Abstract
Endogenous estradiol and estrone are linked causally to increased risks of breast cancer. In this study, we evaluated multiple competing hypotheses for how metabolism of these parent estrogens may influence risk. Prediagnostic concentrations of estradiol, estrone, and 13 metabolites were measured in 1298 postmenopausal cases of breast cancer and 1524 matched controls in four separate patient cohorts. Median time between sample collection and diagnosis was 4.4–12.7 years across the cohorts. Estrogen analytes were measured in serum or urine by liquid chromatographic-tandem mass spectrometry. Total estrogen levels (summing all 15 estrogens/estrogen metabolites) were associated strongly and positively with breast cancer risk. Normalizing total estrogen levels, we also found that a relative increase in levels of 2-hydroxylation pathway metabolites, or in the ratio of 2-hydroxylation:16-hydroxylation pathway metabolites, were associated inversely with breast cancer risk. These associations varied by total estrogen levels, with the largest risk reductions occurring in women in the highest tertile. With appropriate validation, these findings suggest opportunities for breast cancer prevention by modifying individual estrogen metabolism profiles through either lifestyle alterations or chemopreventive strategies.
Keywords: Estrogens / Metabolism, Breast Neoplasms, Postmenopause, Cohort Studies, Meta-Analysis
Introduction
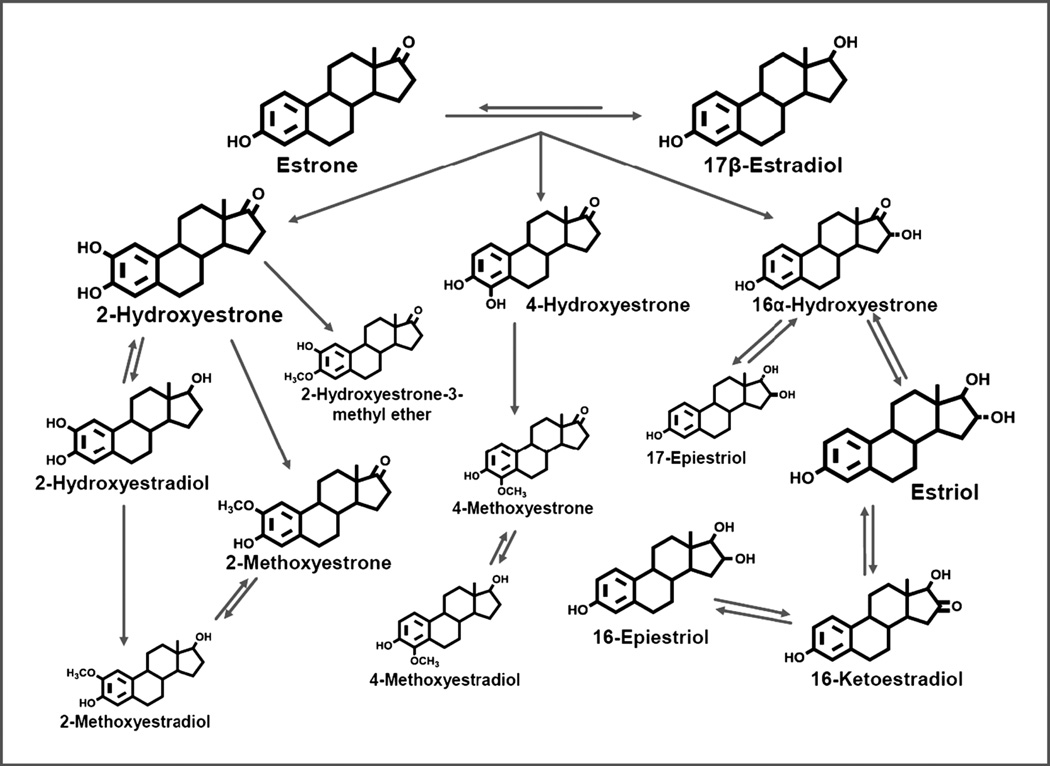
Extensive epidemiological, laboratory, and clinical evidence have demonstrated that elevated endogenous estrogen levels are associated with increased postmenopausal breast cancer risk (1–5). Laboratory studies have assessed the roles of parent estrogens (estradiol and estrone) and their metabolites in carcinogenesis (2,4,5). Estrone and estradiol are irreversibly hydroxylated at the C-2, C-4, or C-16 positions of the steroid ring, leading to a cascade of metabolites (Figure 1). As reviewed by Yager and Davidson (4), two major hypotheses about the carcinogenicity of estrogen metabolites have emerged from decades of experimental research: 1) 16-hydroxylation metabolites, and particularly 16α-hydroxyestrone, can bind covalently to the estrogen receptor (ER) with moderate to strong mitogenic effects; and 2) 2- and 4-hydroxylation catechol estrogen metabolites can be oxidized into mutagenic quinones that form DNA adducts and lead to oxidative DNA damage (1,2,4,6–8). While there have been spirited debates as to whether mitogenic or mutagenic mechanisms are more important in estrogen-mediated carcinogenesis, both are thought to be likely to contribute (2,4).
Figure 1.
Schematic of estrogen metabolic pathway. Sizes of chemical structures as depicted are based on the relative abundance of the estrogen/estrogen metabolite.
Prospective epidemiologic studies over the last two decades have consistently linked elevated circulating estradiol and estrone to increased postmenopausal breast cancer risk (3). However, until very recently none has comprehensively addressed the specific hypotheses noted above or overall relationships of estrogen metabolism to breast cancer risk (9). This has been primarily because there were no accurate, reliable biochemical methods to measure the full range of these metabolites in human populations, particularly at low concentrations characteristic of postmenopausal women (9).
Recently, highly sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have been developed to simultaneously measure parent estrogens and 13 of their metabolites in serum (10) and urine (11). We used this assay in four case-control studies of postmenopausal breast cancer conducted within prospective cohorts (12–15). Although risk estimates for individual estrogen metabolites varied, each study suggested that enhanced 2-hydroxylation of parent estrogens was associated with reduced breast cancer risk. Given the relatively small sample sizes in each study (215–407 cases), analysis and interpretation were limited. Thus, we pooled individual data from these studies in an analysis including 1298 cases and 1524 controls to more robustly test the role of estrogen metabolism in breast carcinogenesis.
Materials and Methods
Data Collection
Four published studies (12–15) of breast cancer risk in postmenopausal women used the same comprehensive assay of endogenous estrogens and their metabolites, allowing individual participant data to be pooled in this analysis. The studies were based on cohorts with prospectively collected serum or urine from women not currently taking exogenous hormones:
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), a multicenter US cancer screening study, randomized 39,116 women to the screening arm (1993–2001). Within this cohort a nested case-control study was conducted of 277 cases and 423 controls, matched on age and period of baseline blood collection (12).
The Columbia Missouri Serum Bank recruited 6,915 women living in or around Columbia, MO (1977–1987) as part of the NCI’s Biological Markers Project to identify serum markers of breast cancer. Within this cohort a nested case-control study was conducted of 215 cases and 215 controls, matched on age, season and time of day of blood collection, and type of and years since menopause (13).
The Breast and Bone Follow-up to the Fracture Intervention Trial (B~FIT), is comprised of 15,595 postmenopausal women screened for FIT, a randomized study of alendronate, at 10 US clinical centers where baseline blood samples were collected (1992–1993). The long-term follow-up for breast cancer identified 407 breast cancer cases and 487 controls, randomly selected within 10-year age and clinical center strata (14).
The Shanghai Women’s Health Study (SWHS), a population-based cohort, enrolled 74,942 women from seven communities in Shanghai, China and collected spot urines (1997–2000). A nested case-control study was conducted of 399 cases and 399 controls, matched on age, date and time of urine collection, time since last meal and antibiotic use (15).
All cases comprised invasive breast cancers, with the exception of Columbia, MO and B~FIT which included 15 and 54 in-situ cancers, respectively. Details of study design, informed consent, ethics approvals, and covariate definitions are in the original study publications (12–15). Additional ethics approval was not required for this analysis.
Laboratory assay
Stable isotope dilution LC-MS/MS was used to measure total (conjugated, including both glucuronidated and sulfated, plus unconjugated) concentrations of 15 estrogens (two parent estrogens and 13 estrogen metabolites) at a single laboratory (Cancer Research Technology Program, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, MD), as previously described (10,11,16,17). While PLCO, Columbia, and B~FIT measured serum, SWHS measured urine and normalized to creatinine levels. In all four studies, laboratory coefficients of variation from masked quality control samples were <5% for all individual analytes (12–15).
Statistical Methods
For each study, we used logistic regression to model linear associations between the log-concentration of each analyte and postmenopausal breast cancer risk. We report odds ratios (ORs) and 95% confidence intervals (CIs) comparing 90th vs. 10th percentiles. ORs were obtained by fitting a model with log-analyte level as an independent continuous variable and multiplying the resulting coefficient by q90–q10(qX=Xth percentile of log-analyte level in controls). We also computed ORs (95% CIs) comparing the 2nd, 3rd, 4th, and 5th quintiles of each analyte’s concentration, as assessed in controls, with the lowest quintile (reference). We adjusted models for covariates collected at baseline that have previously been associated with breast cancer risk (Table 1), date of sample collection (in six month intervals), and for Columbia, MO and SWHS, time between blood draw and diagnosis (or diagnosis of matched case). For our primary analyses, we combined individual study results by a fixed effects meta-analysis. In secondary analyses, reported in the supplementary material, we combined individual study results by a random effects meta-analysis. Heterogeneity was assessed by Cochran’s Q value. We summed concentrations of metabolites across and within pathways, repeating analyses for “Total”, “Parent”, “Path-2”, “Path-4”, and “Path-16” concentrations.
Table 1.
Baseline characteristics.
| PLCO | Columbia, MO | B~FIT | SWHS | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Controls N = 423 |
Cases N = 277 |
Controls N = 215 |
Cases N = 215 |
Controls N = 487 |
Cases N = 407 |
Controls N = 399 |
Cases N = 399 |
| Age (years) | ||||||||
| <55 | 0%ⱡ | 0% | 29% | 28% | 0% | 0% | 22% | 2% |
| 55–<65 | 55% | 55% | 43% | 48% | 34% | 29% | 52% | 53% |
| ≥65 | 45% | 45% | 28% | 24% | 66% | 71% | 26% | 25% |
| BMI kg/m2 | ||||||||
| <25 | 38% | 31% | 43% | 40% | 39% | 34% | 56% | 50% |
| 25–<30 | 35% | 41% | 38% | 41% | 34% | 35% | 35% | 41% |
| ≥30 | 26% | 26% | 19% | 18 | 27% | 30% | 9% | 10% |
| Ever Pregnant† | ||||||||
| No | 9% | 9% | 16% | 17% | 9% | 13% | 4% | 5% |
| Yes | 91% | 91% | 84% | 83% | 91% | 87% | 96% | 95% |
| Age at First Birth | ||||||||
| <20 | 16% | 13% | 16% | 11% | 10% | 9% | 14% | 9% |
| 20–24 | 47% | 44% | 31% | 40% | 43% | 39% | 37% | 35% |
| 25–29 | 19% | 22% | 27% | 23% | 25% | 30% | 34% | 37% |
| ≥30 | 9% | 12% | 10% | 9% | 13% | 10% | 10% | 14% |
| Never pregnant | 9% | 9% | 16% | 17% | 9% | 13% | 4% | 5% |
| Age at Menopause | ||||||||
| < 45 | 10% | 10% | 24% | 25% | 26% | 23% | 16% | 13% |
| 45–50 | 23% | 23% | 29% | 25% | 26% | 28% | 43% | 38% |
| ≥50 | 50% | 48% | 47% | 51% | 47% | 48% | 40% | 46% |
| Age at Menarche | ||||||||
| ≤13 | 71% | 76% | 72% | 73% | 71% | 74% | 18% | 24% |
| >13 | 29% | 24% | 28% | 27% | 25% | 24% | 82% | 76% |
| Ever HT Use | ||||||||
| No | 61% | 67% | 95% | 95% | 67% | 63% | 96% | 94% |
| Yes | 39% | 33% | 5% | 5% | 33% | 37% | 4% | 6% |
| Ever smoke | ||||||||
| No | 60% | 53% | 74% | 73% | 52% | 53% | 95% | 97% |
| Yes | 40% | 47% | 26% | 27% | 47% | 46% | 5% | 3% |
| Race | ||||||||
| White | 88% | 88% | 100% | 100% | 95% | 95% | 0% | 0% |
| Other | 12% | 12% | 0% | 0% | 5% | 5% | 100% | 100% |
PLCO and B-FIT report number of live births, while Columbia, MO and SWHS report number of pregnancies.
Percentages are reported as the percentage of the total population, where the total includes those individuals missing values. The table does not include “Missing” categories, and therefore percentages need not sum to 100%.
Abbreviations: BMI, body mass index; HT, hormone therapy.
To measure the effect of increasing each analyte’s level and reducing levels of remaining analytes, while holding total estrogen concentration constant, we divided levels of a metabolite or pathway by total estrogen levels. We modeled relationships between resulting ratios and risk by logistic regression, adjusting for total estrogen (natural spline, 5 degrees of freedom [df], applied to total estrogen level). We again calculated ORs for 90th vs 10th percentiles, adjusting for covariates, and combined study-specific results with a fixed effects meta-analysis.
Prior findings from the individual studies suggested that enhanced 2-hydroxylation of parent estrogens, as measured by the ratio of Path-2:Path-16 concentrations, was associated with reduced breast cancer risk (12–15). Therefore, we used logistic regression to model the relationship between the Path-2:Path-16 ratio and risk, after adjusting for total estrogen (natural spline, 5df) and covariates. To illustrate that the effect of Path-2:Path-16 ratio was not a surrogate for lower parent estrogen levels, we repeated analyses adjusting for parent estrogen concentrations (natural spline, 5df) instead of and in addition to total estrogen. We similarly evaluated a previous finding (12) concerning the ratio of 4-hydroxylation pathway catechols:4-hydroxylation pathway methylated catechols.
We tested whether known risk factors modified the association between breast cancer risk and Path-2:Path-16 and Path-2:Total ratios by the interaction terms of ratio with known risk factors. For the interaction terms, age, BMI, and total estrogen were coded as study percentiles, while parity (≥1), age at first birth (≥25), ages at menarche (≥14) and menopause (≥50), and previous MHT use (yes/no) were coded as binary variables. To test whether the ratio was associated with any known risk factors, we scaled the ratio by dividing by its standard deviation and regressed this value on each risk factor, in turn, using linear regression and adjusting for total estrogen (natural spline, 5df) and case-control status.
P-values of <0.05 were considered statistically significant. All tests of significance were two-tailed. All statistical analyses were performed using R (18).
Results
The characteristics of breast cancer cases (N=1298) and controls (N=1524) in this analysis are presented in Table 1. Within each study, cases and controls had similar characteristics, but cases tended to have slightly older ages at first birth, younger ages at menarche, and higher BMIs than controls. Median time between blood/urine collection and breast cancer diagnosis was 4.4, 5.6, 6.2, and 12.7 years for PLCO, SWHS, B~FIT, and Columbia, respectively.
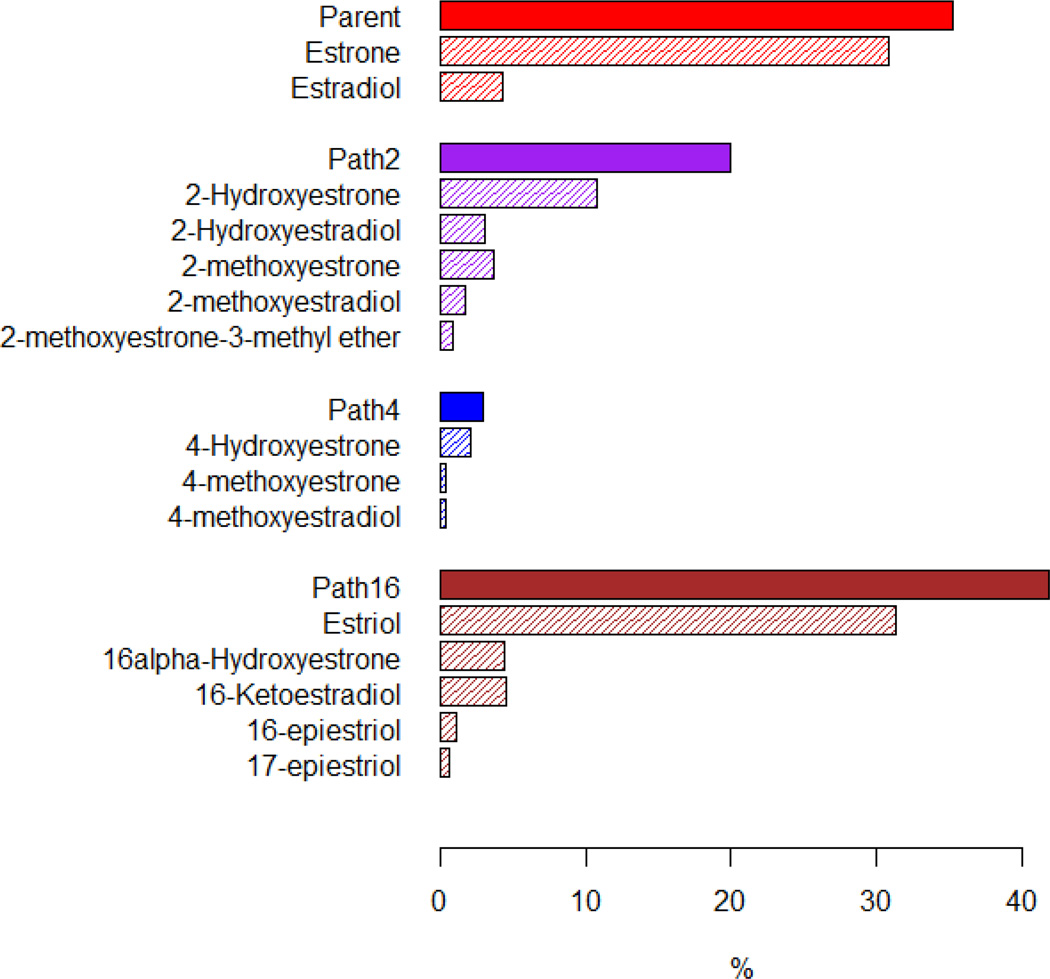
Median (10th–90th percentile) concentrations of two parent estrogens and 13 metabolites are provided in Table 2. Parent estrogens, Path-2, Path-4, and Path-16 metabolites comprised 35%, 20%, 3%, and 42% of total estrogen (sum of all 15 concentrations), respectively (Figure 2).
Table 2.
Median (10th – 90th percentile) metabolite concentrations are reported for each study. Colombia, B~FIT, and PLCO report serum concentrations in pmol/L, while SWHS reports urine concentrations in pMol/mg creatinine
| PLCO | Columbia, MO | B~FIT | SWHS | |
|---|---|---|---|---|
| Estrogen/Estrogen Metabolite | Median (Inter-decile range) | Median (Inter-decile range) | Median (Inter-decile range) | Median (Inter-decile range) |
| Estrone | 393.5 (365.1–444.1) | 242.5 (101.6–702.1) | 306.4 (118.7–828.8) | 5.8 (2.4–14.2) |
| Estradiol | 36.2 (32.7–38) | 25 (10.9–77.2) | 41.2 (19–95) | 1.2 (0.5–3) |
| 2-Hydroxyestrone | 69 (64.3–74.9) | 74.4 (64.4–146.5) | 64.3 (38.7–142) | 4.9 (2.3–11) |
| 2-Hydroxyestradiol | 35.9 (33.6–39) | 23.6 (12.3–47.9) | 12.5 (7.5–25.8) | 1.2 (0.6–2.7) |
| 2-Methoxyestrone | 12.5 (11.6–13.4) | 42.5 (22.4–92.1) | 29.2 (14–62.1) | 1.1 (0.4–2.7) |
| 2-Methoxyestradiol | 7.7 (7.1–8.2) | 14.3 (6.7–34.3) | 13.5 (7–26.9) | 0.5 (0.2–1.5) |
| 2-Methoxyestrone-3-methyl ether | 3.4 (3.2–3.7) | 6 (2–16.5) | 4.1 (2.4–12.4) | 0.3 (0.1–0.7) |
| 4-Hydroxyestrone | 22.1 (20.7–24.1) | 18.5 (13.8–34) | 10.7 (6.5–21.6) | 0.7 (0.3–1.6) |
| 4-Methoxyestrone | 3.5 (3.2–3.8) | 3.5 (1.2–7.3) | 3.9 (2.2–10.1) | 0.1 (0.1–0.4) |
| 4-Methoxyestradiol | 2.8 (2.6–3.1) | 1.9 (1–5.4) | 3 (1.3–6.2) | 0.1 (0–0.2) |
| Estriol | 440 (404.6–487.9) | 278.2 (166.5–597.2) | 255.1 (133.1–620.2) | 5.5 (2.6–12.4) |
| 16α-Hydroxyestrone | 39.2 (36.1–42.6) | 37.5 (27.6–78.9) | 28.6 (17.8–70.3) | 1.5 (0.7–3.2) |
| 16-Ketoestradiol | 36.5 (34.4–39.9) | 31.9 (20.1–67.8) | 32.8 (18.3–104.2) | 1.6 (0.7–3.8) |
| 16-Epiestriol | 3.5 (3.2–3.8) | 5.7 (2.1–19.7) | 4.2 (2–16.1) | 0.6 (0.2–1.5) |
| 17-Epiestriol | 1.3 (1.3–1.5) | 2.3 (1.1–7.6) | 3.5 (1.4–11) | 0.4 (0.1–0.9) |
Figure 2.
Percentage of total estrogen (sum of 15 estrogens/estrogen metabolites) in each of the metabolic pathway groups and in each of the individual estrogens/estrogen metabolites. The proportions for the parent estrogens, 2-hydroxylation pathway (Path2), 4-hydroxylation pathway (Path4), and 16-hydroxylation pathway (Path16) estrogen metabolites sum to 100%.
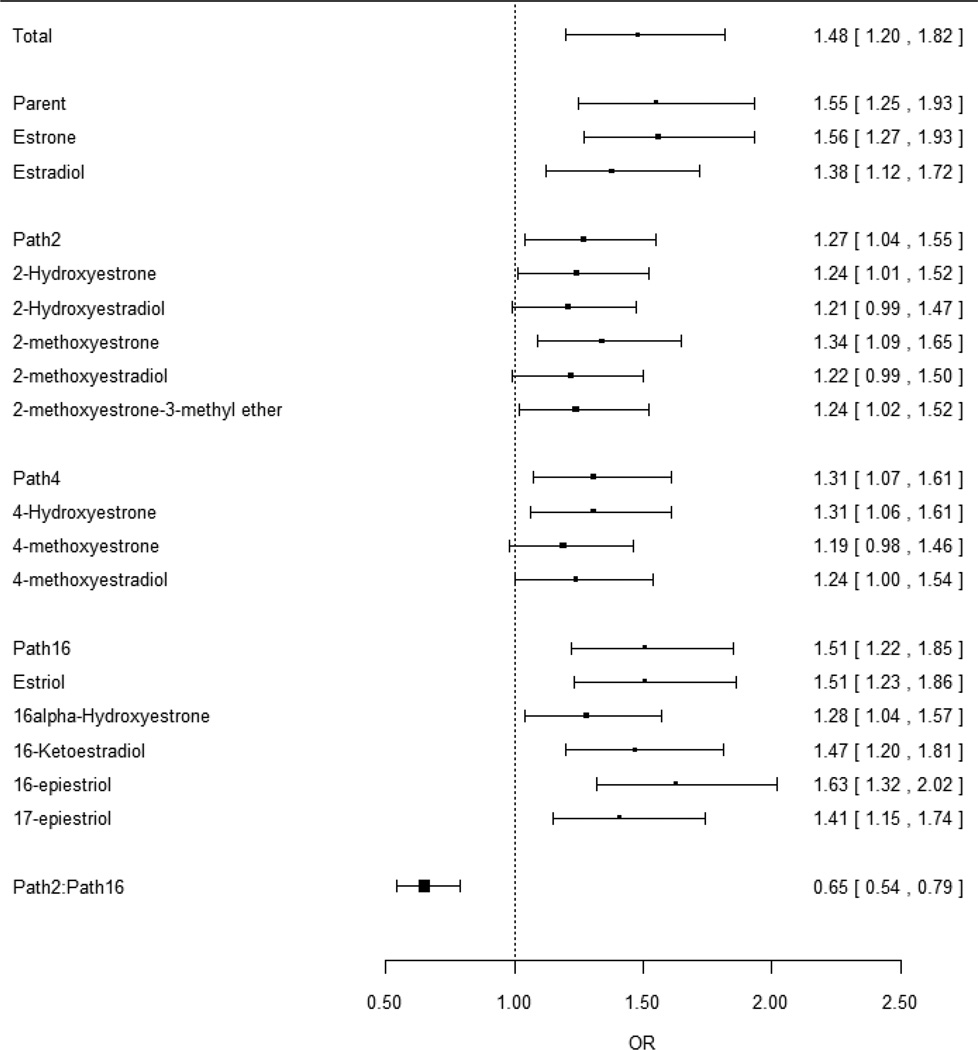
Higher total estrogen levels were strongly associated with increased postmenopausal breast cancer risk (P<0.001). The OR of breast cancer, when comparing women in 90th vs. 10th percentiles of total estrogen, was 1.48 (95%CI:1.20–1.82; Figure 3). Excluding BMI from the model produced similar results (OR=1.52, 95%CI:1.25–1.86). Most of the 15 individual analytes were also significantly associated with risk, consistent with the observation that most analytes were highly correlated with each other (median Pearson correlation coefficient=0.67; interquartile range:0.57–0.76; Supplementary Tables 1A–B). Comparing the highest to the lowest quintile of total estrogen, the OR for women in the highest quintile was 1.69 (95%CI:1.30–2.21; Supplementary Table 2A), consistent with and somewhat higher than that estimated in Figure 3 under the assumption of linearity. The ORs for women in the 2nd, 3rd, and 4th quintiles were 1.17 (0.9–1.53), 1.36 (1.05–1.78), and 1.31 (1–1.72), respectively (Supplementary Table 2A).
Figure 3.
Odds ratios and 95% confidence intervals comparing the risk of breast cancer in individuals with a higher analyte or pathway concentration (90th percentile) to individuals with a lower concentration (10th percentile). Odds ratios were modeled by logistic regression with log-concentration as the independent variable and without adjusting for total estrogen level.
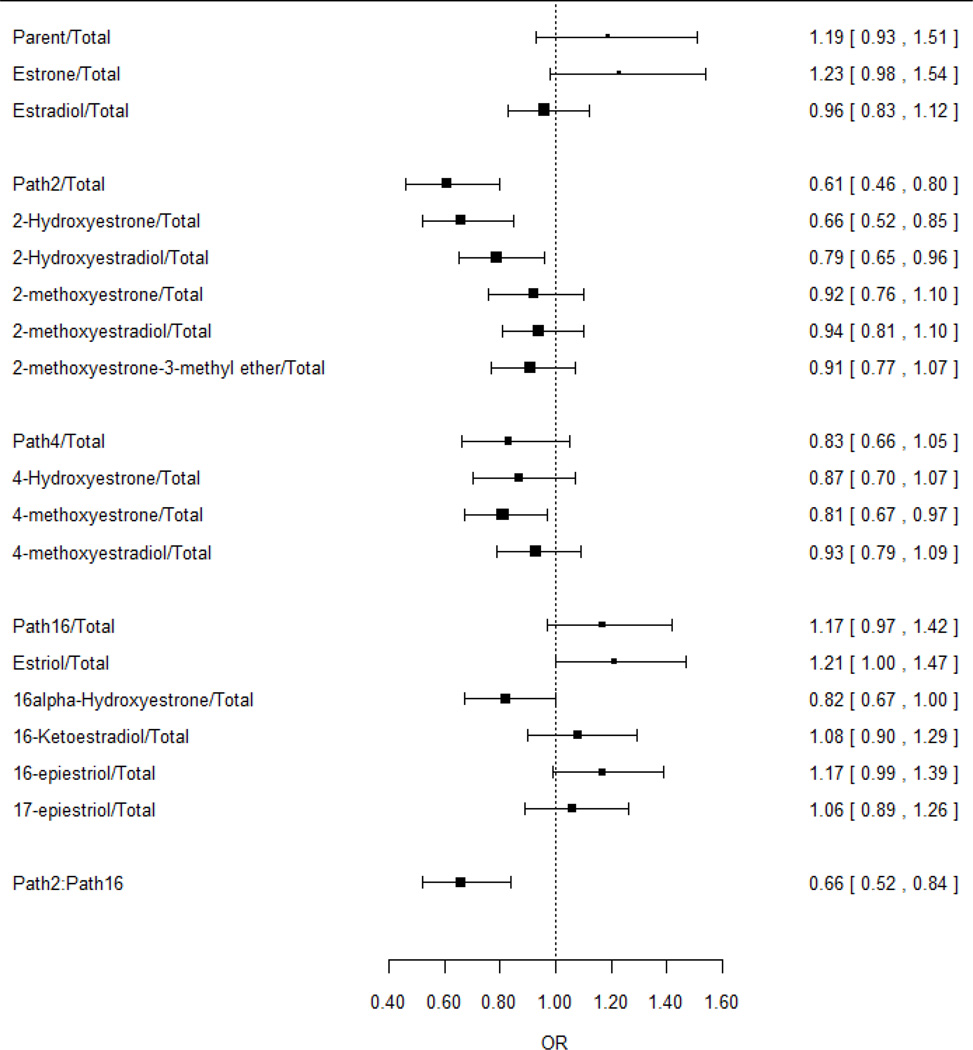
We examined the effect of changing the proportion of each metabolite or pathway, relative to total estrogen level, while holding total estrogen concentration constant (Supplementary Table 2B). In a model that adjusted for total estrogen level, we found that an elevated Path-2:Total ratio was associated with a statistically significant reduced breast cancer risk (OR for 90th vs. 10th percentile=0.61, 95%CI:0.46–0.80, P<0.001; Figure 4). Elevated proportions of Path-4 and Path-16 metabolites were associated with non-statistically significant reductions and increases in risk, respectively. These patterns were seen in each study individually (Supplementary Table 3), in analyses with single studies removed (Supplementary Table 4), and in random effects meta-analysis (Supplementary Table 5).
Figure 4.
Odds ratios and 95% confidence intervals comparing the risk of breast cancer in individuals with a higher analyte:total or pathway:total ratio (90th percentile) to individuals with a lower ratio (10th percentile). Odds ratios were modeled by logistic regression with ratio as the independent variable and adjusting for total estrogen level.
We also considered the Path-2:Path-16 ratio, a common metric in prior studies (9), and found that individuals with higher ratios had a significant reduction in risk both before (OR for 90th vs. 10th percentile=0.65, 95%CI:0.54–0.79, P<0.001; Figure 3) and after (OR=0.66, 95%CI:0.52–0.84, P<0.001; Figure 4) adjusting for total estrogen. This pattern was seen in each study individually (Supplementary Table 3), in analyses with single studies removed (Supplementary Table 4), and in random effects meta-analysis (Supplementary Table 5). We also found an inverse correlation between the Path-2:Path-16 ratio and total estrogen in each study (Supplementary Figure 1). Risk estimates were similar after adjusting for only parent estrogens, in which the OR comparing 90th vs. 10th percentiles of the Path-2:Path-16 ratio was 0.69 (95%CI:0.55–0.87); adjusting for both parent and total estrogen levels, the OR was 0.67 (95%CI:0.53–0.85). The ratio of 4-hydroxylation pathway catechols to 4-hydroxylation pathway methylated catechols was not strongly associated with risk (OR=1.15, 95%CI:0.98–1.36) (Supplementary Table 3).
We tested if the inverse relation of either the Path-2:Path-16 or Path-2:Total ratio with breast cancer risk varied by total estrogen concentration or breast cancer risk factors. We present the results only for the Path-2:Path-16 ratio here, as results for the Path-2:Total ratio were similar. Total estrogen was the only factor to have a statistically significant effect (P-interaction=0.003), with the strongest inverse association between the Path-2:Path-16 ratio and breast cancer risk in women with the highest total estrogen levels (Supplementary Table 6). Among women in the bottom, middle, and highest tertiles of total estrogen, the respective ORs (95%CI) were 1.08 (0.68–1.71), 0.70 (0.44–1.12), and 0.43 (0.27–0.69) (Supplementary Table 6). This pattern was also apparent in the nine groups of women defined by tertiles of total estrogen and tertiles of the Path-2:Path-16 ratio (Supplementary Table 7). BMI was the only risk factor identified as potentially being associated with Path-2:Path-16 ratio (p=0.04), with the inverse association strongest in the SWHS population (p=0.003) (Supplementary Table 8).
Discussion
We evaluated the role of endogenous estrogen metabolism in postmenopausal breast cancer risk using data collected from four studies with prospectively collected serum or urine samples. All studies found that increased parent and total estrogen concentrations were strongly associated with increased breast cancer risk. Our analysis further demonstrated that for a given level of total estrogen, increased levels of 2-hydroxylation pathway metabolites were associated with reduced risk. While the association between elevated parent estrogens and increased postmenopausal breast cancer risk has been well established, our robust findings of reduced risk associated with estrogen metabolism into the 2- as opposed to the 16-hydroxylation pathway also support the long-standing hypothesis that much of the carcinogenicity of estrogens may relate to their mitogenic effects in enhancing cell proliferation, either leading to increased opportunity for random mutations or acting as late-stage promoters. While previous studies have attempted to address mitogenic vs. mutagenic hypotheses of estrogen-mediated breast carcinogenesis, they only measured two metabolites (2-hydroxyestrone and 16α-hydroxyestrone via direct enzyme immunoassays), were unable to comprehensively measure pathways, and produced conflicting and generally non-significant results (9).
The lack of any evidence of a particular adverse effect of catechol estrogens in our analysis is inconsistent with the other long-standing hypothesis that the 2- and 4-hydroxylation pathways produce genotoxic metabolites that can form DNA adducts, thereby acting as direct carcinogens (8). Two (12,13) of the four (12–15) cohort studies in this analysis showed some evidence of increasing risk with an increasing ratio of 4-hydroxylation pathway catechols:4-hydroxylation pathway methylated catechols (Supplementary Table 3), which would be consistent with this hypothesis (i.e., removal of genotoxicity by conversion of one hydroxy group to a methoxy group). However, the other two studies (14,15) and this combined analysis did not confirm this association; this issue requires further attention.
The lower breast cancer risk associated with higher 2-hydroxylation pathway metabolite levels, conditioned on total estrogen, is promising on several fronts. First, it is a strong association, with women in the highest decile of the 2-hydroxylation:16-hydroxylation ratios having ~30–40% lower risk than those in the lowest decile. In addition, the beneficial effect appears to increase with increasing total estrogen levels. If confirmed, the ~50% risk reduction associated with extensive 2-pathway hydroxylation among women with the highest total estrogen levels might offer an opportunity for targeted intervention. Levels of these ratios may be modifiable by lifestyle changes as they have been inversely associated with BMI (19) and positively associated with physical activity (20,21). Moreover, the higher ratios in Asian populations, with lower breast cancer risk (15), decline when these populations migrate West (22). Our findings require replication and further investigation to assess potential causality in these relationships, but these estrogen metabolite ratios could be modifiable risk factors and targets for prevention.
Major strengths of this study are the prospectively collected biologic samples from four high-quality cohort studies, and the opportunity to assay metabolites in the same laboratory. Furthermore, we measured total concentrations of estrogens, not just unconjugated concentrations, using newly developed, reliable, sensitive and specific LC-MS/MS assay for estrogens and estrogen metabolites. The sample preparation method for the LC-MS/MS assay employed in our studies removed sulfate and glucuronide moieties from conjugated forms of the estrogens and estrogen metabolites and released unconjugated estrogens, such as estradiol, from sex hormone binding globulin (SHBG). As we did not measure serum SHBG in these cohorts, we were unable to estimate non-SHBG bound estradiol as has been reported in prior studies using conventional immunoassays (3). One limitation is the variability in absolute values of analytes across studies. However, this is not unusual for metabolite analyses and has less of an impact on ratios. Another limitation is that one (15) of the four (12–15) studies measured urinary, rather than serum, estrogens. However, studies of parent estrogens have consistently found similar relative risks for breast cancer in blood or urine (23). While most risk factors and other metrics were similar among the four studies, there were exceptionally strong correlations between all of the analytes and a comparatively narrow range of total estrogen levels within the PLCO study. These differences may be attributable to variability in LC-MS/MS calibration and standardization, but may be due to other factors as well. In sensitivity analyses excluding each of the studies one at a time, findings were consistent with those observed for all studies combined, suggesting robust results. The four studies had different procedures for selecting controls. While Columbia, MO and SWHS matched each case with a control alive at the time of diagnosis, PLCO selected controls among women alive at the end of the study and B~FIT selected controls among all women, with the latter two selection procedure selections leading to potential survivor bias. Finally, hormone receptor status was obtained only on a fraction of cases. Among postmenopausal women in prior reports, the strongest effects for endogenous estradiol have been observed for ER-positive tumors (24–26). About 81% of breast cancers among US women aged 50+ years are ER-positive (27). Additional studies will be needed to address heterogeneity of estrogen metabolite associations by ER status.
We measured estrogens in a single specimen prior to clinical disease, consistent with the methods for the majority of postmenopausal breast cancer studies (3). A single measure of estradiol was recently found to predict postmenopausal breast cancer risk for up to 16–20 years (24). The timing of sampling in our studies, most ranging from 4–13 years prior to diagnosis, seems ideal to characterize the possible role of metabolites as tumor promoting mitogens. If initiation of breast cancer occurs decades before diagnosis, then our design may not provide a strong test of metabolic profiles associated with initiation. Future laboratory research along with work in large epidemiologic cohorts would be needed to clarify the genetic determinants of estrogen metabolism as well as the mechanisms by which estrogen metabolites may contribute to each phase of the carcinogenic process.
In summary, we demonstrated that among postmenopausal women, relative increases in levels of 2-hydroxylation pathway metabolites are significantly associated with reduced breast cancer risk after adjusting for total estrogen levels. Whether this relationship is causal remains to be determined, but plausible biologic explanations are supported by laboratory, epidemiological and clinical evidence (1–5). If judged to be causal, these findings could support the notion of altering estrogen metabolism through lifestyle modifications or chemopreventive strategies as a means of breast cancer prevention.
Supplementary Material
Acknowledgments
The authors are indebted to the study participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, Columbia Missouri Serum Bank, Fracture Intervention Study, and Shanghai Women’s Health Study and to the physicians, nurses, technologists, and interviewers for their efforts in the field. The authors thank Maya Palakal and Preethi Raj for research assistance.
Financial Support:
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute and National Cancer Institute federal funds awarded under Contract No. HHSN261200800001E to SAIC-Frederick, Inc. The Shanghai Women’s Health Study is supported by R37CA70867 (W. Zheng) and UM1CA182910 (W. Zheng). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
RGZ and XX hold patents and receive royalties pertaining to the estrogen metabolite assay.
Footnotes
Conflict of Interest: The remaining co-authors have no conflicts to disclose.
References
- 1.Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. doi: 10.1093/carcin/17.11.2279. [DOI] [PubMed] [Google Scholar]
- 2.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 3.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 4.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 5.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci U S A. 1988;85:7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeger H, Wallwiener D, Kraemer E, Mueck AO. Comparison of possible carcinogenic estradiol metabolites: effects on proliferation, apoptosis and metastasis of human breast cancer cells. Maturitas. 2006;54:72–77. doi: 10.1016/j.maturitas.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2015;99:61–66. doi: 10.1016/j.steroids.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids. 2015 doi: 10.1016/j.steroids.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhrman BJ, Xu X, Falk RT, Dallal CM, Veenstra TD, Keefer LK, et al. Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2014;23:2649–2657. doi: 10.1158/1055-9965.EPI-14-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A Liquid Chromatography-Mass Spectrometry Method for the Simultaneous Measurement of 15 Urinary Estrogens and Estrogen Metabolites: Assay Reproducibility and Interindividual Variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–339. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res. 2013;15:R34. doi: 10.1186/bcr3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV, Jr, Cauley JA, et al. Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis. 2014;35:346–355. doi: 10.1093/carcin/bgt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore SC, Matthews CE, Ou Shu X, Yu K, Gail MH, Xu X, et al. Endogenous Estrogens, Estrogen Metabolites, and Breast Cancer Risk in Postmenopausal Chinese Women. J Natl Cancer Inst. 2016;108:djw103. doi: 10.1093/jnci/djw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 18.R_Core_Team. R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 19.Schairer C, Fuhrman BJ, Boyd-Morin J, Genkinger JM, Gail MH, Hoover RN, et al. Quantifying the Role of Circulating Unconjugated Estradiol in Mediating the Body Mass Index-Breast Cancer Association. Cancer Epidemiol Biomarkers Prev. 2016;25:105–113. doi: 10.1158/1055-9965.EPI-15-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews CE, Fortner RT, Xu X, Hankinson SE, Eliassen AH, Ziegler RG. Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. J Clin Endocrinol Metab. 2012;97:3724–3733. doi: 10.1210/jc.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallal CM, Brinton LA, Matthews CE, Pfeiffer RM, Hartman TJ, Lissowska J, et al. Association of Active and Sedentary Behaviors with Postmenopausal Estrogen Metabolism. Med Sci Sports Exerc. 2016;48:439–448. doi: 10.1249/MSS.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk RT, Fears TR, Xu X, Hoover RN, Pike MC, Wu AH, et al. Urinary estrogen metabolites and their ratio among Asian American women. Cancer Epidemiol Biomarkers Prev. 2005;14:221–226. [PubMed] [Google Scholar]
- 23.Thomas HV, Reeves GK, Key TJ. Endogenous estrogen and postmenopausal breast cancer: a quantitative review. Cancer Causes Control. 1997;8:922–928. doi: 10.1023/a:1018476631561. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137:883–892. doi: 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James RE, Lukanova A, Dossus L, Becker S, Rinaldi S, Tjønneland A, et al. Postmenopausal Serum Sex Steroids and Risk of Hormone Receptor–Positive and -Negative Breast Cancer: a Nested Case–Control Study. Cancer Prevention Research. 2011;4:1626–1635. doi: 10.1158/1940-6207.CAPR-11-0090. [DOI] [PubMed] [Google Scholar]
- 26.Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562–570. doi: 10.1093/jnci/djr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) Research Data (SEER 18 Registries for 2000–2012), National Cancer Institute, DCCPS, Surveillance Research Program. Surveillance Systems Branch
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.