Abstract
Objective
If bowels and other structures are in the pathway of high-intensity focused ultrasound (HIFU) beam during magnetic resonance image-guided HIFU (MRgFUS) therapy, filling to the bladder and the rectum and then emptying the bladder (i.e., the BRB technique) is used to avoid them. A modified BRB technique might be useful method to using a uterine elevator method or by inducing uterus downward traction to lower the position of the uterus.
Methods
A total of 156 patients who had undergone MRgFUS surgery treatment for uterine fibroids from March 2015 to February 2016 were included in this retrospective study. Of the 156 patients, 40 were treated using a uterine elevator while 29 were treated using downward traction of uterus. HIFU was performed using Philips Achieva 1.5 Tesla MR and Sonalleve HIFU system.
Results
MRgFUS surgery was feasible with modified BRB technique in 69 cases. Using uterine elevator method, the intensity of HIFU for group with antefletxio uteri was significantly lower than that for the group without antefletxio uteri (105.37±17.62 vs. 118.71±26.88 W). The group with downward traction of uterus induced was found to have significantly lower intensity of HIFU compared to the group without downward traction of uterus induced (110.26±22.60 vs. 130.51±27.81 W).
Conclusion
Modified BRB technique was useful in avoiding bowels and other structures located in HIFU beam pathway during MRgFUS treatment to ablate uterine fibroids.
Keywords: BRB, High-intensity focused ultrasound, Magnetic resonance image-guided high-intensity focused ultrasound, Uterine fibroids
Introduction
Magnetic resonance image-guided high-intensity focused ultrasound (MRgFUS) is a newly emerged non-invasive treatment method for uterine fibroid. MRgFUS can be performed as an outpatient procedure without requiring general anesthesia or skin incision. In addition, it can be used to treat large and multiple lesions. Furthermore, it can be performed repeatedly. However, when bowels and other structures are located in the pathway of the high-intensity focused ultrasound (HIFU) beam, some methods have been used to avoid any bowel injury by adjusting beam probe or using water balloon. However, these methods have limits due to angular adjustability and difficulty in consistently modulating ultrasound intensity, resulting in difficulties in treatment [1]. To solve such problem, bladder and rectum filling and bladder emptying (BRB technique) has been used by filling the bladder with saline solution or filling the rectum with glycerin to push the rectum upward to move the bowel out of the beam pathway. Despite those measures, problem still exists when the bowel is located at the anterior of the uterus or remains at the edge of the beam pathway. To solve such problem, we either induced the flexion of uterus forward by using a uterine elevator or induced downward traction of uterus in order to change the location of uterus to avoid bowel or other structures in the beam pathway. We named this method modified BRB technique. The objective of this study was to review the intensity of HIFU, treatment time, non-perfused volume (NPV) ratio, volumetric change in uterine fibroid, change in hemoglobin, and change in symptom severity score (SSS) when uterine elevator was used or when downward traction of uterus was used to determine the clinical effectiveness of the modified BRB technique.
Materials and methods
1. Study population and method
This retrospective study was conducted in a total of 156 patients in the department of obstetrics and gynecology of Hwanmyeong Ilsin Christian Hospital who had undergone MRgFUS-utilized treatment from March 2015 to February 2016 after obtaining approval from the institutional review board of the hospital. Of the 156 patients, 40 were treated by using uterine elevator and 29 were treated by inducing downward traction of uterus.
All procedures of the HIFU treatment were conducted by the HIFU team consisting of four physicians from the department of obstetrics and gynecology, two doctors from the department of radiology, two radiology technicians, and one nurse.
For magnetic resonance imaging (MRI) and the HIFU, the team used Philips Achieva 1.5 Tesla MR (Philips Healthcare, Best, The Netherlands) and Sonalleve HIFU system. Patient information was collected through retrospective analyses based on hospitalization records, out-patient care record, and surgery record. The following patient information was collect: age of patient, history of child birth, Funaki type uterine fibroid and multiple fibroids, pretreatment findings of modified BRB including the number of uterine fibroid, the procedures were done, treatment time of HIFU, whether uterine elevator was used, whether downward uterus traction was induced, the mean output of HIFU, differences of changes in blood test results at pretreatment, 1 month, and 3 months posttreatment, presence/absence of posttreatment complications such as uterine bleeding, pain and skin burn, bowel injury and bladder injury, volumetric changes in the uterine fibroid, and post-treatment symptomatic changes in clinical symptoms.
Uterine fibroids were defined in comparison to skeletal muscle signals in pretreatment T2-weighted image as follows. When image showed low intensity compared to skeletal muscle signals, it was defined as Funaki type I. When image showed higher intensity than skeletal muscle signals but lower than myometrium signals, it was defined as Funaki type II. When image showed equal to or higher than myometrium signals, it was defined as Funaki type III [2].
To evaluate the degree of symptom of the patient, SSS was used. The degrees of symptoms at pretreatment, 1 week, 1 month, and 3 months after treatment were measured [3]. Treatment time was defined as the period from the first performance of HIFU to the last performance of HIFU. Complications were defined as conditions that had occurred due to HIFU therapy as a state that required additional medication or treatment, including uterus bleeding for at least 5 days or longer, fever over 3 days, problem of the urogenital system such as hematuria, intestinal damage, nerve damage, and skin burns. Transient hematuria and degree 1 skin burns less than 2 cm were excluded.
2. Surgical procedures
All patients visited the out-patient department after maintaining eight hours of fasting. They were prepared with general arrangements for surgery. The patient was asked to take posterior lithotomy to expand the cervix. A uterine manipulator (Koh Colpotomizer, Colpo-Pneumo Occluder, RUMI; Cooper Surgical, Trumbull, CT, USA) was then inserted and the position of uterus was made to flexion. The cervix was sutured by using Vicryl 1-0 in the method of figure of eight followed by the preparation for downward traction of uterus. Later, the patient was moved to a MRI room and asked to lie on the MRI bed in prone position. A HIFU coil was installed and MRI scanning was done with adjustment to obtain the exact focus location.
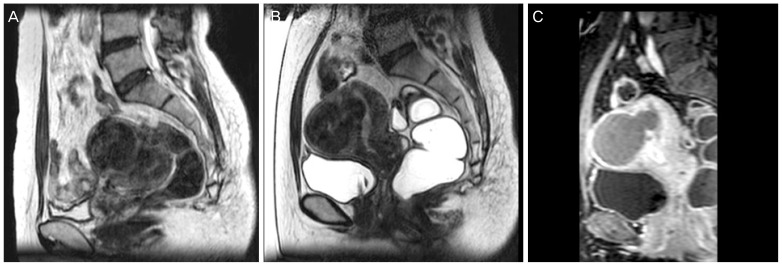
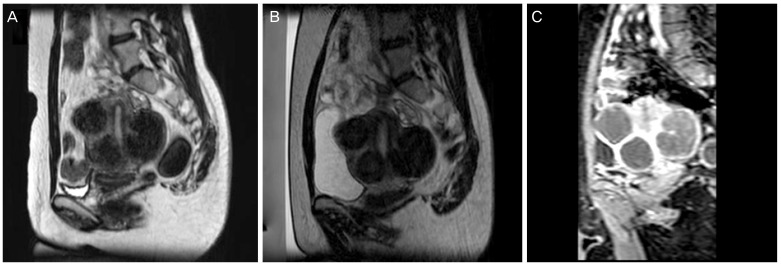
After the first MRI scanning immediately before the treatment, if bowel was found to be located in front of uterus, BRB or modified BRB technique was used to avoid damages to the bowel induced by high-intensity ultrasound, including the following: filling the rectum with glycerin to change bowel position, filling or emptying the bladder with saline, induce uterine flexion by using a uterine elevator, and induce downward traction of the uterus by pulling the Vicryl linked to the cervix (Figs. 1, 2).
Fig. 1. Modified BRB technique using with a uterine elevator. (A) Uterus was state of retroversion, bowel was located anterior of uterine fibroid, and uterine fibroid was located at the posterior state in the pelvis. (B) Uterine fibroid was located at anterior state than previous state after using uterine elevator. (C) Non-perfused volume was seen on contrast enhanced T1-weighted image after high-intensity focused ultrasound.
Fig. 2. Modified BRB technique inducing downward traction of the uterus. (A) Bowel was located anterior of uterine fibroid. It was located in the pathway of high intensity ultrasound beam. (B) The bowel was located upward than previous state after filling the bladder and downward traction of uterus. (C) Non-perfused volume was seen on contrast enhanced T1-weighted image after high-intensity focused ultrasound.
Fentanyl (Fentanyl injection 500 µg/10 mL) and ketorolac (Keromin injection 30 mg/1 mL) were injected through an infusion pump to control pain for patients. When the operating time became longer than 60 minutes, an epidural catheter was inserted. Fentanyl and bupivacaine (Bupivacaine MYM injection 50 mg/20 mL) were administered to control the pain.
For safety, emergency stop button was placed in the hands of the patient. HIFU therapy under real-time MRI scan was done subsequently. The HIFU therapy was carried out under quasi-real time MRI in conjunction with Philips Achieva 1.5 Tesla MR Sonalleve HIFU system of the hospital. With a volumetric heating algorithm, HIFU-induced temperature changes of the focus area and the temperature ripple effects to the adjacent organs were observed by thermometry. To maintain the temperature of focal area at 57℃ for more than 1 second and to increase over 240 equivalent minutes (EM, thermal dose of 240 EM is a power that causes irreversible coagulative necrosis), the hospital applied various target cell sizes and minimized skin burns by using the minimal output power that could reach the temperature of the focal area.
Pretreatment T2-, T1-weighted MRI, and contrast enhanced T1-weighted MRI were taken for all patients. In addition, all patients underwent contrast enhanced T1-weighted MRI immediately after treatment. The MRI scanning was ended after confirming NPV.
3. Statistical analyses
PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses of study results. Chi-square test and t-independent test were used. When significance P-value was less than 0.05, it was considered as statistically significant.
Results
A total of 156 patients underwent MRgFUS with the attempt to ablate uterine fibroids. Of the 156 patients, 40 underwent MRgFUS with uterine elevator (Table 1). Using uterine elevator method, the intensity of HIFU for group with antefletxio uteri was significantly lower than that for the group without antefletxio uteri (105.37±17.62 vs. 118.71±26.88 W). However, no correlation was found among treatment time, NPV ratio, change in SSS, volumetric changes in uterine, or presence/absence of complications (Fig. 3).
Table 1. Clinical characteristics of 40 patients who underwent MRgFUS by using uterine elevator.
| Clinical characteristics | Use | Not use |
|---|---|---|
| Number | 40 | 116 |
| Age of patients (yr) | 41.1±5.5 | 42.4±6.4 |
| Parity | 1.3±0.5 | 1.4±0.5 |
| No. of fibroids | 4.5±3.7 | 4.1±3.1 |
| Size of fibroids (longest diameter, cm) | 6.8±2.3 | 7.5±2.7 |
| Volume of fibroids (cm3) | 140.8±133.2 | 186.4±226.7 |
| Sonication power (W) | 105.4±17.6 | 118.7±26.9* |
| Treatment time (min) | 94.6±62.2 | 105.0±54.0 |
| NPV ratio | 67.9±21.6 | 64.8±23.1 |
| SSS before MRgFUS | 26.9±4.7 | 27.3±4.8 |
| Ratio of SSS change after MRgFUS in 1 month later | 0.7±0.1 | 0.7±0.1 |
| Ratio of SSS change after MRgFUS in 3 months later | 0.4±0.2 | 0.5±0.2 |
| Volume change of fibroids after MRgFUS (%) | ||
| Ratio of volume changes in 1 month later | 48.4±24.6 | 39.6±20.3* |
| Ratio of volume changes in 3 months later | 39.3±19.0 | 34.1±23.6 |
| Hb before MRgFUS | 11.9±1.7 | 12.0±2.0 |
| Hb change after MRgFUS in 1 month later | 0.1±1.1 | 0.5±2.0 |
| Hb change after MRgFUS in 3 months later | 0.3±4.9 | 0.6±1.4 |
| Complication(%) | 6 (15.0) | 14 (17.2) |
| Re-intervention(%) | 2 (5.0) | 11 (9.5) |
Values are presented as mean±standard deviation or number (%); Chi-square test and t-independent test were used.
MRgFUS, magnetic resonance image-guided high-intensity focused ultrasound; NPV, non-perfused volume; SSS, symptom severity score; Hb, hemoglobin.
*P<0.05.
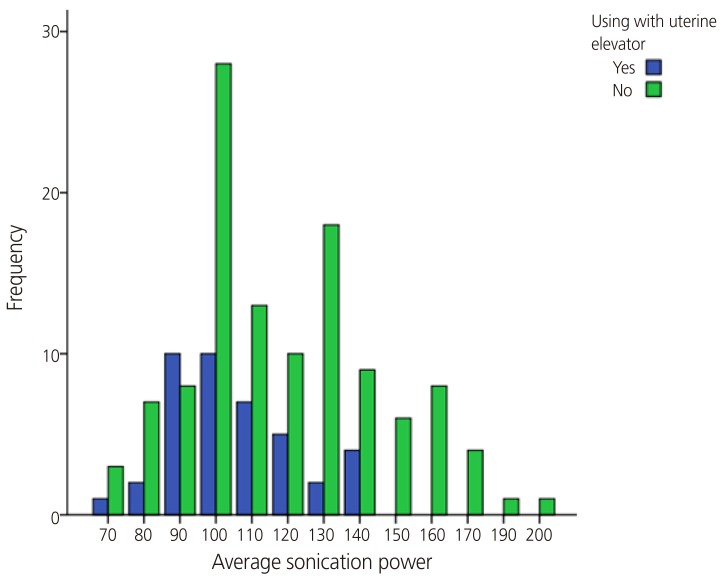
Fig. 3. Correlation between average sonication power, treatment time, and using uterine elevator. Spearman correlation coefficient were 0.224 between the average sonication power and using of uterine elevator, 0.106 between the treatment time and using of uterine elevator in magnetic resonance image-guided high-intensity focused ultrasound; relationship with the average sonication power was only statistically significant.
The shorter the reaching depth of the HIFU, the more the intensity of ultrasound was reinforced. Since fibroids were located at the anterior in the intraperitoneal cavity when uterine flexion was induced, even if the intensity of HIFU was set low, the temperature might have reached target temperature sufficiently.
Of the 156 patients, downward traction of uterus was induced in 29 cases. The group with downward traction of uterus induced was found to have significantly lower intensity of HIFU compared to the group without downward traction of uterus induced (110.26±22.60 vs. 130.51±27.81 W). In addition, the treatment time for the group with downward traction of uterus induced was significantly shorter than that for the group without downward traction of uterus induced (94.68±54.53 vs. 127.76±53.63 min) (Table 2, Fig. 4).
Table 2. Clinical characteristics of 29 patients who underwent MRgFUS by inducing downward traction of uterus.
| Clinical characteristics | Use | Not use |
|---|---|---|
| Number | 29 | 127 |
| Age of patients | 42.1±6.5 | 42.0±5.2 |
| Parity | 1.4±0.5 | 1.4±0.5 |
| No. of fibroids | 4.2±3.4 | 4.3±3.1 |
| Size of fibroids (longest diameter, cm) | 7.4±2.7 | 6.9±2.3 |
| Volume of fibroids (cm3) | 186.9±225.5 | 141.3±135.8 |
| Sonication power (W) | 110.3±22.6 | 130.5±27.8* |
| Treatment time (min) | 94.7±54.5 | 127.8±53.6* |
| NPV ratio | 67.3±21.6 | 59.9±25.3 |
| SSS before MRgFUS | 27.5± 5.0 | 26.5±3.8 |
| Ratio of SSS change after MRgFUS in 1 month later | 0.7±0.1 | 0.7±0.4 |
| Ratio of SSS change after MRgFUS in 3 months later | 0.4±0.2 | 0.5±0.2* |
| Volume change of fibroids after MRgFUS (%) | ||
| Ratio of volume changes in 1 month later | 43.1±22.2 | 38.8±21.2 |
| Ratio of volume changes in 3 months later | 38.6±22.6 | 28.5±21.6 |
| Hb before MRgFUS | 12.0±1.9 | 11.9±2.0 |
| Hb change after MRgFUS in 1 month later | 0.6±1.9 | 0.7±1.7 |
| Hb change after MRgFUS in 3 months later | 0.7±2.0 | 0.2±1.8 |
| Complication (%) | 3 (10.3) | 17 (13.4) |
| Re-intervention (%) | 10 (34.5) | 3 (2.4) |
Values are presented as mean±standard deviation or number (%); Chi-square test and t-independent test were used.
MRgFUS, magnetic resonance image-guided high-intensity focused ultrasound; NPV, non-perfused volume; SSS, symptom severity score; Hb, hemoglobin.
*P<0.05.
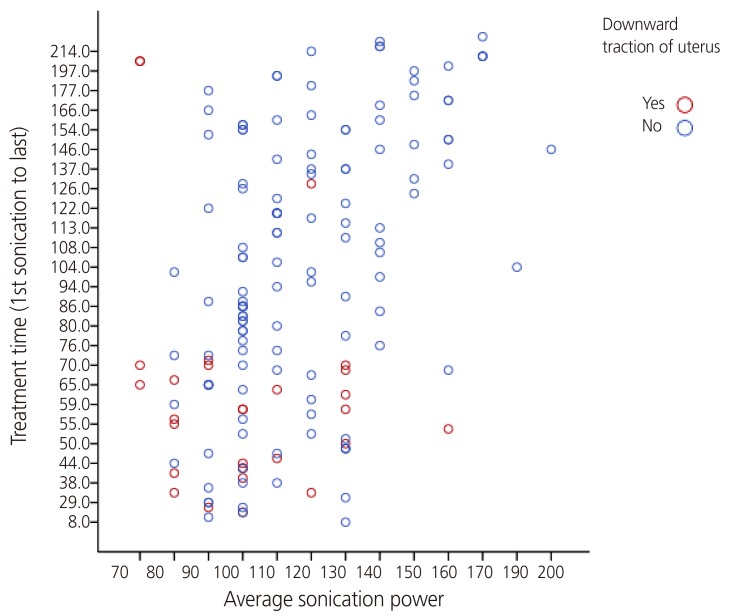
Fig. 4. Correlation between average sonication power, treatment time, and downward traction of uterus. Spearman correlation coefficient were 0.272 between the average sonication power and downward traction of uterus, 0.375 between the treatment time and downward traction of uterus in magnetic resonance image-guided high-intensity focused ultrasound; both were statistically significant.
The NPV ratio of the group with downward traction of uterus induced was higher than that of the group without the induction of downward traction of uterus (67.30±21.59 vs. 59.87±25.27). However, the difference was not significant. The SSS at 3 months after treatment was found to be significantly lower in the group with downward traction of uterus induced compared to that in the group without downward traction of uterus induced. It is possible that the downward traction of the uterus might have increased the effectiveness of treatment and induced improvement of symptoms in a more efficient manner. There was no significant difference in hemoglobin, volumetric change in uterine fibroid, presence/absence of complications, or presence/absence of re-intervention between the two groups.
By moving the fibroids from posterior side to the center within the abdominal cavity through uterine traction, it is possible to set the intensity of HIFU to be low so that bowel located in front of fibroids can be easily avoided, resulting in reduction of operation time. Our results also confirmed that higher NPV ratio indicated high level of treatment effectiveness and more improvement of symptoms.
There was only 1 case that both uterine elevator and downward traction of uterus were performed simultaneously. Majority (88 cases, 68.8%) did not need them (Table 3). This indicated that in most cases, if not all, bowel located in front of the uterus could be avoided by using BRB method. However, for 40 cases (26.1%), it was impossible to avoid the bowels located at the HIFU beam pathway by using the existing BRB method. For them, it was possible to avoid the bowels either by uterine flexion using uterine elevator or by inducing downward traction of uterus.
Table 3. Relationship between inducing downward traction of uterus and using uterine elevator.
| Use with uterine elevatora) | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| Downward traction of uterus | Yes | 1 (3.4) | 28 (96.7) | 29 |
| No | 39 (31.3) | 88 (68.8) | 127 | |
| Total | 40 (26.1) | 116 (73.9) | 156 | |
Values are presented as number (%) or number; Chi-square test was used.
a)P-value 0.002, Pearson R −0.246 (0.002), Spearman correlation −0.246 (0.002).
Discussion
Recently, HIFU has been introduced as a non-invasive treatment for uterine fibroids. It has been attempted in various ways. HIFU has been used as a therapeutic heat treatment to heat the focal area so that the temperature can reach 57℃ to 80℃ to induce coagulation cell necrosis. It does not require anesthesia or hospitalized treatment. It does not require skin incision either. It can be used to treat large or multiple lesions too. In addition, it can be performed repeatedly [4]. Unlike ultrasound image-guided ultrasound image-guided HIFU, MRgFUS can monitor the ripple effects of heat through thermometry during the treatment, which enables us to predict and avoid any damage to surrounding organs more accurately. In addition, we can confirm the therapeutic effectiveness from MRI scanning immediately after the treatment. However, MRgFUS also has it disadvantage. Since it is a quasi-real time, in cases when ultrasound guidance thermometry is not allowed, it is difficult to observe adjacent organs or monitor deep areas. However, its main advantage is that the treatment can be performed in a relatively short time [5]. MRgFUS has been reported to effective treatment for ablating uterine fibroids in several studies. Gizzo et al. [6] have reported that the NPV ratio after MRgFUS treatment of the uterine fibroids is 16.3% to 98% and the volume of uterine fibroids is decreased by 19% to 80%. The more NPV ratio is increased, the more the volume of uterine fibroids is reduced. They have also reported that when uterine fibroids have over 80% of ablation in 3 months, the volume of uterine fibroids is reduced by 50% to 60% and 30% to 40% at 3 and 12 months, respectively. In addition, the improvement state of symptoms has been measured by them using uterine fibroids symptom and health-related quality of life questionnaire. As results, the symptoms are improved from 56.3 down to 31.0 at six months after the treatment. In addition, the higher the NPV ratio, the more improvement in the symptoms. When NPV ratio is increased more than symptom improvement, we report them as a visible improvement. Therefore, there is a significant correlation between symptom improvement and volumetric reduction after the treatment.
Yoon et al. [7] have reported that percentage of symptomatic improvement is 83% at three months and 90% at 6 months with NPV of 42.1±17.6% based on a study conducted in 29 patients in 2008. Morita et al. [8] have also reported a reduction of 33% of the volume of uterine fibroids after observing 48 patients who underwent HIFU therapy for six months. They have also reported a significant correlation between increased NPV ratio and reduction of fibroids volume immediately after treatment.
After a 24-month observation study, Funaki et al. [9] have also reported that the NPV ratio is increased more than 50%. They found that patients with uterine fibroids of Funaki type I and II showed significant volumetric reduction (a total of 36.5%) during the first six months after MRgFUS treatment. When observed for 14 months, the volumetric reduction was a total of 39.5%. In the case of Funaki type III, uterine fibroids were reported to not show any significantly decrease during the first six months (9.1%). Afterward, they were reduced little by little for 24 months. However, after the HIFU therapy, significant reduction in SSS was found in all patients in the first three months with improved symptoms.
Regardless of MRgFUS or ultrasound image-guided HIFU, if bowels or other structures are in the beam pathway of HIFU, different methods such as beam probe angle adjustment or pushing up the bowels using water balloon or BRB technique are used. BRB technique is a useful method to avoid bowels in the beam pathway by filling the bladder with saline solution or by filling the rectum with glycerin and pushing the bowel upward to move the bowel out of the beam pathway. However, despite those measures, problem still exists if the bowel is located at the anterior of uterus or if it remains at the edge of the beam pathway. Thus, it is important to have guidelines for patient selection. When fibroid ablation is performed by using MRgFUS, the guideline on patient selection proposed by Yoon et al. [10] has commented that the following cases are difficult to treat: sub serosal type fibroid, fibroid greater than 10 cm, fibroid with distance from the skin greater than 12 cm, six or more multiple fibroids, any obstacle such as bowel or bone or air-containing tissue at HIFU path, and high vascularity of the uterine fibroids. That guideline also indicates that treatment is difficult for irregular shape of rectal muscle with thick subcutaneous fat because they may cause beam aberration. In addition, it says that diffuse adenomyosis is difficult to treat and the possibility of malignancy should be ruled out. When deciding whether the contrast agent should be increased or decreased before MRgFUS treatment, if necrosis of uterine fibroids is progressed, the therapeutic effect will be reduced. We had overcome those cases mentioned in the above guidelines such as fibroid with distance from the skin greater than 12 cm and any obstacle such as bowel or bone or air-containing tissue at HIFU path by using the modified BRB technique.
When uterine elevator was used and uterine flexion was performed, the distance to the skin was reduced, making the intensity of the ultrasound more reinforced. The intensity of HIFU was found to be significantly low, even when downward traction of uterus was induced, the intensity of HIFU and treatment time were found to be significantly lower.
If there are bowels or other structures in the beam pathway of HIFU in MRgFUS therapy, BRB technique is used to avoid them by filling the bladder with saline solution or filling the rectum with glycerin. In this study, when bowel or other structures were found in the beam pathway of HIFU therapy as an emerging MRgFUS treatment, BRB technique was commonly used. However, when such method could not solve the problem, we had used uterine elevator or downward traction of uterus to change the locations of bowels and other structures. As a result, MRgFUS was feasible. In addition, the intensity from the HIFU therapy was lower and the treatment time was reduced. Therefore, the modified BRB method is considered to be a useful method in feasibility of MRgFUS. However, it should be confirmed furthermore through prospective studies that limits dependent variables by doctor, age, birth history, type of fibroids, and size of fibroid. In addition, further study with MRgFUS in the form of volumetric ablation on its therapeutic effectives against adenomyosis is needed. When conducting MRgFUS in women with child bearing potential, additional studies on injury to the endometrium and other factors that might have impact on infertility are merited.
Acknowledgments
This article was supported by 2017 PNUH clinical research fundation.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Zhang L, Chen WZ, Liu YJ, Hu X, Zhou K, Chen L, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73:396–403. doi: 10.1016/j.ejrad.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–184.e6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 4.Hill CR, Rivens I, Vaughan MG, ter Haar GR. Lesion development in focused ultrasound surgery: a general model. Ultrasound Med Biol. 1994;20:259–269. doi: 10.1016/0301-5629(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 5.Park MJ, Kim YS, Keserci B, Rhim H, Lim HK. Volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids: treatment speed and factors influencing speed. Eur Radiol. 2013;23:943–950. doi: 10.1007/s00330-012-2665-1. [DOI] [PubMed] [Google Scholar]
- 6.Gizzo S, Saccardi C, Patrelli TS, Ancona E, Noventa M, Fagherazzi S, et al. Magnetic resonance-guided focused ultrasound myomectomy: safety, efficacy, subsequent fertility and quality-of-life improvements, a systematic review. Reprod Sci. 2014;21:465–476. doi: 10.1177/1933719113497289. [DOI] [PubMed] [Google Scholar]
- 7.Yoon SW, Kim KA, Hwang YY, Lee C, Cha SH, Lee SY, et al. Magnetic resonance imaging-guided focused ultrasound surgery for uterine fibroids: initial experience in Korea. Korean J Obstet Gynecol. 2008;51:982–987. [Google Scholar]
- 8.Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids: early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139:199–203. doi: 10.1016/j.ejogrb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SW, Lee C, Cha SH, Yu JS, Na YJ, Kim KA, et al. Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol. 2008;18:2997–3006. doi: 10.1007/s00330-008-1086-7. [DOI] [PubMed] [Google Scholar]