Abstract
A 73-year old man with a history of multiple genitourinary malignancies was found to have a left retroareolar soft tissue mass on CT assessment of disease, and dedicated breast imaging was recommended. Diagnostic mammography and ultrasonography confirmed a solid mass, for which biopsy was recommended. Pathologic analysis demonstrated a spindle cell neoplasm with an immunoreactivity pattern consistent with myofibroblastoma. While this entity is benign, nonspecific imaging features necessitate tissue sampling for pathologic diagnosis, and, given pathologic rarity, open communication between the radiologist and pathologist is important to establish the correct diagnosis and to recommend appropriate management.
Keywords: myofibroblastoma, genitourinary malignancy, diagnostic mammography, breast ultrasonography
1. History
A 73-old man with a medical history significant for multiple genitourinary malignancies was noted to have a soft tissue mass of the left retroareolar chest wall on CT assessment of disease status. The patient’s cancer history includes left-sided papillary renal cell carcinoma, treated with laparoscopic partial nephrectomy (2004); prostatic adenocarcinoma, treated with robotic prostatectomy (2011); and left-sided high-grade papillary urothelial carcinoma (diagnosed April 2016), treated with neoadjuvant chemotherapy and robotic-assisted laparoscopic nephroureterectomy, with ongoing cystoscopic, cytologic, and imaging surveillance. The patient’s family history was notable for breast cancer in his mother, who was postmenopausal at the time of her diagnosis.
2. Radiology and pathology findings
Contrast-enhanced computed tomography (CT) of the chest, abdomen and pelvis performed for extent of disease evaluation following a course of neoadjuvant chemotherapy for urothelial carcinoma demonstrated a 7 mm soft tissue mass within the subcutaneous fat of the left retroareolar chest wall (Fig. 1). Retrospective review of prior positron emission tomography (PET)-CT performed two months earlier (not shown) demonstrated the mass to be 7 mm in size at that time as well without associated FDG-avidity. Given the patient’s history of multiple malignancies and the potential impact of distant metastatic disease on management, dedicated breast imaging was recommended for further evaluation.
Figure 1. Detection of a soft tissue mass on CT assessment of disease.
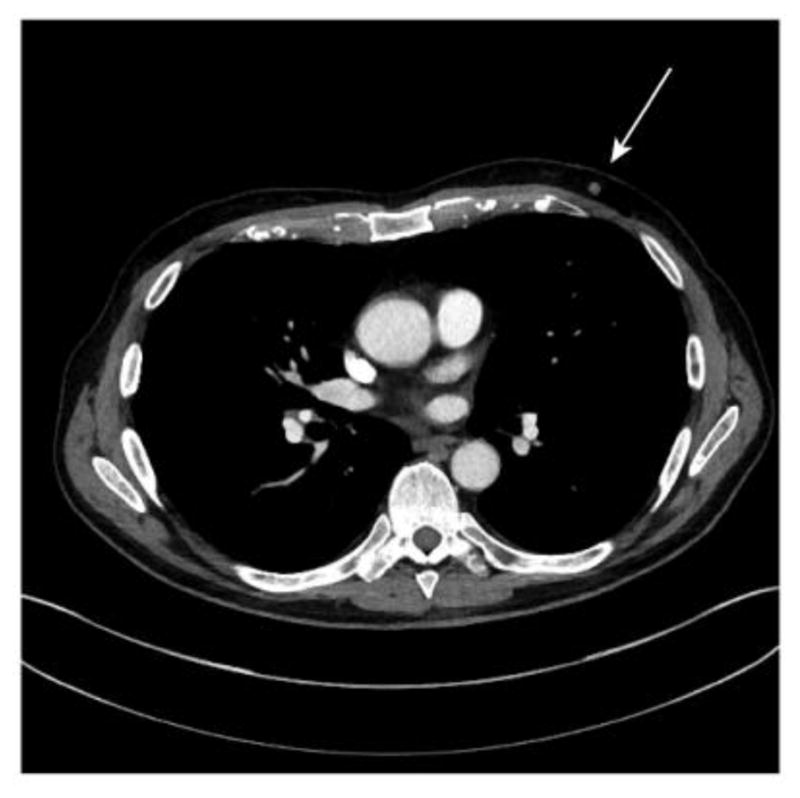
73 year-old male with contrast enhanced CT chest (axial imaging) demonstrating a soft tissue nodule within the subcutaneous fat over the left chest wall (arrow).
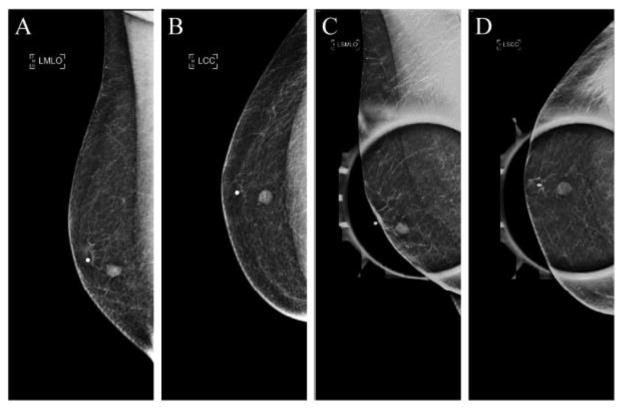
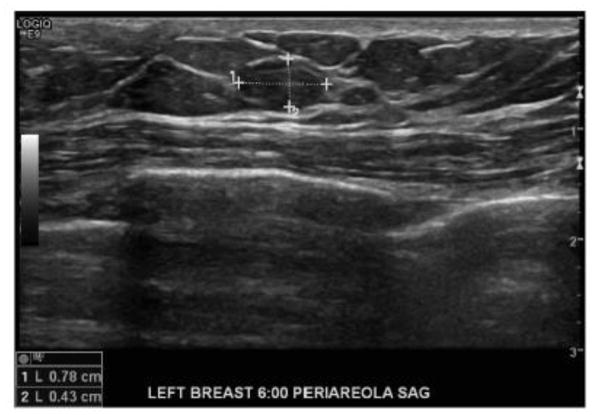
A diagnostic bilateral mammogram utilizing tomosynthesis was performed demonstrating in the 6:00 axis of the left breast a focal asymmetry on routine mediolateral oblique (MLO) and craniocaudal (CC) views, which persisted on spot compression views as a 8 mm oval circumscribed mass (Figs. 2A–2D); the right breast mammogram did not demonstrate any abnormality. Left breast ultrasound was then performed, demonstrating in the 6:00 axis, periareolar region, a 8 × 4 × 7 mm oval parallel circumscribed hypoechoic solid mass with intrinsic vascularity (Fig. 3), corresponding with the mammographic area of concern. Together, mammography and ultrasound imaging was consistent with an American College of Radiology Breast Imaging-Reporting and Data System (ACR BI-RADS) Category 4 assessment. Moreover, although certain imaging features of the mass -- round shape and circumscribed margins – suggested a benign etiology in terms of the differential diagnosis, the presence of a breast mass in a male patient with history of multiple malignancies raised the possibility of new primary cancer versus metastatic disease. An ultrasound-guided needle biopsy was recommended, and, utilizing a lateral approach, a total of 5 biopsy specimens were collected and sent for pathologic analysis.
Figure 2. Diagnostic mammography demonstrates a focal asymmetry of the left breast.
Left breast mammogram demonstrates a focal asymmetry in the 6:00 axis on routine MLO (A) and CC (B) mammographic views, which persists on MLO (C) and CC (D) spot compression views as a 8 mm oval circumscribed mass.
Figure 3. Breast ultrasonography demonstrates a solid mass of the left breast.
Left breast ultrasound demonstrates in the 6:00 axis, periareolar region, a 8 × 4 mm oval parallel circumscribed hypoechoic mass corresponding with the mammographic area of concern.
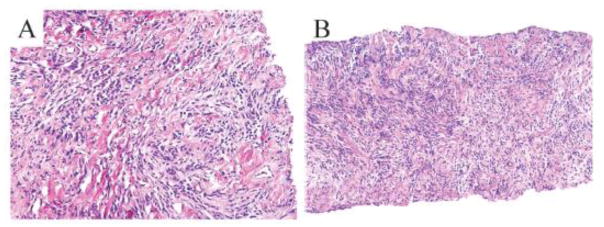
Pathologic analysis of the collected specimens demonstrated a benign, spindle cell neoplasm, with strong and diffuse immunoreactivity for desmin, CD34, and estrogen receptor (ER), most consistent with mammary myofibroblastoma (Figs. 4A and 4B). The pathology results were deemed concordant with the imaging findings. Surgical consultation advised wide local excision under needle localized guidance on an ambulatory basis. Following surgical excision, gross specimen analysis demonstrated a 9 × 7 × 5 mm firm, well-circumscribed mass, and final pathologic analysis demonstrated a 9 mm myofibroblastoma, completely excised.
Figure 4. Pathologic analysis of needle core biopsy is consistent with mammary myofibroblastoma.
The tumor comprises of neoplastic spindle cells that are interspersed by bands of collagen. The neoplastic cells display bland nuclei and eosinophilic cytoplasm. No mitotic figures are evident (A and B; H&E).
3. Discussion
While mammary myofibroblastoma is pathologically classified as a benign spindle cell tumor of the mammary stroma [1, 2], nonspecific imaging features at presentation necessitate tissue sampling for pathologic diagnosis [3, 4]. This was particularly true for the patient in this case, as metastatic carcinoma and primary breast neoplasm were also on the differential diagnosis given his personal history of multiple malignancies and family history of breast cancer. Moreover, pathologic analysis must carefully evaluate collected specimens as immunohistochemical and morphological variants of mammary myofibroblastoma include entities resembling malignancies [3], including epithelioid and deciduoid cell variants. Together, these properties indicate a critical need for awareness of this benign neoplasm among radiologists and pathologists alike, with open communication between the two to establish the correct diagnosis and to recommend appropriate management.
Myofibroblastoma is a mesenchymal tumor derived from stromal fibroblasts most commonly found within the breast parenchyma [3, 4], with case reports also indicating extramammary locations [3], particularly within the genitourinary soft tissues. This is of interest in this particular case, given our patient’s history of multiple genitourinary neoplasms. Although mammary myofibroblastoma has been described as a lesion predominantly found in the male breast [2, 5], as was the case with this patient, overall, it occurs with similar prevalence in both men and women, typically at older ages, with a median age of presentation of 55 years [4, 5]. Previous publications have also reported myofibroblastoma in the setting of prior malignancies [6, 7], including pancreatic, renal, and prostatic carcinoma. Again, this is of interest in this particular case, given our myofibroblastoma patient’s history of renal and prostatic carcinoma, suggestive of a common underlying etiology or predilection in the setting of genitourinary malignancy.
Clinically, mammary myofibroblastoma tends to present as a unilateral, firm, mobile, painless mass that may demonstrate slow, steady growth over a period of months to years [4, 5]. An association with gynecomastia has been reported but was not present in this patient [8]. Myofibroblastomas have also been reported at surgical scar sites following breast cancer excision [9], which may be secondary to the migration and transformation of fibroblasts to the tissue injury location [10]. Imaging features of myofibroblastoma are nonspecific. Mammography tends to demonstrate a well-circumscribed, round to oval, dense mass with rare course calcifications [11, 12]. Sonographically, this neoplasm presents as a well-circumscribed, round to oval mass as well, with variable echogenicity [11, 13]. Thus, nonspecific imaging features necessitate tissue sampling for pathologic diagnosis.
Pathologically, the classic type of mammary myofibroblastoma is composed of bundles of slender, uniform, spindle-shaped cells, typically arranged in clusters that are separated by broad bands of hyalinized collagen, as seen in this case. The majority of the myofibroblastomas are immunoreactive for CD34, actin, CD10 and desmin. They also usually express estrogen receptor (ER) and progesterone receptor (PR), while variably expressing androgen receptor (AR) [14]. They are not immunoreactive for cytokeratins, EMA, S100, HMB-45, and CD117 [5]. In this report, pathologic analysis demonstrated expression of desmin, CD34, and ER, most consistent with myofibroblastoma. Variant forms of myofibroblastoma including collagenized, cellular, infiltrative, myxoid, lipomatous, epitheloid and deciduoid variants have been noted. Myofibroblastomas are surgically managed with wide local excision and is considered curative, without local or metastatic detection up to a period of 15 years [15].
Genetically, myofibroblastoma has been shown to exhibit deletion at the chromosome 13q region [16], with most cases exhibiting 13q14 deletion [3], similar to other benign mesenchymal and stromal neoplasms including spindle cell lipoma [17] and cellular angiofibroma [18, 19]. Immunohistochemical evidence of variable expression of sex hormone receptors further suggests that hormonal signaling may also play a role in the etiology of myofibroblastoma [14, 20]. While not always observed in men presenting with myofibroblastoma, concomitant gynecomastia suggests that estrogen signaling may play a role. Reported increased intensity of AR staining suggests an etiologic role of androgen signaling while requiring further research to determine causality [20].
In conclusion, nonspecific imaging findings of myofibroblastoma necessitate biopsy and pathologic analysis for correct diagnosis, which is particularly critical in the patient with a history of multiple malignancies. Close communication between radiologists and pathologists is necessary to assess for concordance of radiologic and pathologic findings, with surgical consultation always advised as management consists of wide local excision. The long term prognosis is excellent, as this is a benign neoplasm with surgical management considered curative. This case report also provides further evidence of myofibroblastoma in the setting of prior genitourinary malignancy. Interestingly, chromosomal 13q14 deletions have been reported in highly diverse malignancies [21, 22], including one-third of prostate tumors [23, 24], at times involving those of high tumor grade and stage [25]. Chromosomal deletion of a region including 13q14 has also been shown to be a common finding in breast cancer [26], underscoring the critical need for radiologic and pathologic diagnosis in this case report. While alterations of the 13q14 region evidently result in highly diverse patient presentations, further research of this region may demonstrate if there is indeed a predilection for myofibroblastoma in patients with a history of genitourinary malignancy.
Highlights.
A male patient presenting with a rare, benign neoplasm of the mammary parenchyma
Nonspecific imaging findings of this neoplasm necessitate biopsy and pathologic analysis, which is of particular importance in the setting of prior malignancy
Careful pathologic analysis is required for correct diagnosis due to variants of myofibroblastoma
Open communication between radiologists and pathologists is critical to establish the correct diagnosis and to recommend appropriate medical management
Acknowledgments
The authors acknowledge with gratitude Syed Hoda MD, who served as the senior consultant pathologist. John D. Comer was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program [grant number T32GM007739]. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toker C, Tang CK, Whitely JF, Berkheiser SW, Rachman R. Benign spindle cell breast tumor. Cancer. 1981;48(7):1615–22. doi: 10.1002/1097-0142(19811001)48:7<1615::aid-cncr2820480724>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol. 1987;11(7):493–502. doi: 10.1097/00000478-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Magro G. Mammary myofibroblastoma: an update with emphasis on the most diagnostically challenging variants. Histol Histopathol. 2016;31(1):1–23. [PubMed] [Google Scholar]
- 4.Omar LA, Rojanapremsuk T, Saluja K, Merchant KA, Sharma PB. Radiologic and histologic presentation of male mammary myofibroblastoma. Proc (Bayl Univ Med Cent) 2016;29(3):321–2. doi: 10.1080/08998280.2016.11929453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magro G. Mammary myofibroblastoma: a tumor with a wide morphologic spectrum. Arch Pathol Lab Med. 2008;132(11):1813–20. doi: 10.5858/132.11.1813. [DOI] [PubMed] [Google Scholar]
- 6.McMenamin ME, Fletcher CD. Mammary-type myofibroblastoma of soft tissue: a tumor closely related to spindle cell lipoma. Am J Surg Pathol. 2001;25(8):1022–9. doi: 10.1097/00000478-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rosen PP. Myofibroblastoma. In: Rosen PP, editor. Rosen’s Breast Pathology. 2. Philadelphia PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 8.Yoo CC, Pui JC, Torosian MH. Myofibroblastoma associated with bilateral gynecomastia: a case report and literature review. Oncol Rep. 1998;5(3):731–3. doi: 10.3892/or.5.3.731. [DOI] [PubMed] [Google Scholar]
- 9.Gocht A, Bosmuller HC, Bassler R, Tavassoli FA, Moinfar F, Katenkamp D, et al. Breast tumors with myofibroblastic differentiation: clinico-pathological observations in myofibroblastoma and myofibrosarcoma. Pathol Res Pract. 1999;195(1):1–10. doi: 10.1016/S0344-0338(99)80087-9. [DOI] [PubMed] [Google Scholar]
- 10.Metry M, Shaaban M, Youssef M, Carr M. Myofibroblastoma of the Breast: Literature Review and Case Report. Case Rep Oncol Med. 2016;2016:1714382. doi: 10.1155/2016/1714382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg JS, Kaplan SS, Grady C. Myofibroblastoma of the breast in women: imaging appearances. AJR Am J Roentgenol. 1998;171(1):71–2. doi: 10.2214/ajr.171.1.9648767. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi N, Oda K, Yokoi S, Kanda H, Hayakawa S, Tang X, et al. Myofibroblastoma of the breast: report of a case. Surg Today. 1996;26(9):727–9. doi: 10.1007/BF00312094. [DOI] [PubMed] [Google Scholar]
- 13.Dockery WD, Singh HR, Wilentz RE. Myofibroblastoma of the male breast: imaging appearance and ultrasound-guided core biopsy diagnosis. Breast J. 2001;7(3):192–4. doi: 10.1046/j.1524-4741.2001.007003192.x. [DOI] [PubMed] [Google Scholar]
- 14.Magro G, Bisceglia M, Michal M. Expression of steroid hormone receptors, their regulated proteins, and bcl-2 protein in myofibroblastoma of the breast. Histopathology. 2000;36(6):515–21. doi: 10.1046/j.1365-2559.2000.00907.x. [DOI] [PubMed] [Google Scholar]
- 15.Magro G, Bisceglia M, Michal M, Eusebi V. Spindle cell lipoma-like tumor, solitary fibrous tumor and myofibroblastoma of the breast: a clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch. 2002;440(3):249–60. doi: 10.1007/s00428-001-0572-y. [DOI] [PubMed] [Google Scholar]
- 16.Pauwels P, Sciot R, Croiset F, Rutten H, Van den Berghe H, Dal Cin P. Myofibroblastoma of the breast: genetic link with spindle cell lipoma. J Pathol. 2000;191(3):282–5. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH635>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Dal Cin P, Sciot R, Polito P, Stas M, de Wever I, Cornelis A, et al. Lesions of 13q may occur independently of deletion of 16q in spindle cell/pleomorphic lipomas. Histopathology. 1997;31(3):222–5. doi: 10.1046/j.1365-2559.1997.2450851.x. [DOI] [PubMed] [Google Scholar]
- 18.Flucke U, van Krieken JH, Mentzel T. Cellular angiofibroma: analysis of 25 cases emphasizing its relationship to spindle cell lipoma and mammary-type myofibroblastoma. Mod Pathol. 2011;24(1):82–9. doi: 10.1038/modpathol.2010.170. [DOI] [PubMed] [Google Scholar]
- 19.Maggiani F, Debiec-Rychter M, Vanbockrijck M, Sciot R. Cellular angiofibroma: another mesenchymal tumour with 13q14 involvement, suggesting a link with spindle cell lipoma and (extra)-mammary myofibroblastoma. Histopathology. 2007;51(3):410–2. doi: 10.1111/j.1365-2559.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MB, Pitha JV. Myofibroblastoma of the breast revisited: an etiologic association with androgens? Hum Pathol. 1998;29(4):347–51. doi: 10.1016/s0046-8177(98)90114-9. [DOI] [PubMed] [Google Scholar]
- 21.Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45(10):1134–40. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machiela MJ, Zhou W, Caporaso N, Dean M, Gapstur SM, Goldin L, et al. Mosaic 13q14 deletions in peripheral leukocytes of non-hematologic cancer cases and healthy controls. J Hum Genet. 2016;61(5):411–8. doi: 10.1038/jhg.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooney KA, Wetzel JC, Merajver SD, Macoska JA, Singleton TP, Wojno KJ. Distinct regions of allelic loss on 13q in prostate cancer. Cancer Res. 1996;56(5):1142–5. [PubMed] [Google Scholar]
- 24.Yin Z, Spitz MR, Babaian RJ, Strom SS, Troncoso P, Kagan J. Limiting the location of a putative human prostate cancer tumor suppressor gene at chromosome 13q14.3. Oncogene. 1999;18(52):7576–83. doi: 10.1038/sj.onc.1203203. [DOI] [PubMed] [Google Scholar]
- 25.Dong JT, Boyd JC, Frierson HF., Jr Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. The Prostate. 2001;49(3):166–71. doi: 10.1002/pros.1131. [DOI] [PubMed] [Google Scholar]
- 26.Osborne RJ, Hamshere MG. A genome-wide map showing common regions of loss of heterozygosity/allelic imbalance in breast cancer. Cancer Res. 2000;60(14):3706–12. [PubMed] [Google Scholar]