Abstract
Inflammation is a consequence of chronic kidney disease (CKD) and is associated with adverse outcomes in many clinical settings. Inflammation stimulates production of fibroblast growth factor 23 (FGF23), high levels of which are independently associated with mortality in CKD. Few large-scale prospective studies have examined inflammation and mortality in patients with CKD, and none tested the interrelationships between inflammation, FGF23 and risk of death. Therefore, we conducted a prospective investigation of 3875 participants in the Chronic Renal Insufficiency Cohort (CRIC) study with CKD stages 2 to 4 to test the associations of baseline plasma interleukin-6, high sensitivity C-reactive protein, and FGF23 levels with all-cause mortality, censoring at the onset of end-stage renal disease. During a median follow-up of 6.9 years, 550 participants died (20.5/1000 person-years) prior to end stage renal disease. In separate multivariable-adjusted analyses, higher levels of interleukin-6 (hazard ratio per one standard deviation increase of natural log-transformed levels) 1.35 (95% confidence interval, 1.25–1.46), C-reactive protein 1.28 (1.16–1.40), and FGF23 1.45 (1.32–1.60) were each independently associated with increased risk of death. With further adjustment for FGF23, the risks of death associated with interleukin-6 and C-reactive protein were minimally attenuated. Compared to participants in the lowest quartiles of inflammation and FGF23, the multivariable-adjusted hazard ratio of death among those in the highest quartiles of both biomarkers was 4.38 (2.65–7.23) for interleukin-6 and FGF23, and 5.54 (3.04–10.09) for C-reactive protein and FGF23. Thus, elevated levels of interleukin-6, C-reactive protein and FGF23 are independent risk factors for mortality in CKD.
Keywords: FGF23, Inflammation, CKD, Mortality
Introduction
Chronic kidney disease (CKD) is a growing public health problem that affects millions of people worldwide.1 Presence of CKD is a powerful independent risk factor for death,2, 3 but the underlying pathophysiological mechanisms remain incompletely understood. Multiple lines of evidence support a direct pathogenic role for inflammation. In experimental settings, inflammation contributes mechanistically to CKD progression, insulin resistance, oxidative stress, endothelial dysfunction, atherosclerosis, arterial calcification, and osteodystrophy.4–12 Supportive human studies demonstrate that inflammatory markers are elevated in patients with CKD and are associated with malnutrition, atherosclerosis, coronary artery calcification, left ventricular hypertrophy, atrial fibrillation, and heart failure, each of which are risk factors for mortality.13–21 Nonetheless, few observational studies tested the association between inflammation and mortality in CKD stages 2–4. One study reported increased risk of death in association with elevated C-reactive protein (CRP) levels in a secondary analysis of the MDRD trial of non-diabetic and predominantly Caucasian CKD patients.22 Another study reported increased risk of mortality in association with elevated interleukin-6 (IL-6) levels in a small cohort of 125 Caucasian patients with few deaths.23 Data are lacking from large prospective cohorts that are more broadly representative of ethnically diverse populations of CKD due to heterogeneous causes.
Elevated levels of fibroblast growth factor 23 (FGF23) are independently associated with increased risk of death in patients with CKD,24, 25 perhaps in part, by activating fibroblast growth factor receptor 4 (FGFR4) on cardiac myocytes to promote pathological left ventricular hypertrophy and intra-myocardial fibrosis that predispose to heart failure and arrhythmia.26–29 Elevated FGF23 levels are also independently associated with higher levels of IL-6 and CRP in patients with CKD stages 2–4.30 Recent experimental data demonstrate that inflammation directly stimulates osteocyte production of FGF23,31 and that elevated FGF23 can induce production of inflammatory mediators by activating FGFR4 on hepatocytes.32 Together, these data suggest that elevated FGF23 and its consequences may be an additional novel mechanism of mortality associated with inflammation. We performed a prospective cohort study of individuals with CKD stages 2–4 who enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study to test the hypotheses that elevated levels of inflammatory markers are independent risk factors for death prior to the onset of end-stage renal disease (ESRD), and that elevated FGF23 levels mediate an important component of inflammation-associated risk of mortality.
Results
Baseline Characteristics
The study population included 3875 of the total 3939 CRIC Study participants who had baseline measurement of IL-6 and FGF23; 3873 of these participants also had baseline measurements of high-sensitivity CRP. Baseline characteristics of the study population according to IL-6 quartiles are presented in Table 1. Mean estimated glomerular filtration rate (eGFR) was 44.3 ± 15.0 mL/min/1.73 m2, median IL-6 was 1.9 pg/ml (IQR, 1.2–3.2), median high-sensitivity CRP was 2.6 mg/l (IQR, 1.1–6.5), and median FGF23 was 145.6 RU/ml (IQR, 95.8–239.3). Compared to participants in the lowest quartile of IL-6, those with levels in the highest quartile were 4.5 years older, had 9.8 mmHg higher systolic blood pressure and 11 ml/min/1.73m2 lower eGFR. History of diabetes and cardiovascular disease, and levels of CRP, FGF23 and urinary albumin-creatinine ratio (ACR) increased across quartiles of IL-6, while serum albumin levels decreased (Table 1). Baseline characteristics according to quartiles of FGF23 are shown in Supplemental Table 1.
Table 1.
Baseline characteristics of the study population according to quartiles of interleukin-6 levels
| IL-6, pg/ml | Quartile 1 N = 970 ≤ 1.2 |
Quartile 2 N = 968 > 1.2 and ≤ 1.9 |
Quartile 3 N = 969 > 1.9 and ≤ 3.2 |
Quartile 4 N = 968 > 3.2 |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 54.5 ± 11.7 | 57.7 ± 11.0 | 59.4 ± 10.3 | 59.0 ± 10.3 |
| Female, N (%) | 405 (41.8) | 448 (46.3) | 437 (45.1) | 446 (46.1) |
| Black, N (%) | 326 (33.6) | 377 (39.0) | 449 (46.3) | 467 (48.2) |
| Hispanic, N (%) | 70 (7.2) | 127 (13.1) | 143 (14.8) | 155 (16.0) |
| Cardiovascular risk factors | ||||
| Hypertension, N (%) | 729 (75.2) | 843 (87.1) | 873 (90.1) | 890 (91.9) |
| Diabetes, N (%) | 305 (31.4) | 463 (47.8) | 555 (57.3) | 556 (57.4) |
| Coronary artery disease, N (%) | 119 (12.3) | 204 (21.1) | 244 (25.2) | 285 (29.4) |
| Congestive heart failure, N (%) | 38 (3.9) | 75 (7.8) | 109 (11.3) | 154 (15.9) |
| Stroke, N (%) | 62 (6.4) | 97 (10.0) | 108 (11.2) | 118 (12.2) |
| Peripheral vascular disease, N (%) | 20 (2.1) | 66 (6.8) | 75 (7.7) | 97 (10.0) |
| Current smoking, N (%) | 94 (9.7) | 128 (13.2) | 116 (12.0) | 170 (17.6) |
| Body mass index, kg/m2 | 28.9 ± 5.7 | 31.7 ± 7.0 | 33.5 ± 7.9 | 34.3 ± 9.1 |
| Systolic blood pressure, mm Hg | 122.1 ± 18.9 | 128.9 ± 21.5 | 131.5 ± 23.5 | 131.9 ± 23.3 |
| Medication use | ||||
| Aspirin, N (%) | 363 (37.6) | 395 (41.2) | 470 (48.9) | 422 (43.8) |
| B -blockers, N (%) | 325 (33.7) | 462 (48.2) | 546 (56.8) | 564 (58.6) |
| Statins, N (%) | 459 (47.6) | 548 (57.2) | 583 (60.7) | 534 (55.5) |
| ACE inhibitors or ARBs, N (%) | 604 (62.6) | 702 (73.3) | 686 (71.4) | 656 (68.1) |
| Phosphate binders, N (%) | 65 (6.7) | 69 (7.2) | 67 (7.0) | 69 (7.2) |
| Active vitamin D, N (%) | 22 (2.3) | 22 (2.3) | 34 (3.5) | 46 (4.8) |
| Nutritional Vitamin D, N (%) | 130 (13.5) | 92 (9.6) | 84 (8.7) | 91 (9.5) |
| Steroids, N (%) | 94 (9.7) | 87 (9.1) | 83 (8.6) | 122 (12.7) |
| CKD Specific Risk Factors | ||||
| Creatinine, mg/dl | 1.6 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.6 | 2.0 ± 0.7 |
| eGFR, ml/min/1.73m2 | 51.0 ± 15.9 | 44.4 ± 14.0 | 41.9 ± 13.5 | 39.9 ± 14.2 |
| Urinary ACR, mg/g | 17.0 (5.3 – 176.2) | 48.5 (8.0 – 427.7) | 73.1 (11.8 – 649.0) | 117.4 (17.9 – 810.5) |
| Hemoglobin, g/dl | 13.2 ± 1.7 | 12.7 ±1.7 | 12.4 ± 1.8 | 12.1 ± 1.8 |
| Albumin, g/dl | 4.1 ± 0.4 | 4.0 ± 0.5 | 3.9 ± 0.5 | 3.8 ± 0.5 |
| LDL, mg/dl | 106.3 ± 33.5 | 104.1 ±35.8 | 101.6 ± 36.7 | 98.3 ± 34.9 |
| Mineral Metabolites | ||||
| Calcium, mg/dl | 9.3 ± 0.5 | 9.2 ± 0.5 | 9.1 ± 0.5 | 9.1 ± 0.5 |
| Phosphate, mg/dl | 3.6 ± 0.6 | 3.7 ± 0.7 | 3.8 ± 0.7 | 3.8 ± 0.7 |
| Parathyroid hormone, pg/ml | 41.3 (29.0 – 63.0) | 52.9 (34.0 – 82.0) | 58.0 (38.5 –102.4) | 71.0 (43.0 – 115.6) |
| FGF23, RU/ml | 103.0 (73.6 – 150.5) | 141.3 (94.2 – 210.2) | 160.9 (109.7 – 243.5) | 217.3 (127.3 – 364.0) |
| Inflammatory markers | ||||
| IL-6, pg/ml | 0.8 (0.6 – 1.0) | 1.5 (1.3 – 1.7) | 2.4 (2.1 – 2.7) | 4.8 (3.7 – 7.2) |
| HS-CRP, mg/l | 1.2 (0.6 – 2.4) | 2.0 (1.0 – 4.1) | 3.4 (1.4 – 7.2) | 6.7 (2.8 – 13.1) |
| TNF-α, pg/ml | 1.7 (1.2 – 2.4) | 2.2 (1.5 – 3.1) | 2.4 (1.7 – 3.4) | 2.8 (2.0 – 4.0) |
| Fibrinogen, g/l | 3.4 (3.0 – 4.0) | 3.9 (3.4 – 4.5) | 4.3 (3.6 – 5.0) | 4.7 (4.0 – 5.6) |
Values expressed as mean ± SD or median (interquartile range).
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; ACR, albumin to creatinine ratio; LDL, low-density lipoprotein; FGF23, fibroblast growth factor 23; IL-6, interleukin-6; CRP, high sensitive C-reactive protein; TNF-α, tumor necrosis factor alpha.
Individual Effects of Inflammatory Markers and FGF23 on Mortality
During a median follow-up of 6.9 years (IQR, 4.2–8.2), 550 participants died (20.5/1000 person-years) prior to the onset of ESRD. Median levels of IL-6, CRP and FGF23 were significantly higher in participants who died compared to those who remained event-free prior to the onset of ESRD: IL-6, 2.7 (IQR, 1.7–4.6) versus 1.8 (IQR, 1.1–2.9) pg/ml, p < .0001; CRP, 4.0 (IQR, 1.6–8.6) versus 2.4 (IQR, 1.0–6.0) mg/l, p < .0001; FGF23, 192.1 (IQR, 117.7–324.9) versus 139.2 (IQR, 93.6–225.7) RU/ml, p < .0001.
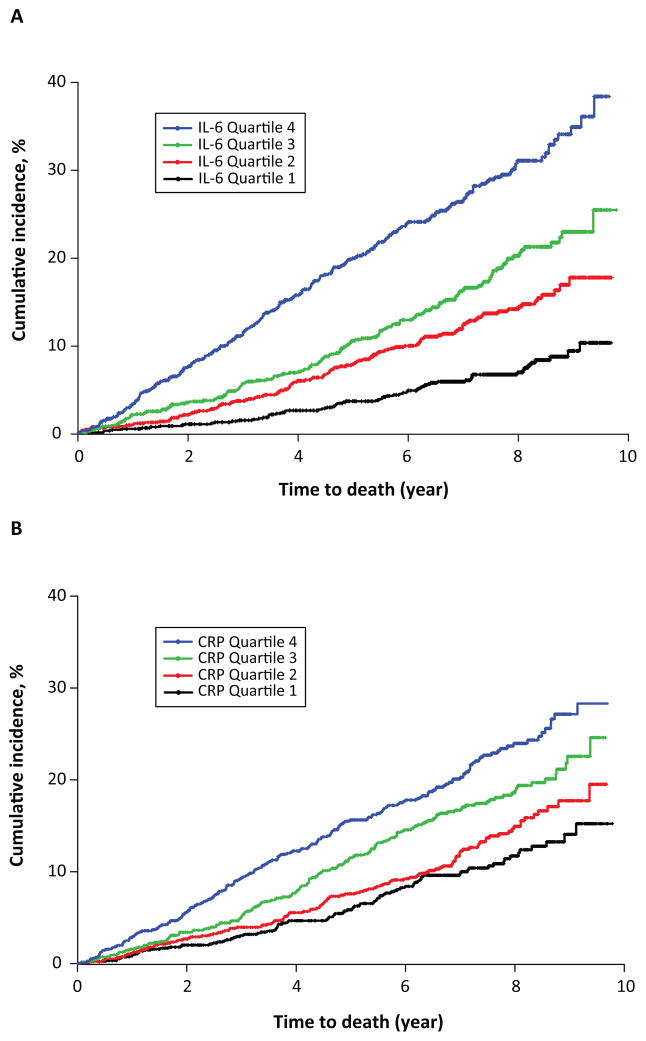
Higher levels of IL-6 and CRP, on the continuous scale and in quartiles, were each independently associated with increased risk of death in unadjusted analyses and in multivariable models that adjusted for conventional cardiovascular risk factors, and CKD- and inflammation-related factors (Figure 1, Table 2). TNF-α was independently associated with increased mortality in crude and adjusted models, but fibrinogen was associated with increased mortality only in unadjusted analyses of the continuous variable and in the uppermost quartile of the categorical analyses (Supplement Table 2). FGF23 was also independently associated with increased risk of death in all unadjusted and multivariable-adjusted analyses (Table 2). In their separate fully adjusted models, participants in the highest quartiles of IL-6, CRP and FGF23 had 2.65-, 1.89- and 2.48-fold higher risks of death than participants in the respective lowest quartiles of each exposure. These results were qualitatively unchanged when we substituted GFR measured directly as the clearance of iothalamate (iGFR) for eGFR in the subset of 1408 participants with available iGFR measurements (Supplement Table 3). There was no effect modification by age, sex, black race, Hispanic ethnicity, diabetes, history of cardiovascular disease, or baseline eGFR (p for interaction in all adjusted analyses ≥ 0.05).
Figure 1. Kaplan–Meier analysis of cumulative incidence of mortality according to quartiles of inflammatory markers.
(A) IL-6
(B) CRP
Table 2. Individual effects of inflammatory markers and FGF23 on all-cause mortality.
For each exposure, results are presented per 1 standard deviation (SD) increase in the overall population and according to quartiles.
| Hazard Ratio (95% Confidence Interval) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (total) | N (events) | Unadjusted | P* | Model A | P* | Model B | P* | Model C | P* | |
| IL-6 | ||||||||||
| Per 1 SD lnIL-6 | 3875 | 550 | 1.52 (1.43–1.61) | < .0001 | 1.38 (1.28–1.49) | < .0001 | 1.38 (1.28–1.48) | < .0001 | 1.35 (1.25–1.46) | < .0001 |
| Quartile 1 ≤ 1.2 | 970 | 64 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 |
| Quartile 2 ≤ 1.9 | 968 | 113 | 2.03 (1.49–2.75) | 1.43 (1.04–1.97) | 1.39 (1.01–1.92) | 1.34 (0.97–1.85) | ||||
| Quartile 3 ≤ 3.2 | 969 | 145 | 2.80 (2.08–3.75) | 1.74 (1.28–2.37) | 1.73 (1.27–2.36) | 1.69 (1.24–2.31) | ||||
| Quartile 4 ≤ 3.2 | 968 | 228 | 5.00 (3.79–6.60) | 2.93 (2.17–3.95) | 2.83 (2.09–3.83) | 2.65 (1.95–3.60) | ||||
| CRP | ||||||||||
| Per 1 SD lnCRP | 3873 | 550 | 1.39 (1.27–1.51) | < .0001 | 1.31 (1.19–1.43) | < .0001 | 1.28 (1.17–1.40) | < .0001 | 1.28 (1.16–1.40) | < .0001 |
| Quartile 1 ≤ 1.1 | 974 | 91 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 |
| Quartile 2 ≤ 2.6 | 965 | 115 | 1.26 (0.96–1.66) | 1.18 (0.90–1.57) | 1.19 (0.90–1.58) | 1.20 (0.90–1.59) | ||||
| Quartile 3 ≤ 6.5 | 969 | 154 | 1.71 (1.32–2.21) | 1.57 (1.20–2.04) | 1.53 (1.17–2.00) | 1.51 (1.15–1.97) | ||||
| Quartile 4 >6.5 | 965 | 190 | 2.25 (1.75–2.89) | 1.97 (1.51–2.56) | 1.87 (1.43–2.44) | 1.89 (1.44–2.48) | ||||
| FGF23 | ||||||||||
| Per 1 SD lnFGF23 | 3875 | 550 | 1.64 (1.53–1.76) | < .0001 | 1.53 (1.40–1.67) | < .0001 | 1.51 (1.38–1.65) | < .0001 | 1.45 (1.32–1.60) | < .0001 |
| Quartile 1 ≤ 95.8 | 969 | 83 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 | Reference | < .0001 |
| Quartile 2 ≤ 145.6 | 969 | 107 | 1.41 (1.06–1.88) | 1.17 (0.87–1.57) | 1.16 (0.86–1.57) | 1.15 (0.84–1.56) | ||||
| Quartile 3 ≤239.3 | 969 | 146 | 2.25 (1.72–2.95) | 1.63 (1.22–2.19) | 1.65 (1.23–2.22) | 1.54 (1.14–2.09) | ||||
| Quartile 4>239.3 | 968 | 214 | 4.21 (3.26–5.43) | 2.84 (2.11–3.82) | 2.79 (2.07–3.77) | 2.48 (1.81–3.39) | ||||
Model A: Stratified by site and adjusted for age, sex, race, ethnicity, body mass index, diabetes, smoking status, history of cardiovascular disease, systolic blood pressure, estimated glomerular filtration rate, urine albumin-to-creatinine ratio categories, serum albumin, hemoglobin, low-density lipoprotein
Model B: Model A plus use of use of aspirin, beta blockers, statins, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, active vitamin D, nutritional vitamin D, phosphate binders, and steroids.
Model C: Model B plus serum calcium, phosphate and parathyroid hormone.
For quartile analyses, p values correspond to tests for linear trend
IL-6, interleukin-6; CRP, high-sensitivity C-reactive protein; SD, standard deviation
Dual Effects of Inflammatory Markers and FGF23 on Mortality
To test whether elevated levels of inflammatory markers and FGF23 influence mortality in CKD through shared, separate or synergistic pathways, we added FGF23 to the separate crude and multivariable-adjusted IL-6 and CRP models to determine whether adjusting for FGF23 would weaken the association between the inflammatory markers and death. The point estimates that summarize the associations of IL-6, CRP and FGF23 with death were only modestly attenuated in bivariate and multivariable-adjusted models that included each individual inflammatory marker plus FGF23 on their continuous scales (Table 3). In all models, higher levels of IL-6, CRP and FGF23 remained significantly associated with increased risk of death prior to onset of ESRD. In unadjusted and adjusted tests of interaction, we found no evidence that FGF23 modified the effect of IL-6 or CRP on risk of death (p for interactions ≥ 0.05 for each).
Table 3.
Dual effects of inflammatory markers and FGF23 on all-cause mortality
| Hazard Ratio (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | P | Model A | P | Model B | P | Model C | P | |
| IL-6 and FGF23 | ||||||||
| Separate Cox proportional hazards models of each individual exposure (from Table 2) | ||||||||
| per 1 SD of lnIL-6 | 1.52 (1.43–1.61) | < .0001 | 1.38 (1.28–1.49) | < .0001 | 1.38 (1.28–1.48) | < .0001 | 1.35 (1.25–1.46) | < .0001 |
| per 1 SD of lnFGF23 | 1.64 (1.53–1.76) | < .0001 | 1.53 (1.40–1.67) | < .0001 | 1.51 (1.38–1.65) | < .0001 | 1.45 (1.32–1.60) | < .0001 |
| Single Cox proportional hazards model that includes both IL-6 and FGF23 | ||||||||
| per 1 SD of lnIL-6 | 1.39 (1.30–1.48) | < .0001 | 1.30 (1.20–1.41) | < .0001 | 1.30 (1.20–1.41) | < .0001 | 1.29 (1.18–1.40) | < .0001 |
| per 1 SD of lnFGF23 | 1.47 (1.36–1.58) | < .0001 | 1.42 (1.30–1.55) | < .0001 | 1.40 (1.28–1.54) | < .0001 | 1.36 (1.23–1.50) | < .0001 |
| CRP and FGF23 | ||||||||
| Separate Cox proportional hazards models of each individual exposure (from Table 2) | ||||||||
| per 1 SD of lnCRP | 1.39 (1.27–1.51) | < .0001 | 1.31 (1.19–1.43) | < .0001 | 1.28 (1.17–1.40) | < .0001 | 1.28 (1.16–1.40) | < .0001 |
| per 1 SD of lnFGF23 | 1.64 (1.53–1.76) | < .0001 | 1.53 (1.40–1.67) | < .0001 | 1.51 (1.38–1.65) | < .0001 | 1.45 (1.32–1.60) | < .0001 |
| Single Cox proportional hazards model that includes both CRP and FGF23 | ||||||||
| per 1 SD of lnCRP | 1.26 (1.15–1.37) | < .0001 | 1.23 (1.13–1.35) | < .0001 | 1.22 (1.11–1.33) | < .0001 | 1.22 (1.11–1.33) | < .0001 |
| per 1 SD of lnFGF23 | 1.58 (1.46–1.70) | < .0001 | 1.48 (1.35–1.62) | < .0001 | 1.46 (1.34–1.60) | < .0001 | 1.41 (1.28–1.55) | < .0001 |
Model A: Stratified by site and adjusted for age, sex, race, ethnicity, body mass index, diabetes, smoking status, history of cardiovascular disease, systolic blood pressure, estimated glomerular filtration rate, urine albumin-to-creatinine ratio categories, serum albumin, hemoglobin, low-density lipoprotein
Model B: Model A plus use of use of aspirin, beta blockers, statins, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, active vitamin D, nutritional vitamin D, phosphate binders, and steroids.
Model C: Model B plus serum calcium, phosphate and parathyroid hormone.
IL-6, interleukin-6; CRP, high-sensitivity C-reactive protein; SD, standard deviation
Since frailty is common in patients with CKD and is associated with increased risk of mortality,33 we tested whether elevated FGF23 and inflammation might share a causal pathway between frailty and mortality risk. We classified participants as frail based on their self-report of fair or poor health in response to question 1 in the Kidney Disease Quality Of Life (KDQOL) questionnaire. In an unadjusted analysis, frailty was associated with increased risk of mortality [HR 1.94 (95%CI, 1.64 – 2.30)], but the strength of association decreased following adjustments for FGF23 and IL-6 [HR 1.53 95%CI, 1.29 – 1.82], whereas FGF23 and IL6 remained leading predictors of mortality in the fully adjusted model that also included frailty (data not shown). Since infection affects inflammation, we conducted additional sensitivity analyses using leukocytosis as a surrogate of occult infection. Only 137 (3.5%) participants had leukocytosis (white blood cell [WBC] count >10.5 ·109 cells/L), and the results were unchanged in models that adjusted for WBC on the continuous scale or in analyses restricted to participants without leukocytosis (data not shown).
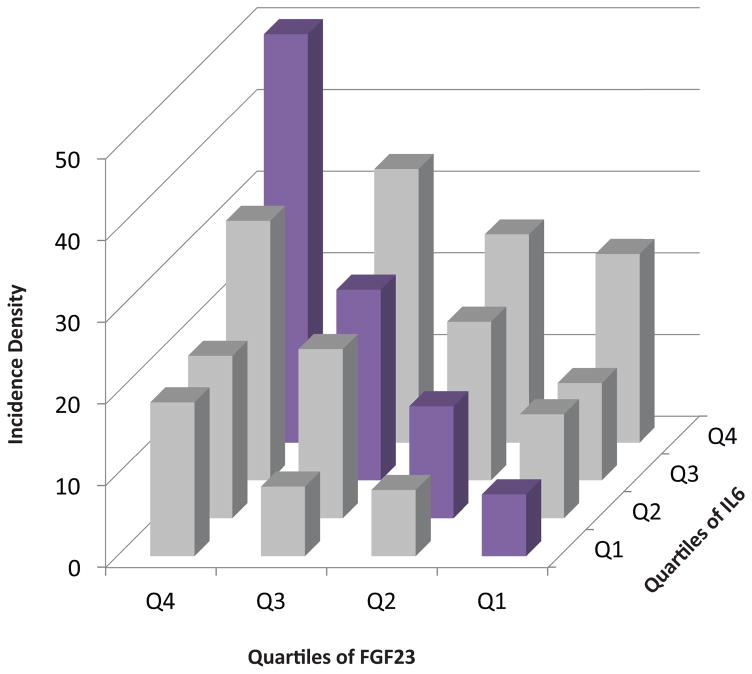
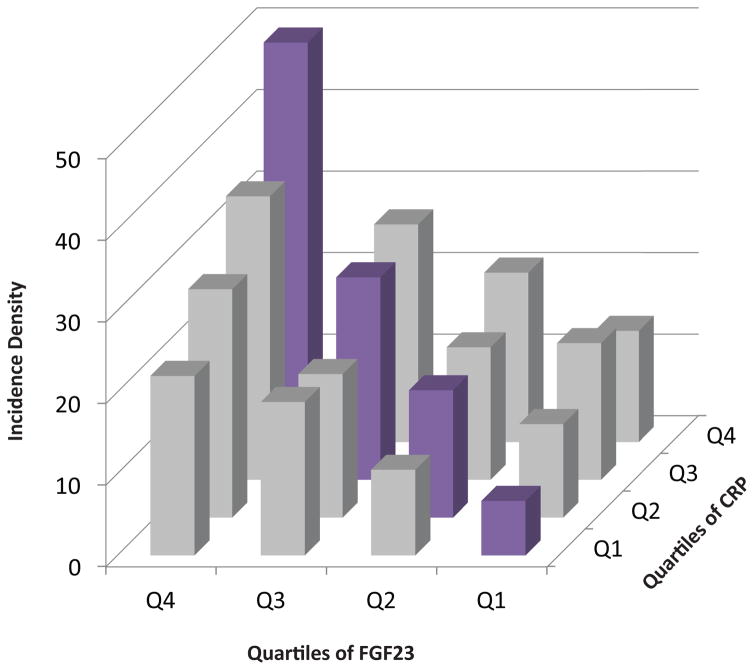
To quantify the dual effects of inflammation and FGF23 in clinically relevant terms, we categorized participants into sixteen groups according to their quartiles of IL-6 and FGF23, and then separately, CRP and FGF23. Membership in ascending quartiles of both IL-6 and FGF23 was associated with additively increased risk of mortality (Figure 2A). Adjusted analyses in which individuals in the lowest quartiles of both IL-6 and FGF23 served as the referent group confirmed additive effects of ascending IL-6 and FGF23 levels on risk of mortality (Table 4). We observed qualitatively similar results for parallel analyses of CRP and FGF23 (Figure 2B, and Table 5). Compared to participants in the lowest quartiles of each, those in the highest quartiles of IL-6 and FGF23 had a 4.38-fold higher adjusted risk of mortality (95%CI, 2.65–7.23), and those in the highest quartiles of CRP and FGF23 had a 5.54-fold higher adjusted risk of mortality (95%CI, 3.04–10.09).
Figure 2. Additive effects of elevated levels of inflammatory markers and FGF23 on risk of mortality.
(A) Crude mortality rates according to combined quartiles of IL-6 and FGF23.
(B) Crude mortality rates according to a baseline quartiles of CRP and FGF23
Table 4.
Multivariable-adjusted hazard ratios of mortality according to combined quartiles of IL-6 and FGF23.
| Total N | Deaths, N | HR (95%CI) | ||
|---|---|---|---|---|
| FGF23 Quartile 1 | IL6 Quartile 1 | 420 | 24 | Reference |
| IL6 Quartile 2 | 256 | 24 | 1.15 (0.63–2.11) | |
| IL6 Quartile 3 | 171 | 15 | 1.12 (0.58–2.17) | |
| IL6 Quartile 4 | 122 | 20 | 1.83 (0.98–3.44) | |
| FGF23 Quartile 2 | IL6 Quartile 1 | 293 | 18 | 0.93 (0.49–1.74) |
| IL6 Quartile 2 | 251 | 25 | 1.08 (0.59–1.97) | |
| IL6 Quartile 3 | 252 | 34 | 1.64 (0.95–2.83) | |
| IL6 Quartile 4 | 173 | 30 | 2.15 (1.22–3.78) | |
| FGF23 Quartile 3 | IL6 Quartile 1 | 172 | 11 | 0.89 (0.43–1.86) |
| IL6 Quartile 2 | 270 | 38 | 1.94 (1.13–3.35) | |
| IL6 Quartile 3 | 296 | 47 | 1.99 (1.17–3.38) | |
| IL6 Quartile 4 | 231 | 50 | 2.65 (1.55–4.51) | |
| FGF23 Quartile 4 | IL6 Quartile 1 | 85 | 11 | 1.94 (0.90–4.15) |
| IL6 Quartile 2 | 191 | 26 | 1.91 (1.03–3.53) | |
| IL6 Quartile 3 | 250 | 49 | 2.75 (1.60–4.74) | |
| IL6 Quartile 4 | 442 | 128 | 4.38 (2.65–7.23) |
Stratified by site and adjusted for age, sex, race, ethnicity, body mass index, diabetes, smoking status, history of CVD, systolic blood pressure, estimated glomerular filtration rate, urine albumin-to-creatinine ratio categories, serum albumin, hemoglobin, low-density lipoprotein; use of aspirin, beta blockers, statins and angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers, active vitamin D, nutritional vitamin D, phosphate binders, and steroids; serum calcium, phosphate and parathyroid hormone.
Table 5.
Multivariable-adjusted hazard ratios of mortality according to combined quartiles of CRP and FGF23.
| Total N | Deaths, N | HR (95%CI) | ||
|---|---|---|---|---|
| FGF23 Quartile 1 | CRP Quartile 1 | 299 | 15 | Reference |
| CRP Quartile 2 | 274 | 23 | 1.51 (0.77–2.99) | |
| CRP Quartile 3 | 208 | 26 | 2.19 (1.13–4.26) | |
| CRP Quartile 4 | 188 | 19 | 1.72 (0.84–3.53) | |
| FGF23 Quartile 2 | CRP Quartile 1 | 259 | 20 | 1.33 (0.67–2.66) |
| CRP Quartile 2 | 269 | 30 | 1.70 (0.88–3.26) | |
| CRP Quartile 3 | 257 | 30 | 1.95 (1.02–3.73) | |
| CRP Quartile 4 | 183 | 27 | 2.32 (1.19–4.51) | |
| FGF23 Quartile 3 | CRP Quartile 1 | 231 | 30 | 2.02 (1.05–3.87) |
| CRP Quartile 2 | 245 | 30 | 1.98 (1.03–3.82) | |
| CRP Quartile 3 | 243 | 41 | 2.85 (1.52–5.33) | |
| CRP Quartile 4 | 250 | 45 | 2.87 (1.54–5.36) | |
| FGF23 Quartile 4 | CRP Quartile 1 | 185 | 26 | 2.86 (1.45–5.65) |
| CRP Quartile 2 | 177 | 32 | 3.32 (1.71–6.43) | |
| CRP Quartile 3 | 261 | 57 | 3.36 (1.80–6.25) | |
| CRP Quartile 4 | 344 | 99 | 5.54 (3.04–10.09) |
Stratified by site and adjusted for age, sex, race, ethnicity, body mass index, diabetes, smoking status, history of CVD, systolic blood pressure, estimated glomerular filtration rate, urine albumin-to-creatinine ratio categories, serum albumin, hemoglobin, low-density lipoprotein; use of aspirin, beta blockers, statins and angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers, active vitamin D, nutritional vitamin D, phosphate binders, and steroids; serum calcium, phosphate and parathyroid hormone.
Discussion
In this prospective cohort study of 3875 participants with CKD enrolled in the CRIC Study, elevated levels of IL-6 and CRP were each independently associated with increased risk of death prior to onset of ESRD. Similar results were observed across levels of age, gender, race, ethnicity, and CKD stage, and among individuals with or without a history of prior cardiovascular disease or diabetes. Interestingly, the strength of association between the inflammatory markers and mortality was only modestly attenuated by adjustment for FGF23, and there was no evidence that FGF23 modified the effects of the inflammatory markers. Instead, elevated levels of inflammatory markers and FGF23 independently increased risk of mortality. These data suggest that elevated levels of inflammatory markers and FGF23 increased risk of mortality mostly through seemingly distinct pathways despite their regulatory effects on one another.
Our results are broadly consistent with prior reports of increased mortality risk in association with elevated levels of inflammatory markers in patients undergoing hemodialysis,34–36 and in earlier stages of CKD,22, 23 but the underlying mechanisms are incompletely understood. Inflammation may increase mortality by aggravating injury of glomeruli, the tubular-interstitial compartment, and vascular endothelium that collectively accelerate CKD progression.5, 37, 38 Indeed, transgenic mice engineered to constitutively overexpress and secrete IL-6 develop progressive glomerulosclerosis and tubular damage.39 Epidemiological studies support an association between inflammation and CKD progression.40–42 Alternatively, inflammation may increase mortality by accelerating atherosclerosis and arterial calcification, and increasing risks of arrhythmias and sudden death.10, 17, 19–21 In support of a direct causal effect of inflammation on cardiovascular disease events in CKD, a recent Mendelian randomization study demonstrated that a functional polymorphism in the IL-6 promoter that is associated with increased IL-6 levels was also associated with increased risk of atherosclerotic cardiovascular events.43
An elevated circulating level of FGF23 has emerged as a strong risk factor for death in patients with CKD.24, 25, 44 In a previous study of 3851 participants in the CRIC Study with CKD stages 2–4, we reported that elevated FGF23 was associated with higher levels of CRP, IL-6, TNF-α and fibrinogen, independent of renal function and other known correlates of FGF23 and inflammation.30 Other studies corroborated these findings.45–48 Inflammation directly induces FGF23 transcription and protein expression in osteocyte cell lines, and increases circulating FGF23 levels in animal models with and without CKD, in part, through a hypoxia inducible factor 1α-dependent mechanism.31 Conversely, elevated FGF23 was sufficient to induce hepatic production of IL-6 and CRP via an FGFR4-dependent but α-Klotho independent mechanism.32 Based on these experimental studies that suggest a positive feedback loop between inflammation and elevated FGF23 levels in CKD, we hypothesized that FGF23 and inflammation would have overlapping effects on mortality that would manifest as attenuation of each exposure’s individual effects when they were combined in a single multivariable model. Contrary to our hypothesis, we found independent effects of FGF23 and inflammatory markers on risk of mortality. We also found no evidence that inflammation and elevated FGF23 were dual downstream consequences of frailty or occult infection. We speculate that while FGF23 and inflammation may directly increase one another, they may have distinct downstream effects that account for their mostly additive impact on mortality. For example, FGF23 might preferentially increase risks of cardiovascular and infectious death due to consequences of left ventricular hypertrophy, heart failure, and impaired leukocyte function,49, 50 whereas inflammation might preferentially increase risk due to occlusive atherosclerotic events.43
This observational study has limitations. We adjusted for numerous factors that affect inflammation, FGF23, and mortality, but cannot exclude the possibility of residual confounding by unmeasured covariates. Misclassification of true GFR by its estimation is one potential source of residual confounding, however, subgroup analyses of participants with directly measured iGFR yielded congruent results. Lack of vitamin D levels, which are modified by FGF23 and may have effects on inflammatory markers, is another limitation. The single measurements of FGF23 and inflammatory markers that we analyzed may not accurately reflect long-term exposure to these risk factors. However, since random misclassification due to biological variability will usually lead to underestimation of true associations, this limitation is unlikely to explain our findings. Furthermore, inflammation may downregulate renal expression of α-Klotho, which is the co-receptor for FGF23 in the kidney.51 Klotho expression is reduced in CKD,52 but in the absence of a validated assay for soluble Klotho, we could not test whether Klotho downregulation due to inflammation contributed to increased mortality associated with elevated levels of inflammatory markers and FGF23.
In conclusion, elevated levels of IL-6, CRP and FGF23 are independent risk factors for mortality in CKD. Despite the independent association between elevated levels of inflammatory markers and FGF23 in patients with CKD, FGF23 neither potentiates nor markedly attenuates the relationship between inflammation and death. Future studies should investigate the impact of FGF23-lowering interventions on inflammatory markers; the impact of anti-inflammatory therapies on FGF23 levels, for example, pentoxifylline and bariticinib, which reduce proteinuria in diabetic kidney disease possibly by anti-inflammatory mechanisms;53, 54 and potential additive effects of combining such therapeutic strategies to improve clinical outcomes in CKD.
Methods
Study population
The CRIC Study is a prospective cohort study of risk factors for cardiovascular disease, CKD progression and mortality among individuals with moderate CKD at enrollment. Adult participants aged 21–74 years with an age-stratified eGFR of 20 – 70 ml/min/1.73m2 at the screening visit were enrolled from seven clinical centers across the United States between 2003 and 2008.55, 56 Participants were excluded for pregnancy, New York Heart Association class III–IV heart failure, human immunodeficiency virus, cirrhosis, myeloma, renal cancer, recent chemotherapy or immunosuppressive therapy, polycystic kidney disease, organ transplantation, or previous treatment with dialysis for at least one month. The study was approved by the institutional review boards at each participating clinical center, and all participants provided written informed consent.
Exposure and Outcomes
The primary exposures were plasma IL-6, high-sensitivity CRP and FGF23. The primary outcome was all-cause mortality prior to the onset of ESRD. Participants’ follow up was censored at the time of voluntary withdrawal from the study, loss to follow-up, onset of ESRD, or database locking in mid-2013. In secondary analyses, we investigated tumor necrosis factor (TNF)-α and fibrinogen as additional inflammatory exposures.
Data collection and measurements
Data collected at the baseline visit included demographics, past medical history, smoking status, body mass index (BMI), use of medications, and laboratory test results. Using batched assays of stored plasma samples, the CRIC Study Central Laboratory at the University of Pennsylvania measured IL-6 and TNF-α by enzyme-linked immunosorbent assay (ELISA; R&D systems, Minneapolis, MN; coefficient of variation [CV] <15%); high-sensitivity CRP by particle enhanced immunonephelometry (Dade Behring – Siemens Healthcare, CV<5%); fibrinogen by immunochemical reaction (Dade Behring - Siemens Healthcare, CV<5%); second generation C-terminal FGF23 by ELISA (Immutopics, San Clemente, CA; CV <5%); and parathyroid hormone (PTH) using a total PTH assay that detects both the 1–84 molecule and 7–84 fragment (Scantibodies, Santee, CA; CV<5%). Standard assays were used to measure comprehensive metabolic panels. Glomerular filtration rate was estimated based on the CKD-Epidemiology Collaboration equation (CKD-EPI)57 and by direct measurement of iGFR in a subgroup of 1408 participants. Albuminuria was quantified as the urinary ACR.
Statistical Analysis
We used descriptive statistics to compare clinical characteristics after categorizing participants in quartiles of baseline IL-6 levels. We chose IL-6 as the inflammatory marker for the descriptive analyses since inflammatory markers are highly inter-correlated and because IL-6 was most strongly associated with mortality and with FGF23 in prior studies of CKD populations.23, 30, 35 Continuous variables were summarized as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical variables were expressed as frequencies and proportions. Due to their skewed distributions, we natural log (ln)-transformed IL-6, CRP, FGF23, and PTH.
We used Kaplan-Meier curves to estimate cumulative incidence of mortality according to quartiles of inflammatory markers. We used time-to-event analyses to examine ESRD-censored hazard ratios (HR) of mortality according to one SD increment in baseline ln-transformed IL-6, CRP and FGF23 levels on the continuous scale, and in quartiles, defining the lowest quartile as the reference group. We analyzed IL-6, CRP and FGF23 in separate Cox proportional hazards models using an identical multivariable modeling strategy. These models were adjusted for conventional cardiovascular risk factors, and CKD- and inflammation-related factors. In model A, we adjusted for demographics (age, sex, race, ethnicity), CKD factors (eGFR, urinary ACR categories [< or ≥300 mg/g, or missing in 3.5% of participants], hemoglobin, serum albumin), and cardiovascular disease risk factors (diabetes, systolic blood pressure, BMI, smoking status, low-density lipoprotein, prior history of cardiovascular disease). In model B, we further adjusted for use of cardio- and reno-protective medications (aspirin, beta blockers, statins, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers), and for use of medications that may alter levels of FGF23 or inflammatory markers (nutritional vitamin D, active vitamin D, phosphate binders, corticosteroids). In model C, we adjusted for the same covariates as in model B plus serum calcium, phosphate and PTH levels.
Since inflammation is associated with sarcopenia,58 which can reduce the reliability of eGFR as an estimate of true GFR, we performed a sensitivity analysis in which we repeated the main analyses but adjusted for directly measured iGFR in place of eGFR in the 1408 participants in whom iGFR was measured. We used the response to question 1 in the KDQOL questionnaire as a surrogate measure of frailty: In general, would you say your health is: excellent, very good, good, fair, or poor. Participants who answered fair or poor were classified as frail, and we examined models that sequentially adjusted for frailty, FGF23 and inflammation. We adjusted for WBC count and performed a sensitivity analyses that excluded participants with leukocytosis (WBC > 10.5·109 cells/L). We tested for effect modification by age, sex, black race, Hispanic ethnicity, diabetes, history of cardiovascular disease, and baseline eGFR. All models were stratified by study site to account for possible regional variability in baseline hazards. We used the same strategy in the secondary analyses of TNF-α and fibrinogen as the inflammatory exposures.
To quantify the dual effects of inflammation and FGF23 on risk of mortality in clinically interpretable terms, we categorized participants into sixteen groups defined by their quartiles of IL-6 and FGF23 (4 by 4 groups). We calculated unadjusted mortality rates per 1000-person-years of follow-up for each group, and multivariable-adjusted HRs for mortality using the same covariates as in model C. The referent group for these analyses included those participants in the lowest quartiles of IL-6 and FGF23. We repeated the analytic strategy for CRP and FGF23. Two-sided p-values <0.05 were considered statistically significant for all tests, including tests for interaction. P values were not adjusted for multiple comparisons. We analyzed the data using SAS, version 9.4 (SAS Institute, Cary, NC) and R version 3.2.2 (2015-08-14) (http://cran.r-project.org).
Supplementary Material
Acknowledgments
*CRIC Study Investigators include: Lawrence J. Appel, MD, MPH, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, and Raymond R. Townsend, MD.
This study was supported by grants R01DK081374-S1 (JMM), R01DK080033 (SER), R01DK073665 (DSR), R01DK081374 (MW), K24DK093723 (MW), and a Strategically Focused Research Network Center Grant from the American Heart Association (MW).
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003; Johns Hopkins University UL1 TR-000424; University of Maryland GCRC M01 RR-16500; Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research; Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433; University of Illinois at Chicago CTSA UL1RR029879; Tulane University Translational Research in Hypertension and Renal Biology P30GM103337; and Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Matsushita K, Woodward M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggins JE, Patel SR, Shedden KA, et al. NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J Am Soc Nephrol. 2010;21:587–597. doi: 10.1681/ASN.2009060663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman DL, Ruef C. Interleukin-6: an autocrine regulator of mesangial cell growth. Kidney Int. 1992;41:604–606. doi: 10.1038/ki.1992.91. [DOI] [PubMed] [Google Scholar]
- 6.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 7.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansson GK. Immune and inflammatory mechanisms in the development of atherosclerosis. Br Heart J. 1993;69:S38–41. doi: 10.1136/hrt.69.1_suppl.s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La Mata J, Uy HL, Guise TA, et al. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Invest. 1995;95:2846. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadra I, Mason JC, Philippidis P, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 13.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 15.Gupta J, Dominic EA, Fink JC, et al. Association between inflammation and cardiac geometry in chronic kidney disease: Findings from the CRIC Study. PLoS One. 2015;10:e0124772. doi: 10.1371/journal.pone.0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Mohler ER, 3rd, Xie D, et al. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am J Cardiol. 2012;110:136–141. doi: 10.1016/j.amjcard.2012.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Stenvinkel P, Heimbürger O, Jogestrand T. Elevated interleukin-6 predicts progressive carotid artery atherosclerosis in dialysis patients: association with Chlamydia pneumoniae seropositivity. Am J Kidney Dis. 2002;39:274–282. doi: 10.1053/ajkd.2002.30546. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Odamaki M, Takita T, et al. Association between interleukin-6 and carotid atherosclerosis in hemodialysis patients. Kidney Int. 2002;61:1143–1152. doi: 10.1046/j.1523-1755.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 20.Stompór T, Pasowicz M, Sułowicz W, et al. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41:203–211. doi: 10.1053/ajkd.2003.50005. [DOI] [PubMed] [Google Scholar]
- 21.Amdur RL, Mukherjee M, Go A, et al. Interleukin-6 Is a Risk Factor for Atrial Fibrillation in Chronic Kidney Disease: Findings from the CRIC Study. PLoS One. 2016;11:e0148189. doi: 10.1371/journal.pone.0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabner A, Amaral AP, Schramm K, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Met. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2015 doi: 10.1038/ki.2015.290. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Grabner A, Yanucil C, et al. FGF23 directly targets hepatocytes to promote inflammation in CKD. Kidney Int. 2016 doi: 10.1016/j.kint.2016.05.019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilhelm-Leen ER, Hall YN, Tamura MK, et al. Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. The American journal of medicine. 2009;122:664–671. e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanner C, Zimmermann J, Schwedler S, et al. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002:99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 35.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Rao M, Guo D, Perianayagam MC, et al. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2005;45:324–333. doi: 10.1053/j.ajkd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Miller D, Ruoslahti E, et al. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int. 1992;41:1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- 38.Bertani T, Abbate M, Zoja C, et al. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134:419–430. [PMC free article] [PubMed] [Google Scholar]
- 39.Fattori E, Della Rocca C, Costa P, et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. 1994;83:2570–2579. [PubMed] [Google Scholar]
- 40.Bash LD, Erlinger TP, Coresh J, et al. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2009;53:596–605. doi: 10.1053/j.ajkd.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 42.Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spoto B, Mattace-Raso F, Sijbrands E, et al. Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol. 2015;10:232–240. doi: 10.2215/CJN.07000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manghat P, Fraser WD, Wierzbicki AS, et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2010;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 47.Ashikaga E, Honda H, Suzuki H, et al. Impact of fibroblast growth factor 23 on lipids and atherosclerosis in hemodialysis patients. Ther Apher Dial. 2010;14:315–322. doi: 10.1111/j.1744-9987.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 48.Singh R, Chennasamudram S, Sheth S, et al. Correlation of fibroblast growth factor 23 with markers of inflammation and endothelial dysfunction in end-stage renal disease and type 2 diabetes patients on peritoneal dialysis. J Diabetes Metab. 2014;5:2. [Google Scholar]
- 49.Rossaint J, Oehmichen J, Van Aken H, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126:962–974. doi: 10.1172/JCI83470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chonchol M, Greene T, Zhang Y, et al. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. J Am Soc Nephrol. 2016;27:227–237. doi: 10.1681/ASN.2014101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno JA, Izquierdo MC, Sanchez-Niño MD, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu MC, Shiizaki K, Kuro-o M, et al. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navarro JF, Mora C, Rivero A, et al. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis. 1999;33:458–463. doi: 10.1016/s0272-6386(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 54.Tuttle K, Adler S, Kretzler M. Baricitinib in diabetic kidney disease: results from a phase 2, multicenter, randomized, double-blind, placebocontrolled study. American Diabetes Association Meeting; Boston MA. 2015. [Google Scholar]
- 55.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 56.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaap LA, Pluijm SM, Deeg DJ, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e529–526.e517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.