Abstract
Determining candidacy for live kidney donation among obese individuals remains challenging. Among healthy non-donors, body mass index (BMI) above 30 is associated with a 16% increase in risk of end-stage renal disease (ESRD). However, the impact on the ESRD risk attributable to donation and living with only one kidney remains unknown. Here we studied the risk of ESRD associated with obesity at the time of donation among 119 769 live kidney donors in the United States. Maximum follow-up was 20 years. Obese (BMI above 30) live kidney donors were more likely male, African American, and had higher blood pressure. Estimated risk of ESRD 20 years after donation was 93.9 per 10 000 for obese; significantly greater than the 39.7 per 10 000 for non-obese live kidney donors. Adjusted for age, sex, ethnicity, blood pressure, baseline estimated glomerular filtration rate , and relationship to recipient, obese live kidney donors had a significant 86% increased risk of ESRD compared to their non-obese counterparts (adjusted hazard ratio 1.86; 95% confidence interval 1.05–3.30). For each unit increase in BMI above 27kg/m2 there was an associated significant 7% increase in ESRD risk (1.07, 1.02–1.12). The impact of obesity on ESRD risk was similar for male and female donors, African American and Caucasian donors, and across the baseline estimated glomerular filtration rate spectrum. These findings may help to inform selection criteria and discussions with persons considering living kidney donation.
Keywords: living kidney donation, kidney transplantation, obesity, end-stage renal disease (ESRD)
INTRODUCTION
The demand for transplantable kidneys remains high, and living kidney donors (LKDs) continue to be a critical source of organs facilitating both timely transplantation and excellent outcomes.1 Historically, living kidney donors were free of isolated medical abnormalities (IMA) at the time of donation. However, in response to the organ shortage and changes in the general population, transplant centers now commonly approve donors with IMAs such as obesity.2–7 Currently, more than 25% of all living kidney donors are considered obese at the time of donation compared to fewer than 8% in the 1970’s.6 Relaxation of selection criteria to include obese living kidney donors has occurred despite a paucity of supporting safety data.
The National Institutes of Health defines obesity as a body mass index (BMI) of greater than 30kg/m2.8 According to data from the National Health and Nutrition Examination Survey (NHANES), the prevalence of obesity has increased in the US from 27.5% in 1999 to 36.5% in 2011–2014,9 and in parallel with these general trends, the mean BMI of living kidney donors in the US has increased over time, from 24.3kg/m2 in the 1970’s to 27.3kg/m2 in the 2000’s.6 Obesity is strongly correlated with increased risk for cardiovascular disease, diabetes mellitus, and chronic kidney disease (CKD).10–14 Data from a population-based case control study conducted in Sweden as well as two US studies (The Framingham Offspring cohort and the Hypertension Detection and Follow-up Program) have shown that higher weight for height was associated with increased risk of new onset CKD.15–17
Beyond CKD, obesity has been linked with increased risk for end stage renal disease (ESRD). In the general population, Hsu et al found that compared to persons considered normal weight (BMI: 18.5–24.9kg/m2), obese individuals (BMI ≥ 30kg/m2) were at 3.57-fold higher risk of developing ESRD (adjusted RR: 3.57, 95%CI: 3.05–4.18).18 However, donors are not drawn from the general population, but are very carefully screened, and the impact of obesity might be different in these healthier individuals. In fact, in a multi-cohort study of individuals healthy enough to be potential donors (healthy non-donors), the adjusted risk of ESRD associated with obesity was only 1.16 (95%CI: 1.04–1.29).19 The true risk among living donors likely falls somewhere in between these estimates: living donors are healthy at baseline, but then they lose half of their nephron mass, and the impact of obesity in this context remains unclear.
Unfortunately, the four recent major studies of living kidney donors that establish the existing evidence of living donor risk for ESRD were done in the context of standard selection criteria, healthy and IMA-free, and did not address the impact of obesity, highlighting a remaining gap in our knowledge of living donor outcomes.20–23 To better understand ESRD risk in obese living kidney donors, and to better inform selection criteria for potential obese kidney donors, we performed a national study of the association between BMI and post-donation risk of ESRD among living kidney donors, adjusting for potential confounders and exploring potential effect modifiers of this association.
RESULTS
The mean BMI among obese donors was 32.7 kg/m2 compared to 24.8 kg/m2 among non-obese donors. At the time of donation, obese and non-obese living donors were similar with regard to age, baseline eGFR, smoking history, insurance status, and relationship to the recipient. However, compared to their non-obese counterparts, obese living donors were more commonly male (43.1% vs. 39.2%), African American (16.4% vs. 11.1%), and had higher mean systolic (124.1 mmHg vs. 119.9 mmHg) and diastolic blood pressures (75.6 mmHg vs. 72.9 mmHg) (Table 1).
Table 1.
Demographics of living kidney donors by obesity status at time of donation (10/1/1987–6/30/2013).
| Donor characteristic | Obese (BMI ≥ 30 kg/m2) (N=20 588) | Non-obese (BMI < 30 kg/m2) (N=58 004) | Missing BMI (N=41 177) |
|---|---|---|---|
| Age- years, mean (SD) | 40.7 (10.7) | 40.8 (11.4) | 38.5 (10.9) |
| Sex, N(%) | |||
| Male | 8,864 (43.1) | 22,763 (39.2) | 17,744 (43.1) |
| Female | 11,724 (56.9) | 35,241 (60.8) | 23,433 (56.9) |
| Ethnicity | |||
| African American | 3,374 (16.4) | 6,450 (11.1) | 5,485 (13.3) |
| Non-African American | 17,214 (83.6) | 51,554 (88.9) | 35,692 (86.7) |
| BMI- kg/m2, mean (SD)* | 32.7 (3.2) | 24.8 (2.9) | - |
| Systolic BP, mean (SD)* | 124.1 (13.1) | 119.9 (13.3) | 121.2 (14.0) |
| Diastolic BP, mean (SD)* | 75.6 (9.3) | 72.9 (9.4) | 74.1 (9.3) |
| eGFR- mL/min/1.73m2, mean (SD)* | 96.8 (18.9) | 97.2 (18.5) | 95.5 (20.0) |
| Ever smoked cigarettes* | 3,192 (15.5) | 8,926 (15.4) | 278 (0.7) |
| Insured* | 8,981 (43.7) | 25,996 (44.8) | 1,360 (3.3) |
| Related to recipient* | 12,953 (62.9) | 35,482 (61.2) | 34,124 (82.9) |
not collected for the entire cohort
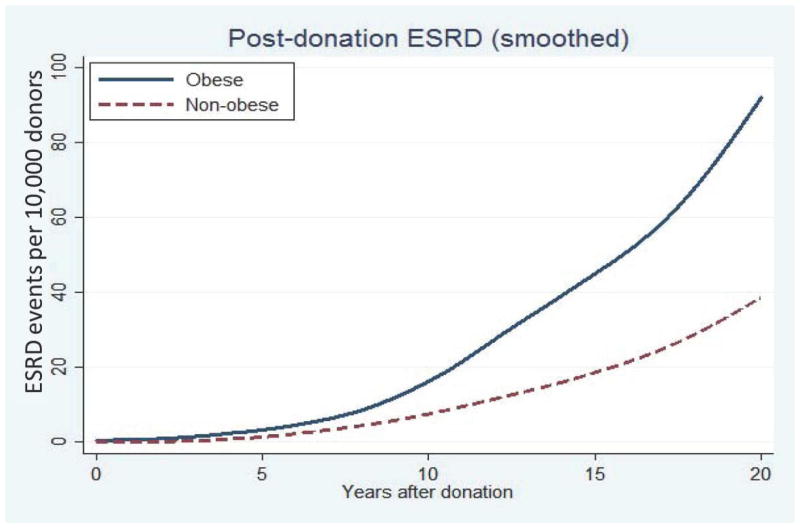
The cumulative incidence of ESRD per 10 000 living donors was 3-fold greater among living kidney donors who were obese at the time of donation compared to their non-obese counterparts. At 20-years post-donation, obese living kidney donors had a cumulative incidence of ESRD of 93.9 per 10 000 compared to 39.7 per 10 000 among their non-obese living donor counterparts (Table 2 & Figure 1).
Table 2.
Cumulative incidence of ESRD events per 10 000 living donors estimated from the multiple imputation analyses (10/1/1987–6/30/2013).
| Obesity status | 5-years | 10-years | 15-years | 20-years |
|---|---|---|---|---|
| Obese (BMI ≥ 30 kg/m2) | 3.2 | 15.2 | 42.5 | 93.9 |
| Non-obese (BMI < 30 kg/m2) | 1.0 | 7.4 | 17.5 | 39.7 |
Figure 1.
Cumulative incidence of post-donation ESRD events among living kidney donors by obesity status at time of donation.
After controlling for multiple risk factors, including age at donation, ethnicity, gender, baseline eGFR and blood pressure, and relationship to the recipient, the only potentially modifiable factor that remained independently associated with increased risk for development of ESRD post-donation was obesity. Compared to non-obese living kidney donors, obese living kidney donors had a 1.9-fold increased risk of ESRD post-donation (adjusted hazard ratio (aHR): 1.86, 95%CI: 1.05–3.30, p=0.04) (Table 3). This finding was consistent with the results seen with analyses performed only among donors who had complete data available (Table 4). On stratified analyses, for each one unit increase in BMI above 27 kg/m2 there was an associated 7% increase in the risk of ESRD (aHR: 1.07, 95%CI: 1.02–1.12, p=0.004). This same effect was not observed for those with a BMI ≤ 27 kg/m2. The potential for effect modification was explored using interaction term analyses. No significant interactions were observed between obesity and sex (aHR: 1.09, 95%CI: 0.51–2.34, p=0.82), African American ethnicity (aHR: 0.89, 95%CI: 0.41–1.92, p=0.75), relationship to the recipient (aHR: 1.15, 95%CI: 0.45–2.93, p=0.76), or eGFR (aHR: 1.00, 95%CI: 0.98–1.02, p= 0.99).
Table 3.
Adjusted risk of ESRD among living kidney donors estimated from multiple imputation analyses (10/1/1987–6/30/2013).
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| Obese (ref= non-obese) | 1.86 | 1.05–3.30 | 0.04 |
| Age, per 1-year increase | 0.99 | 0.98–1.01 | 0.37 |
| Female | 0.51 | 0.39–0.66 | < 0.001 |
| African American (ref=non-African American) | 4.62 | 3.46–6.16 | < 0.001 |
| Systolic BP ≥ 120 or diastolic BP ≥ 80 mmHg | 1.29 | 0.73–2.26 | 0.37 |
| eGFR, per 1 mL/min/1.73m2 increase | 0.97 | 0.95–0.98 | < 0.001 |
| Related to recipient | 1.51 | 1.00–2.28 | 0.05 |
Table 4.
Adjusted risk of ESRD among living kidney donors estimated from complete case (2/3 of donors in whom no baseline risk factors were missing) analyses (10/1/1987–6/30/2013).
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| Obese (ref= non-obese) | 2.26 | 1.30–3.92 | 0.004 |
| Age, per 1-year increase | 1.00 | 0.98–1.03 | 0.74 |
| Female | 0.56 | 0.32–0.98 | 0.04 |
| African American (ref=non-African American) | 3.17 | 1.72–5.86 | < 0.001 |
| Systolic BP ≥ 120 or diastolic BP ≥ 80 mmHg | 1.64 | 0.83–3.24 | 0.6 |
| eGFR, per 1 mL/min/1.73m2 increase | 0.99 | 0.97–1.00 | 0.09 |
| Related to recipient | 1.27 | 0.67–2.41 | 0.47 |
DISCUSSION
In this national study of 119 769 living kidney donors linked to CMS data for ascertainment of ESRD, we estimated that approximately 40 non-obese and 94 obese living donors per 10 000 developed ESRD within 20 years of kidney donation. While the absolute risk for post-donation ESRD was low, donor obesity was independently associated with increased risk for ESRD 20 years after kidney donation. Compared to non-obese living donors, obese donors had a 1.9-fold increased risk for post-donation ESRD, and for each one unit increase in pre-donation BMI above 27 kg/m2 there was an associated 7% increased risk of ESRD post-donation.
Studies from the general US population have demonstrated the relationship between excess weight or obesity and risk of ESRD. These cohorts, however, included individuals with baseline comorbidities such as diabetes and hypertension, conditions known to be along the CKD/ESRD causal pathway.18, 24 While our cohort of living kidney donors were free of diabetes and hypertension at the time of donation, and beyond their baseline obesity were otherwise healthy, we too found that obesity was independently associated with increased risk for ESRD. Moreover, we found this association began with living donor baseline BMIs greater than 27 kg/m2. In other words, while the greatest risk for post-donation ESRD may have been observed among obese living kidney donors, the increased risk for ESRD post-donation began among living kidney donors who would be labeled merely overweight at baseline. These findings have important implications for current living donor selection practices with regard to BMI cutoffs used as absolute contraindications for donation.
These data also suggest that it may be prudent to enroll potential obese living kidney donors in a pre-donation rehabilitation or weight loss program, as short-term weight loss has been associated with improvements in cardiovascular risk factors.25–30 However, it is likely that long-term maintenance of weight loss would be required to mitigate the risk for ESRD associated with obesity. Studies have shown that even in the setting of an intense rehabilitation program with regular treatment contact most participants regain 30–50% of their initial weight loss over a 2–3 year period,31, 32 and as such, pre-donation weight loss may do little to mitigate long-term ESRD risk associated with obesity. Encouragingly, however, recent data from the Look AHEAD trial, a randomized trial of intensive lifestyle interventions compared to a control condition in overweight/obese individuals with type 2 diabetes, found no negative associations of losing and regaining weight relative to not having lost weight; and individuals with large initial weight loss but full regain of weight still had greater improvements in diabetes control than those with no initial weight loss.33 These data lend some support for pre-donation weight loss for obese potential living kidney donors as an effort to modify short-term risk for post-donation ESRD; however, the impact of weight loss on long-term risk for ESRD remains uncertain. To date, no study has examined weight loss/gain among living kidney donors and its impact on risk for post-donation ESRD.
The primary strengths of our approach were the inclusion of every known living kidney donor in the US since the establishment of the OPTN in 1987 and the highly reliable linkage-based ESRD ascertainment. The large samples size of more than 100 000 actual living kidney donors enabled us to estimate the incidence of a rare event and to make inferences specific to the subgroup of obese living donors. These data provide critical information for those considering donation as well as the more than 100 000 individuals known to have already donated a kidney for the purposes of living donor transplantation. Despite these strengths, our study does have some important limitations. One third of the cohort was missing information on pre-donation BMI, and as such multiple imputation was utilized to allow us to include those who donated in years prior to when BMI was routinely captured for LKDs. This methodology generated 20 datasets for analysis in which some donors with missing BMI were imputed as obese but may not have been in other imputations. As such the median time to development of post-donation ESRD could not be estimated. However, it is important to note that the inferences presented from the imputed data set were confirmed in the complete-case analyses (2/3 of donors in whom no baseline risk factors were missing). Moreover, median follow-up for the study was only 10.7 years and may not have permitted us to fully understand the long-term risk of post-donation ESRD, and it is likely that the overall, lifelong risk for ESRD among obese donors observed in our study is an underestimate. In addition, the data lacked granularity with regard to pre-donation lipids, and as such, we were not able to identify those obese donors with metabolic syndrome, a condition known to be associated with increased risk for CKD.34 As with any other retrospective cohort study, there may be residual confounding from failing to include factors not collected or not reliably captured by the OPTN (e.g. socioeconomic status, medication use). Finally, the incremental risk of ESRD directly attributable to living donation was not assessed in this study, and it remains to be determined what additional risk for development of ESRD, if any, kidney donation poses in the setting of obesity.
This is the first national study to examine risk for ESRD among a cohort of obese living kidney donors. Our findings indicate that, while the absolute risk for post-donation ESRD remains low, obese living kidney donors have a 1.9-fold higher risk for ESRD compared to their non-obese counterparts. These data have important implications for donor selection, pre-donation management of living donor candidates, and informed consent discussions with obese persons considering living donation. Further research is needed to better understand the relationships between living donor pre-and post-donation weight trajectories and long-term risk for ESRD post-donation.
METHODS
The study used data from the Scientific Registry of Transplant Recipients (SRTR) which includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors. All adult live kidney donors reported to the OPTN between October 1, 1987 and June 30, 2013 were included in this study (N=119 769). Maximum time at risk was twenty years (median: 10.7 years, interquartile range (IQR): 6.0–16.0), with a maximum of 26.8 years since donation.
Linkage to medical evidence Form 2728 Centers for Medicare & Medicaid Services (CMS) permitted ascertainment of ESRD.23 ESRD was defined as earliest of initiation of maintenance dialysis, placement on the renal transplant waiting list, or receipt of a living or deceased donor kidney transplant. The OPTN did not begin collecting social security numbers until April 1, 1994; for donors whose date of donation occurred on or after April 1, 1994, linkage was performed using a combination of Social Security number (SSN), last name, first name, middle name, or all three; date of birth; and sex. For donors whose date of donation occurred prior to April 1, 1994, linkage to CMS was performed using identifiers listed other than SSN. Ascertainment of ESRD outcomes via the CMS 2728 form began in April 1994; therefore, donors whose date of donation occurred prior to April 1, 1994 were considered late entries. These donors were assumed to be free of ESRD prior to that date, and as such were left truncated (e.g. did not begin contributing time at risk until April 1, 1994).
Data collection on living donor registration forms by the OPTN has varied over time, with changes to the number and format of variables collected, resulting in a wide range in the prevalence of missing data. The probability of a particular set of variables missing for an individual was assumed to not depend on the values themselves, conditional on the observed values of other variables. Therefore, multiple imputation using chained equations35, 36 was used to impute values that were missing for BMI, pre-operative systolic and diastolic blood pressure, baseline eGFR, insurance status, relationship to the recipient, and smoking status. Missing values were imputed based on outcome, age, sex, year of donation, and baseline hazard, with imputed values of BMI restricted to the range of 10 to 70 kg/m2. Twenty imputations were run, each with 20 burn-in periods, and trace file plots were used to assess convergence of the imputations.
Donors were defined as obese at the time of donation if their BMI was ≥ 30 kg/m2. Donor characteristics were compared by obesity status at donation in the non-imputed dataset. Continuous variables were analyzed using analysis of variance (ANOVA), and categorical variables were examined using chi-squared tests of independence. Martingale residuals, spline terms, and stratification were used in the complete case data to examine the functional form of BMI and to explore the presence of a point at which BMI becomes significantly hazardous. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-epi equation. While there are limitations associated with assessing renal function using calculated eGFR, particularly in an obese population, data reported to the OPTN on kidney function are limited, and as such the calculated eGFR was the most reliable method available for assessing donor baseline kidney function (a factor known to be associated with risk for ESRD). Furthermore, CKD-epi has been demonstrated to be superior to the MDRD equation for eGFR among individuals with creatinine clearance expected to be between 60–120 cc/min (represents the typical living kidney donor); and importantly during the development and validation of the CKD-epi equation it was determined that the addition of weight did not significantly improve model performance.37
All survival analyses were performed using imputed data, estimating the parameters from all imputed datasets and adjusting coefficients and standard errors for the variability between imputations. Cumulative incidence of ESRD was estimated using Kaplan-Meier methods and compared between obese and non-obese donors using log-rank tests and displayed graphically using Loess smoothed survival curves. Risk of ESRD by obesity status at donation was estimated using Cox proportional hazards models, adjusting for donor age, sex, race, blood pressure, baseline estimated glomerular filtration rate (eGFR), and relationship to recipient.
Potential effect modification was investigated by testing for interactions, although no interaction terms were included in the final model due to lack of statistical significance. The proportional hazards assumption was assessed and verified using time-dependent variables.
The final model was then applied to the complete case cohort (2/3 of donors in whom no baseline risk factors were missing). Inferences from both the imputed and complete case data were congruent. Multiple additional sensitivity analyses were also performed. Full models were produced using all factors examined on univariate analysis; systolic and diastolic blood pressures and BMI were examined as continuous measures; blood pressure was examined as an ordinal variable; and donation year was accounted for. Results across all models were congruent and inferences were confirmed.
All analyses were performed using Stata 12.0 (Stata Corp), and all hypothesis tests were 2-sided with a significance level of α=0.05.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health grant numbers K23-DK103918 (PI: Locke), R01-DK096008 (PI: Segev), and K24-DK101828 (PI: Segev) and the American Society of Transplantation Clinical Scientist Faculty Development Grant (PI: Locke). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health, SRTR, OPTN, or the American Society of Transplantation.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. [Accessed January 30, 2014];Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. http://optn.transplant.hrsa.gov/data/. Published 2013.
- 2.Gopalakrishnan G, Gourabathini SP. Marginal kidney donor. Indian J Urol. 2007;23:286–293. doi: 10.4103/0970-1591.33726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brar A, Jindal RM, Abbott KC, et al. Practice patterns in evaluation of living kidney donors in United Network for Organ Sharing-approved kidney transplant centers. Am J Nephrol. 2012;35:466–473. doi: 10.1159/000338450. [DOI] [PubMed] [Google Scholar]
- 4.Caliskan Y, Yildiz A. Evaluation of the medically complex living kidney donor. J Transplant. 2012;2012:450471. doi: 10.1155/2012/450471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelbrot DA, Pavlakis M, Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7:2333–2343. doi: 10.1111/j.1600-6143.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 6.Taler SJ, Messersmith EE, Leichtman AB, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant. 2013;13:390–398. doi: 10.1111/j.1600-6143.2012.04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelbrot DA, Pavlakis M. Living donor practices in the United States. Adv Chronic Kidney Dis. 2012;19:212–219. doi: 10.1053/j.ackd.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health: National Heart Lung and Blood Institute. [Accessed July 8, 2016];Overweight and Obesity. http://www.nhlbi.nih.gov/health/health-topics/topics/obe/diagnosis.html. Published 2012.
- 9.Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 10.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol. 2006;17:1501–1502. doi: 10.1681/ASN.2006040327. [DOI] [PubMed] [Google Scholar]
- 11.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 12.Abdullah A, Amin FA, Hanum F, et al. Estimating the risk of type-2 diabetes using obese-years in a contemporary population of the Framingham Study. Glob Health Action. 2016;9:30421. doi: 10.3402/gha.v9.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and metaanalysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 15.Ejerblad E, Fored CM, Lindblad P, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 16.Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 17.Kramer H, Luke A, Bidani A, et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Sang Y, Levey AS, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med. 2016;374:411–421. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–732. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherikh WS, Young CJ, Kramer BF, et al. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11:1650–1655. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 23.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood PD, Stefanick ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 28.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 29.Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 30.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 32.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Action for Health in Diabetes Study Group. Association of Weight Loss Maintenance and Weight Regain on 4- Year Changes in CVD Risk Factors: the Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care. 2016;39:1345–1355. doi: 10.2337/dc16-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panwar B, Hanks LJ, Tanner RM, et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int. 2015;87:1216–1222. doi: 10.1038/ki.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghunathan TWLJ, Van Hoewyk J, Solenbeger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 36.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 37.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Neph Dial Trans. 2010;25(2):449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.