Abstract
A major impediment to the development of effective treatments for metastatic or unresectable pheochromocytomas and paragangliomas has been the absence of valid models for pre-clinical testing. Attempts to establish cell lines or xenografts from human pheochromocytomas and paragangliomas have previously been unsuccessful. NOD-scid gamma (NSG) mice are a recently developed strain lacking functional B-cells, T-cells and NK cells. We report here that xenografts of primary human paragangliomas will take in NSG mice while maintaining their architectural and immunophenotypic characteristics as expressed in the patients. In contrast to grafts of cell lines and of most common types of primary tumors, the growth rate of grafted paragangliomas is very slow, accurately representing the growth rate of most pheochromocytomas and paragangliomas even in metastases in humans. Although the model is therefore technically challenging, primary patient derived xenografts of paragangliomas in NSG mice provide a potentially valuable new tool that could prove especially valuable for testing treatments aimed at eradicating the small tumor deposits that are often numerous in patients with metastatic paraganglioma.
Keywords: paraganglioma, pheochromocytoma, NSG mouse, xenograft, PDX
Introduction
Ten to 15% of pheochromocytomas and up to 40% of extra-adrenal paragangliomas give rise to metastases, with metastatic risk depending on tumor location and patient genotype [1] [2]. There is currently no highly effective treatment after metastases occur, and many patients die from chemotherapy complications, excess catecholamine secretion or a large tumor burden [3]. In the absence of metastases, local invasion or complications resulting from surgical resection can produce extreme morbidity, requiring treatment strategies that balance iatrogenic injury against risk of metastasis [4] [5].
A major impediment to the development of effective treatments has been the absence of valid experimental models for pre-clinical testing. Although mouse models [6], rodent pheochromocytoma cell lines [7–9] and other cell lines [10] have yielded important information, there is no model representing both the genotype and the fully developed phenotypes of actual tumors that are most likely to metastasize or cause local morbidity, particularly tumors that occur in patients with germline mutations of the SDHB gene, which encodes a subunit of succinate dehydrogenase [6]. Attempts over many years to establish human pheochromocytoma and paraganglioma cell lines directly from primary cell cultures have been unsuccessful, as have attempts to xenograft primary tumors in nude mice and other immunosuppressed mouse strains [11].
NOD-scid gamma (NSG) mice are a recently developed strain lacking functional B-cells, T-cells and NK cells [12]. It is reported that most types of primary human tumors will take as xenografts in these mice, while maintaining their characteristics as expressed in the patients [12]. We therefor undertook a study both to evaluate NSG mice as potential recipients of grafts from pheochromocytomas and paragangliomas and to begin using these grafts as an intermediate step in establishing human cell lines.
Materials and Methods
The study was performed with stored cryopreserved aliquots of viable cells from 13 primary human pheochromocytomas and paragangliomas representing both SDHB-mutated and SDHB-wild type genotypes. Studies of the banked tumor samples were approved by the Institutional Review Boards of the National Institutes of Health and Tufts Medical Center. Patients provided written informed consent. The characteristics of the tumors are summarized in Table 1. Prior to cryopreservation the tumor cells were dissociated in collagenase followed by trypsin as previously described [13]. Each aliquot contained a heterogeneous mixture of cell types including chief cells, sustentacular cells and endothelial cells reflecting the proportions of cell types in the intact tumor tissue. Replicate aliquots from each tumor had previously been tested unsuccessfully in attempts to establish cell lines directly from primary cultures.
Table 1.
Patient and tumor data and xenograft results
| Patient and Tumor Data | Mouse Data | |||||
|---|---|---|---|---|---|---|
| Age | Sex | Tumor Type | Mutated Gene | Mouse # | Tumor Take | Months of Observation |
| 9 | F | PGL | SDHB | 1 | − | 12 |
| 13 | F | PGL | SDHB | 2 | − | 12 |
| 37 | F | PGL | Not Tested | 3 | − | 12 |
| 45 | M | PGL | SDHB | 4 | + | 12 |
| 52 | M | PGL | SDHB | 5 | − | 11 |
| 53 | M | PGL | SDHB | 6 | − | 11 |
| 61 | F | PGL | Sporadic | 7 | + | 11 |
| 27 | M | PGL | SDHB | 8 | − | 10 |
| 46 | F | PCC | SDHB | 9 | − | 10 |
| 55 | M | PGL-liver metastasis | SDHB | 10 | + | 10 |
| 29 | F | PCC | SDHB | 11 | − | 9 |
| 41 | M | PGL | Not Tested | 12 | − | 9 |
| 54 | M | PGL | SDHB | 13 | − | 6 |
The cryopreserved cells were rapidly thawed, resuspended in RPMI1640 medium with 15 % fetal bovine serum, centrifuged and resuspended in fresh medium after aspirating the supernatant. Approximately 500,000 cells were injected subcutaneously into both rear flanks of NSG mice in vehicle consisting of 1 part medium: 2 parts Matrigel. The mice were maintained in sterile cages with periodic monitoring. Because large tumor masses did not form, the mice were kept until general health issues required them to be euthanized.
At necropsy, injection sites that contained tumor were resected en bloc, fixed in 10% neutral buffered formalin, dehydrated in a graded ethanol series and embedded in paraffin. For comparison to sections of the intact tumor tissue, sections were stained immunohistochemically with antibodies directed against chromogranin A (CgA) (# LK2H10, Ventana, pre-diluted), S100 (# 4C4.9, Ventana, pre-diluted), Ki 67 (# 30.9, Ventana, pre-diluted), tyrosine hydroxylase (TH) (#22941, Immunostar, 1:4000), and succinate dehydrogenase B (SDHB)(#HP0028668, Sigma). In addition, sections of the xenografts were stained for CD31 using an antibody that reacts with human but not murine endothelium (Cell Marque # 1A10, pre-diluted). This permitted us to assess the relative contributions of human and mouse endothelium to vascularization of the grafts. The Mach 4 system from Biocare Medical was used for detection.
Results
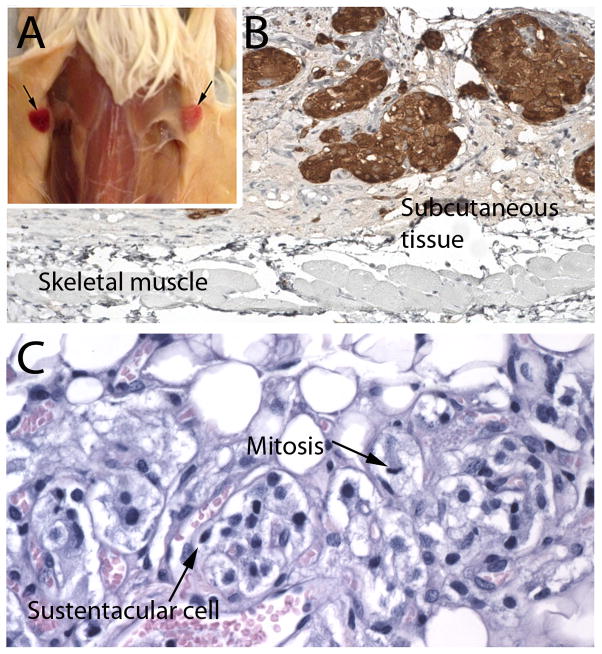
There were tumor takes in 3 of the 13 mice, euthanized at 12, 11, and 10 months (#’s 4, 7 and 10 in Table 1). No takes were present before 10 months. Engraftment was successful in both male and female mice, and with both SDHB-mutated and SDHB-wild type tumors. In every case, the tumor implants were bilateral and appeared nearly identical grossly and microscopically, indicating that the grafting procedure was reproducible and success was not random (Figure 1).
Figure 1.
A. Representative bilateral subcutaneous grafts of SDHB-mutated paraganglioma in a NSG mouse (Mouse 10). B. TH expression in subcutaneous tumor nodules from a sporadic, TH-positive paraganglioma (mouse 7). C. Zellballen architecture, sustentacular cell and mitotic figure in a SDHB-mutated paraganglioma (mouse 4)
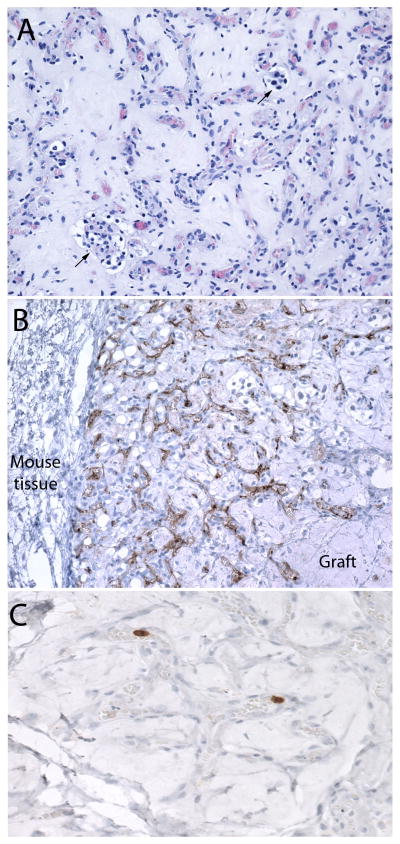
Histologically, the grafts recapitulated the morphological and immunophenotypic features of their parent tumors, including the same pattern of retained or lost tyrosine hydroxylase, chromogranin and SDHB. The tumor architecture exhibited a prominent mesh of capillaries and variable numbers of tumor cells including chief cells and sustentacular cells arranged in small nests in subcutaneous fibroadipose tissue and within a proteinaceous matrix consistent with residual matrigel (Figs. 1 and 2). In one tumor (Fig. 2), capillaries were very prominent while tumor cells were extremely rare. Immunoreactivity for CD31 was demonstrated in the majority of tumor vessels but not in peritumoral mouse vessels. Because the antibody employed was human-specific, this indicated a major contribution of human endothelium to vascularization of the grafts. Ki-67, which was also a human-specific antibody, showed occasional labeled chief cells and endothelial cells (Fig. 2), while mitoses were extremely rare. The low proliferative activity of the grafts mirrored that in the parent tumors.
Figure 2.
A. Xenograft of a SDHB-mutated paraganglioma showing an area with only sparse nests of tumor cells (arrows) in a proteinaceous background containing a web of numerous capillaries resembling the vascular architecture of paragangliomas in vivo. B. Immunohistochemical stain for human CD31 demonstrating that many of the vessels within the graft are human-derived. Vessels in the adjacent mouse tissue are unstained. C. Ki-67 labelling of tumor-derived endothelial cells in an area devoid of tumor cells.
Discussion
This report is the first to describe successful xenografts of human pheochromocytomas or paragangliomas in any mouse strain. Because the tumors are rare they are difficult to study. Our laboratory maintains an archive of cryopreserved cells from primary pheochromocytomas and paragangliomas dating back several decades. The availability of replicate aliquots of cells in the archive permits experiments to be repeated and new ideas to be tested. All tumors in this study had been previously tested in unsuccessful attempts to establish pheochromocytoma and paraganglioma cell lines from primary monolayer cultures. Although chief cells from several of the tumors survived for more than one year in cell cultures, there was no evidence of proliferation at any time, as confirmed by the absence of bromodeoxyuridine labelling [14]. In contrast to cell cultures, chief cells both survived and proliferated in NSG mice, as evident from the presence of mitotic figures, Ki-67 labeling and formation of grossly visible tumors.
An additional characteristic of the tumor xenografts was reconstitution of the tumor microarchitecture, including the formation of prominent tumor vasculature and Zellballen with a typical relationship between sustentacular cells and chief cells. This is a particularly intriguing finding because the nature and origin of sustentacular cells have been debated. Some evidence suggests a non-neoplastic origin from a peritumoral or intratumoral population of benign glial-like cells [15], while countervailing evidence suggests that they are integral parts of tumors, possibly contributing to tumor development. The latter is supported by their reported expression of stem cell markers in both normal [16,17] and neoplastic [18] paraganglionic tissue. This issue could not be resolved in the present study because small cell clusters were undoubtedly present in the initially injected cell suspensions, and we could not determine whether the sustentacular cells were ever completely separate from the chief cells.
An important additional finding was that the tumor vasculature consisted in large part of human rather than mouse-derived endothelium. This suggests that the optimal grafting protocol in future studies should employ heterogeneous rather than purified tumor cell populations. The finding that some areas of tumor contained Ki-67-labelled tumor vessels without any tumor cells is consistent with the fact that endothelium in some paragangliomas in vivo can also show more robust labelling than the neoplastic chief cells.
The ready acceptance of primary tumor xenografts by NSG mice and other new mouse strains has led to increasing use of a new approach to pre-clinical drug testing employing “patient–derived xenografts” (PDXs) [12]. A PDX model can consist of tumor from an individual patient or multiple representative tumors with a very limited number of serial passages, the latter often being commercially available. The rationale for this approach is that primary xenografts or early in vivo passages are likely to resemble a patient’s tumor more closely than established cell lines, and may therefore be more valid predictors of drug response. This study provides proof of principle for the potential use of NSG mice in PDX models of pheochromocytoma and paraganglioma. Although the growth rate of the grafted cells was slow and the tumors were small, the slow growth accurately represents the growth rate of most pheochromocytomas and paragangliomas even in metastases [19]
It should be noted that the numbers of tumor cells injected in this study were small because the aliquots were originally apportioned for cell culture studies. Both the tumor size and incidence of successful engraftment are likely to be increased in future investigations by increasing the number of injected cells, which will also shorten the waiting period before studies can be conducted in a practical manner. This model could be especially suited for use in conjunction with exquisitely sensitive imaging techniques such as 68Ga-DOTATATE PET/CT [20] for testing the effects of potential therapies on small, slowly growing tumor deposits that typify most metastases.
While PDX models are a potentially important new pre-clinical tool, there is still a pressing need for human pheochromocytoma and paraganglioma cell lines to provide a reliable homogeneous source of cells for both basic and translational research. The present findings suggest that xenografts in NSG mice will further the goal of establishing cell lines by means of repeated alternate passaging of cells between cultures and mice. This time-tested technique, which selects for increasingly rapid proliferation in culture and in vivo, was used to establish both the PC12 rat pheochromocytoma and MPC mouse pheochromocytoma line [7,8], currently the most widely studied pheochromocytoma models.
Acknowledgments
Funding: This study was funded by PheoPara Alliance.
This work was supported by the Intramural Research Program of the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development
Footnotes
Compliance with ethical standards: Studies of the banked tumor samples were approved by the Institutional Review Boards of the National Institutes of Health and Tufts Medical Center. Patients provided written informed consent.
All procedures performed in studies involving animals were in accordance with the ethical standards of Tufts Medical Center.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Pacak K, Wimalawansa SJ. Pheochromocytoma and paraganglioma. Endocr Pract. 2015;21:406–12. doi: 10.4158/EP14481.RA. [DOI] [PubMed] [Google Scholar]
- 2.Benn DE, Robinson BG, Clifton-Bligh RJ. 15 YEARS OF PARAGANGLIOMA: Clinical manifestations of paraganglioma syndromes types 1–5. Endocr Relat Cancer. 2015;22:T91–T103. doi: 10.1530/ERC-15-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudin E, Habra MA, Deschamps F, et al. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol. 2014;171:R111–22. doi: 10.1530/EJE-14-0113. [DOI] [PubMed] [Google Scholar]
- 4.Jansen JC, van den Berg R, Kuiper A, van der Mey AG, Zwinderman AH, Cornelisse CJ. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88:2811–6. [PubMed] [Google Scholar]
- 5.Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E. The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. 2014;260:158–62. doi: 10.1097/SLA.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepoutre-Lussey C, Thibault C, Buffet A, et al. From Nf1 to Sdhb knockout: Successes and failures in the quest for animal models of pheochromocytoma. Mol Cell Endocrinol. 2016;421:40–8. doi: 10.1016/j.mce.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers JF, Evinger MJ, Tsokas P, et al. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302:309–20. doi: 10.1007/s004410000290. [DOI] [PubMed] [Google Scholar]
- 9.Martiniova L, Lai EW, Elkahloun AG, et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26:239–50. doi: 10.1007/s10585-009-9236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lussey-Lepoutre C, Hollinshead KE, Ludwig C, et al. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun. 2015;6:8784. doi: 10.1038/ncomms9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tischler AS, Powers JF, Alroy J. Animal models of pheochromocytoma. Histol Histopathol. 2004;19:883–95. doi: 10.14670/HH-19.883. [DOI] [PubMed] [Google Scholar]
- 12.Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014;2014:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers JF, Picard KL, Tischler AS. RET expression and neuron-like differentiation of pheochromocytoma and normal chromaffin cells. Horm Metab Res. 2009;41:710–4. doi: 10.1055/s-0029-1224136. [DOI] [PubMed] [Google Scholar]
- 14.Tischler AS, Ruzicka LA, Riseberg JC. Immunocytochemical analysis of chromaffin cell proliferation in vitro. J Histochem Cytochem. 1992;40:1043–5. doi: 10.1177/40.7.1351491. [DOI] [PubMed] [Google Scholar]
- 15.Douwes Dekker PB, Corver WE, Hogendoorn PC, van der Mey AG, Cornelisse CJ. Multiparameter DNA flow-sorting demonstrates diploidy and SDHD wild-type gene retention in the sustentacular cell compartment of head and neck paragangliomas. chief cells are the only neoplastic component. J Pathol. 2004;202:456–62. doi: 10.1002/path.1535. [DOI] [PubMed] [Google Scholar]
- 16.Mazzatenta A, Marconi GD, Macchi V, et al. Coexpression of Galanin and Nestin in the Chemoreceptor Cells of the Human Carotid Body. Adv Exp Med Biol. 2016;885:77–82. doi: 10.1007/5584_2015_189. [DOI] [PubMed] [Google Scholar]
- 17.Rubin de Celis MF, Garcia-Martin R, Wittig D, et al. Multipotent Glia-Like Stem Cells Mediate Stress Adaptation. Stem Cells. 2015 doi: 10.1002/stem.2002. [DOI] [PubMed] [Google Scholar]
- 18.Oudijk L, Neuhofer CM, Lichtenauer UD, et al. Immunohistochemical Expression of Stem Cell Markers in Pheochromocytoma/Paraganglioma is Associated with SDHx-mutations. Eur J Endocrinol. 2015 doi: 10.1530/EJE-14-1164. [DOI] [PubMed] [Google Scholar]
- 19.Hescot S, Leboulleux S, Amar L, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:4006–12. doi: 10.1210/jc.2013-1907. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Blanchet EM, Adams K, et al. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2015;21:3888–95. doi: 10.1158/1078-0432.CCR-14-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]