sinusoids. As one gets older, these SMC continue to degrade and disappear. When approximately 15% of these cells have been impacted, it results in an inability of the corporal tissue to retain and/or prevent the blood from “leaking” out of the corporal sinusoids into the systemic veins. However, the corporal SMC themselves begin to combat this aging process by expressing the inducible nitric oxide synthase (iNOS) enzyme to make nitric oxide (NO) in an attempt to quench the high intracellular oxidative stress responsible for the SMC apoptosis. When this iNOS pathway is then pharmacologically upregulated, reversal of these aging related changes in the corpora with correction of the venous leakage is observed. Since we believe that aging related ED is pathologically the same disorder as essential hypertension, the development of a therapeutic regimen that can halt, delay or possibly reverse the cellular processes that lead to aging related ED should also be applicable to those patients diagnosed with essential hypertension.
Keywords: Erectile dysfunction (ED), aging, nitric oxide (NO), inducible nitric oxide synthase (iNOS)
Introduction
For many a man, their potency defines their joie de vivre. It is well established that men who engage in sexual activity are happier (1), live longer (2), are less depressed and definitely do experience a better quality of life (3-7). It is generally assumed in today’s society which values youth over old age that it is only the elderly who are at risk of losing their potency; however, recent data suggest that this may be a gross misconception (8,9) and could explain why some of us believe that “grumpy old men” seem to be getting younger and younger. Thus, it seems reasonable to ask the question: what can medicine do to prevent or, more reasonably, delay the onset of impotency or erectile dysfunction (ED) in the hopes that this affliction which so defines many a man does not raise its ugly head until one’s very end? Like most quests in medicine, the solution to an affliction usually lies in an understanding of its cause. Along this reasoning, this review will highlight what is currently known about the epidemiology, physiology and pathophysiology of ED and from this knowledge we will try to identify therapeutic options that could lead to a potential solution to our aforementioned million dollar question. Since the penis is considered the window for what is ongoing within the cardiovascular system and since we believe that both aging related ED and essential hypertension are pathologically the same disorder, we hypothesize that any treatment that is effective in either preventing and/or delaying the onset of aging related ED should also be effective in the same manner against essential hypertension.
Every man, if he lives long enough, is destined to develop what Masters and Johnson called aging related impotence (10) which we now term aging related erectile dysfunction (ARED). This is exemplified by the data from the Massachusetts Male Aging Study (MMAS) which indicated that about 40% of men in their 40’s will have some form of ED and this prevalence will increase about 10% per decade such that a man in his 50’s has about a 50% chance of having ED while a man in his 60’s has about a 60% chance of having ED, etc. (11). These prevalence data from the MMAS as well as others (8) intuitively suggest that the physiological processes that cause 40% of men in their 40’s to have some form of ED must have begun at an earlier age. If this intuition is indeed correct, it would explain why we physicians do see in our office some men in their 20’s and 30’s complaining of ED that ultimately turns out to be due a primary physiological and not a primary psychological cause.
Pathophysiology of ARED
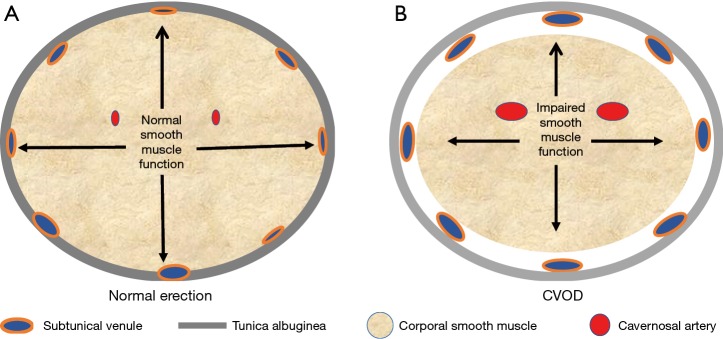
ED is defined as the inability of a man to either attain and/or maintain his erection long enough to complete the sexual act (12). It turns out that the development of an erection is a simple sequential two step mechanical event: the initial step is for fluid (blood) to be transported into an expanding receptacle (the cavernosal sinusoids) which results in the enlargement and rigidity of the penis. The second event involves the maintenance of that enlargement and rigidity, a process that is dependent on the ability of the corporal bodies to prevent the blood that has come into the expanded sinusoids from then “leaking” out through the veins draining these sinusoids, before the sexual act is completed. The inflow of blood into the corporal sinusoids via the cavernosal arteries that are located within the corporal bodies themselves, as well as the expansion of the corporal sinusoids which provides a space into which this increasing blood will pool is primarily dependent on the relaxation of the smooth muscle located both within the arterial system and corporal sinusoids, respectively (13,14). When the blood from the arterial system pools into the expanding corporal sinusoids, the intra-corporeal pressure will begin to rise and at a certain level the intra-corporeal pressure will passively compress shut the venous channels that egress from those corporal bodies under the less distensible tunica albuginea (Figure 1). It is this compression of the veins by the attainment of an intra-corporeal pressure that is high enough to accomplish this that prevents the sinusoidal blood from leaking out into the venous channels (15,16). When arterial inflow is low (trouble attaining the erection), we term this arterial insufficiency and when venous outflow is too high (trouble maintaining the erection), we term this cavernosal veno-occlusive dysfunction (CVOD) or simply “venous leakage”. Both arterial insufficiency and CVOD are the two forms of vasculogenic dysfunction and either one by itself or a combination of both can lead to symptomatic ED.
Figure 1.
Schematic representation of the corporal tissue in the (A) normal erect state and (B) partially erect state where CVOD is present. Note the compression of the subtunical veins in A and inadequate compression of the veins in B.
Etiology of ARED
All patients with ED will have as their primary cause either a psychological or a physiological reason. All forms of physiological ED other than what is due to a structural anomaly such as a chordee can be relegated to either a vasculogenic, neurogenic and/or a hormonal cause. When men of various ages from 18 to 80 years are studied to determine the cause of their ED, the most common etiology identified regardless of age is vasculogenic, specifically CVOD (17,18). This high prevalence of CVOD when compared to that of arterial disease (or defective inflow of blood into the penis) is most striking in the younger population i.e., in men younger than 40 years of age (18,19). However, once middle age begins to set in and the onset of hypertension and diabetes mellitus and other middle age maladies become more prevalent, the incidence of arterial disease as a cause of ED begins to follow suit (20). Nevertheless, despite this increase in the incidence of arterial disease as men age, CVOD or venous leakage can still be identified in about 67% to 75% of men complaining of ED, regardless of whether they are young, middle aged or elderly (17).
For those of us who specialize in seeing men with ED, particularly men younger than 50 years of age, the most common first symptomatic complaint of their ED is that they are either unable to keep their erection lasting as long as they previously could or that they simply “lost” their erection during the sexual act. These complaints would suggest, in the Oslerian way of listening to what the patient is saying, that the patient was telling us that he was “losing the blood from his erect penis” i.e., heralding the onset of symptomatic venous leakage or CVOD. So why does this venous leakage seem to signal the onset of ED in most men and why does it begin at an early age in some men? As mentioned earlier, the ability to attain an erection is due to the increase in blood going into the corporal sinusoids via the arterial tree in tandem with pooling of this blood within the corporal sinusoids. The increase in blood flow via the arterial vessels as well as the increase in the size of corporal sinusoids is due to the relaxation of the smooth muscle within both the arterial vessels and the corporal sinusoids, a process that is regulated by the release of nitric oxide (NO) (21) synthesized by its enzyme, the neuronal isoform of nitric oxide synthase (nNOS), that is actually located outside the SMC within the terminal axons of the nerve (Figure 2) innervating this corporal smooth muscle (22). The NO from nNOS rapidly enters the SMC and initiates the process of smooth muscle relaxation.
Figure 2.
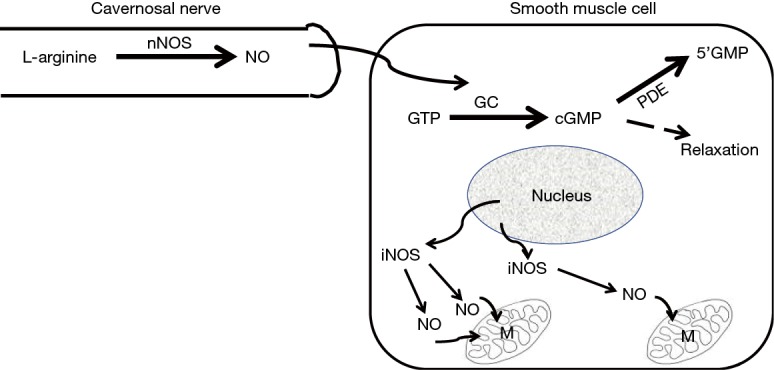
Schematic representation of the difference between nNOS and iNOS in the corporal smooth muscle cell. nNOS is involved in the neurogenic stimulation of nitric oxide (NO) within the nerve terminals. This neurally derived NO rapidly traverses from the nerve terminal into the cytoplasm of the underlying smooth muscle cell to activate guanylyl cyclase to form cGMP from GTP and induce smooth muscle relaxation (the erectile state). cGMP is endogenously degraded by PDE into 5’-GMP. iNOS originates from the smooth muscle cell itself and the NO from iNOS combats the oxidative stress that is ongoing within the mitochondria (M).
As long as the blood that enters the sinusoidal spaces can be retained within the sinusoids to allow the attainment of an intra-corporeal pressure that is high enough to compress the veins exiting the corporal bodies, the erection itself will be maintained. The length of time a man can keep his erection without losing it before the completion of the sexual act is then dependent on how long the corporal smooth muscle can be maintained in its relaxed state. If the corporal smooth muscle tires easily or if there are not enough SMC functioning normally within the sinusoids to achieve that high intra-corporeal pressure necessary to compress those egressing veins, the sinusoidal blood will “leak out” and the erection will be “lost”. It is estimated that one only needs to lose approximately 15% of the function of the corporal smooth muscle mass for symptomatic venous leakage to occur (23). Based on our own observations with dynamic infusion cavernosometry over the past 30 years (17,20) in tandem with the known anatomical relationship between the cavernosal artery and the corporal sinusoids (Figure 1), the intra-corporeal pressure that is required to compress the veins exiting the corpora and maintain one’s erection is probably somewhere around the mean arterial pressure of that individual.
As men age, it is recognized that there is an aging dependent decrease in the amount of the functioning corporal smooth muscle. The mechanism(s) underlying this aging related loss of the normal smooth muscle within the corporal bodies is believed to be due mainly to an apoptotic process that is primarily triggered by oxidative stress (24). When about 15% of the functioning corporal smooth muscle mass has been impacted, it can lead to symptomatic ED and this theoretically can occur at any age since it is believed the apoptotic process simply due to the aging process is most likely genetically determined in each individual. Support for the concept that this aging related apoptotic process and subsequent dysfunction of the corporal smooth muscle can and does occur at an early age, is based on the clinical observation that the refractory period of the penis, that time period between the attainment of two subsequent and separate erectile episodes, begins to increase in most men sometime during their 20’s and continues to progress with the aging process. By inference, it is safe to state that when the patient (who has normal arterial inflow) “recognizes” the onset of the inability to maintain his erection, it most likely indicates that the aging process within the corpora has already begun.
Additional clinical evidence to support the belief that the aging related apoptotic process of the corporal smooth muscle begins at an age much earlier than when symptomatic ED occurs lies in the response of young potent men to the ingestion of PDE5 inhibitors used for treating ED. By inhibiting phosphodiesterase, PDE5 inhibitors enhance the relaxation of the corporal smooth muscle by preventing the breakdown of cGMP within the smooth muscle cells (25) and as a result this should theoretically allow one to maintain his erection for longer periods of time. cGMP itself is formed within the smooth muscle cell from guanosine triphosphate (GTP) by the enzyme, guanylyl cyclase (26) which is the enzyme that is targeted by NO to begin the production of cGMP. In the penis, the NO that initiates the erectile response is formed from the nNOS enzyme located within the axons of the erectile nerves which is located outside its target, the corporal smooth muscle cells. When young men who are documented to have normal erectile function are then given oral PDE5 inhibitors, the only observed outcome in these “normal men” is a decrease in their refractory period (27,28) without any significant effect on the rigidity of their erection as measured by the IIEF (international index of erectile function) score (29). Therefore, the reported enhancement of the erectile response to oral PDE5 inhibitors in young men who claim to have normal erectile function would theoretically only occur in those men whose corporal smooth muscle function has already begun to deteriorate.
In fact, it is safe to state that most men who respond to these PDE5 inhibitors, if they live long enough, will at some time later on their life ultimately fail to respond to these drugs (30,31). Logic then dictates that when this lack of responsiveness occurs, and barring any loss of arterial inflow, it could only be due to either (I) progression of the process within the penile tissues that is causing the ED or (II) tachyphylaxis of the PDE5 inhibitor. Since it has been demonstrated unequivocally that these PDE5 inhibitors do not undergo tachyphylaxis (32,33), one can only conclude that for those men who are suffering from ARED the subsequent diminution in their response to these PDE5 inhibitors would have to be by default the progression of the aging related processes in particular the SMC apoptosis that continues to forge ahead on as one ages. Once the PDE5 inhibitors become incapable at its highest dose of inducing sufficient tumescence to allow sexual activity to occur where it was previously able to do so, it merely identifies the time has been reached when either the remaining functioning corporal smooth muscle is incapable via the oral route of drug administration of achieving sufficient relaxation to allow the attainment of an intra-corporeal pressure high enough to compress the subtunical veins (increasing venous leakage) or there has been a concomitant decrease in the inflow of blood into the penis which is incapable of providing enough blood to the corporal sinusoids (arteriogenic dysfunction) to allow for any veno-occlusion to occur or it could be due to a little bit of both of these processes. Since an erection is simply a mechanical event requiring a dynamic balance between inflow and outflow of blood within the corporal sinusoids, the determination of whether one or both processes are functioning normally in an individual patient requires an individual evaluation of each of these processes (17,20).
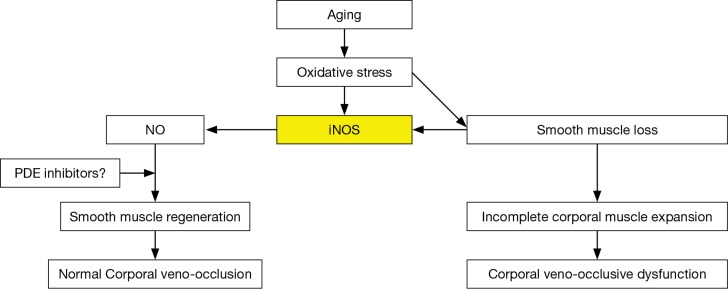
Within the last decade it was observed in the laboratory that when this aging dependent apoptosis of the corporal smooth muscle occurred, the corporal tissues themselves responded to the related causative oxidative stress by increasing the production of NO within the SMC itself (34) via a process that is different from the NO that is released into the SMC from the terminal axons of the erectile nerves by the nNOS enzyme (Figure 2). This NO that is synthesized within the cytosol of the SMC is produced by another NOS isoform called inducible NOS (iNOS) which is one of three NO producing enzymes in the body. iNOS is normally produced only in the macrophages and Kupffer cells (35) and under the appropriate stimulus, can produce high local levels of NO. Interestingly, these high intracellular levels of NO produced by iNOS have been shown in some systems and tissues to be both noxious, by inducing apoptosis, as well as protective, by being anti-apoptotic (36,37). However, with respect to the corporal smooth muscle undergoing its aging related apoptosis, laboratory data suggest that the SMC of the corpora begin the process of expressing iNOS, which produces NO within the cell itself and this appears to be an attempt by the SMC itself to combat the oxidative stress associated with the ongoing apoptotic process (38). In theory, this production of NO from iNOS in the aging penis can be viewed as an attempt by the tissue itself to retard or reverse the ongoing deterioration of the corporal smooth muscle. This can be observed in animal models of ED where this anti-apoptotic effect of NO from iNOS has been shown to be enhanced by compounds that either boost iNOS production (39) or inhibit the breakdown of cGMP (40) without any obvious detrimental effects to any other organ systems in these animals. Indeed, when PDE5 inhibitors which inhibit the breakdown of cGMP and therefore complement the effect of NO are given for long periods of time on a daily and continuous basis to aged animals with ED, the aging related apoptotic process in the corpora seems to be retarded, the corporal smooth muscle cell content within the corporal tissue surprisingly seems to increase and normal corporal veno-occlusion can be achieved (Figure 3) (40).
Figure 3.
Aging related events ongoing in the vascular smooth muscle cell. Aging induces oxidative stress which leads to apoptosis of the smooth muscle cells and induction of iNOS. NO from iNOS combats the oxidative stress and helps retard the apoptotic process within the cell.
Penis and CV system
The penis is considered by many to be the window to the cardiovascular system. Indeed, it was the observation that the smooth muscle cell of the media of the arterial system is indistinguishable physiologically from that of the smooth muscle cell of the corporal tissue that provided us initially with the clue that the corporal SMC of the penis were an NO dependent tissue (41). Therefore, one could infer that any endogenous systemic influence such as the aging process that affects one vascular smooth muscle tissue should also affect all other vascular smooth muscle tissue within that organism. This is exactly what Ferrini et al. observed in the laboratory rat (42). In this animal model which does not normally develop atherosclerosis with aging, there appeared to be similar histological changes in the media of the peripheral vascular system and the corpora with aging (42). Since the loss or dysfunction of SMC within the arterial media leads to a “stiffer” artery and such “stiffer” vessels (arteriosclerosis or arterial stiffness) are a hallmark of hypertension, this experimental observation in the rat provided the basis for the statement that essential hypertension, which could be considered an aging related loss or dysfunction of smooth muscle from the media of the peripheral vascular system, is essentially the same disorder as ARED within the penis, i.e., both are due to the smooth muscle loss and/or dysfunction that occurs as a result of the aging process (43,44). This would certainly explain why hypertension is found to be the most common medical condition associated with ED and why the aging prevalence of both conditions parallel each other decade for decade (11,45).
Most recently, it has been proposed that it is actually hypertension, the most common of all the cardiovascular diseases, that is the major risk factor for ED (46) rather than the common held view that ED is a predictor for the development or presence of cardiovascular disease (47-49). If hypertension, including the aging related form of it, is indeed the major risk factor for ED and if hypertension is one of the major risk factors for endothelial dysfunction, the presumptive cause of ED (50), this would suggest that our focus on the treatment of two of the most common maladies to afflict a man (hypertension and ED) should be directed towards their common presumptive cause i.e., preventing the aging related changes occurring in our vascular SMC. Theoretically then, if iNOS is the synthesizer of the signaling molecule, NO, and one proposes to upregulate its production and subsequent effect in order to help retard or delay the aging related apoptosis seen in the cavernosal smooth muscle, it is potentially possible that such a systemic treatment could also benefit the aging related apoptosis simultaneously ongoing within the peripheral arterial media. From a practical point of view, a long-term treatment that is not linked to the induction of an erection but to achieve constant moderately higher intracellular levels of NO and/or cGMP, targeted to mimic the effect of iNOS (to produce both more NO and cGMP) or a treatment, like with PDE5i, that inhibits the breakdown of the cGMP, derived from this iNOS pathway (to enhance the effect of NO from iNOS) or a treatment that does both, may be operative in preventing or delaying the onset of aging related ED and/or essential hypertension. Preliminary results using PDE5 inhibitors in humans to accomplish this in a more accelerated version of aging related ED i.e., when the cavernosal nerves that innervate the corporal smooth muscle are surgically transected, support such a therapeutic trial (51).
If the actual defective cell in ARED is the corporal SMC, what about regenerative medicine in this condition? Since the first report by Bahk et al. in 2010 describing the use of stem cells from umbilical cord blood to treat ED (52), there have not been any published evidence based studies in humans supporting the use of any of the various types of stem cells that have shown efficacy in rodents (53) although most of the anecdotal and early reports in humans have been mainly in elderly men where it is suspected that they have both arterial disease and CVOD.
Conclusions
The most common cause of ED, regardless of the age of the patient, is vasculogenic due to CVOD or venous leakage. Pathologically, this is due to an aging related apoptosis of the corporal SMC similar to what is believed to also occur in the media of the peripheral vasculature. The apoptotic process in the penis appears to begin early in a man’s life and can be first identified by the increase in the refractory period most men will experience usually sometime around their 3rd decade of life. The progression of this increase in the refractory period over time will reach a stage, depending on the patient’s genetics and co-morbidities, where maintenance of the erection becomes problematic and symptomatic ED is apparent. In response, the SMC begin to fight the oxidative stress and apoptosis associated with these aging related changes by producing NO from iNOS. Preliminary data suggest that products that upregulate this NO producing pathway and/or pharmacologically release NO, and/or protect its product, cGMP, show promise in halting or reversing the cellular changes associated with this aging process. It remains to be determined whether such therapy will also be found to be effective on similar aging related changes that occur in the media of the peripheral vasculature.
Acknowledgements
Funding: This work was supported from the National Institute of Neurological Disorders and Stroke and the National Institute of General Medicine NINDS/NIGMS SC1NS064611, the National Institute on Minority Health and Health Disparities (NIMHD) 5U54MD00 7598-06 and by NIMHD S21 MD000103 and from KLRM, LLC (Long Beach, CA, USA).
Footnotes
Conflicts of Interest: JR is one of the founders of KLRM, LLC, Long Beach, CA, USA. The other authors have no conflicts of interest to declare.
References
- 1.Palmore EB. Predictors of the longevity difference: a 25-year follow-up. Gerontologist 1982;22:513-8. 10.1093/geront/22.6.513 [DOI] [PubMed] [Google Scholar]
- 2.Davey Smith G, Frankel S, Yarnell J. Sex and death: are they related? Findings from the Caerphilly Cohort Study. BMJ 1997;315:1641-4. 10.1136/bmj.315.7123.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snaith RP. Depression and impotence. BMJ 1994;308:1439-40. 10.1136/bmj.308.6941.1439b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gheorghiu S, Godschalk M, Gentili A, et al. Quality of life in patients using self-administered intracavernous injections of prostaglandin E1 for erectile dysfunction. J Urol 1996;156:80-1. 10.1016/S0022-5347(01)65945-7 [DOI] [PubMed] [Google Scholar]
- 5.Araujo AB, Durante R, Feldman HA, et al. The relationship between depressive symptoms and male erectile dysfunction: cross-sectional results from the Massachusetts Male Aging Study. Psychosom Med 1998;60:458-65. 10.1097/00006842-199807000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Shabsigh R, Klein LT, Seidman S, et al. Increased incidence of depressive symptoms in men with erectile dysfunction. Urology 1998;52:848-52. 10.1016/S0090-4295(98)00292-1 [DOI] [PubMed] [Google Scholar]
- 7.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med 1998;13:159-66. 10.1046/j.1525-1497.1998.00050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capogrosso P, Colicchia M, Ventimiglia E, et al. One patient out of four with newly diagnosed erectile dysfunction is a young man--worrisome picture from the everyday clinical practice. J Sex Med 2013;10:1833-41. 10.1111/jsm.12179 [DOI] [PubMed] [Google Scholar]
- 9.Reed-Maldonado AB, Lue TF. A syndrome of erectile dysfunction in young men? Transl Androl Urol 2016;5:228-34. 10.21037/tau.2016.03.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masters WH, Johnson VE. Sexual Inadequacy in the Aging Male.In, Human Sexual Inadequacy, Chapter 12. Ishi Press International 1970:316-22. ISBN 4-87187-701-9. [Google Scholar]
- 11.Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [DOI] [PubMed] [Google Scholar]
- 12.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83-90. 10.1001/jama.1993.03510010089036 [DOI] [PubMed] [Google Scholar]
- 13.Lue TF, Takamura T, Schmidt RA, et al. Hemodynamics of erection in the monkey. J Urol 1983;130:1237-41. [DOI] [PubMed] [Google Scholar]
- 14.Lue TF, Tanagho EA. Physiology of erection and pharmacological management of impotence. J Urol 1987;137:829-36. [DOI] [PubMed] [Google Scholar]
- 15.Fournier GR, Jr, Juenemann KP, Lue TF, et al. Mechanisms of venous occlusion during canine penile erection: an anatomic demonstration. J Urol 1987;137:163-7. [DOI] [PubMed] [Google Scholar]
- 16.Banya Y, Ushiki T, Takagane H, et al. Two circulatory routes within the human corpus cavernosum penis: a scanning electron microscopic study of corrosion casts. J Urol 1989;142:879-83. [DOI] [PubMed] [Google Scholar]
- 17.Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol 1988;140:69-71. [DOI] [PubMed] [Google Scholar]
- 18.Donatucci CF, Lue TF. Erectile dysfunction in men under 40: etiology and treatment choice. Int J Impot Res 1993;5:97-103. [PubMed] [Google Scholar]
- 19.Rajfer J, Valeriano J, Sinow R. Early onset erectile dysfunction is usually not associated with abnormal cavernosal arterial Inflow. Int J Impot Res 2013;25:217-20. 10.1038/ijir.2013.17 [DOI] [PubMed] [Google Scholar]
- 20.Lue TF, Mueller SC, Jow YR, et al. Functional evaluation of penile arteries with duplex ultrasound in vasodilator-induced erection. Urol Clin North Am 1989;16:799-807. [PubMed] [Google Scholar]
- 21.Ignarro LJ, Bush PA, Buga GM, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun 1990;170:843-50. 10.1016/0006-291X(90)92168-Y [DOI] [PubMed] [Google Scholar]
- 22.Burnett AL, Lowenstein CJ, Bredt DS, et al. Nitric oxide: a physiologic mediator of penile erection. Science 1992;257:401-3. 10.1126/science.1378650 [DOI] [PubMed] [Google Scholar]
- 23.Nehra A, Goldstein I, Pabby A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol 1996;156:1320-9. 10.1016/S0022-5347(01)65578-2 [DOI] [PubMed] [Google Scholar]
- 24.Grünewald T, Beal MF. NOS knockouts and neuroprotection. Nat Med 1999;5:1354-5. 10.1038/70918 [DOI] [PubMed] [Google Scholar]
- 25.Rajfer J, Aronson WJ, Bush PA, et al. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med 1992;326:90-4. 10.1056/NEJM199201093260203 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta 1993;1178:153-75. 10.1016/0167-4889(93)90006-B [DOI] [PubMed] [Google Scholar]
- 27.Aversa A, Mazzilli F, Rossi T, et al. Effects of sildenafil (Viagra) administration on seminal parameters and post-ejaculatory refractory time in normal males. Hum Reprod 2000;15:131-4. 10.1093/humrep/15.1.131 [DOI] [PubMed] [Google Scholar]
- 28.Mondaini N, Ponchietti R, Muir GH, et al. Sildenafil does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225-8. 10.1038/sj.ijir.3901005 [DOI] [PubMed] [Google Scholar]
- 29.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 30.Mumtaz FH, Khan MA, Morgan RJ. Tolerance to sildenafil: possible mechanism. BJU Int 2000;85:14.10619937 [Google Scholar]
- 31.El-Galley R, Rutland H, Talic R, et al. Long-term efficacy of sildenafil and tachyphylaxis effect. J Urol 2001;166:927-31. 10.1016/S0022-5347(05)65866-1 [DOI] [PubMed] [Google Scholar]
- 32.Musicki B, Champion HC, Becker RE, et al. In vivo analysis of chronic phosphodiesterase-5 inhibition with sildenafil in penile erectile tissues: no tachyphylaxis effect. J Urol 2005;174:1493-6. 10.1097/01.ju.0000173006.47623.2c [DOI] [PubMed] [Google Scholar]
- 33.Vernet D, Magee T, Qian A, et al. Phosphodiesterase type 5 is not upregulated by tadalafil in cultures of human penile cells. J Sex Med 2006;3:84-94; discussion 94-5. 10.1111/j.1743-6109.2005.00197.x [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Cadavid NF, Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol 2004;39:1705-12. 10.1016/j.exger.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 35.Buttery LD, Evans TJ, Springall DR, et al. Immunochemical localization of inducible nitric oxide synthase in endotoxin-treated rats. Lab Invest 1994;71:755-64. [PubMed] [Google Scholar]
- 36.Budziñski M, Misterek K, Gumulka W, et al. Inhibition of inducible nitric oxide synthase in persistent pain. Life Sci 2000;66:301-5. 10.1016/S0024-3205(99)00421-X [DOI] [PubMed] [Google Scholar]
- 37.Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 2006;49:170-82. 10.1016/j.neuint.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 38.Ferrini M, Magee TR, Vernet D, et al. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod 2001;64:974-82. 10.1095/biolreprod64.3.974 [DOI] [PubMed] [Google Scholar]
- 39.Ferrini MG, Hlaing SM, Chan A, et al. Treatment with a combination of ginger, L-citrulline, muira puama and Paullinia cupana can reverse the progression of corporal smooth muscle loss, fibrosis and veno-occlusive dysfunction in the aging rat. Andrology (Los Angel) 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrini MG, Kovanecz I, Sanchez S, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod 2007;76:915-23. 10.1095/biolreprod.106.059642 [DOI] [PubMed] [Google Scholar]
- 41.Krall JF, Fittingoff M, Rajfer J. Characterization of cyclic nucleotide and inositol 1,4,5-trisphosphate-sensitive calcium-exchange activity of smooth muscle cells cultured from the human corpora cavernosa. Biol Reprod 1988;39:913-22. 10.1095/biolreprod39.4.913 [DOI] [PubMed] [Google Scholar]
- 42.Ferrini MG, Davila HH, Valente EG, et al. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovasc Res 2004;61:796-805. 10.1016/j.cardiores.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 43.Rajfer J. The aging penis: what is it trying to tell us? Transl Androl Urol 2012;1:58-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clavijo RI, Miner MM, Rajfer J. Erectile Dysfunction and Essential Hypertension: The Same Aging-related Disorder? Rev Urol 2014;16:167-71. [PMC free article] [PubMed] [Google Scholar]
- 45.Wolz M, Cutler J, Roccella EJ, et al. Statement from the National High Blood Pressure Education Program: prevalence of hypertension. Am J Hypertens 2000;13:103-4. 10.1016/S0895-7061(99)00241-1 [DOI] [PubMed] [Google Scholar]
- 46.Ning L, Yang L. Hypertension might be a risk factor for erectile dysfunction: a meta-analysis. Andrologia 2016. [Epub ahead of print]. 10.1111/and.12644 [DOI] [PubMed] [Google Scholar]
- 47.Kloner RA, Speakman M. Erectile dysfunction and atherosclerosis. Curr Atheroscler Rep 2002;4:397-401. 10.1007/s11883-002-0078-3 [DOI] [PubMed] [Google Scholar]
- 48.Vlachopoulos C, Rokkas K, Ioakeimidis N, et al. Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol 2005;48:996-1002; discussion 1002-3. 10.1016/j.eururo.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 49.Salem S, Abdi S, Mehrsai A, et al. Erectile dysfunction severity as a risk predictor for coronary artery disease. J Sex Med 2009;6:3425-32. 10.1111/j.1743-6109.2009.01515.x [DOI] [PubMed] [Google Scholar]
- 50.Maas R, Schwedhelm E, Albsmeier J, et al. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med 2002;7:213-25. 10.1191/1358863x02vm429ra [DOI] [PubMed] [Google Scholar]
- 51.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol 2004;171:771-4. 10.1097/01.ju.0000106970.97082.61 [DOI] [PubMed] [Google Scholar]
- 52.Bahk JY, Jung JH, Han H, et al. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant 2010;8:150-60. [PubMed] [Google Scholar]
- 53.Alwaal A, Zaid UB, Lin CS, et al. Stem cell treatment of erectile dysfunction. Adv Drug Deliv Rev 2015;82-83:137-44. 10.1016/j.addr.2014.11.012 [DOI] [PubMed] [Google Scholar]