Abstract
The induction of localized pro-inflammatory niches in the periphery is instrumental in metastasis. In order to better understand how tumors engage distal sites and activate a pro-inflammatory response we utilized syngeneic breast cancers as a model and showed that soluble factors from the neoplastic epithelium activate the expression of the monocyte chemoatractive protein (MCP) chemokines of the mouse 11C cluster that include Ccl1, Ccl2, Ccl7, Ccl8, Ccl11 and Ccl12. Tissues such as the lungs and the brain, that are more prone to colonization by breast cancer cells, were more sensitive to MCP cluster chemokine induction than others such as the liver. Subsequent analyses involving chemokine arrays in breast cancer cells and media followed by functional validation assays in in vitro and in vivo identified the cytokine Ccl3 as the principle mediator of the communication between the neoplastic epithelium and the peripheral tissues in terms of MCP cluster chemokine induction. Our results show that MCP chemokines are activated in peripheral tissues of breast cancer-bearing mice, by a mechanism that involves breast cancer cell-derived Ccl3. Interference with the expression of cancer cell-derived Ccl3 may find application in the management of breast cancer metastases.
Keywords: CCL3, chemokine, breast cancer, inflammation, periphery, metastasis
1. Introduction
The establishment of a network of soluble growth and chemoattractive factors between the cancer cells, the stroma and the periphery is essential for the promotion of tumorigenesis and the seeding of distal sites during metastasis [1, 2]. During this process several different cell types are being engaged that ultimately promote cancer growth and facilitate organ –specific metastases [3, 4]. In breast cancer, organs such as the lungs and the brain represent the site of preference for metastatic growth, an event that signifies the onset of the incurable form of the disease [5]. The development of the metastatic disease is associated with the induction of systemic inflammation that besides increasing the baseline levels of tumor promoting factors in the circulation also creates locally, within certain peripheral tissues, pro-inflammatory niches at which circulating cancer cells may preferably establish local outgrowths and eventually develop into metastatic sites [6].
Chemokines of the MCP region of the 11C cluster in mice are chemoattractive cytokines that include Ccl2, Ccl7, Ccl11, Ccl12, Cc8 and Ccl1 [7]. In humans the cluster is localized in chromosome 17q12 and instead of Ccl12 contains the chemokine Ccl13 [8]. All these chemokines have been associated before, directly or indirectly with metastasis, primarily by mechanisms involving the recruitment of cells of the immune system and the development of a pro-inflammatory niche [9–18].
Recently we showed that peripheral tissues such as the brain and the lung, that are preferably colonized by breast cancer cells during metastasis, exhibited elevated levels of Ccl8 in breast tumor-bearing mice [16]. In view of the tumor promoting activities of Ccl8 this observation prompted us to explore if MCP cluster chemokine activation in the periphery reflects a more generalized feature during breast tumorigenesis. As a model we used the syngeneic EO771 breast tumors that allow the growth of breast cancers in immune-competent wild type C57BL6 mice [19].
2. Materials and Methods
2.1 Cell culture
NHI/3T3 mouse embryonic fibroblasts were maintained in DMEM supplemented with 10% Fetal Bovine Serum (Corning). The C57BL/6-derived mouse mammary adenocarcinoma cell line EO771 was kindly provided by Dr. Frank C. Marini (Wake Forest School of Medicine, NC) and cultured in RPMI-1640 medium supplemented with 10% FBS. Cell lines were regularly tested for mycoplasma contamination using commercially available Mycoplasma detection kit (Myco Alert kit; Lonza).
2.2 Conditioned media experiments
For conditioned media preparation, EO771 cells were plated on 6 well plates at a density of 5×104 cells/well and cultured for 96 hours. Conditioned media were collected, centrifuged for 5min at 1500 rpm and added for 48h in 3T3 fibroblasts that had been cultured for 16 hours in serum free media on 12-well plates at a density of 5×105 cells/well. Experiments were performed in triplicates, and similar results were obtained.
For Ccl3 and Cx1 neutralization, antibodies were added in the conditioned media after their collection at concentrations 20 μg/ml for mouse monoclonal Ccl3 (MAB450; R&D Systems) and 1ug/ml for mouse monoclonal Cxcl1 (MAB453; R&D Systems).
2.3 RNA and Protein assays
Total RNA extraction from cells was performed using RNeasy Mini Kit (Qiagen, Valencia, CA) and from tissues using Trizol reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. cDNA was prepared with a total of 1 ug RNA using iScript™ cDNA Synthesis Kit (Biorad), according to the manufacturer’s protocol.
For real-time quantitative PCR, cDNA was amplified using the iTaq Universal SYBR Green Supermix (Biorad) in a total reaction volume of 20 μL, using an Applied Biosystems 7300 real time instrument (Applied Biosystems) as described [16].
For cytokine secretion analysis, conditioned media from 3T3 and EO771 at a density of 4×105 cells/well were collected after 20 hours of culture in serum-free media and cytokine secretion was detected using Mouse Cytokine Array C2000 (Ray Biotech, Inc., Norcross GA, USA) according to the manufactures’ instructions. The data were quantified by densitometry using Image J software (National Institutes of Health, Bethesda, MD) and presented as expression ratio of the signal intensity between EO771 and 3T3.
For detection of cytokines, 3T3 cells were plated at a density of 5×104 cells/well, serum starved for 16 hours and treated with 1 ng/ml and 10 ng/ml of mouse recombinant proteins Ccl2, Ccl3, Ccl5, Cxcl1, IL3, IL10 (Tonbo Biosciences). Cell supernatants were collected 24 hours later and Ccl8 protein levels were assessed by Mouse CCL8/MCP-2 DuoSet ELISA Development kit (R&D Systems) according to manufacturer’s protocol.
2.4 Animal studies
C57B6 mice were obtained from Jackson lab and maintained at the USC. Six- to eight-week-old, female animals were used for all experiments.
For tumor reconstitution experiments, 1×105 EO771 cells were resuspended in 0.1 mL of serum-free RPMI and then injected subcutaneously into mice. EO771 cells used in animal studies were tested for viral pathogens and mycoplasma contamination (IDEXX Bioresearch, Columbia, MO). For conditioned media administration in mice, EO771 and 3T3 cells were plated on 6 well plates in duplicates at a density of 4×105 cells/well and cultured in serum free media for 20 hours. Supernatants from two wells were collected and pooled and concentrated to 200 μl using vacuum centrifuge. Samples were filter sterilized and injected i.p. in mice every other day for 5 total injections. For Ccl3 neutralization, mouse monoclonal Ccl3 antibody (MAB450; R&D Systems) was added in the conditioned media at concentration 20 μg/ml and incubated for 30 min at room temperature prior to mice injection. For mouse recombinant Ccl3 administration, mice received an i.p. injection of 1.6 μg Ccl3 diluted in PBS.
Immunostaining was performed in formalin fixed, paraffin embedded sections of tissue by using the Dako EnVision+ System-HRP (DAB) (K4041), following the manufacturer’s instructions by using rat monoclonal Neutrophil Marker (NIMP-R14) sc-59338 (Santa Cruz Biotechnology) 1:50, rabbit monoclonal [SP115] anti-F/480 (ab111101), by Abcam; 1:250. Negative controls included non-immune serum instead of antibody. The number of stained cells was quantified using Image-J software (National Institutes of Health, USA). Evaluation of samples was performed blindly.
2.5 Histology
For histological analyses, tumors were fixed in 10% formalin, paraffin-embedded, serially sectioned, and stained with hematoxylin/eosin.
2.6 Statistics
All data are presented as average values of samples, error bars correspond to standard error of the mean (SEM) unless otherwise stated. Statistical analysis of the results was performed using Student’s two tailed t test. The results were considered statistically significant when p-value <0.05. Survival data were obtained from http://www.compbio.iupui.edu/proggene [20]. Cutoffs were set at median.
3. Results
3.1 Breast tumor-bearing mice activate MCP cluster chemokines in the periphery
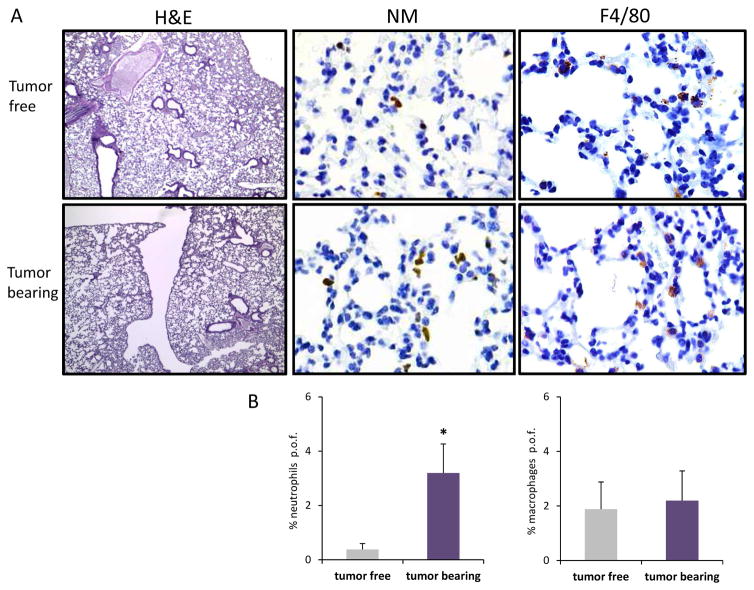
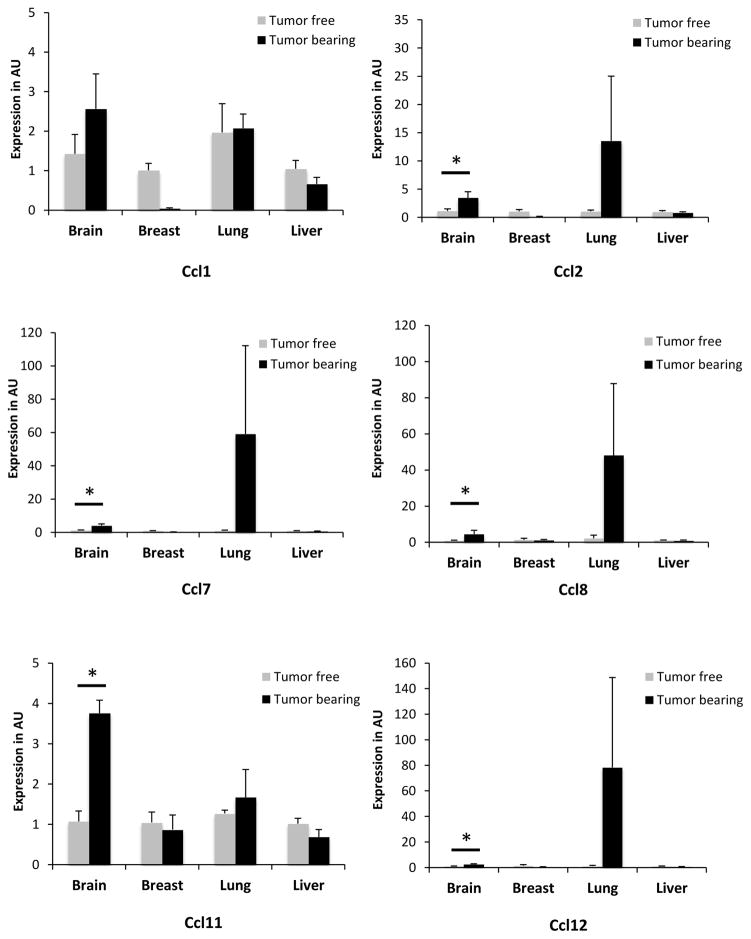
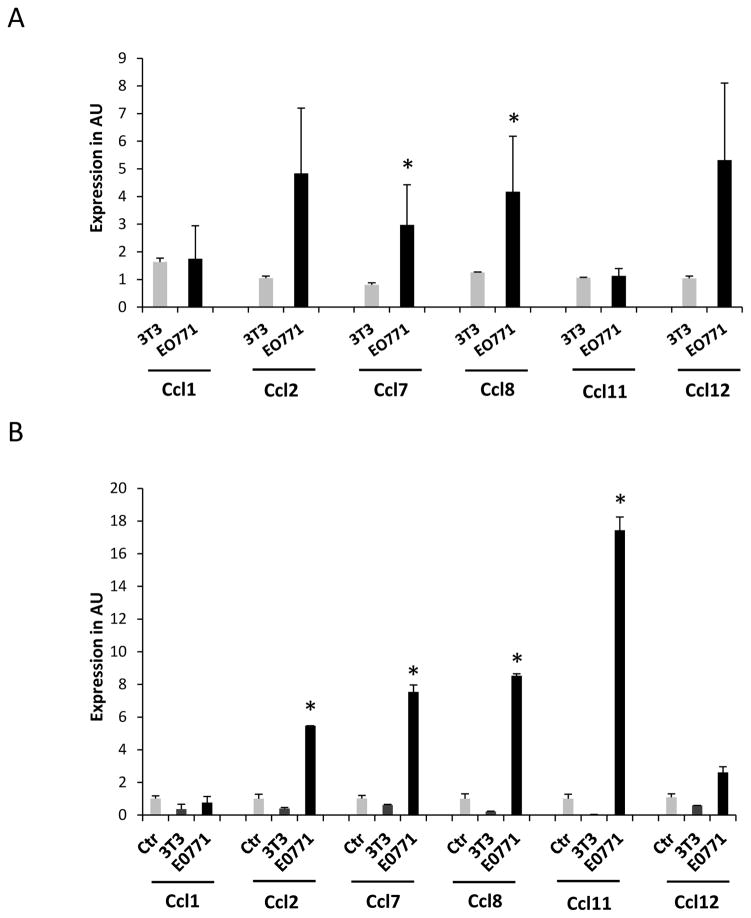
In order to test if this distal activation of Ccl8 in the periphery is limited to this particular chemokine or also extended to other chemokines of the same cluster we monitored MCP chemokines expression levels in peripheral tissues during breast tumor growth. Initially we implanted EO771 tumors in the mammary fat pad of 6–8 weeks female C57BL6 mice and when tumors reached a volume of about 200mm3 animals were sacrificed and the opposite breast tissue, the lungs, the brain and the kidney were harvested. Detailed histopathological analysis consisting of serial tissue sectioning and evaluation for the presence of metastatic foci didn’t reveal any evidence of metastatic disease in any of the tissues examined (Fig. 1). However recruitment of leukocytes and particularly neutrophils was reported in the lung parenchyma suggesting the initiation of an acute inflammatory response triggered by the breast tumors [21, 22]. In order to test if this response was associated with the activation of the MCP chemokines, the expression of Ccl2, Ccl7, Ccl11, Ccl12, Cc8 and Ccl1 was examined in the tumor free breast tissue as well as in the lung, brain and liver of mice and was compared to their expression in tumor free mice. The analysis revealed a clear trend for elevated expression of all chemokines of the cluster in the brain and the lung of the tumor-bearing animals (Fig. 2). Conditioned media from EO771 cells were also injected every other day in tumor free animals and chemokine expression was assessed after 10 days of treatment. Expression was evaluated in comparison with that of animals receiving conditioned media from 3T3 fibroblasts as control. As shown in Figure 3A, EO771 conditioned media stimulated the expression of all chemokines tested in the lungs, with the exception of Ccl11, with this stimulation reaching significance for Ccl7 and Ccl8. In view of our earlier findings that certain MCP cluster chemokines can be activated in stromal fibroblasts by paracrine mechanisms by the cancer cells we asked if EO771 conditioned media stimulate these chemokines in 3T3 fibroblasts in vitro. As shown in Figure 3B, fibroblasts cultured in media conditioned by EO771 cells exhibited elevated expression of Ccl2, Ccl7, Ccl8 and Ccl11, as compared to media from 3T3 cells suggesting that at least in part, the chemokine activation recorded is due to the direct effects of cancer cell derived factors in the fibroblasts.
Figure 1. Neutrophil recruitment in the lungs of EO771 tumor-bearing mice.
(A). Hematoxilin and eosin (H&E) (4X), neutrophil marker (NM) and F4/80 (40X), representative images of the staining for neutrophils and macrophages in the lungs of tumor bearing and tumor free animals (B). Quantification of positive cells per optic field (p.o.f.) of the results described above.
Figure 2. MCP cluster chemokine gene expression in tumor bearing mice.
Expression of MCP cluster chemokines in tumor free and tumor bearing animals (n=3) in the tissues indicated. Results are expressed in arbitrary units (AU) as average + SEM. *, p<0.05.
Figure 3. MCP cluster chemokine gene expression in fibroblasts and mouse lungs treated with EO771 conditioned media.
Expression of MCP cluster chemokines in the lungs of tumor-free animals (n=3) treated with EO771 or 3T3 conditioned media (A) or 3T3 fibroblasts cultured in EO771 or 3T3 conditioned media for 48 hours (B). Results are expressed in arbitrary units (AU) as average + SEM. *, p<0.05.
3.2 Comparison in the secretion profile of EO771 and 3T3 cells
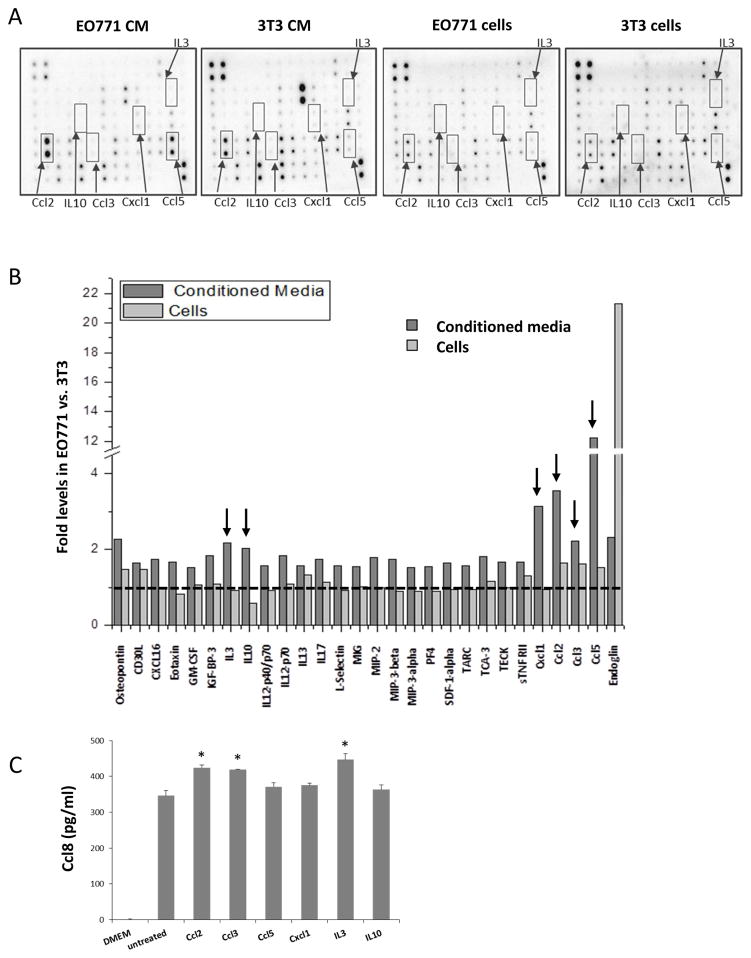
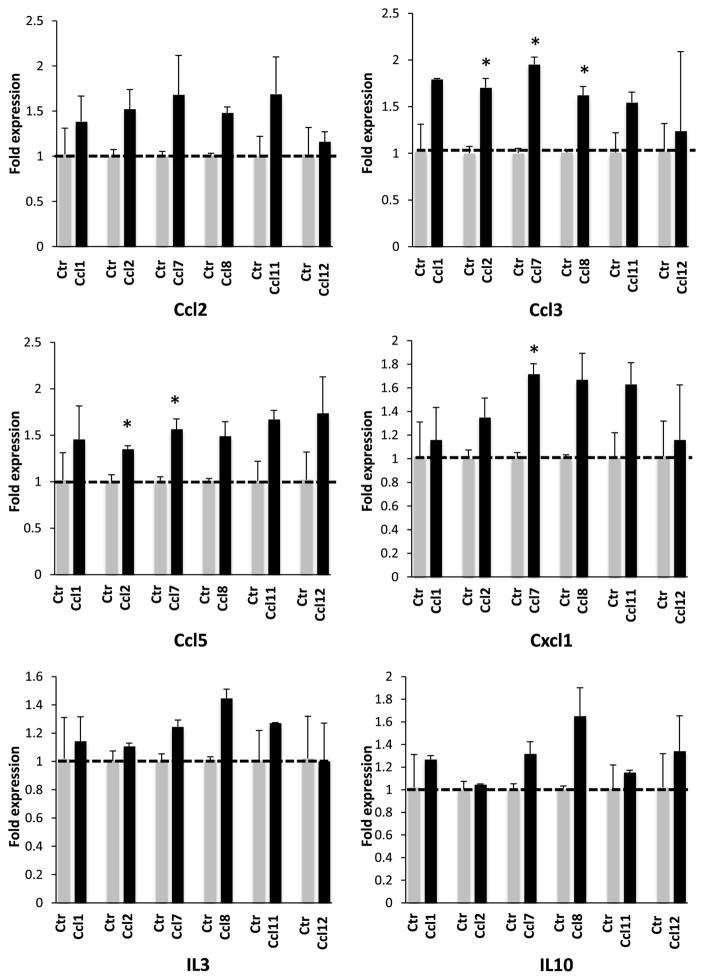
The fact that EO771 breast cancer cells and 3T3 fibroblasts exhibit differential paracrine activity in vitro in terms of MCP cluster chemokines’ activation provides a reporter system at which soluble factors produced by each of these cell types can be assessed. Cytokine secretion analysis between EO771 and 3T3 cells as well as of their corresponding conditioned media revealed several soluble factors were upregulated in EO771 cells (Fig. 4A, B). We proceeded by focusing on a set of such targets that exhibited the higher and more consistent stimulation in the EO771 cells and media including Ccl2, Ccl3, Ccl5, Cxcl1, IL3 and IL10. The criteria used for candidate target selection is to exhibit elevated expression both the media (at least 2-fold) and the cells with the former surpassing that of the latter reflecting presumably secretion. Additional factors also fulfilled these criteria, such as MMP-3 and osteopontin however they were excluded from further analyses because they have an established role in tumor microenvironment but their systemic effects appear unlikely [23, 24]. Albeit elevated only in the media but not in the cell extracts IL10 was included because of its established role in inflammation [25]. In order to validate them as chemokine activators in fibroblasts we undertook in parallel two approaches. First, we tested their effects in Ccl8 levels released in the supernatants of 3T3 fibroblasts. This strategy showed that a moderate though significant elevation of Ccl8 in the media was attained by Ccl2, CCl3, and IL3 exposure (Fig. 4C). Then we tested their effects in the expression of MCP chemokines at the level of transcription. As shown in Figure 5, all soluble factors under evaluation stimulated chemokine expression in 3T3 cells with Ccl3 producing significant activation of Ccl7, Ccl8 and Ccl11. Ccl3 has been shown to be involved in leukemia progression and to regulate the microenvironment in the lymph nodes [26, 27]. Furthermore, Ccl3 has been linked with the promotion of breast cancer metastases in different experimental models [9, 28]. Thus, we considered Ccl3 as the most promising target for subsequent validation. For subsequent studies we also included Cxcl1 due to its previously established pivotal role in metastasis of breast cancer cells [29, 30].
Figure 4. Cytokine secretion analysis of EO771 cells and media.
(A) Representative images of cytokine arrays of EO771 and 3T3 cell extracts and conditioned media. Arrows indicate cytokines upregulated in EO771 as compared to 3T3, >2 fold for media and >1 fold for cells. (B) Expression ratio of cytokines with >1.5 fold induction in the media. Data for MMP-3 and osteopontin are not shown. (C) Ccl8 levels in the supernatants of 3T3 cells exposed to the factors indicated at concentration 10 ng/ml for 24 hours. Results are expressed as average + SEM. *, p<0.05.
Figure 5. Effect of various soluble factors in the RNA levels of the MCP cluster chemokines.
3T3 cells were treated with 1 ng/ml of the factors for 24 hours. Results are expressed in arbitrary units (AU) as average + SEM. *, p<0.05.
3.3 Stimulation of the expression of the MCP cluster chemokines is Ccl3-dependent
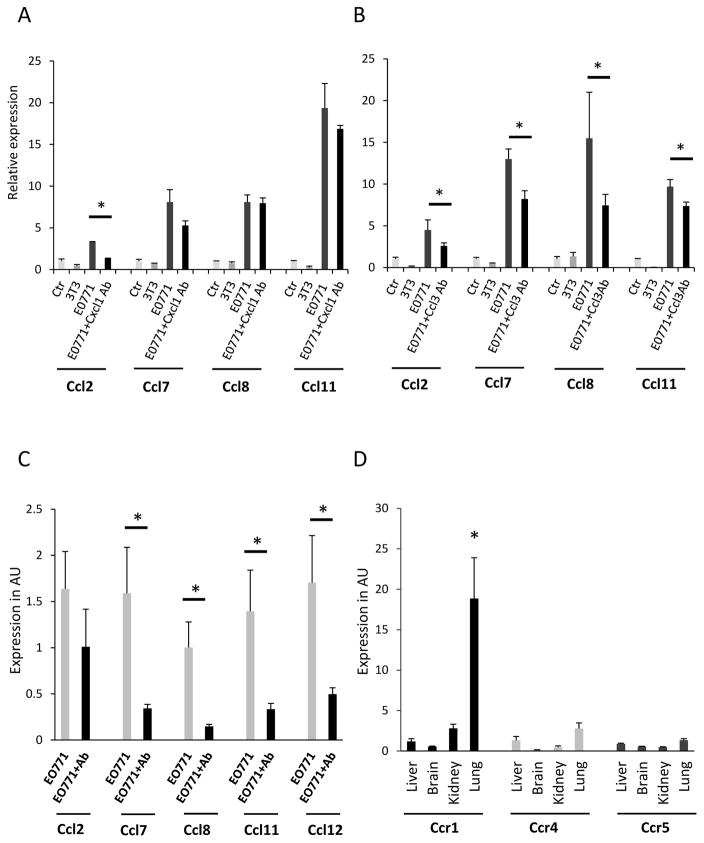
In order to test if the stimulatory activity of EO771 conditioned media is Ccl3 or Cxcl1-dependent we cultured fibroblasts in the presence of control (unconditioned) media, conditioned media for 3T3 or EO771 as well as EO771 conditioned media in the presence of a neutralizing antibody for Cxcl1 or Ccl3. After 48 hours of culture, fibroblasts were assessed for the expression of the chemokines Ccl2, Ccl7, Ccl8 and Ccl11. As shown in Figure 6A neutralization of Cxcl1 activity inhibited significantly only the expression of Ccl2. However, exposure of fibroblasts with the EO771 conditioned media in combination with the anti-Ccl3 antibody significantly suppressed the expression of Ccl2, Ccl7, Ccl8 and Ccl11 chemokines in 3T3 fibroblasts (Fig. 6B). Thus we concluded that while Cxcl1 may also contribute to MCP chemokine activation, Ccl3 emerges as the most promising candidate. In line with this notion are the results of an in vivo study in tumor -free C57BL6 mice that received for 10 days EO771 conditioned media alone or in combination with a neutralizing antibody for Ccl3. As shown in Figure 6C, inactivation of Ccl3 in the media resulted in strong inhibition in the expression of Ccl2, Ccl7, Ccl8, Ccl11 and Ccl12 in the lungs. In addition, administration of Ccl3 in tumor free mice resulted in an increase in the numbers of neutrophils infiltrating the lungs (Fig. 7). Thus Ccl3, at least in part, mediates the induction of MCP cluster chemokines in the lungs.
Figure 6. Inhibition of Cxcl1 or Ccl3 affects the expression of MCP cluster chemokines in vitro and in vivo.
3T3 fibroblasts were cultured for 48 hours, in 96 hours conditioned media from 3T3 cells or EO771 cells alone or in combination with a neutralizing antibody for Cxcl1 (A) or Ccl3 (B) at concentrations 1ug/ml and 20ug/ml respectively and the expression of MCP chemokines was evaluated. (C) Induction of MCP cluster chemokines in the lungs by EO771 conditioned media is Ccl3-dependent. Mice received every other day EO771 media alone or in combination with a neutralizing antibody for Ccl3 at concentration 20 ug/ml and MCP cluster chemokine levels were evaluated in the lungs. (D) Expression of Ccl3 receptors in peripheral tissues. The expression of Ccr1, Ccr4 and Ccr5 was evaluated in the liver, brain lung and kidney of tumor free mice. Results are expressed in arbitrary units (AU) as average + SEM. *, p<0.05.
Figure 7. Neutrophil recruitment in the lungs of tumor-free mice 30 min or 4h after Ccl3 administration.
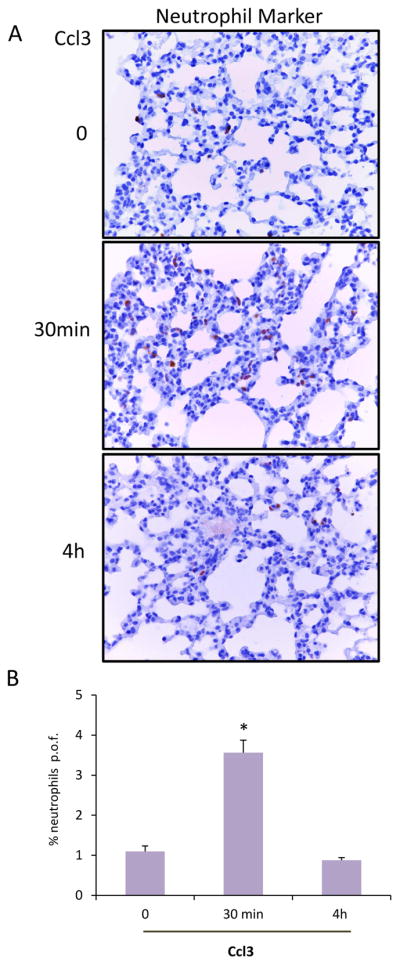
(A). Representative images (40X), of neutrophil marker-stained lungs of tumor free animals that received Ccl3 (1.6 μg per mouse, n=3). (B). Quantification of positive cells per optic field (p.o.f.) of the results described above. (*, p <0.001)
3.4 The receptors of Ccl3 are expressed differentially in peripheral tissues
Our results are consistent with a mechanism according to which cancer cell –produced Ccl3 acts preferentially in the lung to promote the production of the pro-inflammatory MCP cluster chemokines. This hypothesis predicts that the receptor(s) for Ccl3 will be overexpressed in the tissues at which Ccl3 stimulatory activity is more prominent. To test this hypothesis we evaluated the expression of the major receptors of Ccl3, being Ccr1, Ccr4 and Ccr5 [31] in peripheral organs such as the liver, the brain, the kidney and the lung. As shown in Figure 6D, Ccr1 was overexpressed in the lung as compared to other tissues which is in line with the strong activating activity of the MCP cluster chemokines by Ccl3 in the lung.
3.5 High Ccl3 expression is associated with worse outcome in breast cancer
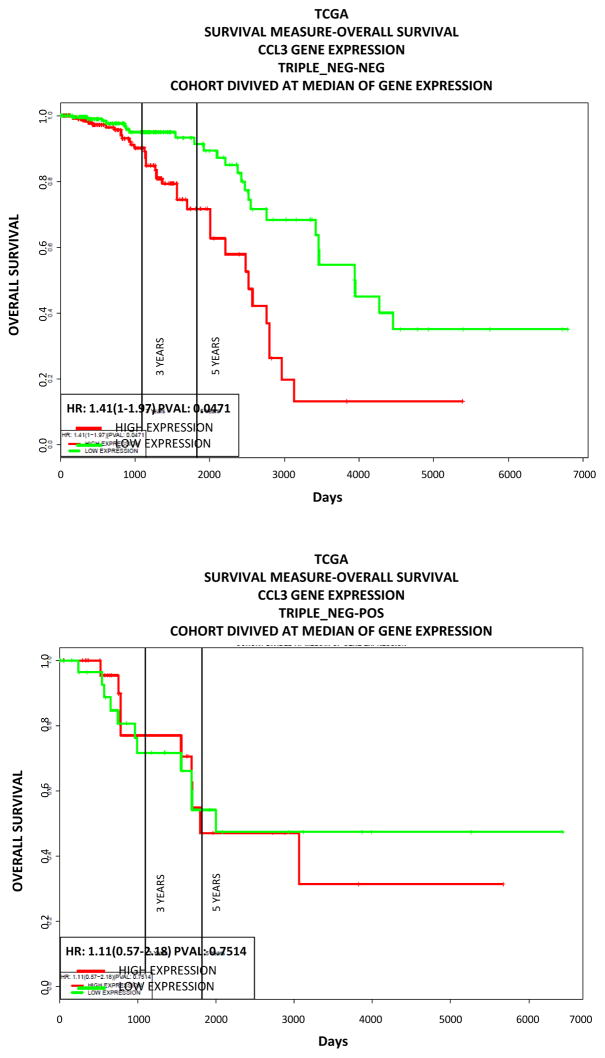
In order to explore the clinical relevance of Ccl3 as an activator of chemoattractive cytokines in the lung in the context of primary breast cancer we investigated the prognosis of breast tumor patients in relation to the Ccl3 levels of expression in the tumors. For our analysis we used publicly available data from http://www.compbio.iupui.edu/proggene [20]. Our analysis showed that high CCL3 expression is associated with less favorable outcome of the disease for the non-triple negative breast cancers (Fig. 8).
Figure 8. Survival of breast cancer patients’ in relation to tumoral CCL3 expression.
High CCL3 expression is associated with worse prognosis. Data were obtained from http://www.compbio.iupui.edu/proggene. High expression is indicated in red, low expression in blue, cutoffs were set at median.
4. Discussion
In the present study, we identified the chemokines of the mouse MCP region of 11C cluster, 17q12 in humans, as potential mediators of this process since they exhibit a profile of activation that simulates the organ –specificity of metastasis of breast cancer cells. Specifically we have found that in tumor –bearing mice most of the MCP chemokines were significantly activated in the brain but not in breast and liver which implies the operation of organ specificity. The same trend was also detected in the lung of the same animals however variation in the induction levels prevented significance. The neutrophil accumulation that was recorded in the lungs of the tumor-bearing animals was consistent with the induction of acute inflammation. However, under the present experimental conditions, it did not progress into full-fledged inflammation exemplified by macrophage recruitment. The relatively short period of tumor growth may account for that observation nevertheless, MCP chemokines had been activated suggesting their significant role during the early stages of tumor growth.
Administration of conditioned media from EO771 cells to tumor-free mice attained similar effects in chemokine induction in the lung suggesting that soluble factors from the tumor, via the systemic circulation, act in the lung promoting MCP chemokine induction. For this experiment we analyzed lung and not brain, despite the significant effects attained in the latter after the implantation of the EO771 cells, because the blood-brain barrier may have interfered with the action of the soluble factors in the conditioned media [32].
At least in part, fibroblasts account for these effects as pointed by the elevated expression of the MCP chemokines in 3T3 cells cultured in the presence of EO771 media. This also suggests that transcriptional induction in addition to the accumulation of immune cells accounts for the induction of chemokines’ expression.
Comparison of the secreted factors between EO771 cells that activate MCP chemokines and 3T3 that do not, identified several candidates that may mediate these effects. Among these factors Ccl3 was validated as a major regulator of MCP cluster induction. This notion was based on the observation that antibody-mediated neutralization of Ccl3 activity was sufficient to inhibit the induction of Ccl2, Ccl7, Ccl8 and Ccl11 triggered by EO771 media in vitro and the induction of Ccl7, Ccl8, Ccl11 and Ccl12 triggered by EO771 media in vivo. Consistent with these findings is the elevated levels of CCR1 receptor that mediates the effects of Ccl3 in the lung as compared to other organs.
These findings are consistent with a model according to which cancer cells produce Ccl3 that acts in the periphery and induces the expression of the MCP cluster chemokines to trigger a pro-inflammatory and tumor promoting cascade. In addition to the direct effects of CCl3 in fibroblasts additional soluble factors may act alone or in combination to trigger the expression of MCP chemokines. Besides the effects in gene transcription the progressive recruitment of immune and other stromal cells alters locally the profile of chemokines’ expression. Nevertheless, an important role for Ccl3 in mammary tumorigenesis should be considered, a notion that is also supported by the worse prognosis of breast cancer patients with elevated Ccl3 levels in the tumors. These findings further confirm and extend earlier studies suggesting that CCL3 is involved in metastatic breast cancer (9,28). Noteworthy this association was prevalent for non-triple negative breast cancers only implying a relation between Ccl3 and estrogen signaling. In view of these findings modulation of Ccl3 should be considered for the management of breast cancer development and progression.
Highlights.
The chemokines of the monocyte chemoattractive protein (MCP) cluster were upregulated in the lungs and brains of mice bearing breast tumors.
Cancer cell-derived Ccl3 was identified as stimulator of stromal chemokine expression.
Inhibition of Ccl3 activity suppressed MCP expression in vivo and in vitro by paracrine mechanisms.
Acknowledgments
Financial support: This study was supported by a pilot grant 5P30GM103336-02 from NIH
This study was supported by a pilot grant 5P30GM103336-02 from NIH.
Footnotes
Funder’s participation
The authors declare that the funder did not participate in the writing of this manuscript.
Conflict of interest statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–21. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Kopfstein L, Christofori G. Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci. 2006;63:449–68. doi: 10.1007/s00018-005-5296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic Acids Res. 2014;42:D749–55. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–59. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roblek M, Strutzmann E, Zankl C, Adage T, Heikenwalder M, Atlic A, et al. Targeting of CCL2-CCR2-Glycosaminoglycan Axis Using a CCL2 Decoy Protein Attenuates Metastasis through Inhibition of Tumor Cell Seeding. Neoplasia. 2016;18:49–59. doi: 10.1016/j.neo.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Lee Y, Kim SY, Song SJ, Hong HK, Lee Y, Young OhB, et al. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016 doi: 10.18632/oncotarget.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolen BM, Lokshin AE. Targeting CCL11 in the treatment of ovarian cancer. Expert Opin Ther Targets. 2010;14:157–67. doi: 10.1517/14728220903512983. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal M, He C, Siddiqui J, Wei JT, Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013;73:573–81. doi: 10.1002/pros.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajaram M, Li J, Egeblad M, Powers RS. System-wide analysis reveals a complex network of tumor-fibroblast interactions involved in tumorigenicity. PLoS Genet. 2013;9:e1003789. doi: 10.1371/journal.pgen.1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmaki E, Chatzistamou I, Kaza V, Kiaris H. A CCL8 gradient drives breast cancer cell dissemination. Oncogene. 2016 doi: 10.1038/onc.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–28. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung MY, Kim SH, Cho D, Kim TS. Analysis of the expression profiles of cytokines and cytokine-related genes during the progression of breast cancer growth in mice. Oncol Rep. 2009;22:1141–7. doi: 10.3892/or_00000547. [DOI] [PubMed] [Google Scholar]
- 19.Casey AE, Laster WR, Jr, Ross GL. Sustained enhanced growth of carcinoma EO771 in C57 black mice. Proc Soc Exp Biol Med. 1951;77:358–62. doi: 10.3181/00379727-77-18779. [DOI] [PubMed] [Google Scholar]
- 20.Goswami CP, Nakshatri H. PROGgene: gene expression based survival analysis web application for multiple cancers. J Clin Bioinforma. 2013;3:22. doi: 10.1186/2043-9113-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issekutz AC, Movat HZ. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980;42:310–7. [PubMed] [Google Scholar]
- 22.Ryan GB, Majno G. Acute inflammation. A review Am J Pathol. 1977;86:183–276. [PMC free article] [PubMed] [Google Scholar]
- 23.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–46. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10:71–81. doi: 10.2174/156652410791065381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 26.Baba T, Tanabe Y, Yoshikawa S, Yamanishi Y, Morishita S, Komatsu N, et al. MIP-1alpha/CCL3-expressing basophil-lineage cells drive the leukemic hematopoiesis of chronic myeloid leukemia in mice. Blood. 2016;127:2607–17. doi: 10.1182/blood-2015-10-673087. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann EM, Rudelius M, Burger JA, Rosenwald A. CCL3 chemokine expression by chronic lymphocytic leukemia cells orchestrates the composition of the microenvironment in lymph node infiltrates. Leuk Lymphoma. 2016;57:563–71. doi: 10.3109/10428194.2015.1068308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol. 2008;181:6384–93. doi: 10.4049/jimmunol.181.9.6384. [DOI] [PubMed] [Google Scholar]
- 29.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su SB, Mukaida N, Wang J, Nomura H, Matsushima K. Preparation of specific polyclonal antibodies to a C-C chemokine receptor, CCR1, and determination of CCR1 expression on various types of leukocytes. J Leukoc Biol. 1996;60:658–66. doi: 10.1002/jlb.60.5.658. [DOI] [PubMed] [Google Scholar]
- 32.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]