Abstract
An intermolecular condensation of alkynols and terminal alkynes is reported. Using IPrAuNTf2, an efficient Au-catalyzed cyclization-alkynylation strategy furnishes (2-arylalkynyl) cyclic ethers in moderate to excellent yields (up to 94%). This strategy is extended to the synthesis of functionalized 2,3-dihydrooxepines via the sequential Au-catalyzed ring expansion of the cyclic ether substrates.
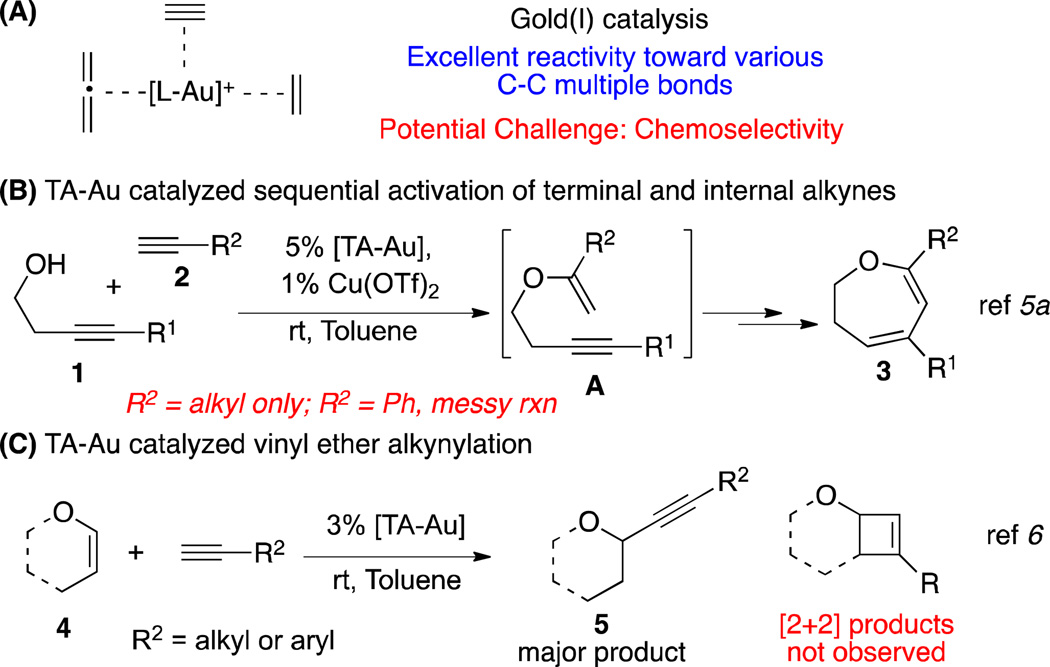
Gold(I) catalysis has developed rapidly in recent years and the resulting methodologies have proved useful for the facile installation of molecular complexity.1 Gold’s intrinsic π-acid mode of activation has rendered it synthetically valuable in promoting reactions involving substrates bearing unsaturated C-C moieties (alkyne, allene, and alkene; Scheme 1A). Consequently, the design of selective catalytic systems is often problematic when multiple reactive functional groups are present.2 Furthermore, additional challenges in attaining selectivity arise if these catalytic systems are a composite of intra- and inter- molecular reactions.3
Scheme 1.
Gold-catalyzed C-C multiple bond activation
Over the past several years, our group has demonstrated the application of 1,2,3-triazole gold(I) (TA-Au) catalysts in promoting these challenging transformations.4 One relevant example is the intermolecular condensation between homo-propargyl alcohols and terminal aliphatic alkynes to afford 2,3-dihydrooxepines (Scheme 1B).5 Within this reaction pathway, a vinyl ether intermediate is selectively formed via the intermolecular gold-catalyzed hydroalkoxylation of the terminal alkyne. Moreover, in exploring the reactivity of vinyl ethers with terminal alkynes under triazole gold-catalytic conditions, we later developed an intermolecular alkynylation of vinyl ethers.6
Despite these recent advances, certain concerns regarding the mechanisms of the reactions have not been completely addressed. First, the enyne cycloisomerization reaction between homo-propargyl alcohol 1 and terminal alkyne 2 was tolerated only by aliphatic alkynes (Scheme 1B). This result was unexpected considering that gold π-acid activation of aryl-substituted terminal alkynes is extensively documented.7 A messy reaction mixture was obtained when aryl-substituted terminal alkynes such as phenylacetylene were used. Second, for the alkynylation of vinyl ether 4, aliphatic alkynes such as 1-hexyne resulted in slightly lower yields in comparison with aromatic alkynes (Scheme 1C). Based on these observations, changing the substrate of either reaction has the potential to dramatically alter the reaction pathway, and this phenomenon warrants further investigation.
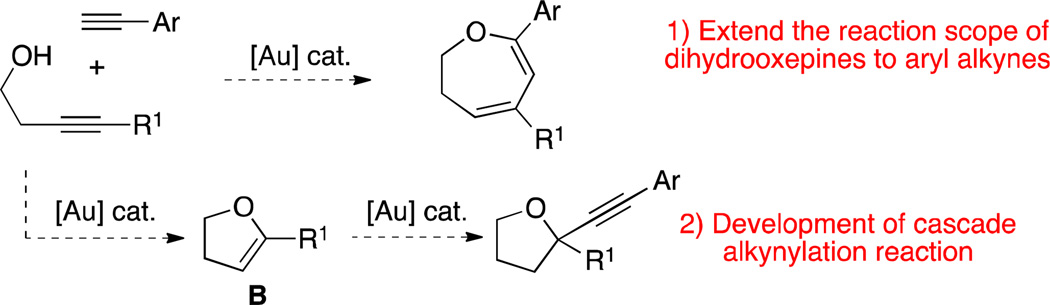
Our strategy in exploring this unique catalytic system was focused on: 1) extending the reaction scope of the 2,3-dihydrooxepine synthesis to include aryl-substituted terminal alkynes and, 2) exploring the possibility of a cascade process for the alkynylation of vinyl ether B (Scheme 2).8–9
Scheme 2.
Proposed reaction pathways
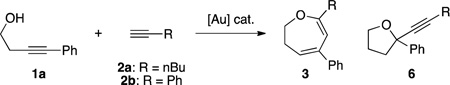
As shown in Table 1, the reaction of 1a with aromatic alkyne 2b under our previously reported conditions resulted in a messy reaction, with no formation of dihydrooxepine 3a (entry 2). To explore the plausibility of a cascade process, we screened various gold catalysts (see ESI†). Interestingly, when 5% RuPhosAuNTf2 was used as the catalyst, alkyne 6b was observed, albeit in low yield (31%, entry 3). Since the formation of 6b requires gold π-acid activation of the internal alkyne of 1a, we increased the reaction temperature with the objective of promoting the intramolecular cyclization of 1a over intermolecular hydroalkoxylation. Gratifyingly, the reaction yield was improved to 61% (entry 4). Finally, ligand screening revealed IPrAuNTf2 as the optimal catalyst, affording the desired product 6b in excellent yield (5% loading, 91% yield, entry 5; 4% loading, 87% yield, entry 6). Having identified the optimal conditions, we turned our attention to exploring the reaction scope (Table 2).
Table 1.
General conditions: 1 (0.2 mmol), 2 (eq), and gold catalyst (mol %) in CHCl3 (0.8 mL) under Ar;
The yield was determined by 1H-NMR using 1,3,5-trimethoxybenzene as the internal standard.
Table 2.
General conditions: 1 (0.2 mmol), 2 (3 eq), IPrAuNTf2 (3 mol %) in CHCl3 (0.8 mL) under Ar at 40 °C. Yields of isolated products are given. Reference the ESI for detailed conditions.
Reaction heated at 60 °C
Ratio determined via 1H-NMR.
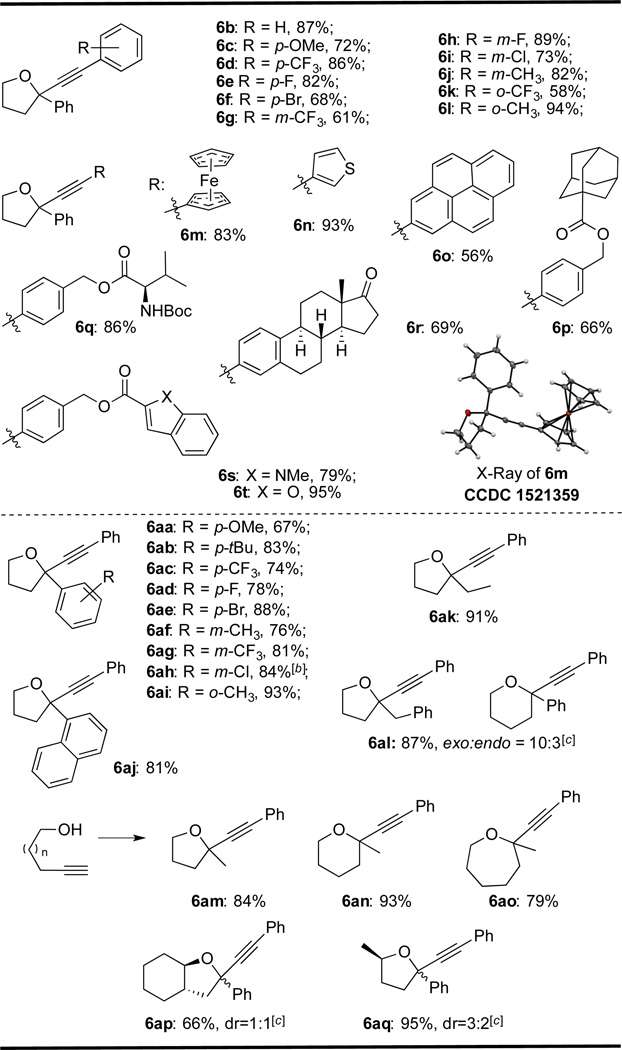
A variety of phenylacetylene derivatives were subjected to the optimized reaction conditions and subsequently afforded the desired products in good yield (6b–6l). Other aromatic alkynes such as ferrocenyl (6m, structure confirmed by X-ray), thiophenyl (6n), and pyrenyl (6o) derivatives could also be utilized in the reaction. Additionally, alkynes bearing adamantyl (6p), indole (6s), and benzofuran (6t) moieties were tolerated without significant decomposition of the product. The amino acid derivative 6q and estrone derivative 6r were also prepared, demonstrating the tolerance of the reaction conditions to a variety of functional groups. Conversely, non-aromatic terminal alkynes such as cyclopentyl acetylene, 1-hexyne, and TMS acetylene, did not form the desired alkynylation product.
To fully investigate the scope of this alkynylation reaction, different alkynols were also assessed. Substitution at the para (6aa–6ae), meta (6af–6ah), and ortho (6ai) position of the benzene ring on the homopropargyl alcohol afforded good yields. Additionally, the naphthyl derivative 6aj could be furnished in good yield (81%). To probe the endo/exo selectivity of the cyclization step, we used a series of extended-chain internal alkynols bearing aliphatic or phenyl substitution. Aliphatic substitution selectively yielded the exo product (6ak), while phenyl substitution resulted in a 10:3 mixture of exo:endo isomers (6al). Terminal alkynes were tolerated under the reaction conditions, yielding the 5-exo (6am), 6-exo (6an), and 7-exo (6ao) products. In contrast, the terminal alkyne substrate for 8-exo cyclization gave trace formation of the desired product. To determine the diastereoselectivity of the alkynylation reaction, we utilized enantiomerically pure alkynols. However, when an α-methyl homopropargyl alcohol is used (6aq, dr = 3:2) or an α,β-di-substituted homopropargyl alcohol (6ap, dr = 1:1), low diastereoselectivity was exhibited. Next, we diverted our focus to the development of a strategy for the synthesis of the corresponding 2,3-dihydrooxepine 3b.
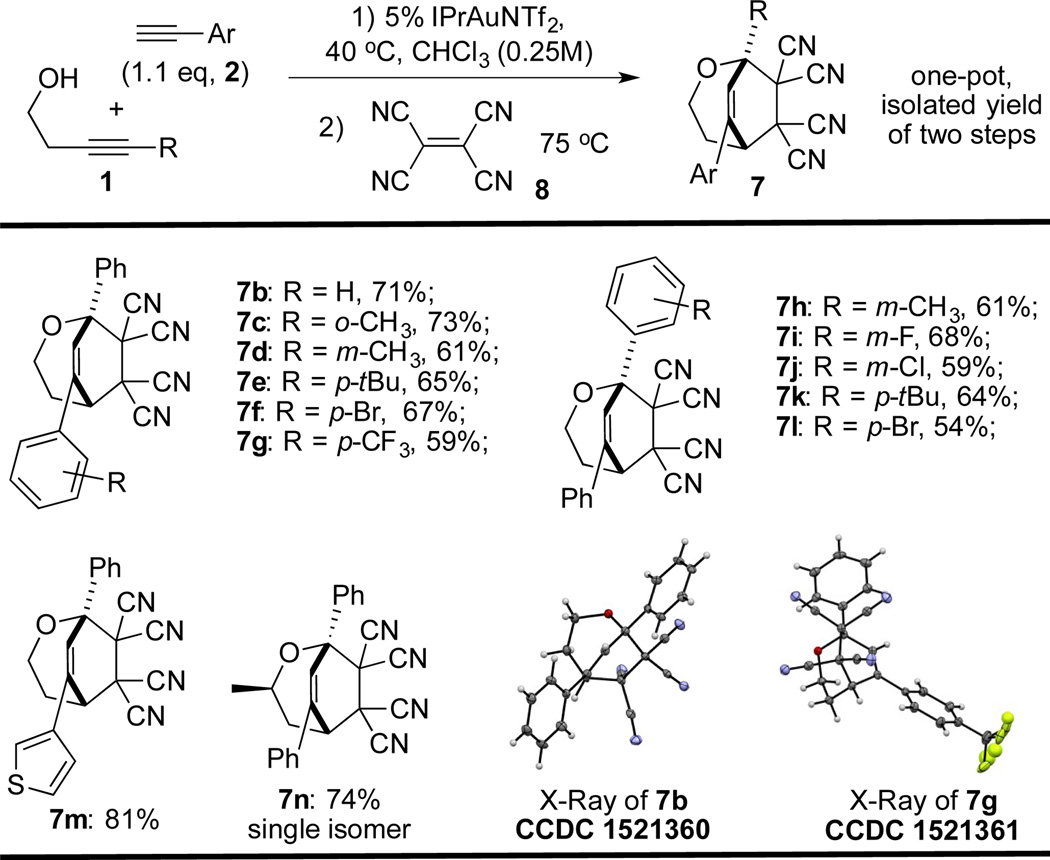
During optimization of the reaction conditions (Table 1), we observed that if 2 equivalents of terminal alkyne 2b were used, the yield of the desired product 6b was reduced. Monitoring the reaction using 1H-NMR revealed the formation of dihydrooxepine 3b as the major by-product (see ESI†). Reducing the amount of phenylacetylene to 1.1 equivalents resulted in the formation of 3b as the major product in 74% yield. Notably, 3b was unstable upon chromatographic isolation. Therefore, the dienophile tetracyanoethylene was used to trap the product via a Diels Alder cycloaddition10 and the adduct 7b was obtained in 71% isolated yield over two steps. The structure of 7b was unambiguously characterized by X-ray crystallography (see ESI†). With these new optimized conditions for the synthesis of 2,3-dihydrooxepines, various aromatic alkynes were subjected to the reaction and moderate yields of the derivatives were obtained (Table 2).
Table 2.
Reaction scope of oxepine adductsa
General conditions: 1 (0.4 mmol), 2 (1.1 eq), and IPrAuNTf2 (5 mol %) in dry CHCl3 (1.6 mL) under Ar atmosphere at 40 °C. The crude was passed through silica, 8 (1.5 eq) was added, and the reaction was heated at 75 °C. Yields of isolated products are given.
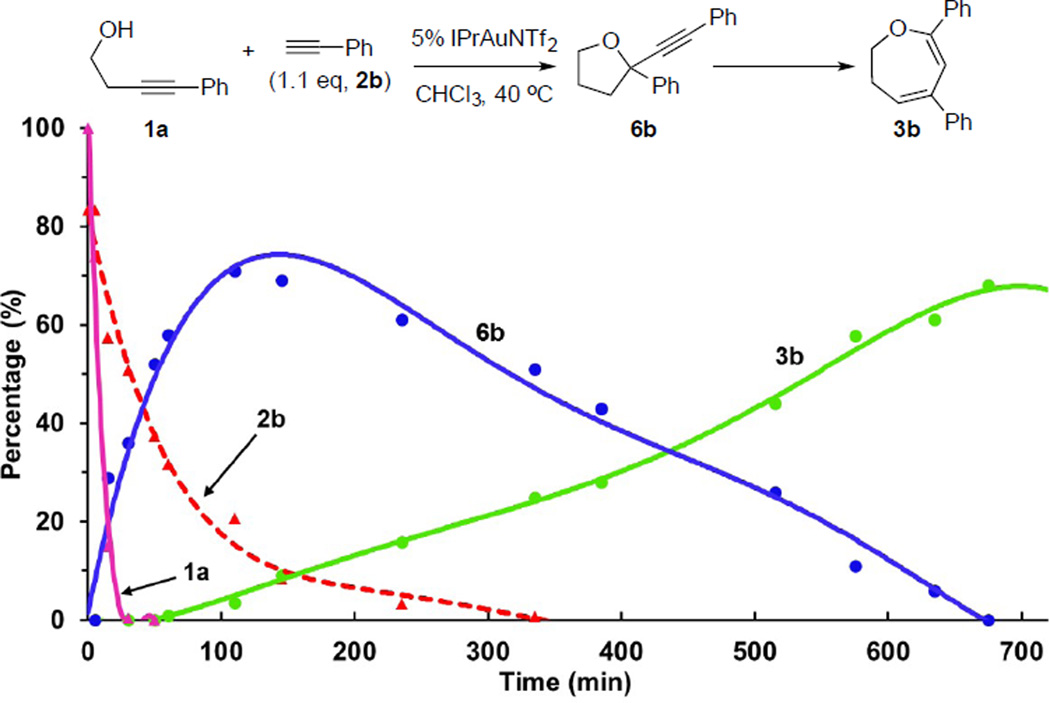
Accordingly, to explore the progression of this process, we monitored the kinetics of the reaction with 1H-NMR. Interestingly, within approximately 15 minutes, homo-propargyl alcohol 1a was completely converted to the corresponding ketone 1a’ via gold-catalyzed hydrolysis.11 Thus, 1a’ serves as a viable intermediate in the formation of 6b. After the full conversion of 1a’ to 6b, a slow conversion of 6b occurs with concomitant formation of 3b. Monitoring the analogous reaction in the presence of 3 equivalents of phenylacetylene indicated low yield of 3b (<30%) after 48 hours (Figure 1).
Figure 1.
Reaction kinetic profile
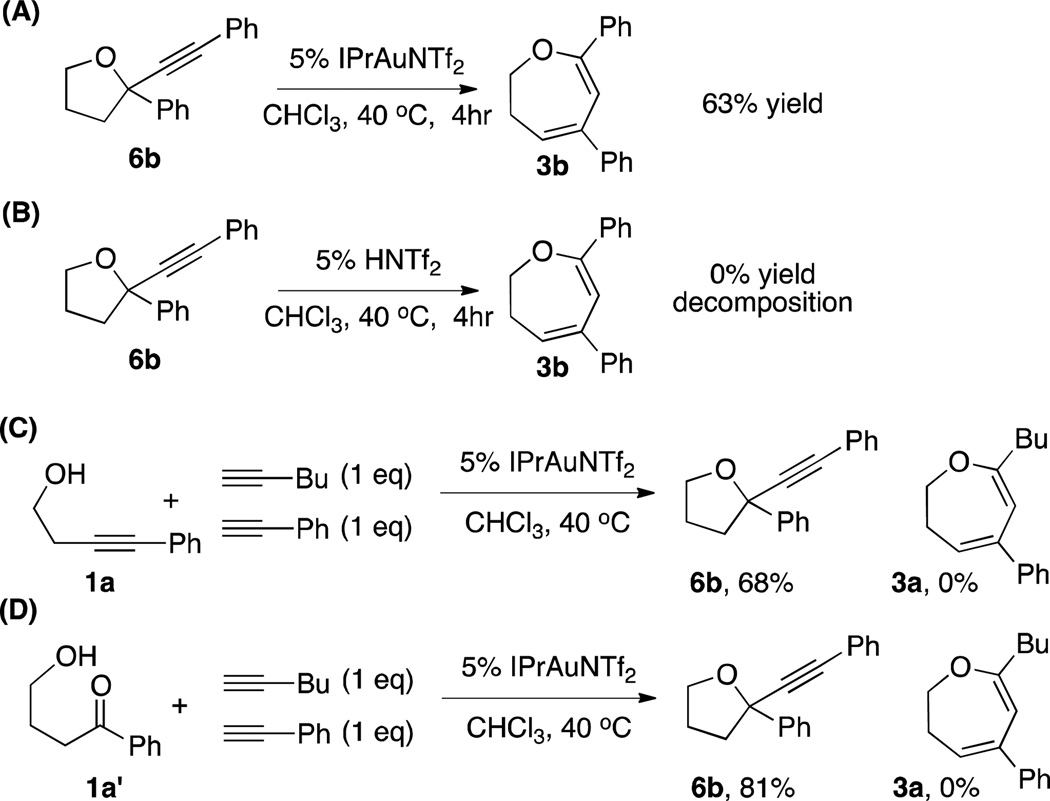
Therefore, this kinetic profile and the intermediary nature of 6b suggest that, unlike the analogous reaction involving aliphatic terminal alkynes, oxepine 3b was formed via rearrangement of alkyne 6b.12 To confirm this hypothesis, 6b was isolated and subjected to the reaction conditions (5% IPrAuNTf2, 40 °C). Within 6 hours, full conversion of 6b was attained, with oxepine 3b formed in 63% yield (Figure 2A). Notably, treating 6b with a catalytic amount of HNTf2 (5%) led to decomposition of the substrate with no observation of oxepine (Figure 2A). These results confirmed that this ring-expansion rearrangement is a gold-catalyzed process.
Figure 2.
Exploration of reaction mechanism
An additional mechanistic concern is the reactivity difference between aliphatic and aryl-substituted terminal alkynes. As shown in Figure 2B, treating either alkyne 1a (Figure 2C) or ketone 1a’ (Figure 2D) with an equimolar ratio of aliphatic and aromatic alkyne afforded alkyne 6b as the major product, with no aliphatic oxepine or alkynylation observed. This data suggests that gold-acetylides formed from either aliphatic or aryl-substituted terminal alkynes exhibit divergent reactivity within the catalytic system based on alkyne substitution. Therefore, the intramolecular cyclization of homo-propargyl alcohol 1a efficiently out-competes the intermolecular hydroxylation of the terminal alkyne, selectively forming the alkynylation product 6b.
This result contrasts with our previously reported system in which intermolecular hydroalkoxylation was preferred. Furthermore, by utilizing the N-heterocyclic carbene gold catalyst IPrAuNTf2,13 the internal alkyne functionality of compound 6 can be activated to form oxepine 3 through a ring-expansion rearrangement (a detailed mechanistic study is currently under investigation). Consequently, aryl-substituted terminal alkynes are successfully implemented into a highly efficient gold-catalyzed synthesis of 2,3-dihydrooxepines for the first time.
Herein, we report the gold-catalyzed intermolecular condensation of alkynols and aromatic terminal alkynes. It was discovered that the initial alkynylated substrates undergo a subsequent gold-catalyzed ring expansion to afford the corresponding 2,3-dihydrooxepines. This reaction represents a complementary synthesis to our previously reported enyne cycloisomerization. Additionally, the transformation is an illustration of reaction divergence that is tuned by altering the structure of the substrate. As a result of the mild reaction conditions, expedient functional group modification, and mechanistic insight gained, this study holds significant value in the advancement of gold-catalysis for the synthesis of complex molecules.
Supplementary Material
Acknowledgments
We thank the NIH (1R01GM120240-01), NSF (CHE-1362057) and NSFC (21629201) for financial support. CAS thanks NSF (HRD-1400837) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and spectroscopic data of new compounds. CCDC 1521359, 1521360, and 1521361. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c000000x/
Notes and references
- 1.For a collection of recent reviews, see: Pflasterer S, Hashmi ASK. Chem. Soc. Rev. 2016;45:1331–1367. doi: 10.1039/c5cs00721f. Dorel R, Echavarren AM. Chem. Rev. 2015;115:9028. doi: 10.1021/cr500691k. Rudolph M, Hashmi ASK. Chem. Soc. Rev. 2012;41:2448. doi: 10.1039/c1cs15279c. Krause N, Winter C. Chem. Rev. 2011;111:1994. doi: 10.1021/cr1004088. Fürstner A. Chem. Soc. Rev. 2009;38:3208. doi: 10.1039/b816696j.
- 2.(a) Chen G-Q, Fang W, Wei Y, Tang X-Y, Shi M. Chem. Sci. 2016;7:4318. doi: 10.1039/c6sc00058d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fang W, Tang X-Y, Shi M. RSC Adv. 2016;6:4047. [Google Scholar]; (c) Tomás-Mendivil E, Toullec PY, Díez J, Conejero S, Michelet V, Cadierno V. Org. Lett. 2012;14:2520. doi: 10.1021/ol300811e. [DOI] [PubMed] [Google Scholar]; (d) Alcaide B, Almendros P, Cembellín S, del Campo TM, Fernández I. Chem. Commun. 2013;49:1282. doi: 10.1039/c2cc37872h. [DOI] [PubMed] [Google Scholar]; (e) Meiss R, Kumar K, Waldmann H. Chem. Eur. J. 2015;21:13526. doi: 10.1002/chem.201502843. [DOI] [PubMed] [Google Scholar]; (f) Miró J, Sánchez-Roselló M, González J, del Pozo C, Fustero S. Chem. Eur. J. 2015;21:5459. doi: 10.1002/chem.201406224. [DOI] [PubMed] [Google Scholar]; (g) Rao WD, Koh MJ, Kothandaraman P, Chan PWH. J. Am. Chem. Soc. 2012;134:10811. doi: 10.1021/ja304964s. [DOI] [PubMed] [Google Scholar]; (h) Brady PB, Carreira EM. Org. Lett. 2015;17:3350. doi: 10.1021/acs.orglett.5b01607. [DOI] [PubMed] [Google Scholar]
- 3.Wei Y, Shi M. ACS Catal. 2016;6:2515. [Google Scholar]
- 4.(a) Motika SE, Wang Q, Akhmedov NG, Wojtas L, Shi X. Angew. Chem., Int. Ed. 2016;55:11582. doi: 10.1002/anie.201604986. [DOI] [PubMed] [Google Scholar]; (b) Yang Y, Qin A, Zhao K, Wang D, Shi X. Adv. Synth. Catal. 2016;358:1433. [Google Scholar]; (c) Wang Q, Motika SE, Akhmedov NG, Petersen JL, Shi X. Angew. Chem., Int. Ed. 2014;53:5418. doi: 10.1002/anie.201402614. [DOI] [PubMed] [Google Scholar]; (d) Duan H, Sengupta S, Petersen JL, Akhmedov NG, Shi X. J. Am. Chem. Soc. 2009;131:12100. doi: 10.1021/ja9041093. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hosseyni S, Wojtas L, Li M, Shi X. J. Am. Chem. Soc. 2016;138:3994. doi: 10.1021/jacs.6b00882. [DOI] [PubMed] [Google Scholar]; (b) Snieckus V, Frota LC. Synfacts. 2016;12:573. [Google Scholar]
- 6.Hosseyni S, Smith CA, Shi X. Org. Lett. ASAP; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wang W, Hammond GB, Xu B. J. Am. Chem. Soc. 2012;134:5697. doi: 10.1021/ja3011397. [DOI] [PubMed] [Google Scholar]; (b) Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- 8.For selected examples of metal-catalyzed oxonium alkynylation, see: Yao X-Q, Li C-J. Org. Lett. 2006;8:1953. doi: 10.1021/ol060645p. Obradors C, Echavarren AM. Chem. Eur. J. 2013;19:3547. doi: 10.1002/chem.201300131. Michalska M, Songis O, Taillier C, Bew SP, Dalla V. Adv. Synth. Catal. 2014;356:2040. Ueda H, Yamaguchi M, Kameya H, Sugimoto K, Tokuyama H. Org. Lett. 2014;16:4948. doi: 10.1021/ol5024695. Ueda H, Yamaguchi M, Tokuyama H. Chem. Pharm. Bull. 2016;64:824. doi: 10.1248/cpb.c16-00193.
- 9.For selected examples of metal-catalyzed iminium alkynylation, see: Koradin C, Polborn K, Knochel P. Angew. Chem., Int. Ed. 2002;41:2535. doi: 10.1002/1521-3773(20020715)41:14<2535::AID-ANIE2535>3.0.CO;2-M. Knöpfel TF, Zarotti P, Ichikawa T, Carreira EM. J. Am. Chem. Soc. 2005;127:9682. doi: 10.1021/ja052411r. Han J, Xu B, Hammond GB. J. Am. Chem. Soc. 2010;132:916. doi: 10.1021/ja908883n.
- 10.(a) Yan J, Tay GL, Neo C, Lee BR, Chan PWH. Org. Lett. 2015;17:4176. doi: 10.1021/acs.orglett.5b01935. [DOI] [PubMed] [Google Scholar]; (b) Borrero NV, DeRatt LG, Barbosa LF, Abboud KA, Aponick A. Org. Lett. 2015;17:1754. doi: 10.1021/acs.orglett.5b00528. [DOI] [PubMed] [Google Scholar]; (c) Gonzalez AZ, Toste DF. Org. Lett. 2010;12:200. doi: 10.1021/ol902622b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TXM, Son-Meniel B, Jullian J-C, Figadere B. Lett. Org. Chem. 6:630. [Google Scholar]
- 12.For recent examples of gold-catalyzed ring expansions, see: Zhu L-L, Li X-X, Zhou W, Li X, Chen Z. J. Org. Chem. 2011;76:8814. doi: 10.1021/jo2015517. Bolte B, Gagosz F. J. Am. Chem. Soc. 2011;133:7696. doi: 10.1021/ja202336p. Kim K-D, Yeom H-S, Shin S, Shin S. Tetrahedron. 2012;68:5241. Yuan W, Dong X, Wei Y, Shi M. Chem. Eur. J. 2012;18:10501. doi: 10.1002/chem.201201161. An J-H, Yun H, Shin S, Shin S. Adv. Synth. Catal. 2014;356:3749. Zhao J, Liu J, Xie X, Li S, Liu Y. Org. Lett. 2015;17:5926. doi: 10.1021/acs.orglett.5b03160. Chen M, Sun N, Xu W, Zhao J, Wang G, Liu Y. Chem. Eur. J. 2015;21:18571. doi: 10.1002/chem.201504165.
- 13.Díez-González S, Marion N, Nolan SP. Chem. Rev. 2009;109:3612. doi: 10.1021/cr900074m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.