Abstract
Long noncoding RNAs (lncRNAs) are important for transcription and for epigenetic or posttranscriptional regulation of gene expression, and may contribute to carcinogenesis. MALAT1 (Metastasis Associated Lung Adenocarcinoma Transcript 1), a lncRNA involved in the regulation of the cell cycle, cell proliferation and cell migration, is known to be deregulated in multiple cancers. Here we analyzed the expression of MALAT1 on 195 cases of benign and malignant thyroid neoplasms by using tissue microarrays for RNA in-situ hybridization (ISH) and real-time PCR. MALAT1 is highly expressed in normal thyroid tissues (NT) and thyroid tumors, with increased expression during progression from NT to papillary thyroid carcinomas (PTCs), but is downregulated in poorly differentiated thyroid cancers (PDCs) and anaplastic thyroid cancers (ATCs) compared to NT. Induction of epithelial to mesenchymal transition (EMT) by TGF beta in a PTC cell line (TPC1) led to increased MALAT1 expression, supporting a role for MALAT1 in EMT in thyroid tumors. This is the first ISH study of MALAT1 expression in thyroid tissues. It also provides the first piece of evidence suggesting MALAT1 downregulation in certain thyroid malignancies. Our findings support the notion that ATCs may be molecularly distinct from low grade thyroid malignancies, and suggest that MALAT1 may function both as an oncogene and as a tumor suppressor in different types of thyroid tumors.
Keywords: In situ hybridization, long non-coding RNA, MALAT1, thyroid carcinoma, papillary thyroid carcinoma, RT-PCR
Introduction
Thyroid cancer is the most common malignancy of endocrine organs with enormous heterogeneity in terms of morphological features and prognosis (1). Although the majority of thyroid carcinoma tends to be biologically indolent and have an excellent prognosis, some are associated with more aggressive clinical behaviors (2). Most thyroid cancers are derived from thyroid follicular epithelial cells, which are further divided in to well-differentiated papillary thyroid carcinoma (PTC) and follicular carcinomas (FC), poorly differentiated carcinoma (PDC)) and anaplastic (undifferentiated) thyroid carcinoma (ATC) based on histological features (1). Despite extensive research on morphological subclassification and immunohistochemistry characters, it remains difficult to accurately predict disease progression and high-grade transformation in various thyroid malignancies.
The transcription of RNAs without obvious coding potential led to the discovery of non-coding RNAs (ncRNAs), which have distinct expression pattern in different tissues/organ/developmental stage and is increasingly recognized as a key regulatory element of proliferation, differentiation and apoptosis (3). Long non-coding RNAs (lncRNAs) are generally defined as ncRNAs that are longer than 200 nucleotides. Compared to other shorter ncRNAs (e.g. miRNAs, tRNAs) that are better studied, lncRNAs are more numerous and diverse. Dysregulation of lncRNAs has been associated with various malignancies of multiple organs, including breast, liver, prostate, bladder, intestine and brain (3). In the thyroid, several lncRNAs have been proposed as susceptibility markers of cancers especially papillary thyroid carcinoma, such as papillary thyroid cancer susceptibility candidate 1 (PTCSC1) (4), PTCSC2 (5) and PTCSC3 (6, 7). In addition, a few lncRNAs may be related to the pathogenesis of thyroid cancers, including BANCR (8) and PVT1 (9)
MALAT1 (Metastasis Associated Lung Adenocarcinoma Transcript 1), a lncRNA that participates in the regulation of cell cycle and migration, is known to be deregulated in malignancies in multiple organs, including lung, uterus, cervix, breast, colon, pancreas, stomach, kidney, bladder and bone (10). In this study, RNA in-situ hybridization (ISH) of MALAT1 was performed on tissue microarrays (TMAs) constructed from 195 cases of normal thyroid tissues, chronic lymphocytic thyroiditis, as well as benign and malignant thyroid neoplasms. The results were further validated by real-time PCR. We found that MALAT1 is highly expressed in thyroid tissues, with upward trend from NT to benign neoplasm then to the well differentiated thyroid cancers (PTCs and FCs). Interestingly, it is downregulated in poorly differentiated thyroid cancers (PDCs) anaplastic thyroid cancers (ATCs). In addition, induction of epithelial mesenchymal transition (EMT) by TGF beta led to increased MALAT1 expression in a PTC cell line. These results may shed new light on the categorization of thyroid neoplasms and the understanding of their pathogenesis.
Materials and Methods
Tissue Microarrays (TMA)
TMAs were constructed as described previously (11, 12) from 195 case samples of formalin-fixed, paraffin-embedded tissues, including normal thyroid (NT, n=10), Hashimoto thyroiditis (HT, n=12), nodular goiters (NG, n=10), follicular adenoma (FA, n=32), follicular carcinoma (FC, n=28), conventional papillary thyroid carcinoma (cPTC n=28), follicular variant of papillary thyroid carcinoma(FV, n=29), poorly differentiated thyroid carcinomas (PDC, n=21 and anaplastic thyroid carcinoma (ATC, n=35). The TMA consisted of triplicate 0.6-mm cores made using a manual TMA (Beecher Instruments, Sun Prairie, WI). The NT consisted of tissues from the opposite (histologically normal) thyroid lobe in patients with follicular or papillary carcinomas.
The study was approved by the Institutional Review Board at the University of Wisconsin–Madison.
In Situ Hybridization
TMAs were probed for MALAT1 expression using the RNAscope 2.0 HD-Brown Manual Assay (Advanced Cell Diagnostics, Newark, Ca. USA) as per manufacturer’s recommendations with the following modifications: antigen retrieval for 15 min, protease digestion for 30 min, probe incubation overnight at 40°C and amplification steps 1–4 only (steps 5 & 6 were omitted due to high expression). The probes used are hs-Malat1 (400811), hs-ACTB (Actin, positive control, 310141) and dapB (negative control, 310043) (Advanced Cell Diagnostics). MALAT1 expression levels were visualized with DAB and quantitated using an Automated Image Acquisition and Analysis system.
Automated Image Acquisition and Analysis
The stained TMA slides were visualized and analyzed with the Vectra slide scanner as previous described (13). Briefly, after image acquisition, any core with tissue folding or loss of tissue was excluded for analysis. InForm 1.4.0 software was used to segment tissue compartments (epithelium vs. non-epithelium) and subcellular compartments (nucleus vs. cytoplasm). MALAT1 expression level from each sample was quantitated as optical density (OD) per unit area (pixel) and normalized to the total pixel counts obtained from each cell. Only nuclear signal was used for analysis. Mean MALAT1 expression level of each case was used for further analysis.
Real-time PCR
Total RNA was extracted from samples with TRIzol reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions, and RNA quality and concentrations were assessed with a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific, Waltham, MA). 1 μg of total RNA was reverse-transcribed using the All-in-One miRNA RT-qPCR detection kit (GeneCopoeia Rockville, MD). RT-qPCR was performed on a CFX96 PCR detection system (Bio-Rad Laboratories, Hercules, CA) using Bullseye EvaGreen qPCR master mix (MIDSCI, St. Louis, MO), normalized to 18S rRNA; relative fold change was determined by the ΔΔ CT method. The PCR primers used are Malat1; Forward 5’-GACGGAGGTTGAGATGAAGC-3’ and Reverse 5’-ATTCGGGGCTCTGTAGTCCT-3’, and 18S; Forward 5’-GTAACCCGTTGAACCCCATT-3’ and Reverse 5’-CCATCCAATCGGTAGTAGCG-3’
Tissue Culture and EMT Induction with TGF-β
The papillary thyroid carcinoma cell line TPC1 was cultured and treated with TGF-β to induce EMT as previously described (14, 15). Briefly, TPC-1 cells were treated with serum free media with and without TGF-β (2ng/ml) (14, 15).
Statistics
Student’s t-test was used to analyze MALAT1 expression data collected from RNA ISH and real-time PCR. Two-tailed P values of <0.05 were considered to be statistically significant. Data are expressed as means ± standard error of the mean (SEM).
Results
ISH of MALAT1
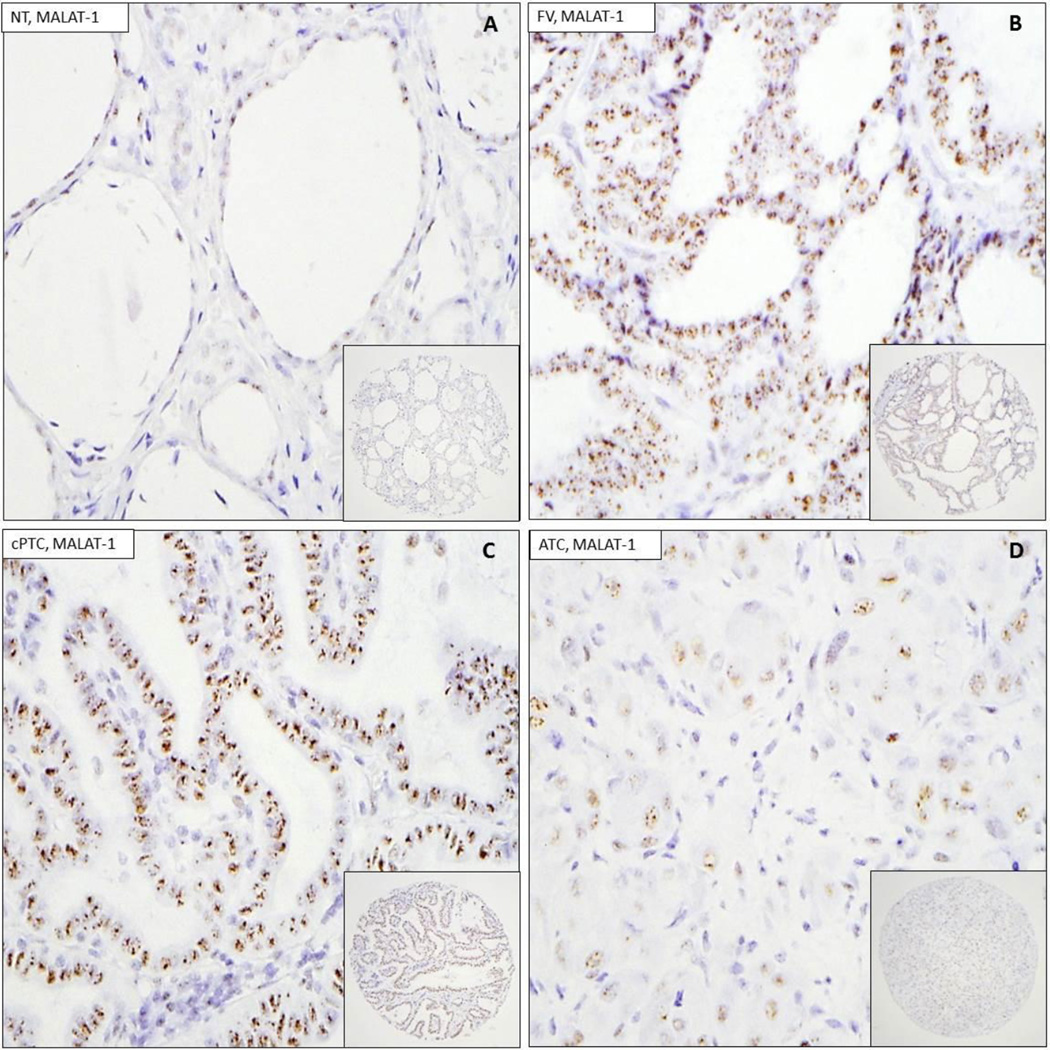
MALAT1 ISH was performed on three TMAs containing 10 NT, 12 HT, 10 NGs, 32 FAs, 28 FCs, 28 cPTCs, 29 FVs, 21 PDCs, and 35 ATCs, and automated image acquisition and analysis was performed as described in Materials and Methods. MALAT1 shows dot-like nuclear staining pattern, which is typical for lncRNAs (Figure 1). Moderate to strong nuclear expression of MALAT1 was seen in NT as well as benign neoplasms and well-differentiated thyroid cancers, including NGs, FAs, FCs and cPTCs. However, the signal for MALAT1 in PDCs and ATCs are low (Figure 1 and 2). The positive control actin probe showed strong cytoplasmic labeling and weaker nuclear stain labeling. The negative controls did not show any labeling in the thyroid tissues. Interestingly, cytoplasmic staining was also present, but was several folds lower than the nuclear staining in all types of thyroid tumors examined.
Figure 1.
Representative ISH images of MALAT-1 on TMA. 1A : normal thyroid tissue (NT). 1B: follicular variant of papillary thyroid carcinoma (FV). 1C: conventional papillary thyroid carcinoma (cPTC). 1D: anaplastic thyroid carcinoma (ATC). Insets show low power view of corresponding TMA cores.
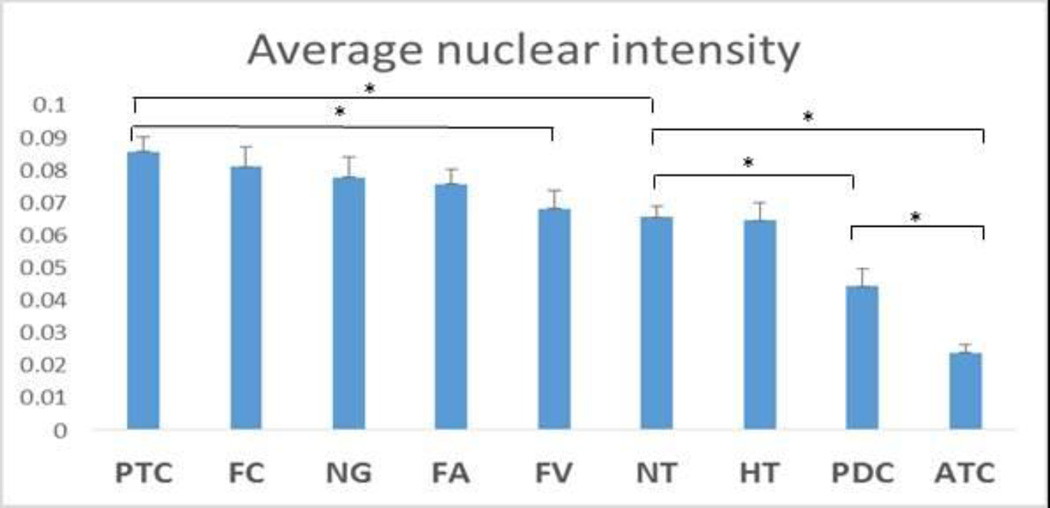
Figure 2.
MALAT1 ISH nuclear expression level via in normal thyroid tissue and thyroid neoplasms. The highest levels of MALAT1 are observed in PTCs which was significantly higher than in NT (p=0.014) and FVPTC (p=0.016). In contrast NT express higher levels of MALAT1 than PDC (p=0.015) or ATC (p<0.001).“┬” indicate SEM; “*” indicates p<0.05.
As shown in Figure 2, a trend of increasing MALAT1 expression was observed from NT and HT to benign neoplasms to well-differentiated cancers, with the highest expression level observed in cPTCs, despite lack of statistically significant difference among cPTCs, FCs, NGs and FAs. MALAT1 expression is significantly higher in cPTCs comparing to NT (p = 0.014). Interestingly, MALAT1 expression level in FV is similar to NT and is significantly lower than cPTCs (p = 0.016). On the other hand, MALAT1 expression in PDCs and ATCs is significantly lower than NT (p < 0.01), with ATCs expressing significantly lower MALAT1 than PDCs (p < 0.01).
Real-time PCR of MALAT1 in thyroid cancers
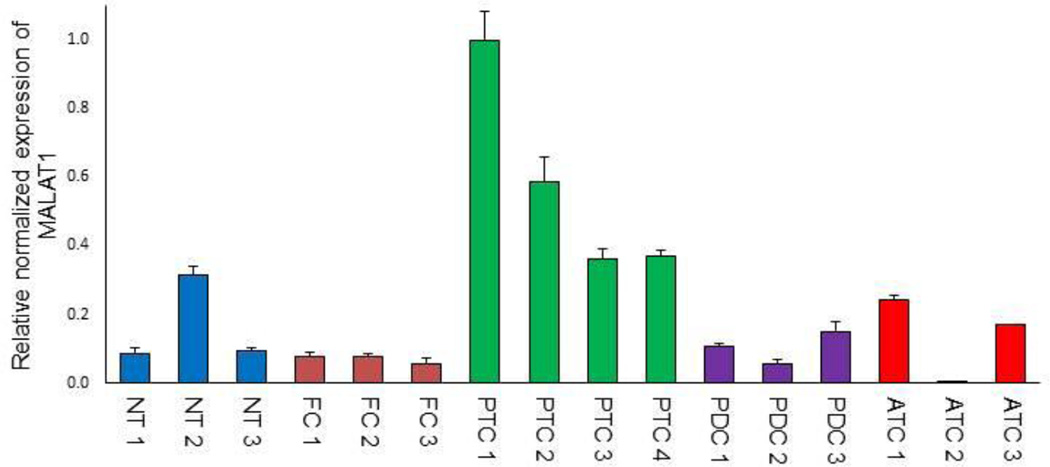
To confirm the ISH findings, MALAT1 expression level was studied using real-time PCR in FFPE tissues from a subset of cPTCs, FCs, PDCs and ATCs used on the TMAs. As seen in Figure 3, MALAT1 expression level was significantly higher in the cPTCs compared to the other groups (p<0.05).
Figure 3.
qRT-PCR analysis of MALAT1 expression in a subset of TMA cases. The PTC cells expressed significantly more MALAT1 compared to NT, HT, NG, FA, FCA, PDC and ATC (p< 0.05) “┬” indicate SEM.
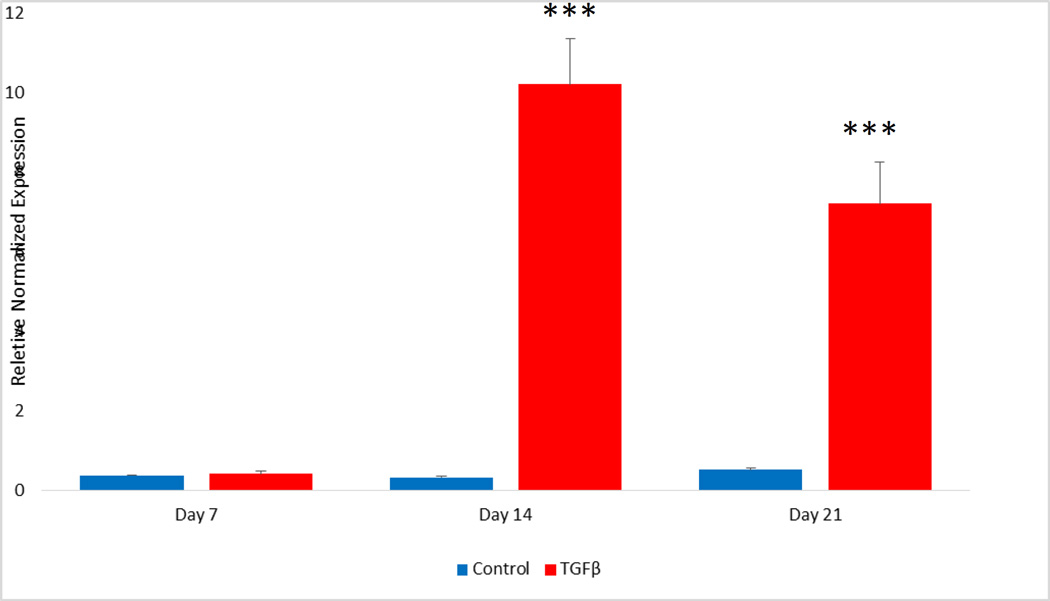
TGF-β treatment
After 14 and 21 days of TGF-β treatment MALAT1 RNA levels were significantly increased compared to the control groups indicating induction of MALAT1 expression. during EMT. Our previous studies had also shown increase in the EMT markers SLUG and OCT4 in the TPC1 cell line with EMT (14, 15).
Discussion
The role of lncRNAs in pathogenesis is increasingly recognized. MALAT1, a well-known lncRNA and possible oncogene (16), is upregulated in malignancies of multiple organs (10). RNA ISH enables direct visualization of lncRNAs, and strong MALAT1 hybridization signals were previous reported in breast malignancies (17). In this study, we examined the expression level of MALAT1 in normal thyroid tissue as well as benign and malignant neoplasms using ISH and real-time PCR validation. We found strong nuclear staining of MALAT1 in both normal thyroid tissues and thyroid neoplasms, with upregulation during progression from NT to benign neoplasm then to the well differentiated thyroid cancers (PTCs and FCs). It is worth noting that HTs, which is an inflammatory condition rather than neoplasm, showed essentially the same MALAT1 expression level as in NT. This further supports that MALAT1 dysregulation is potentially specific for tumorigenesis.
Different from cPTCs, FVs, especially the encapsulated FVs, usually have an indolent disease course. This feature was recently further characterized which led to the adoption of the new terminology "noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP" for a subgroup of benign neoplasms previous categorized into PCTs (18). TMAs used in this study were constructed prior to the introduction of NIFTP terminology, and the possible NIFTPs were not excluded from the FV group. Thus, despite that the low expression of MALAT1 some in FVs could support the notion that FVs might be pathogenetically different from cPTCs and generally behave in benign fashion, we are unable to draw definitive conclusions without separating NIFTPs from FVs in our current study.
MALAT1 expression in PDCs and ATCs are significantly lower than NT, with the expression in ATCs showing the lowest levels. This is the first report of MALAT1 being downregulated in any malignancy. It is worth noting that other relatively well studied ncRNAs in thyroid, such as miR-146b, share similar trends of being upregulated in PTCs and FCs while downregulated in ATCs (12). Interestingly, while more known for its oncogenic features, miR-146b is also proposed to be a tumor suppressor (19). It is possible that similar to miR-146b, depending on the cancer subtype, MALAT1 functions both as an oncogene and as a tumor suppressor.
Various lncRNAs have been shown to have a role in EMT (3). The present study showed that TGF- β treatment, which we have used previously to induce EMT in thyroid cell lines (14, 15) led to increased MALAT1 expression in the PTC cell line. Our previous studies showed that during EMT in thyroid cell lines, there was increased cell proliferation and cell migration as well as increased in tumor growth in immunodeficient mice (14). In addition EMT was also associated with increased in the numbers of cancer stem cells (14, 15), suggesting that MALAT1 may have a role in cancer stem cell proliferation in thyroid tumors.
Although extensive molecular studies have been performed on PTCs including studies of microRNAs expressed by PTCs (20), only limited molecular studies have been done in PDTC and ATCs (21–23). Thus the role of lncRNAs in the regulation of ATCs has not been analyzed. The recent study of Huang et al (24) on thyroid carcinoma cell lines suggested that FC cell line FTC133 expressed higher levels of MALAT1 compared to the SW1736 ATC cell line. Since our studies showed that PTC tumors had higher levels of MALAT1 compared to FCs and ATCs, the molecular mechanisms regulating differences in MALAT1 expression in these various thyroid tumor types must await further studies.
In summary, we characterized the expression pattern of MALAT1 in thyroid tissues and showed that it is dysregulated in thyroid carcinomas in this study. This study also showed that MALAT1 expression is up-regulated in PTC cells during TGF- β-induced EMT. Our results suggest that lncRNAs such as MALAT1 may be important in thyroid cancer pathogenesis and may be useful for the classification of thyroid cancers. Further studies are needed to illustrate the function of MALAT1 and other lncRNAs in thyroid cancer, which may convey diagnostic and prognostic values.
Figure 4.
TGF-β induction of EMT significantly increases MALAT1 expression. TPC1 cells were incubated in serum free media with or without 2 ng/ml TGFβ-1 and analyzed for MALAT1 expression by qPCR on days 7, 14 and 21. TGF-β treatment significantly increased MALAT1 expression on days 14 and 21. (***= p<0.001)
Acknowledgments
We kindly thank Dr. Daniel T. Ruan (Brigham and Women’s Hospital, Boston, MA) for the TPC-1 cell line, the staffs of the Translational Research in Pathology (TRIP) (University of Wisconsin Carbone Cancer Center Cancer Center Support Grant P30 CA014520) for their services. Dr. Ranran Zhang received a research grant from the Department of Pathology and Laboratory Medicine, University of Wisconsin School of Medicine and Public Health.
Footnotes
Presented as a Poster Session at the 105th Meeting of the United States and Canadian Academy of Pathology in Seattle WA, 2016.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- 1.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 2.Livolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(Suppl 2):S1–S9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Wu Z, Fu X, Han W. Long Noncoding RNAs: Insights from Biological Features and Functions to Diseases. Med Res Rev. 2013;33(3):517–553. doi: 10.1002/med.21254. [DOI] [PubMed] [Google Scholar]
- 4.He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, et al. A Susceptibility Locus for Papillary Thyroid Carcinoma on Chromosome 8q24. Cancer Res. 2009;69(2):625–631. doi: 10.1158/0008-5472.CAN-08-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, et al. Genetic Predisposition to Papillary Thyroid Carcinoma: Involvement of FOXE1, TSHR, and a Novel lincRNA Gene, PTCSC2. J Clin Endocrinol Metab. 2015;100(1):E164–E172. doi: 10.1210/jc.2014-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jendrzejewski J, He H, Radomska HS, Li W, Tomsic J, Liyanarachchi S, et al. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc Natl Acad Sci U S A. 2012;109(22):8646–8651. doi: 10.1073/pnas.1205654109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Hardin H, Chen J, Guo Z, Lloyd RV. Non-Coding RNAs in Thyroid Cancer. Endocr Pathol. 2016;27(1):12–20. doi: 10.1007/s12022-016-9417-8. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Wang M, Jiang L, Chu H, Hu J, Ning J, et al. BRAF-activated Long Non-coding RNA Modulates Papillary Thyroid Carcinoma Cell Proliferation through Regulating Thyroid Stimulating Hormone Receptor. Cancer Res Treat. 2015 doi: 10.4143/crt.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Chen J, Feng J, Wang J. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR) Tumour Biol. 2015 doi: 10.1007/s13277-015-4149-9. [DOI] [PubMed] [Google Scholar]
- 10.Ma X-Y, Wang J-H, Wang J-L, Ma CX, Wang X-C, Liu F-S. Malat1 as an evolutionarily conserved lncRNA, plays a positive role in regulating proliferation and maintaining undifferentiated status of early-stage hematopoietic cells. BMC Genomics. 2011;16(676) doi: 10.1186/s12864-015-1881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, et al. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2012;26(10):54–61. doi: 10.1038/modpathol.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z, Hardin H, Montemayor-Garcia C, Asioli S, Righi A, Maletta F, et al. In Situ Hybridization Analysis of miR-146b-5p and miR-21 in Thyroid Nodules: Diagnostic Implications. Endocr Pathol. 2015;26(2):157–163. doi: 10.1007/s12022-015-9363-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Eickhoff JC, Mehraein-Ghomi F, Church DR, Wilding G, Basu HS. Expression of spermidine/spermine N1-acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate. 2015;75(11):1150–1159. doi: 10.1002/pros.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, et al. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184(8):2342–2354. doi: 10.1016/j.ajpath.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardin H, Yu X-M, Harrison AD, Larrain C, Zhang R, Chen J, et al. Generation of Novel Thyroid Cancer Stem-Like Cell Clones Effects of Resveratrol and Valproic Acid. Am J Pathol. 2016;186(6):1662–1673. doi: 10.1016/j.ajpath.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, et al. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75(7):1322–1331. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Weaver DL, Olsen D, Dekay J, Peng Z, Ashikaga T, et al. Long non-coding RNA chromogenic in situ hybridisation signal pattern correlation with breast tumour pathology. J Clin Pathol. 2016;69(1):76–81. doi: 10.1136/jclinpath-2015-203275. [DOI] [PubMed] [Google Scholar]
- 18.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, R Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Khalaf HH, Aboussekhra A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J Biol Chem. 2014;289(45):31433–31447. doi: 10.1074/jbc.M114.593004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun J, Hoang-Vu C, Dralle H, Hutelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29(29):4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 23.Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26(54):7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 24.Huang J-K, Ma L, Song W-H, Lu B-Y, Huang Y-B, Dong H-M, et al. MALAT1 promotes the proliferation and invasion of thyroid cancer cells via regulating the expression of IQGAP1. Biomed Pharmacother. 2016;83:1–7. doi: 10.1016/j.biopha.2016.05.039. [DOI] [PubMed] [Google Scholar]