Abstract
Epithelioid trophoblastic tumor (ETT) is a very rare variant of gestational trophoblastic disease (GTD) which arises in reproductive age women with prior gestational history. Although abnormal vaginal bleeding is the most common symptom of ETT, there are no reported pathognomonic symptoms of ETT because of its rarity. ETT is similar to placental site trophoblastic tumor in terms of its slow growing characteristic and microscopic findings. Therefore, it could be misdiagnosed as placental site trophoblastic tumor or other types of GTD. Unlike other types of GTD, primary treatment of ETT is surgical resection because of its chemo-resistant nature. Accordingly, immunohistochemical staining is essential for accurate diagnosis and appropriate treatment. Here, we report a case of a 42-year-old hysterectomized woman with pelvic masses who suffered from abdominal pain. Through laparotomy, tumors were resected completely and they were diagnosed as ETT through immunohistochemical stain. This report provides more evidence about its clinical features, diagnosis, and treatment including a brief review of the literature.
Keywords: Beta human chorionic gonadotropin, Epithelioid trophoblastic tumor, Gestational trophoblastic disease
Introduction
Gestational trophoblastic disease (GTD) encompasses a group of relevant diseases, which occur after pregnancy. These include premalignant conditions of a partial or complete hydatidiform mole, malignant disorders of invasive mole or choriocarcinoma, and rarely placental site trophoblastic tumor (PSTT) or epithelioid trophoblastic tumor (ETT). ETT is the rarest variant of gestational trophoblastic neoplasia (GTN), which was previously termed atypical choriocarcinoma [1,2,3].
Mostly, ETT presents in reproductive aged women with prior gestational history. The typical clinical manifestation is abnormal vaginal bleeding after normal or abnormal gestation [4,5]. ETT originates from chorionic-type intermediate trophoblasts and it shows similar clinical characteristics to PSTT as slow growing and chemo-resistant feature, which is another type of GTN derived from intermediate trophoblasts [4,5,6,7]. However, it has an aggressive clinical course, when it accompanied with metastasis or extra-uterine disease. Therefore, an early diagnosis is important in ETT patients to achieve complete remission [4,5,6].
Since Shih and Kurman [1] first described ETT in 1998, there have been a few published cases in Korea. Above all, extrauterine ETTs were reported in only 2 cases as isolated pulmonary ETT [8,9]. Still, there is no reported case of extrauterine ETT presenting as extensive pelvic masses in a hysterectomized woman.
Herein, we report a case of extra-uterine ETT with multiple intra-abdominal masses in a premenopausal hysterectomized woman with a systematic review of the literature.
Case report
A 42-year-old, gravida 3, para 2, Korean woman was referred to the Department of Emergency Medicine at Samsung Medical Center in November, 2014, for abdominal pain and fever. Her past medical history was unremarkable, although she had received myomectomy in 2010 and total abdominal hysterectomy in 2011 at another university hospital due to symptomatic uterine leiomyoma. Unfortunately, the registered medical chart or pathology slides of her previous operation specimens were not available. Therefore, we could not review the previous slides of her previous operation.
She had been suffered from abdominal pain since one month ago. Otherwise, there were no urinary or gynecologic symptoms. Even though, she had fever at first, her vital signs relatively stable as follows: systolic and diastolic blood pressure 127 and 76 mmHg, pulse rate 96 beats per minutes, respiratory rate 20 breaths/min, and body temperature 39.1℃. Laboratory test showed no definite abnormal results except for elevated C-reactive protein (25.93 mg/dL). Blood and urine culture results revealed no growth of microorganisms. Routine urine human chorionic gonadotropin (hCG) test for childbearing age women was not performed, because she already had hysterectomy.
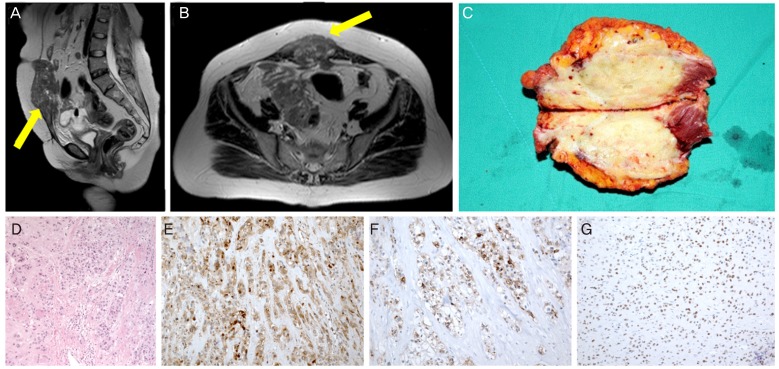
On magnetic resonance imaging and computed tomography (CT) for the abdomino-pelvic region, there were 9-cm-sized soft tissue like lesion in the midline lower abdominal wall (Fig. 1A, B) and another 6.6-cm-sized lesion in the right side of the pelvic cavity which formed a fistula with the distal sigmoid colon. Otherwise, there were no definite abnormal findings on imaging studies.
Fig. 1. Magnetic resonance imaging findings and pathologic evaluations of our patient. (A) Gross finding, midline lower abdominal wall mass with rectus abdominis muscle (photo during operation). (B,C) About 9-cm-sized midline lower abdominal wall soft tissue lesion with low signal intensity in T2W1 image on magnetic resonance imaging (arrowed). (B) Sagittal view and (C) transverse view. (D) The tumor was composed of nests of epithelioid cells with necrotic debris and peritumoral hyaline-like material (H&E, ×20). (E) Positive cytoplasmic staining for β-human chorionic gonadotropin (×100). (F) Positive cytoplasmic staining for inhibin-α (×100). (G) Positive nuclear staining for p63 (×100).
The presumed diagnosis was desmoid tumor or mesenteric sarcoma. Urgent exploratory laparotomy was performed for surgical resection and pathologic confirmation. Midline lower abdominal wall mass was removed by wide excision (Fig. 1C). Through exploration, we identified that the distal sigmoid colon was perforated with massive inflammatory changes and had a fistula connected with the right-side pelvic mass. This mass with the distal sigmoid colon fistula was removed through the Hartmann's procedure. Furthermore, there were three nodular lesions in the sigmoid mesentery and a 4-cm-sized tumor in the terminal ileum, therefore, the general surgeon removed all of these notable masses. Resected tumors were suspected to malignant tumors of mesenchymal origin by frozen section biopsy during surgery.
Grossly, tumors were tan-yellow colored soft masses with necrotic changes. Microscopically, tumors were composed of nests and cords of epithelioid cells with moderate to marked atypia. Peri-tumoral hyaline material and geographic tumor necrosis were observed. Mitotic rate was 12 per 10 high power fields (HPFs) and Ki 67 labelling index was up to 50%. Immunohistochemically, the tumor cells were positive for β-hCG, human placental lactogen (hPL), inhibin-α, and p63 (Fig. 1D-G). The immunohistochemical staining results are summarized in Table 1. Collectively, the histologic features and the immunohistochemical results were compatible with ETT in the final pathologic diagnosis.
Table 1. Immunohistochemical results of the tumor in our patient.
| Antigen | Tumor |
|---|---|
| β-human chorionic gonadotropin | Positive in some tumor cells |
| Human placental lactogen | Positive in some tumor cells |
| p63 | Positive |
| Ki 67 index | Positive in up to 50% |
| CD 34 antigen | Negative |
| S 100 protein | Negative |
| MDM2 | Perinuclear weakly positive |
| Desmin | Negative |
| HMB 45 | Negative |
| Smooth muscle actin | Negative |
| CK* (AE1/AE3) | Positive |
| Inhibin-α | Positive |
MDM2, mouse double minute 2; HMB45, human melanoma black 45; CK*, cytokeratin.
She received intensive post-operative care in the intensive care unit for 2 days and was discharged 20 days later, uneventfully. Her serum β-hCG level was not checked before surgery because there were no clues based on which we could suspect ETT. After pathologic confirmation, her β-hCG level was checked on the post-operative fifteenth day and the value was 1.3 mIU/mL. Fortunately, even though large multiple masses had been existed in her abdominopelvic cavity before surgery, there was no residual tumor or distant metastasis on CT scan which was done at post-operative seventh days. Considered imaging studies, β-hCG results and ETT's chemoresistant nature, she did not need adjuvant chemotherapy.
Two months after surgery, her β-hCG level was checked at the outpatient clinic and it was 0.6 mIU/mL. Three months later, CT scan of her chest and abdomino-pelvis showed no recurrent or remarkable lesion. Nine months later, there was no evidence of recurrence on serum β-hCG and imaging studies.
Discussion
ETT is a very rare variant of GTD (1.39% to 2% of GTD). Since first described by Shih and Kurman in 1998, only 108 cases have been reported through Medline research to date [1,4,5,6]. In general, ETT presents in women of reproductive age like other types of GTD. However, six cases of ETT were diagnosed in postmenopausal women [4,5,6].
Patients with uterine ETT have various symptoms. The most common symptom is abnormal vaginal bleeding which occurs in 57% to 67% of the patients and others complain of amenorrhea, abdominal pain, and abdominal bloating [4,5,6]. Rarely, patients with isolated or metastatic pulmonary ETT have dyspnea or hemoptysis as the primary symptoms. However, it is sometimes asymptomatic; especially in patients who are diagnosed with extra-uterine ETT [8,9,10]. In our case, patient didn't complain of vaginal bleeding or other gynecologic symptom because she already had hysterectomy. Otherwise, she suffered from abdominal pain because of peritonitis caused by sigmoid fistula.
Mostly, about 71% of ETTs arise in the uterus, especially in the lower segment of the uterus. Besides, ETTs also present as an extra-uterine disease or primary uterine disease with metastasis [4,5,6,8]. Metastasis from ETT occurred in 25% to 42.9% of the reported cases; among these cases, lung was the most common site of extra-uterine ETT (19%) [4,5,6,8,10]. Also, ETTs were identified in the small bowel, vagina, fallopian tube, broad ligament, and gallbladder infrequently [4,6].
Unlike other types of GTD, the β-hCG level in patients with ETT is within the normal range or is slightly elevated. As reported by Palmer et al. [6], 69% of the patients diagnosed with ETT showed relatively lower β-hCG levels of less than 2,500 mIU/mL. Davis et al. [4] reported two cases of normal range β-hCG value at presentation and other two atypical cases of β-hCG level elevated to 100,000 mIU/mL or beyond; however, they presumed that latter two cases of ETT were accompanied with other type of GTD such as choriocarcinoma or molar pregnancy.
To date, the mortality rate for ETT has been reported to be 10% to 24% [2,3,4,6]. Extra-uterine ETT is the widely known, most important poor prognostic factor. Also, time interval greater than four years between antecedent pregnancy, age over forty and mitotic count more than 5 per 10 HPFs are one of the acknowleged poor prognostic factors [4,5,6].
On evaluating antecedent pregnancy, Scott et al. [5] reported that prior term deliveries (43%) were most common, followed by molar pregnancies (39%) and abortions (18%). Furthermore, duration between antecedent pregnancy was varied from two months to thirty years.
Grossly, ETT is a well-circumscribed, solid or cystic mass which is tan-yellow to dark brown in color. Microscopically, it has a monomorphic pattern and it comprises of relatively uniform intermediate trophoblastic cells with abundant eosinophilic or clear cytoplasm. These cells are arranged in sheets, nests, cords, and trabeculae in a background of prominent hyaline material and geographic necrosis and show various mitotic indices, but, mitosis is still scarce in most of the tumors [6,11,12].
Occasionally, these microscopic findings are confused with those of squamous cell carcinoma of the cervix, PSTT, or other type of GTD. In order to make an accurate diagnosis, immunohistochemical staining is essential [7]. Li et al. [11] performed immunohistological examination in seven ETT patients. It showed diffuse strong reactivity for cytokeratin 18 in the tumor cells with a cytoplasmic and membranous pattern. Unlike PSTT, these tumors showed patchy reactivity for hPL, β-hCG, Mel-CAM (CD146), and inhibin-α. Also, nuclear p63 expression was observed. But in some cases, there was only weak cytoplasmic and nuclear staining for p16. Median Ki 67 proliferation index was 15.3% (2.3% to 39.8%). Zhang et al. [12] analyzed 78 cases of ETT and they also showed similar results.
There is no established standard treatment for ETT to date. However, considering its chemo-resistant nature, complete surgical resection is the primary therapeutic option. Therefore, in patients in whom the disease is confined to the uterus, hysterectomy is recommended. Even though extra-uterine or metastatic lesions have been recognized, complete surgical resection has a critical role in cure [4,5,6,8].
Chemotherapy is one of the treatment options for patients who had incomplete surgical resection, metastasis, recurrence of tumor or who has poor prognostic factor as we discussed above. The regimens of chemotherapy are not standardized, but regimens such as EMACO (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine) or MAC (methotrexate, actinomycin, and chlorambucil) were used relatively common [3,4,5,6].
Postoperative surveillance to detect recurrence of ETT remains a challenge in the clinical situation. However, considering its clinical features and laboratory findings, follow-up every three months with monthly serum β-hCG and hPL tests was recommended [4,5,6]. In our cases, she received complete surgical resection and CT scan after operation confirmed it. Therefore, chemotherapy was not performed. However, short term follow up is essential considering poor prognostic factor; 42 year old, duration between antecedent pregnancy and pathologic result (mitotic rate 12 per 10 HPFs, tumor necrosis).
Considering the extremely rare incidence of ETT, we think that our clinical experience can provide more information on its clinical features and prognosis. A prospective randomized study can provide the most powerful evidence for deciding the optimal treatment and determining the diagnostic value. However, in reality, it is very difficult to perform such a large study of extremely rare diseases. In this respect, collaborative multicenter or national data collection is crucial.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Shih IM, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. 1998;22:1393–1403. doi: 10.1097/00000478-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203:531–539. doi: 10.1016/j.ajog.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 3.Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204:11–18. doi: 10.1016/j.ajog.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 4.Davis MR, Howitt BE, Quade BJ, Crum CP, Horowitz NS, Goldstein DP, et al. Epithelioid trophoblastic tumor: a single institution case series at the New England Trophoblastic Disease Center. Gynecol Oncol. 2015;137:456–461. doi: 10.1016/j.ygyno.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Scott EM, Smith AL, Desouki MM, Olawaiye AB. Epithelioid trophoblastic tumor: a case report and review of the literature. Case Rep Obstet Gynecol. 2012;2012:862472. doi: 10.1155/2012/862472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer JE, Macdonald M, Wells M, Hancock BW, Tidy JA. Epithelioid trophoblastic tumor: a review of the literature. J Reprod Med. 2008;53:465–475. [PubMed] [Google Scholar]
- 7.Moutte A, Doret M, Hajri T, Peyron N, Chateau F, Massardier J, et al. Placental site and epithelioid trophoblastic tumours: diagnostic pitfalls. Gynecol Oncol. 2013;128:568–572. doi: 10.1016/j.ygyno.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, An S, Jang SJ, Kim HR. Extrauterine epithelioid trophoblastic tumor of lung in a 35-year-old woman. Korean J Thorac Cardiovasc Surg. 2013;46:471–474. doi: 10.5090/kjtcs.2013.46.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn HY, Hoseok I, Lee CH, Jung YJ, Shin NR, Kim KH, et al. Pulmonary mass diagnosed as extrauterine epithelioid trophoblastic tumor. Thorac Cardiovasc Surg. 2013;61:97–100. doi: 10.1055/s-0032-1331264. [DOI] [PubMed] [Google Scholar]
- 10.Lewin SN, Aghajanian C, Moreira AL, Soslow RA. Extrauterine epithelioid trophoblastic tumors presenting as primary lung carcinomas: morphologic and immunohistochemical features to resolve a diagnostic dilemma. Am J Surg Pathol. 2009;33:1809–1814. doi: 10.1097/PAS.0b013e3181b9cd67. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Shi Y, Wan X, Qian H, Zhou C, Chen X. Epithelioid trophoblastic tumor: a clinicopathological and immunohistochemical study of seven cases. Med Oncol. 2011;28:294–299. doi: 10.1007/s12032-010-9419-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Lu W, Lu B. Epithelioid trophoblastic tumor: an outcome-based literature review of 78 reported cases. Int J Gynecol Cancer. 2013;23:1334–1338. doi: 10.1097/IGC.0b013e31829ea023. [DOI] [PubMed] [Google Scholar]