Abstract
Tubulointerstitial fibrosis, tubular atrophy and peritubular capillary rarefaction are major hallmarks of chronic kidney disease. The tubulointerstitium consists of multiple cell components including tubular epithelia, mesenchymal (fibroblasts and pericytes), endothelial, and inflammatory cells. Crosstalk among these cell components is a key component in the pathogenesis of this complex disease. Following severe or recurrent injury, the renal tubular epithelial cells undergo changes in structure and cell cycle which are accompanied by altered expression and productions of cytokines. These cytokines contribute to the initiation of the fibrotic response by favoring activation of fibroblasts, recruitment of inflammatory cells, and loss of endothelial cells. This review focuses on how augmented growth factor and cytokine production induces epithelial crosstalk with cells in the interstitium to promote progressive tubulointerstitial fibrosis after renal injury.
Keywords: interstitium, epithelial cells, fibroblasts, endothelial cells, inflammation, fibrosis
The hallmark of chronic kidney diseases is tubulointerstitial fibrosis (TIF) which has the histopathology features of extracellular matrix (ECM) accumulation, tubular atrophy, inflammatory cell infiltration, and peritubular microvasculature loss. This pathology is the common endpoint of chronic kidney diseases of multiple etiologies including glomerular insults, repeated acute kidney injury, and chronic tubulointerstitial injuries. The tubulointerstitium consists of multiple cell components including tubular epithelia, mesenchymal (fibroblasts and pericytes), endothelial, and inflammatory cells, all of which contribute to fibrosis progression. In addition, altered matrix metalloproteinase enzyme activity and disruptions in the tubular basement membrane can promote growth factor release and communication between the epithelial and interstitial compartments(1). The interplay among these cells is highly complex and, although initially aimed at tubular repair and recovery following injury, may become unregulated and accelerate tubular atrophy and TIF progression.
Tubular epithelia, in particular the proximal tubules, are targeted by acute and chronic injuries. The injured epithelia de-differentiate and proliferate resulting in repair after acute kidney injury(2). When epithelial injury occurs repetitively or persists over time, tubular apoptosis may occur and lead to progressive TIF. This was nicely demonstrated with the diphtheria toxin model in which mice with repeated tubule-specific injury leading to apoptosis developed interstitial fibrosis, tubular atrophy, and inflammation(3). These dying epithelial cells may elicit pro-inflammatory cytokines and other growth factors that promote inflammation and fibrosis, but more research is required to elucidate the mechanisms whereby epithelial apoptosis leads to TIF(4). Tubular apoptosis may be sufficient but not necessary to promote TIF as even sub-lethal epithelial injury alters the structure and function of these tubules in ways that can also lead to progressive renal dysfunction. More than a decade ago, it was postulated that the injured epithelia undergo epithelial to mesenchymal transformation (EMT) and transform into interstitial mesenchymal cells that are responsible for ECM production and ultimately fibrosis(5). Many factors including TGF-β, HIF-1α, and integrin-linked kinase have been implicated in EMT in vivo(6–8), and prevention of TGF-β-mediated signaling by BMP-7 was proposed to be beneficial in reversing TGF-β-mediated tubulointerstitial fibrosis(6). Although the origin of fibroblasts still remains controversial, a recent study showed that only 5% of fibroblasts originate from EMT(9), and lineage tracing studies have shown that fibroblasts derive from resident mesenchymal cells and pericytes that are PDGFRβ positive(10, 11).
Regardless of whether epithelia undergo EMT, the injured proximal tubule clearly de-differentiates and undergoes cell cycle changes. The de-differentiated epithelial cells acquire a partial mesenchymal phenotype which is associated with increased production of pro-fibrotic cytokines. Although injured epithelia are unlikely to be the main producers of ECM, they are important producers of growth factors that have paracrine effects on resident fibroblasts/pericytes. Consistent with this, deletion of Snail and Twist, transcription factors that promote de-differentiation by repressing E-cadherin, reduced TIF after renal injury (12, 13). In addition, chronically injured epithelia become arrested in G2/M, and this cell cycle dysfunction is also associated with excessive production of pro-fibrotic growth factors(14). G2/M cell cycle arrest induced increased JNK (c-jun N-terminal kinase) activity which augmented production of TGF-β and CTGF/CCN2 (connective tissue growth factor)(14). Thus, the chronically injured epithelial cells undergo changes in cell structure and cell cycle which are accompanied by increases in cytokine production (Table 1). This review focuses on how epithelial injury, through augmented growth factor production that induces either autocrine signaling or crosstalk with interstitial cells, promotes progressive TIF after renal injury.
Table 1.
List of cytokines and growth factors produced by epithelial cells described in this review and some of their effects in the progression of chronic kidney disease
| FACTOR | EFFECT | REFERENCES |
|---|---|---|
|
| ||
| pro-fibrotic tubular cell dedifferentiation |
7 | |
| TGF-β | fibroblast to myofibroblast differentiation pro-fibrotic |
23–25,30 |
| PDGF | fibroblast proliferation fibroblast to myofibroblast differentiation pro-fibrotic recruitment of pericytes |
34,36 |
| Hh | fibroblast proliferation pro-fibrotic |
39,40,43 |
| CTGF | fibroblast proliferation pro-fibrotic |
31 |
| Wnt | fibroblast to myofibroblast differentiation | 49,50 |
| MCP-1 | mobilization of macrophages | 74–76 |
| RANTES | mobilization of macrophages | 75,77 |
| CSF-1 | macrophage recruitment and proliferation polarization into an M2 phenotype |
79,80 |
| CX3CL1 | macrophage recruitment and adhesion survival of pro-fibrotic macrophages |
81,82 |
| Thrombospondin-1 | activation of TGF-β anti-angiogenesis |
92 |
| VEGF | endothelial cell proliferation and survival macrophage recruitment |
94–96 |
| TIMP | fibroblast proliferation fibroblast to myofibroblast differentiation |
54,55 |
| Lcn2 | fibroblast production of ECM epithelial production of ECM |
52,56 |
Epithelial/epithelial and epithelial/fibroblast crosstalk
Injured epithelia are potent producers of growth factors and cytokines such as TGF-β, PDGF, hedgehog and Wnt ligands. These growth factors may initially promote regeneration of the injured epithelia but, in persistent injury, have paracrine effects on surrounding cells such as fibroblasts causing them to transform into myofibroblasts. Activated fibroblasts have increased stress fibers, proliferate, and produce ECM components like collagens leading to progressive TIF. Activated fibroblasts are difficult to study, in part, because they were initially defined by ultrastructural features on electron microscopy, and there are no markers that identify these cells specifically(15). The expression of α-SMA is commonly used as an indicator of myofibroblasts, but we and others have found that α-SMA inconsistently marks collagen I-producing fibroblasts after injury(16, 17). Given the heterogeneity of fibroblasts isolated from injured kidneys, it is likely that many different subsets of fibroblasts exist with different expression profiles and functions.
There is strong evidence for epithelial/fibroblast crosstalk through growth factors in vitro, but defining it in vivo is difficult due to the various other cells (e.g. inflammatory cells, endothelial cells) that also produce pro-fibrotic growth factors. In support of epithelial/fibroblast crosstalk, a rat model of tubular injury found that activated fibroblasts surrounded only those proximal tubules with evidence of injury(18). Epithelial/fibroblast crosstalk is important in both renal development and tumorigenesis and is mediated by many of the same growth factors up-regulated after renal injury(19–21). These growth factors include members of the TGF-β superfamily, Notch, Wnt, and Hedgehog pathways, and the importance of developmental signaling pathways in renal fibrosis was recently reviewed (22). These pleiotropic growth factors most likely mediate all TIF regardless of etiology, but we currently have little information on the role of specific growth factors in different disease processes. One of the major reasons is that most studies investigating TIF progression utilize the unilateral ureteral obstruction model (UUO). While this is considered the standard model of TIF, it has many limitations as it induces extreme injury in a short time period resulting in destruction of the kidney without producing functional data such as altered glomerular filtration rate.
TGF-β is considered one of the strongest pro-fibrotic growth factors due to its ability to activate fibroblasts into myofibroblasts and stimulate matrix production(23–25). The injured tubular epithelium is a potent source of TGF-β though other cells (macrophages, fibroblasts, and cells in the vasculature) also produce this growth factor (23–26). There are three TGF-β isoforms (−β1, β2, β3), and TGF-β1 is considered the primary mediator of TIF progression in the kidney. Injured epithelial cells may increase TGF-β, not only through production of ligands, but also by augmented expression of integrin αvβ6, an important activator of latent TGF-β,(27, 28) or through transfer of TGF-β mRNA to fibroblasts via exosomes(29). A transgenic model in which tubule cells overexpress TGF-β resulted in tubular damage as well as fibroblast proliferation and transformation into myofibroblasts, suggesting that epithelial-derived TGF-β has both autocrine and paracrine effects(30). Thus, increased TGF-β produced by injured epithelia may promote TIF through paracrine signaling on surrounding fibroblasts.
Augmented epithelial TGF-β production may also induce crosstalk with fibroblasts through other growth factors. After rodent ischemia-reperfusion (I/R), an acute injury that can lead to TIF, injured tubules in fibrotic tissue had increased TGF-β activity as measured by phosphorylation of downstream Smad2 as well as increased PDGF-B (platelet derived growth factor) and CTGF/CCN2(31). Furthermore, blockade of the TGF-β type I receptor reduced PDGF-B and CTGF protein expression after I/R and suppressed their mRNA levels after LPA (lysophosphatidic acid) stimulation of proximal tubules in vitro(31). Thus, autocrine TGF-β signaling may increase epithelial production of PDGF-B and CTGF/CCN2 that can signal on neighboring fibroblasts and promote TIF progression.
TGF-β is arguably the most potent activator of fibroblasts, but it is not required for fibroblast proliferation and matrix production as deletion of the type II receptor (TβRII), required for signaling, on fibroblasts did not rescue TIF in either the UUO or aristolochic acid models of injury(16). Another group found a modest (29%) reduction in fibrosis after UUO when deleting TβRII in fibroblasts(9). These differences are likely due to differing strategies to target fibroblasts: one group used the COL1A2-Cre and the Tenascin C-Cre, both of which target matrix-producing interstitial cells(16), whereas the other group used the α-SMA-Cre to target myofibroblasts(9). The loss of TβRII in α-SMA+ cells primarily affected proliferation of bone-marrow-derived cells rather than resident fibroblasts; cells that were probably not targeted by COL1A2 or Tenascin-Cre. The surprisingly negative result produced by deleting TβRII with either the COL1A2 or Tenascin-Cre may have been due to recombination efficiency, however, the percentage of cells that underwent recombination was quantitatively equal to the α-SMA positive cells(16). A more likely explanation for the negative finding is that inhibiting TGF-β signaling in fibroblasts may not be sufficient to prevent TIF progression because other growth factor pathways can also activate fibroblasts and stimulate ECM production. Consistent with this recent pre-clinical data, clinical trials targeting TGF-β for the treatment of diabetic nephropathy have not shown a benefit. Both Genzyme and Lilly developed neutralizing antibodies to TGF-β1 (CAT-192 and LY2382770, respectively) that failed to have a benefit in diabetic nephropathy (32). Possible explanations for these disappointing results are that although TGF-β1 is the ligand most strongly linked to renal fibrosis, compensatory upregulation of other isoforms may have negated any benefit. Alternatively, other growth factors (e.g. PDGF, Wnt, hedgehog, EGF) play an important role in promoting CKD progression, suggesting that targeting just one growth factor is insufficient to alter the course of TIF.
The PDGF pathway is another mediator of epithelial/fibroblast crosstalk that may be important in the pathophysiology of CKD. The PDGF-B ligand, implicated in interstitial fibrosis, has increased expression in atrophic, injured tubules but not in healthy renal epithelia(33–35). However, expression of the PDGFRα and PDGFRβ receptors is restricted to interstitial fibroblasts (36). Blockade of PDGF signaling by anti-PDGFR antibody reduced fibroblast numbers, expression of α-SMA, and renal fibrosis in the UUO injury model (36). It is tempting to speculate that PDGF-B (or other ligands) produced by injured epithelia augment PDGFR phosphorylation and subsequent signaling on neighboring fibroblasts, but other cells (endothelial cells, macrophages) also produce PDGF ligands(37, 38). Therefore, studies with tubule-specific ablation of these ligands are necessary to determine whether tubular/fibroblast crosstalk is responsible for PDGF-dependent effects or if another cell type is also involved.
Injured epithelia also utilize the hedgehog pathway, upregulated in injured human and murine kidneys, to signal to neighboring fibroblasts and potentially promote fibrosis progression. The three hedgehog secreted ligands (Hh) bind to the Patched1 (Ptch1) membrane receptor, and this binding releases the protein smoothened (Smo) which facilitates transcription of the Gli1/2 effectors. Immunostaining and reporter mice show that while the Hh ligands are primarily expressed on tubular epithelial cells, the receptor Ptch1 and the Gli transcriptional targets are exclusively expressed by fibroblasts(39–41). The spatially restricted expression pattern of hedgehog ligands and effectors strongly suggests an epithelial/fibroblast paracrine signaling pattern. The hedgehog ligands augment proliferation of fibroblasts in vitro(39, 40). However, pharmacologic inhibition of the hedgehog pathway (Smo inhibitor) led to conflicting effects on renal fibrosis(39, 40). Genetic and pharmacologic inhibition of Gli1/2 have been successful in reducing fibrosis after either UUO or I/R, though there is some evidence that other pathways (e.g. TGF-β) may also regulate this protein(40, 42–44). Thus the hedgehog pathway likely mediates epithelial/fibroblast crosstalk, however the mechanisms whereby it promotes TIF after renal injury requires further investigation.
The Wnt/β-catenin pathway is implicated in epithelial repair, but studies also show that Wnt signaling mediates tubulointerstitial communication that promotes fibrosis(45, 46). The Wnt pathway consists of 19 Wnt ligands which bind to Frizzled and LRP5/6 receptors leading to canonical signaling through β-catenin nuclear localization and stimulation of Wnt target genes. Wnt ligands and receptors are upregulated after renal injury, and β-catenin activity is increased in injured tubules(47, 48). Overexpression of Wnt1 ligand specifically in renal proximal tubule cells is sufficient to cause TIF with evidence of increased Wnt/β-catenin activity in neighboring fibroblasts, suggesting paracrine signaling(49). As the epithelia producing Wnt1 did not show evidence of injury, the TIF was likely a direct effect of Wnt1 ligand on surrounding stromal cells. Genetic stabilization of β-catenin (i.e. β-catenin activation) in interstitial fibroblasts was sufficient to induce TIF without further injury(50). Data showing that epithelial-specific deletion of β-catenin had little effect on renal fibrosis further support the consensus that fibroblasts mediate the deleterious effects of Wnt/β-catenin signaling in injury(51). It is likely that injured tubular epithelia produce Wnt ligands which then activate neighboring fibroblasts to promote TIF, but exactly which Wnt ligands and receptors mediate this critical crosstalk is unclear.
Recently, the transcription factor Stat3 was shown to be a critical mediator of epithelial/fibroblast crosstalk leading to TIF by affecting multiple profibrotic genes (52). Transcriptomic analysis from renal tissues revealed that the Jak/Stat pathway was upregulated in human diabetic nephropathy (53). Stat3 mediates signals of both inflammatory- (e.g. interferon receptors, IL-6R) and growth factor-dependent pathways implicated in fibrogenesis (e.g. PDGFR, FGFR). Tubule-specific deletion of Stat3 in mice protected against TIF after 75% nephron reduction, and this was associated with diminished expression of PDGF-B, Lcn2 (lipocalin/NGAL), and TIMP1(52). As PDGF-B and TIMP-1 have been implicated in fibroblast proliferation (54, 55) and Lcn2 can augment fibroblasts’ production of collagen I (56), Stat3 may mediate pro-fibrotic epithelial/fibroblast crosstalk through multiple targets. Given that the numerous pathways that both activate Stat3 and are downstream of Jak/Stat signaling, Stat3 likely coordinates cellular crosstalk that extends beyond epithelial/fibroblast interactions (e.g. inflammatory/epithelial). A phase II study using the Jak1/2 inhibitor baricitinib reduced proteinuria in patients with diabetic nephropathy (57). Thus targeting the Jak/Stat pathway may reduce tubulinterstitial fibrosis but further data are needed on long term functional changes (e.g. GFR).
Another growth factor produced by tubular cells that plays a role in the response to acute and chronic renal injury is the epidermal growth factor (EGF). However, the overall role of this growth factor and its receptors (e.g., EGF receptor) in tubular kidney injury is controversial. In both ischemic and toxin models of AKI, exogenous EGF promotes renal recovery by enhancing tubular proliferation (58, 59). Consistent with this, both mice expressing functionally hypomorphic EGF receptor (waved-2 mice) and mice with proximal tubule-specific deletion of the EGF receptor had delayed recovery from AKI (60, 61). Although this pre-clinical data suggests that EGF signaling has a beneficial role in AKI, animal models of chronic kidney injury indicate that EGF signaling promotes TIF progression. Genetic or pharmacologic inhibition of the EGF receptor significantly decreased TGF-β-induced proximal tubule fibrogenesis (62). Similarly, the waved-2 mice had reduced fibrosis in both the UUO and a model of chronic renal ischemia (63, 64). Interestingly, EGF has been recently identified as a biomarker of human CKD progression (65). Lower urinary EGF levels correlated with kidney transcript levels and, somewhat surprisingly, higher amounts of tubular atrophy, TIF, and rate of progression (65). However, this study investigated EGF as a biomarker rather than mediator of CKD, and the lower EGF levels may just reflect a diminished number of healthy tubular cells that can produce this growth factor. Another potential explanation for the discrepancy between the pre-clinical CKD data and this human biomarker study is that EGF receptor activation may be detrimental through an EGF-independent ligand (66–68). Clearly, more research is warranted to define how EGF and its receptor contribute to human CKD progression.
In conclusion epithelial-derived growth factors do play a key role in activating tubular cells and/or fibroblasts and stimulating their proliferation, migration, and matrix production. TGF-β is considered the master inducer of myofibroblast formation and matrix production, but other growth factor pathways also play an important role. Multiple growth factor pathways, including some not discussed (e.g. Notch), that regulate renal development become re-expressed or upregulated after injury(69). Many of these pathways have synergistic actions and promote epithelial regeneration and wound healing, but if signaling is prolonged or excessive, can also lead to TIF independent of TGF-β signaling. Although the increased ECM production is in response to epithelial injury, the augmented peritubular fibrosis can potentially exacerbate injury by worsening epithelial hypoxia, further accelerating the progression of TIF. A better understanding of how growth factors interact and which downstream signaling pathways regulate TIF may lead to more therapeutic targets.
Epithelial crosstalk with inflammatory cells
The injured epithelia also produce cytokines that affect interstitial inflammatory cells, specifically macrophages and dendritic cells (DC), which are important modulators of chronic kidney disease. Macrophages/DCs populate the uninjured renal interstitium and act as antigen-presenting cells and macrophages also have an important phagocytic function. After renal injury, these inflammatory cells expand both through local proliferation and infiltration of circulating monocytes and subsequent differentiation. Macrophages/DCs in injured kidneys display considerable plasticity and functional heterogeneity with distinct and overlapping roles. The subsets of macrophages/DCs are classified based upon expression of certain markers (e.g. F4/80, CD11b/c, Ly6C), and has been reviewed by others(70, 71). Although macrophages can be classified into many subsets, they are broadly described as either a pro-inflammatory, M1 (classically activated) or a wound healing and pro-fibrotic M2 (alternatively activated) phenotype(70). In acute kidney injury, M1 promotes inflammation whereas M2 has a reparative, anti-inflammatory effect. However in CKD, M2 macrophages may promote TIF through production of pro-fibrotic growth factors, and further studies with macrophage-specific deletion of growth factors are necessary to better define their importance in TIF progression. The balance of these different macrophage populations in the injured kidney is influenced by crosstalk with tubular epithelia. Although macrophages can play an important role in removing debris and promoting epithelial recovery from acute injury, increased macrophage infiltration is associated with CKD and increased TIF in human biopsies(72).
Injured renal epithelia modulate the inflammatory response by production of chemokines, chemoattractant cytokines that bind to receptors on inflammatory cells and promote migration of macrophages/DCs to the site of injury. Renal epithelia, through injury or growth factor signaling (e.g. TGF-β), express the chemokines monocyte chemoattractant protein-1 (MCP-1) and RANTES (regulated upon activation, normal T cell expressed and secreted) which bind to the receptors CCR2 and CCR5, respectively, to mobilize macrophages/DCs(73–75). Furthermore, the expression of MCP-1 and RANTES by tubular epithelial cells in human glomerular diseases was also associated with CCR5+ interstitial cell infiltrate and TIF(76, 77). Injured proximal tubules can also promote local macrophage proliferation through the production of macrophage colony stimulating factor (CSF-1)(78).
In addition to inducing quantitative changes in macrophage/DC infiltration, injured epithelia also alter the type of inflammatory infiltrate in the renal interstitium. Recent data indicate that epithelial-derived CSF-1 is not only critical for macrophage recruitment and proliferation, but also polarization into a reparative, M2 phenotype(79, 80). Mice with proximal tubule-specific deletion of CSF-1 had reduced numbers of M2 macrophages 5 days after ischemia/reperfusion injury and increased fibrosis four weeks after injury(79). The chemokine fractalkine (CX3CL1) and its CX3CR1 receptor provide another example whereby injured epithelia qualitatively alter the inflammatory infiltrate. The interaction between fractalkine, expressed on injured epithelia, and CX3CR1, expressed on macrophages/DCs, not only directs macrophage recruitment and adhesion, but also increases the survival specifically of pro-fibrotic macrophages (Ly6C−CX3CR1hi)(81, 82). Thus, injured proximal tubule cells modulate the amount and phenotype of infiltrating macrophages/DCs through production of cytokines.
Precisely how this epithelial/inflammatory cell crosstalk affects the progression of CKD depends upon the effect on macrophage phenotype, the timing relative to injury, and microenvironment. Animal models of acute kidney injury, a risk factor for CKD progression, suggest that a pro-inflammatory, M1 macrophage phenotype predominates at 1–3 days post-injury. Depleting macrophages using clodronate or genetic methods may ameliorate injury in this phase. However, at later time points, there is a switch to a reparative, M2 macrophage subtype which promotes recovery from injury. Depletion of macrophages at this later stage can impair renal recovery and lead to CKD(83, 84). In human CKD kidneys, persistent macrophage infiltration portends a poor prognosis, and reducing renal macrophages ameliorated fibrosis in the UUO and diabetic murine models of chronic injury(85–87). In CKD, unlike AKI, the M2 phenotype likely promotes TIF due to production of factors like TGF-β, PDGF, and galectin3(86). Thus, persistent epithelial injury may stimulate TIF progression through chemokine-dependent, increased M2 macrophage infiltration. Although injured epithelia also alter DC polarization through cytokine production, there is less data to support a role for DCs in TIF progression(88, 89). As most of the studies establishing epithelial/macrophage crosstalk were performed in AKI, further studies on macrophage subtypes in CKD are warranted with the caveat that the UUO may not be an ideal model as its inflammatory component is much greater than in the CKD human kidney. We recognize that other immune cells (lymphocytes, natural killer cells) may also play a role in chronic kidney injury, but have focused primarily on macrophages as their role in chronic injury and crosstalk with epithelia is better defined.
Cellular crosstalk within the vascular compartment
In addition to tubular cells, fibroblasts and immune cells, the vascular component has emerged as a key contributor of interstitial fibrosis(90). During chronic kidney disease, peritubular endothelial cells undergo apoptosis which results in peritubular capillary rarefaction and increased tissue hypoxia and oxidative stress(91). Although peritubular capillary rarefaction is often associated with interstitial fibrosis, whether changes in the kidney vasculature are a cause or a consequence of fibrosis is difficult to determine. Altered vascular function as a result of a fibrotic environment could result in hypoxia thus driving the expression of HIF-1α by proximal tubule cells, dedifferentiation of these cells and altered matrix production(7). On the other hand, a fibrotic environment could promote the production of thrombospondin-1 by tubular cells and/or fibroblasts. This glycoprotein could potentiate the fibrotic response both by activating TGF-β and by exerting anti-angiogenic actions, thus leading to loss of microvascular endothelium and generation of a hypoxic environment, thus creating a vicious cycle. In support of thrombospondin-1’s deleterious role in microvascular stability, in vivo inhibition of thrombospondin expression by siRNA suppresses TIF by promoting VEGF production and restoring peritubular capillary density(92).
VEGF is one of the most potent pro-angiogenic factors, but whether its production is beneficial and/or deleterious in the course of TIF has not been directly investigated(93). Injured or hypoxic proximal tubule cells produce VEGF via activation of HIF-1α. This growth factor could enhance peritubular capillaries density by directly promoting endothelial cell survival and proliferation, thus counteracting the anti-angiogenic action of thrombospondin-1. In support of VEGF’s role protecting peritubular capillaries, kidney-derived mesenchymal stem cells home to the injured kidney tubulointerstitium, promote tissue repair, and reduce peritubular capillary rarefaction via secretion of VEGF(94). Moreover, in late stages of the remnant kidney model characterized by loss of VEGF expression by either the glomerular or tubulointerstitial component, VEGF administration results in the preservation of peritubular capillaries and amelioration of tubulointerstitial injury (95, 96). Although these studies support a protective role of VEGF in preventing peritubular capillary rarefaction, the levels of VEGF need to be tightly controlled as VEGF can lead to the formation of functional as well as defective angiogenesis. To this end, excessive production of this growth factor promotes the formation of leaky and nonfunctional vessels, thus resulting in a hypoxic and highly oxidative environment. Moreover, VEGF is also a potent pro-inflammatory mediator and, paradoxically, could exacerbate the fibrotic response by promoting macrophage extravasation to the site of injury. Although a direct link between excessive VEGF production and formation of leaky and non-functional peritubular capillaries has not been made, one could speculate that the levels of VEGF produced by either tubular and/or infiltrating cells might determine the beneficial vs. deleterious effects of this factor in the stability of peritubular endothelium and amelioration of TIF.
In addition to VEGF, other locally produced factors have been shown to exert a ‘double edge sword’ effect on peritubular endothelium stability and enhancement of fibrotic responses. In this regard, PDGF-B secretion by injured tubular cells can ameliorate capillary rarefaction and stability by promoting pericyte recruitment to the vasculature, but, at the same time, it can also promote matrix production thus contributing to fibrosis. Similarly, FGF-2, produced by fibroblasts, tubular or endothelial cells, can enhance both VEGF-mediated pro-angiogenic and fibroblast-driven pro-fibrotic effects. A comprehensive list of factors involved in the regulation of angiogenesis and capillary regression in chronic kidney disease is reviewed by others(91).
Crosstalk between the peritubular endothelium and pericytes is another critical cellular communication that mediates TIF progression. The close proximity between endothelial cells and pericytes, which are in direct contact with endothelial cells through breaks in the capillary basement membrane, facilitates bidirectional crosstalk that supports capillary stability through growth factors like PDGF-B and VEGF-A (97, 98). Pericytes augment VEGF-A signaling within endothelial cells, thus enhancing their survival (99). However, inhibiting the VEGF-A receptor VEGFR2, primarily expressed by endothelial cells, protects against UUO-mediated microvascular rarefaction (100). This paradoxical finding may be explained by the fact that UUO-induced injury changed the specific VEGF isoforms expressed from the pro-angiogenic VEGF164 to the anti-angiogenic VEGF120/188, providing a putative mechanism whereby blocking VEGF signaling protects the injured microvasculature(100).
Endothelial/pericyte crosstalk after injury is also altered by disruptions in angiopoietins 1/2. Pericytes produce angiopoietin 1, a growth factor that stabilizes the microvasculature by acting on the endothelial Tie2 receptor. After renal injury, endothelial-derived angiopoietin-2, an antagonist of angiopoietin-1, increases(101) and this could lead to increased capillary leak and pericyte loss, as observed in cardiac tissue(102). On the other hand, augmenting angiopoietin-1 levels protected the vasculature and ameliorated proteinuria and TIF in murine models of diabetic nephropathy and UUO, respectively(103, 104). Thus, renal injury alters endothelial/pericyte crosstalk through VEGF signaling, angiopoietins, and other factors not discussed in this review (e.g. ephrinB2, TIMP/MMPs) (105) which induces pericyte loss and endothelial damage that likely promotes TIF through capillary rarefaction.
Conclusions
Much recent attention has focused on the role of activated fibroblasts in the progression of TIF as they are the primary producers of extracellular matrix. However, the injured epithelial cell is a potent producer of pro-fibrotic and pro-inflammatory cytokines that promote tubular atrophy and also crosstalk with inflammatory cells, endothelial cells and fibroblasts (Figure 1). Thus, therapeutic approaches that only target mesenchymal cells may not address the important roles these other compartments also play in TIF progression. The growth factors that mediate this injurious crosstalk between injured epithelia and interstitial cells clearly play a vital role in CKD progression. However, most of these cytokines exert both beneficial and harmful effects depending upon their site of production, concentration, and target cell types. Thus, further investigation to elucidate the mechanisms of crosstalk that drive tubular atrophy, ECM accumulation, and loss of capillary density is necessary for more effective therapies to halt TIF progression of CKD.
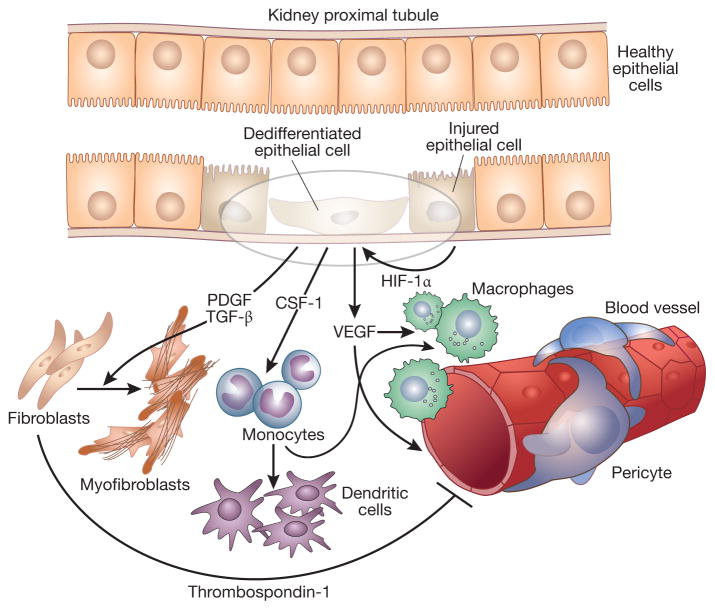
Figure 1. Schematic representation of crosstalk among kidney tubular epithelial cells, fibroblasts, endothelial cells and inflammatory cells that could lead to the progression of chronic kidney disease.
Injured epithelial cells produce HIF-1α that promotes epithelial cell dedifferentiation as well as VEGF production. This growth factor, in turn, promotes macrophage recruitment to the site of injury and stimulates endothelial cell proliferation leading to leaky vessels. Injured epithelial cells can also produce other growth factors such as CSF-1, a potent chemoattractant for monocytes that can then differentiatite into dendritic cells or macrophages. Injured epthelial cells can also produce PDGF and TGF-β which promote fibroblast proliferation and fibroblast to myofibroblast differentiation. Finally, fibroblasts can produce thrombospondin-1 that, in addition to activating TGF-β, inhibits endothelial cell proliferation contributing to capillary rarefaction.
Acknowledgments
This work was supported by a Career Development Award from the Department of Veteran’s Affairs, Veteran’s Health Administration, Office of Research and Development (LG), Veteran’s Affairs Merit Awards 1I01BX002025-01 (AP), 1I01BX002196 (R.Z.), the National Institutes of Health Grants RO1-DK108968-01 (LG), R01DK095761 (AP), RO1-DK-083187 (R.Z.), RO1-DK-075594 (R.Z.), RO1-DK-066921 (R.Z.).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou Y, Fang L, Yu Y, et al. Erythropoietin protects the tubular basement membrane by promoting the bone marrow to release extracellular vesicles containing tPA-targeting miR-144. Am J Physiol Renal Physiol. 2016;310(1):F27–40. doi: 10.1152/ajprenal.00303.2015. [DOI] [PubMed] [Google Scholar]
- 2.Ishibe S, Cantley LG. Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr Opin Nephrol Hypertens. 2008;17(4):379–85. doi: 10.1097/MNH.0b013e3283046507. [DOI] [PubMed] [Google Scholar]
- 3.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–83. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schelling JR. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol. 2016;31(5):693–706. doi: 10.1007/s00467-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature medicine. 2003;9(7):964–8. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 7.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–20. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Yang J, Dai C, et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112(4):503–16. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebleu VS, Taduri G, O’Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nature medicine. 2013;19(8):1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121(10):3981–90. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nature medicine. 2015 doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 13.Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nature medicine. 2015 doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine. 2010;16(5):535–43. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochemistry and cell biology. 2008;130(2):247–62. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neelisetty S, Alford C, Reynolds K, et al. Renal fibrosis is not reduced by blocking transforming growth factor-beta signaling in matrix-producing interstitial cells. Kidney Int. 2015 doi: 10.1038/ki.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun KH, Chang Y, Reed NI, et al. alphaSMA is an inconsistent marker of fibroblasts responsible for force dependent TGFbeta activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016 doi: 10.1152/ajplung.00350.2015. ajplung 00350 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujigaki Y, Muranaka Y, Sun D, et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 2005;446(2):164–76. doi: 10.1007/s00428-004-1155-5. [DOI] [PubMed] [Google Scholar]
- 19.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16(1):55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matise LA, Palmer TD, Ashby WJ, et al. Lack of transforming growth factor-beta signaling promotes collective cancer cell invasion through tumor-stromal crosstalk. Breast cancer research : BCR. 2012;14(4):R98. doi: 10.1186/bcr3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combes AN, Davies JA, Little MH. Cell-cell interactions driving kidney morphogenesis. Curr Top Dev Biol. 2015;112:467–508. doi: 10.1016/bs.ctdb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Edeling M, Ragi G, Huang S, et al. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nature reviews Nephrology. 2016;12(7):426–39. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262(14):6443–6. [PubMed] [Google Scholar]
- 25.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83(12):4167–71. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CF, Chiang WC, Lai CF, et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182(1):118–31. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma LJ, Yang H, Gaspert A, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/ −) mice. Am J Pathol. 2003;163(4):1261–73. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 29.Borges FT, Melo SA, Ozdemir BC, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24(3):385–92. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koesters R, Kaissling B, Lehir M, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 177(2):632–43. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng H, Lan R, Singha PK, et al. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Galphaq-mediated Rho and alphavbeta6 integrin-dependent activation of TGF-beta. Am J Pathol. 2012;181(4):1236–49. doi: 10.1016/j.ajpath.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura M, Asano M, Abe K, et al. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dial Transplant. 2005;20(8):1559–65. doi: 10.1093/ndt/gfh872. [DOI] [PubMed] [Google Scholar]
- 34.Tang WW, Ulich TR, Lacey DL, et al. Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol. 1996;148(4):1169–80. [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T, Kimura M, Asano M, et al. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol. 2001;158(1):75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YT, Chang FC, Wu CF, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80(11):1170–81. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 37.Seifert RA, Alpers CE, Bowen-Pope DF. Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Int. 1998;54(3):731–46. doi: 10.1046/j.1523-1755.1998.00046.x. [DOI] [PubMed] [Google Scholar]
- 38.Kliem V, Johnson RJ, Alpers CE, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49(3):666–78. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- 39.Fabian SL, Penchev RR, St-Jacques B, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180(4):1441–53. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding H, Zhou D, Hao S, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23(5):801–13. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou D, Li Y, Zhou L, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol. 2014;25(10):2187–200. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villegas VE, Rahman MF, Fernandez-Barrena MG, et al. Identification of novel non-coding RNA-based negative feedback regulating the expression of the oncogenic transcription factor GLI1. Mol Oncol. 2014;8(5):912–26. doi: 10.1016/j.molonc.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramann R, Fleig SV, Schneider RK, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest. 2015;125(8):2935–51. doi: 10.1172/JCI74929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramann R, Dirocco DP, Maarouf OH, et al. Matrix Producing Cells in Chronic Kidney Disease: Origin, Regulation, and Activation. Curr Pathobiol Rep. 2013;1(4) doi: 10.1007/s40139-013-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang HM, Huang S, Reidy K, et al. Sox9-Positive Progenitor Cells Play a Key Role in Renal Tubule Epithelial Regeneration in Mice. Cell Rep. 2016;14(4):861–71. doi: 10.1016/j.celrep.2015.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. The Journal of pathology. 2013;229(2):221–31. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 47.He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20(4):765–76. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito S, Tampe B, Muller GA, et al. Primary cilia modulate balance of canonical and non-canonical Wnt signaling responses in the injured kidney. Fibrogenesis Tissue Repair. 2015;8:6. doi: 10.1186/s13069-015-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maarouf OH, Aravamudhan A, Rangarajan D, et al. Paracrine Wnt1 Drives Interstitial Fibrosis without Inflammation by Tubulointerstitial Cross-Talk. J Am Soc Nephrol. 2016;27(3):781–90. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiRocco DP, Kobayashi A, Taketo MM, et al. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24(9):1399–412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou D, Tan RJ, Zhou L, et al. Kidney tubular beta-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep. 2013;3:1878. doi: 10.1038/srep01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bienaime F, Muorah M, Yammine L, et al. Stat3 Controls Tubulointerstitial Communication during CKD. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58(2):469–77. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Y, Liu S, Zhang S, et al. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol Cells. 2011;31(3):225–30. doi: 10.1007/s10059-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovelock JD, Baker AH, Gao F, et al. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288(2):H461–8. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 56.Tarjus A, Martinez-Martinez E, Amador C, et al. Neutrophil Gelatinase-Associated Lipocalin, a Novel Mineralocorticoid Biotarget, Mediates Vascular Profibrotic Effects of Mineralocorticoids. Hypertension. 2015;66(1):158–66. doi: 10.1161/HYPERTENSIONAHA.115.05431. [DOI] [PubMed] [Google Scholar]
- 57.Duffield JS. Beyond EMT: Epithelial STAT3 as a Central Regulator of Fibrogenesis. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2016060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 59.Yen TH, Alison MR, Goodlad RA, et al. Epidermal growth factor attenuates tubular necrosis following mercuric chloride damage by regeneration of indigenous, not bone marrow-derived cells. J Cell Mol Med. 2015;19(2):463–73. doi: 10.1111/jcmm.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Chen JK, Wang SW, et al. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14(12):3147–54. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82(1):45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Chen JK, Nagai K, et al. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol. 2012;23(2):215–24. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu N, Guo JK, Pang M, et al. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23(5):854–67. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang J, Liu N, Tolbert E, et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183(1):160–72. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7(316):316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakopoulou L, Stefanaki K, Boletis J, et al. Immunohistochemical study of epidermal growth factor receptor (EGFR) in various types of renal injury. Nephrol Dial Transplant. 1994;9(7):764–9. [PubMed] [Google Scholar]
- 67.Sis B, Sarioglu S, Celik A, et al. Epidermal growth factor receptor expression in human renal allograft biopsies: an immunohistochemical study. Transpl Immunol. 2004;13(3):229–32. doi: 10.1016/j.trim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Kok HM, Falke LL, Goldschmeding R, et al. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nature reviews Nephrology. 2014;10(12):700–11. doi: 10.1038/nrneph.2014.184. [DOI] [PubMed] [Google Scholar]
- 69.Bielesz B, Sirin Y, Si H, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120(11):4040–54. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers NM, Ferenbach DA, Isenberg JS, et al. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nature reviews Nephrology. 2014;10(11):625–43. doi: 10.1038/nrneph.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottschalk C, Kurts C. The Debate about Dendritic Cells and Macrophages in the Kidney. Front Immunol. 2015;6:435. doi: 10.3389/fimmu.2015.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eardley KS, Kubal C, Zehnder D, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74(4):495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 73.Morrissey JJ, Klahr S. Differential effects of ACE and AT1 receptor inhibition on chemoattractant and adhesion molecule synthesis. Am J Physiol. 1998;274(3 Pt 2):F580–6. doi: 10.1152/ajprenal.1998.274.3.F580. [DOI] [PubMed] [Google Scholar]
- 74.Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48(5):1477–86. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 75.Lai KN, Leung JC, Chan LY, et al. Interaction between proximal tubular epithelial cells and infiltrating monocytes/T cells in the proteinuric state. Kidney Int. 2007;71(6):526–38. doi: 10.1038/sj.ki.5002091. [DOI] [PubMed] [Google Scholar]
- 76.Prodjosudjadi W, Gerritsma JS, van Es LA, et al. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol. 1995;44(3):148–55. [PubMed] [Google Scholar]
- 77.Segerer S, Mac KM, Regele H, et al. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56(1):52–64. doi: 10.1046/j.1523-1755.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 78.Isbel NM, Hill PA, Foti R, et al. Tubules are the major site of M-CSF production in experimental kidney disease: correlation with local macrophage proliferation. Kidney Int. 2001;60(2):614–25. doi: 10.1046/j.1523-1755.2001.060002614.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Chang J, Yao B, et al. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int. 2015;88(6):1274–82. doi: 10.1038/ki.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang MZ, Yao B, Yang S, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122(12):4519–32. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng X, Zhang J, Xiao Z, et al. CX3CL1-CX3CR1 Interaction Increases the Population of Ly6C(−)CX3CR1(hi) Macrophages Contributing to Unilateral Ureteral Obstruction-Induced Fibrosis. J Immunol. 2015;195(6):2797–805. doi: 10.4049/jimmunol.1403209. [DOI] [PubMed] [Google Scholar]
- 82.Kassianos AJ, Wang X, Sampangi S, et al. Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney Int. 2015;87(6):1153–63. doi: 10.1038/ki.2014.407. [DOI] [PubMed] [Google Scholar]
- 83.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107(9):4194–9. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinuesa E, Hotter G, Jung M, et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. The Journal of pathology. 2008;214(1):104–13. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 85.Sung SA, Jo SK, Cho WY, et al. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron Experimental nephrology. 2007;105(1):e1–9. doi: 10.1159/000096859. [DOI] [PubMed] [Google Scholar]
- 86.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172(2):288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ninichuk V, Khandoga AG, Segerer S, et al. The role of interstitial macrophages in nephropathy of type 2 diabetic db/db mice. Am J Pathol. 2007;170(4):1267–76. doi: 10.2353/ajpath.2007.060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machida Y, Kitamoto K, Izumi Y, et al. Renal fibrosis in murine obstructive nephropathy is attenuated by depletion of monocyte lineage, not dendritic cells. J Pharmacol Sci. 2010;114(4):464–73. doi: 10.1254/jphs.10246fp. [DOI] [PubMed] [Google Scholar]
- 89.Kitching AR. Dendritic cells in progressive renal disease: some answers, many questions. Nephrol Dial Transplant. 2014;29(12):2185–93. doi: 10.1093/ndt/gfu076. [DOI] [PubMed] [Google Scholar]
- 90.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13(3):806–16. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 91.Kida Y, Tchao BN, Yamaguchi I. Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease. Pediatr Nephrol. 2014;29(3):333–42. doi: 10.1007/s00467-013-2430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun D, Ma Y, Han H, et al. Thrombospondin-1 short hairpin RNA suppresses tubulointerstitial fibrosis in the kidney of ureteral obstruction by ameliorating peritubular capillary injury. Kidney Blood Press Res. 2012;35(1):35–47. doi: 10.1159/000330718. [DOI] [PubMed] [Google Scholar]
- 93.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26(4):1132–7. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70(1):121–9. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 95.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12(7):1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 96.Schrijvers BF, Flyvbjerg A, Tilton RG, et al. Pathophysiological role of vascular endothelial growth factor in the remnant kidney. Nephron Experimental nephrology. 2005;101(1):e9–15. doi: 10.1159/000086034. [DOI] [PubMed] [Google Scholar]
- 97.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 98.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 99.Franco M, Roswall P, Cortez E, et al. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118(10):2906–17. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin SL, Chang FC, Schrimpf C, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178(2):911–23. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsai YC, Chiu YW, Tsai JC, et al. Association of angiopoietin-2 with renal outcome in chronic kidney disease. PloS one. 2014;9(10):e108862. doi: 10.1371/journal.pone.0108862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ziegler T, Horstkotte J, Schwab C, et al. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013 doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim W, Moon SO, Lee SY, et al. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17(9):2474–83. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- 104.Dessapt-Baradez C, Woolf AS, White KE, et al. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol. 2014;25(1):33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schrimpf C, Teebken OE, Wilhelmi M, et al. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51(4):247–58. doi: 10.1159/000365149. [DOI] [PMC free article] [PubMed] [Google Scholar]