Introduction
There are three important reasons why immune cells enter the mammary gland: to effect general immune surveillance, to participate in post-pubertal mammary gland development and involution, and to contribute immune cells to milk. Our focus is on the latter two mammary-specific processes.
At birth in the mouse, the mammary gland consists of a rudimentary ductal tree. This epithelial tree then expands to reach the outer limits of the mammary fat pad during puberty and young adulthood in response to hormonal signals. Branching morphogenesis of an epithelial tree within the fat pad requires epithelial cell proliferation and apoptosis, and remodeling of the surrounding stroma to allow ductal invasion and expansion [1]. Cells of both the innate and adaptive branches of the immune system have been implicated or proven to play important roles in normal mammary gland development and involution [2,3]. This is most dramatically illustrated by postnatal ablation of hematopoietic cells, which results in a block of mammary development that can be rescued by bone marrow transplantation [4]. More specifically, macrophages, eosinophils and mast cells are crucial to the formation of a branched ductal tree [5,6,2], but are also important players during further development in pregnancy and during involution [6,7]. A specific subset of T cells, in collaboration with antigen-presenting cells, is also a negative regulator of postnatal mammary gland organogenesis [3].
Van Leewenhoek [8] was the first to document the presence of cells in milk. Later, using histological criteria, milk cells were determined to be a combination of epithelial and immune cells [9,10]. In one study of healthy women, the proportions of immune cell types present in milk during the first four days of lactation were determined to be 30-47% macrophages, 40-60% neutrophils, and 5.2-8.9% lymphocytes [11]. In another study, the lymphocytes were further analyzed and 83% were identified as T cells and 6% as B cells [12]. The functional roles attributed to milk immune cells include protection of the mammary gland during lactation when the ducts are open, and protection of the neonate's gastrointestinal tract [13]. The microbicidal and phagocytic properties of macrophages and neutrophils are appropriate to these functions [14,15,16,17], but the role of lymphocytes, given the transit time within the gastrointestinal tract, has been less clear. More recently, evidence that some milk immune cells can pass from the milk across the wall of the gastrointestinal tract and into the neonate has accumulated. This occurs in a variety of species, including rats [18,19], sheep [18,20], pigs [21], baboons [22], and mice [23,24,25]. While many of these studies used concentrated cells and delivery by gavage, others have demonstrated the movement of cells into the neonate under entirely physiological conditions, and gone on to demonstrate that these cells affected subsequent immune responses in the recipients when adult [25,26]. A given milk immune cell, therefore, may contribute to maternal breast health or gastrointestinal or general immune health in offspring.
Even as late as the fourth month of lactation, milk from healthy women has a higher concentration of activated and memory T cells than is present in the general circulation [12,27]. It appears, therefore, that movement of at least these cells into milk is an active and specific process. Immune cells are recruited to the mammary gland from the circulation or a local lymphnode. For mammary gland organogenesis, they are recruited to stromal regions proximal to the developing structures [2], whereas those contributing to the production of a lumen or entering the milk must also cross the ductal basement membrane, move around the processes of myoepithelial cells, and then traverse the luminal epithelial cell layer. In initiating this study, we hypothesized that the luminal epithelial cells would be responsible for production of significant quantities of immune cell chemoattractants.
A related, but distinct, phenomenon is the attraction of IgA-producing cells into mammary tissue. These cells situate themselves close to alveolar epithelial cells in order for IgA to be placed into milk, but do not themselves travel into milk [28,29]. Using in vivo antibody blockade and gene knockout of the chemokine, CCL28, and its receptor, CCR10, this ligand-receptor pair has been shown to be important to recruitment of IgA-producing cells [30,31,32]. Still other antibody blockade studies have demonstrated that vascular adhesion molecule 1, and α4, but not other, integrins are important to IgA+ cell movement into mammary tissue during lactation, although these molecules are thought to be primarily important to efficient exit from the circulation [33].
Prolactin is the most important orchestrator of mammary gland development during pregnancy. Development during pregnancy involves additional ductal branching and the production of lobuloalveoli [34], both of which involve extensive tissue remodeling and therefore immune cells [2]. In mice and a number of other species, prolactin's influences include regulation of ovarian progesterone production as well as direct effects on mammary epithelial cells [35]. The direct effects of prolactin on mammary epithelium are perhaps best illustrated by transplantation studies of prolactin receptor null epithelium into the mammary fat pads of normal mice [36]. The transplanted prolactin receptor null epithelium forms a relatively normal ductal tree that fills the fat pad, but fails to develop further during pregnancy. Prolactin is also a crucial hormone for lactation in most species, responsible for stimulating the production of milk proteins, lipids and carbohydrates by the luminal epithelial cells, and for the movement of large volumes of fluid and ions into the milk [37,38]. Given prolactin's importance in mammary gland development and so many aspects of the production of milk, we hypothesized that prolactin was important to the movement of immune cells into the mammary gland and into milk. Certainly, for the related movement of IgA-secreting cells towards mammary epithelium, prolactin seems to play a role [28]. In addition, there is also some evidence that prolactin may be involved in T cell movement into the mammary gland. Thus, in animals with partial deletion of the desmoglein 4 gene that are secondarily deficient in prolactin, fewer T cells enter the gland during early lactation [39]. However, while generally supportive of a potential role for prolactin, the T cell effect in these animals may be secondary to impaired lactation or even a function related to faulty desmoglein 4.
In the current study, we show 1) that prolactin administration to adult female mice increased immune cell flux through the gland, 2) that mammary epithelial cells produce chemoattractants for multiple immune cell types that are important to gland development as well as those that are found in milk, and 3) that for two identified chemoattractants, prolactin increased their expression in mammary epithelial cells, and their depletion from conditioned medium reduced migration of normally responsive immune cells.
Materials and Methods
Compliance with Ethical Standards
The authors have no conflict of interest regarding any of the work presented in this manuscript. All animal procedures were approved by the University of California, Riverside, institutional animal care and use committee and were in accordance with guidelines from the American Association for Laboratory Animal Care, the United States Department of Agriculture, and the National Institutes of Health.
In vivo continuous prolactin treatment
Eight week-old C57BL6/J female mice were obtained from Jackson Laboratories. Animals were housed in a specific pathogen-free facility and were exposed to a 12-h light/dark cycle. Osmotic mini pumps (Alzet, Cupertino CA) containing prolactin or Dulbecco's phosphate buffered saline (DPBS) as vehicle control were implanted subcutaneously. Treatment was for 28 days and 2.4 μg of prolactin (prepared as previously described [40] was released per day. This dose results in circulating levels of about 25 ng/mL [41].
Vaginal Smears
Vaginal smears were collected daily for the last two weeks of treatment to determine the pattern of estrous cycling for each animal. Smears were stained with 1% Toluidine blue, washed with deionized water, and left to air dry overnight. Estrous cycle stage was determined based on cell types present, as described by Caligioni [42]. Briefly, smears were classified as proestrus if they contained predominantly nucleated epithelial cells, as estrus if they predominantly contained cornified squamous epithelial cells, and as diestrus if they predominantly contained leukocytes. A combination of many leukocytes in the presence of both nucleated and cornified squamous epithelial cells was classified as metestrus.
HC11 cell culture and in vitro prolactin treatment
HC11 cells were a gift from Nancy Hynes (Friedrich Miescher Institutes, Basel, Switzerland). Cultures were maintained in growth medium (RPMI 1640, 10% FBS, 5 μg/mL insulin, 10 ng/mL epidermal growth factor, 100 Units/mL penicillin, 100 μg/mL streptomycin). HC11 cells were plated in growth medium for experiments at 1 × 106 cells/well in a 6 well plate. Cell numbers were determined by hemocytometer counts. Forty eight hours after plating, medium was changed to initial differentiation medium (RPMI 1640, 10% charcoal-stripped horse serum, 10 μg/mL Insulin, 1 μg/mL hydrocortisone, 100 Units/mL penicillin, 100 μg/mL streptomycin). Twenty-four hours later, medium was changed to initial differentiation medium with or without prolactin (100ng/mL). Controls received an equivalent volume of diluent (DPBS). Forty-eight hours later, medium was removed and cells were washed in the same medium lacking serum and then incubated for an additional 24h for a total of 72h with or without prolactin, the last 24 h of which was in the absence of serum. At the end of the 24h incubation, the now conditioned medium was collected for Transwell™ experiments. In some experiments, cells were collected for RNA extraction.
Chemokine Depletion of Conditioned Media
Conditioned medium was depleted of CXCL1 or CCL2 using antibodies previously loaded onto protein A/G Plus-Agarose beads (Santa Cruz Biotechnology, Dallas TX) at a ratio of 10 μL beads to 1μg antibody. Beads were washed before and after antibody loading. Antibodies were purified anti-CXCL1 (R and D systems MAB453, Minneapolis MN) used at 2 μg/mL conditioned medium, anti-CCL2 (R and D systems AF-479) at 5 μg/mL, Rat IgG2a isotype control (R and D systems MAB006) at 2 μg/mL, or goat IgG (R and D systems AB-108) at 5 μg/mL. Incubation with conditioned medium was on a rocking platform overnight at 4°C, after which the beads were pelleted and the depleted conditioned medium was collected and used for downstream applications.
RNA extraction and Reverse transcription PCR
RNA was extracted from cells with Trizol™/chloroform (Life Technologies, Carlsbad CA/Sigma Aldrich, St. Louis MO) separation and further purification with columns (Qiagen, Valencia CA) as per manufacturer recommended protocol. RNase-Free DNase (Qiagen) treatments were used to eliminate any residual genomic DNA. RNA was quantified by nanodrop and equivalent amounts of RNA were reverse transcribed using Oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA), M-MLV Reverse Transcriptase (Invitrogen), RNase OUT (Invitrogen), and 10 mM DNTP mix (Invitrogen) for 1 h at 37 °C. Gene expression was analyzed by Quantitative PCR and Classical PCR using 2× SYBR Green (Biorad, Hercules CA) or 2× Taq Mastermix (Bioland Scientific, Paramount CA) and primers for GAPDH, CCL2, and CXCL1. Gene expression for CCL2 and CXCL1 was normalized to GAPDH. Primer sequences were: GAPDH (forward)-TGCACCACCAACTGCTTAG, (reverse)- GGATGCAGGGATGATGTTC, CCL2 (forward)- AGTTAACGCCCCACTCACCT (reverse)-GAGCTTGGTGACAAAAACTACAGC, CXCL1 (forward)-ACCCAAACCGAAGTCATAGCC (reverse)- TTGTCAGAAGCCAGCGTTCA. Quantitative PCR was performed with Initialization at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C 10 sec and annealing 30 sec. After each run, a melt curve was run to assess appropriately amplified products. For classical PCR, the initialization step was set at 94°C for 5 min, followed by 30 cycles of denaturation at 95°C 30 sec, annealing at 55°C 30 sec, and extension at 72°C 30 sec, and a final elongation at 72°C for 7 min. Products were electrophoresed on a 2% agarose, ethidium bromide gel and imaged on an ultraviolet light box. Relative band intensity was measured using Image J software.
Transwell™ experiments
Serum-free conditioned medium from HC11 cells treated with DPBS or prolactin was collected and aliquoted into the bottom well of 5 μm pore size Transwells™ (Corning, Corning CA). Spleens were collected from adult female C57BL6/J mice. Cells were counted using a Scepter™ (EMD Millipore, Hayward CA), using the 40 μM sensor. 1.5 × 106 splenocytes in 200 μL RPMI1640 were loaded onto the tops of Transwells™. Plates were covered and incubated at 37 °C with 5% CO2. After 2 h, Transwells™ were carefully removed and samples in the bottom well were mixed by pipetting, after which 400 μL was utilized for staining with fluorescently-labeled antibodies to assess cell type by flow cytometry and 200 μL was aliquoted into tubes containing 10 μL of fluorescent count beads (Polysciences, Warrington PA). After mixing, the sample/bead suspensions were run through the flow cytometer and 5,000 gated bead events were recorded for each. The flow cytometry standard files were then analyzed by gating on immune cells and the total number of gated cell events was calculated for each sample. This value provides a rapid, standardized means to quantify the number of cells that migrated. Percentage of the cell type of interest present in input that crossed into the bottom well was calculated and values were then normalized to the non-conditioned medium group given a value of 1.
Flow cytometric analysis
All antibodies were diluted in 0.2% BSA in DPBS (FACS Buffer). Fc receptors were blocked with purified anti-CD16/32 (eBioscience 14-0161, San Diego CA) at a dilution 1:50 for 15 min at 4 °C. Cells were washed with FACS buffer, and resuspended in a mixture of fluorescently-labeled antibodies at the following dilutions: 1:100 rat anti-mouse CD3 (eBioscience 11-0031), 0.5:100 rat anti-mouse CD4 (eBioscience 47-0041), 0.5:100 rat anti-mouse CD8a (eBioscience 17-0081), 0.5:100 rat anti-human/mouse CD11b (Tonbo Biosciences 11-0112, San Diego CA), 0.5:100 rat anti-mouse CD19 (eBioscience 25-0193), 0.5:100 rat anti-mouse CD44 (eBioscience 25-0441), 0.25:100 rat anti-mouse CD45 (eBioscience 11-0451), 1:100 rat anti-mouse CD127 (eBioscience 12-1271), 1:100 rat anti-mouse F4/80 (eBioscience 50-4801), 0.3:100 rat anti-mouse Ly6c (eBioscience 12-5932), 0.25:100 rat anti-mouse Ly6g (eBioscience 45-5931), and 1:100 rat anti-mouse Siglec F (BD Biosciences 562680, San Jose CA), or appropriate isotype controls for 1 h at 4 °C in the dark. Cells were washed and re-suspended in FACS buffer and run live on the BD Facs Canto II. FLOJO software was used for analysis. Representative dot plots for the gating strategy used to define the various cell types discussed in this manuscript are provided in Fig. 1.
Fig. 1. Representative dot plots of the gating strategy used for cell types discussed in this manuscript.
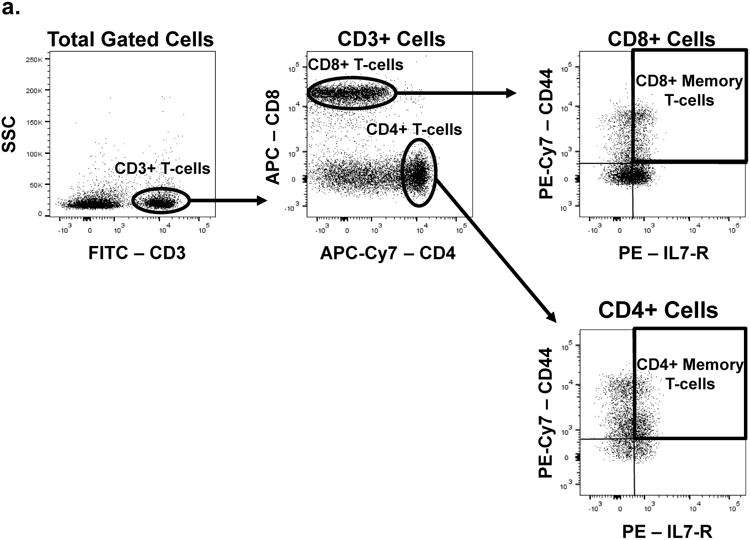
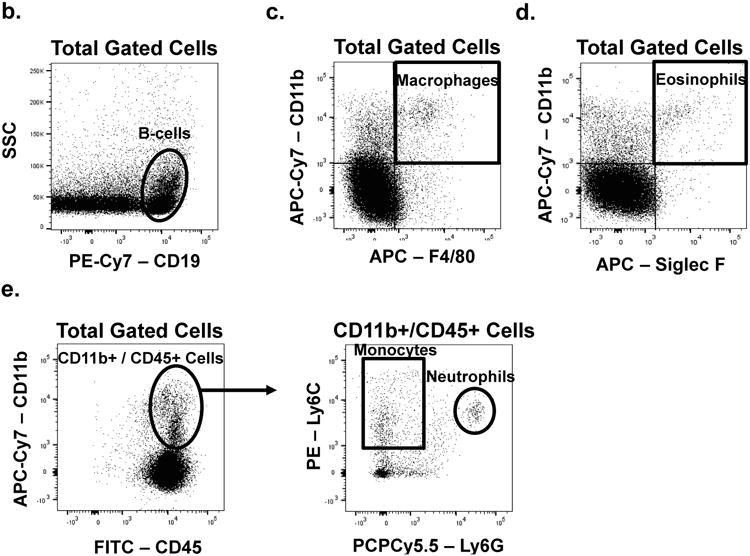
Gates were determined on the basis of isotype control staining.
Statistical Analyses
All statistical analyses were performed utilizing the Mann-Whitney test. Data are presented as mean ± standard error. A p-value of less than 0.05 was considered significant and the levels of significance are designated by asterisks as follows; * P< 0.05, * * P< 0.01, * * * P< 0.0001.
Results
Effects of Administered Prolactin on the Estrous Cycle
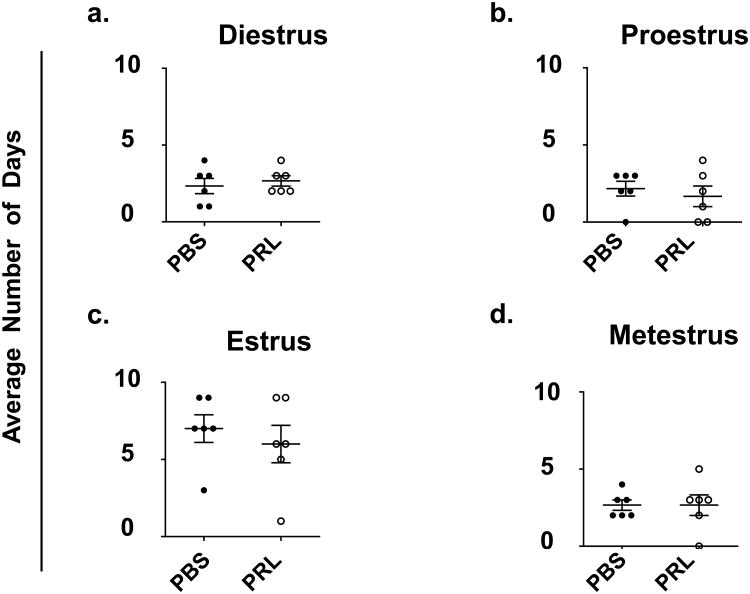
Mice were treated with prolactin for 28 days in order to mimic a duration of exposure equivalent to prolactin and placental lactogens during pregnancy plus the first week of lactation. Mice continuously treated with prolactin enter a pseudopregnant state for two weeks and subsequently return to normal cycling [43,44]. To determine whether prolactin treatment caused any abnormal estrous cycling for the last 2 weeks of treatment (i.e. after an expected return to normal cycling), we assessed the number of days the mice were in proestrus, estrus, metestrus, and diestrus. No difference in cycling during the last 2 weeks of treatment was observed in the prolactin- versus control-treated animals (Fig.2).
Fig. 2. After 14 days, estrous cycling returns to normal in prolactin-treated animals.
Vaginal smears were collected every morning between 8 and 10 am for the last two weeks of a 28-day treatment. Estrous cycle stage was determined on the basis of ratios of cell types present. Data are presented as the average number of days in the 14 day period that mice in each group (n = 6 per group) experienced (A) Diestrus (B) Proestrus (C) Estrus (D) Metestrus. * P < 0.05, ** P < 0.01, and *** P < 0.0001
Prolactin treatment increased lymphocyte numbers in mammary gland-draining lymph nodes
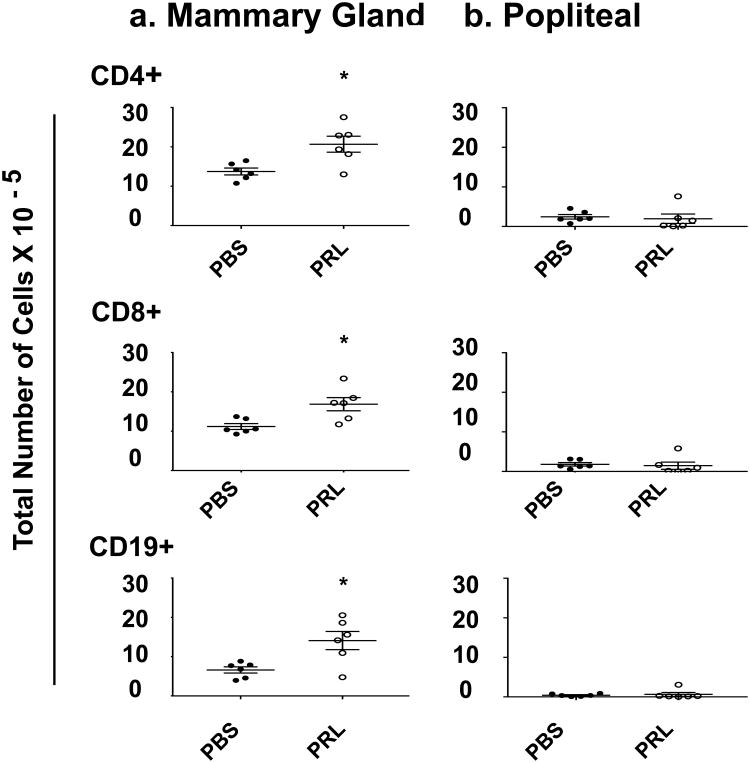
In order to determine the effect of prolactin on the number of cells in the mammary gland, we examined draining lymphnodes. Lymphocytes are long-lived and recirculate through tissues, whereas other immune cell types are either short-lived or terminally differentiate in tissues and do not recirculate. Neutrophils, for example live for less than a day in mice [45] and, once monocytes have become macrophages, they do not reenter the circulation. Our focus was therefore on lymphocytes in the draining lymphnodes. Because the different mammary glands in any animal are so variable in their size and physiology, we combined the axillary and inguinal mammary gland-draining lymphnodes to create a representative sample and compared their contents to those of the popliteal lymphnode. Prolactin treatment increased CD4+ and CD8+ T cells and CD19+ B cells in axillary and inguinal lymph nodes by 60%, 50%, and 100%, respectively (Fig.3a). By contrast, prolactin treatment had no effect on T or B cell numbers in the popliteal lymphnode (Fig.3b).
Fig. 3. Prolactin increased CD4+, CD8+, and CD19+ cell numbers in mammary gland-draining, but not the popliteal lymph nodes.
Numbers of indicated cell types were determined for each animal by total cell counts per lymph node and flow cytometric analysis of the markers. n = 6 per group. * P < 0.05, ** P < 0.01, and *** P < 0.0001
Media conditioned by mammary epithelial cells increased the migration of immune cells
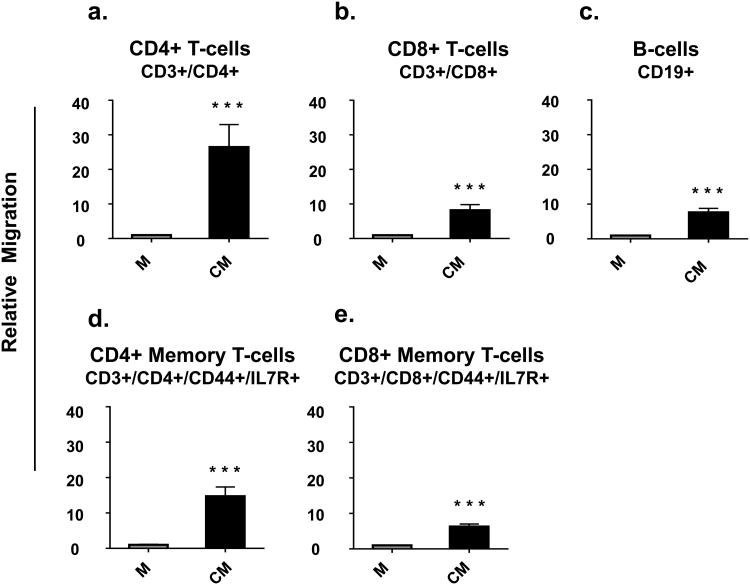
HC11 cells are an immortalized, normal mouse mammary epithelial cell line that has been widely used to study mammary epithelial differentiation [46]. Serum-free conditioned medium from confluent and partially differentiated HC11 cells was used in the bottom of a Transwell™ system and the migration of immune cells through the membrane was monitored by flow cytometric analysis of cells in the lower chamber. Conditioned serum-free medium increased the migration of spleen CD4+ T, CD8+ T and B cells by a striking 26, 7, and 7 fold, respectively, when compared to non-conditioned serum-free medium (Fig.4a, 4b, and 4c).
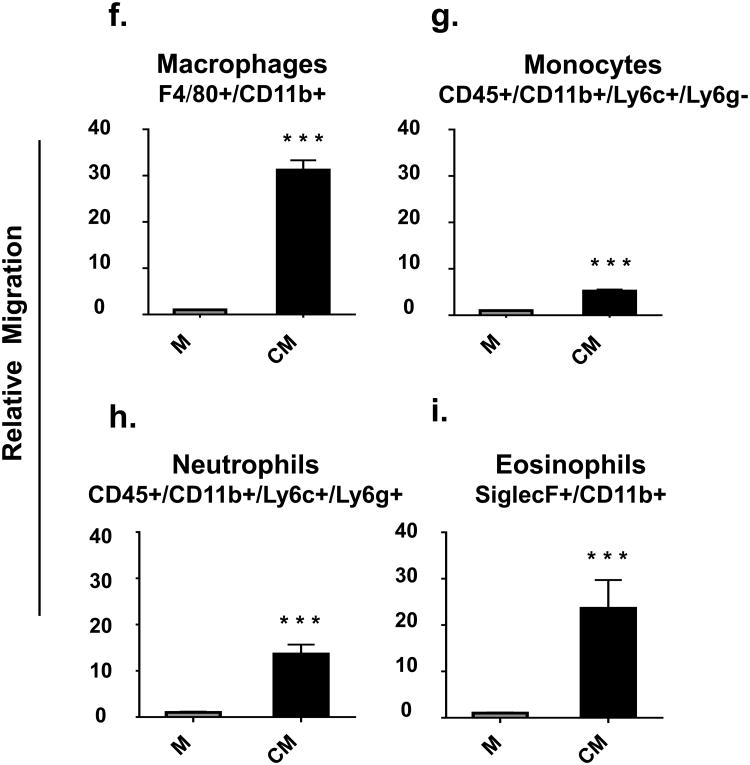
Fig. 4. Media conditioned by mammary epithelial cells increased migration of immune cells.
Splenocytes were loaded into the top of Transwells™ with either serum-free medium (M) or mammary epithelial cell-conditioned serum-free medium (CM) in the bottoms of the Transwells™. Data are presented as relative migration of immune cells compared to control (set at 1). n = 9 per group. P < 0.05, ** P < 0.01, and *** P < 0.0001
Because of the reported concentration of memory T cells in milk [12,27], memory T cell subsets were also examined. Migration towards conditioned serum-free medium by CD4+ memory T cells (CD3+/CD4+/IL7R+/CD44+) and CD8+ memory T cells (CD3+/CD8+/IL7R+/CD44+) was increased by 14 and 5 fold, respectively, compared to non-conditioned serum-free media (Fig.4d and 4e).
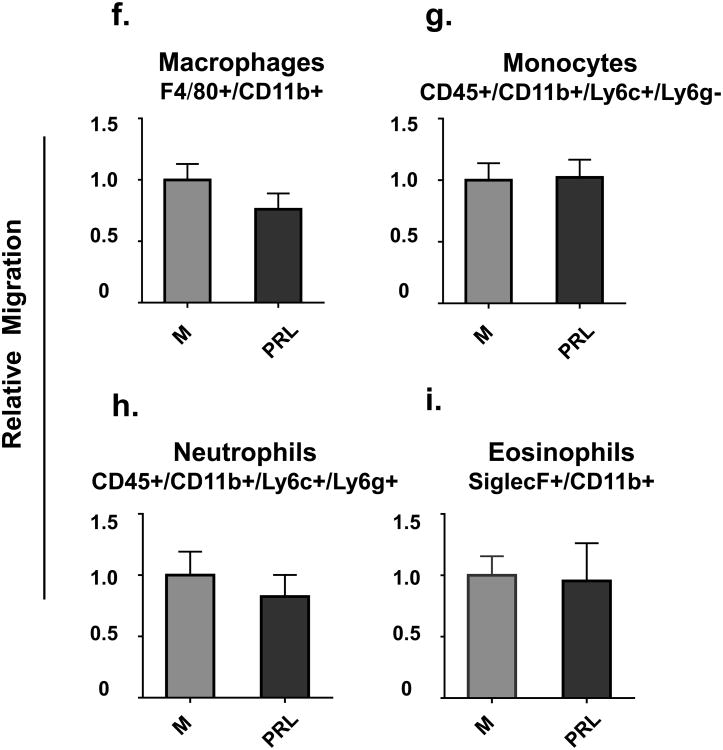
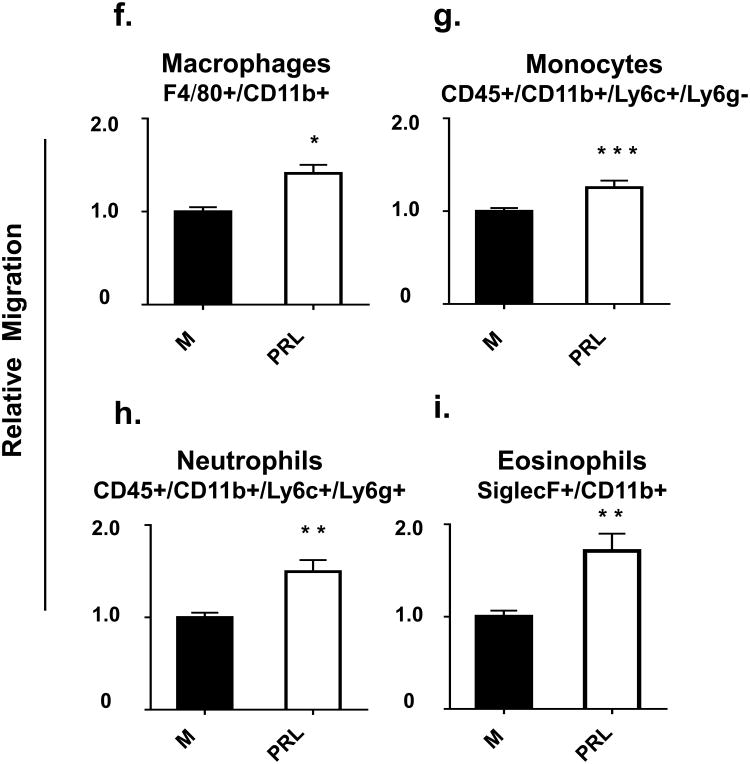
Conditioned serum-free medium also increased the migration of macrophages (CD11b+/F4/80+) and monocytes (CD45+/CD11b+/Ly6c+/Ly6g-) by 30 and 4 fold, respectively. (Fig.4f and 4g). Similarly, conditioned serum-free medium increased migration of neutrophils (CD45+/CD11b+/Ly6c+/Ly6g+) and eosinophils (CD11b+/SiglecF+) by 13 and 23 fold, respectively (Fig.4h and 4i).
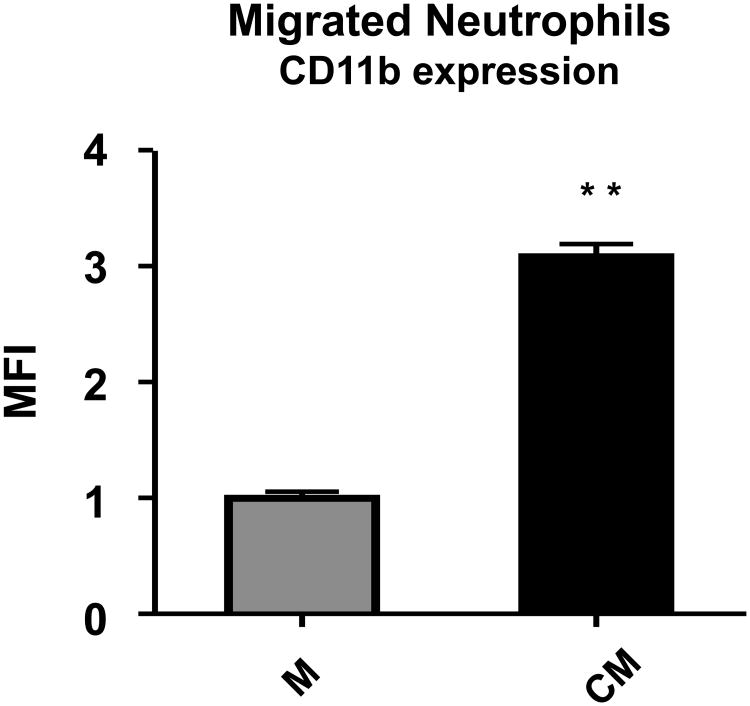
Others have reported that neutrophils in milk display increased CD11b [47,48]. To determine whether mammary epithelial cells were responsible for the homing of neutrophils with increased CD11b expression, we compared the mean fluorescence intensity of cell surface antibody-labeling of CD11b on neutrophils migrating towards control versus conditioned medium. Those responding to products of HC11 cells exhibited a 2.1 fold increased expression of CD11b (Fig.5).
Fig. 5. Neutrophils that migrated towards media conditioned by mammary epithelial cells expressed more CD11b.
Relative mean fluorescence intensity (MFI) of labeled CD11b on neutrophils that migrated to the bottoms of Transwells™ containing either serum-free medium (M) normalized to 1 or mammary epithelial cell conditioned serum-free medium (CM). n = 9 per group. P < 0.05, ** P < 0.01, and *** P < 0.0001
Prolactin treatment of mammary epithelial cells increased migration of most immune cells
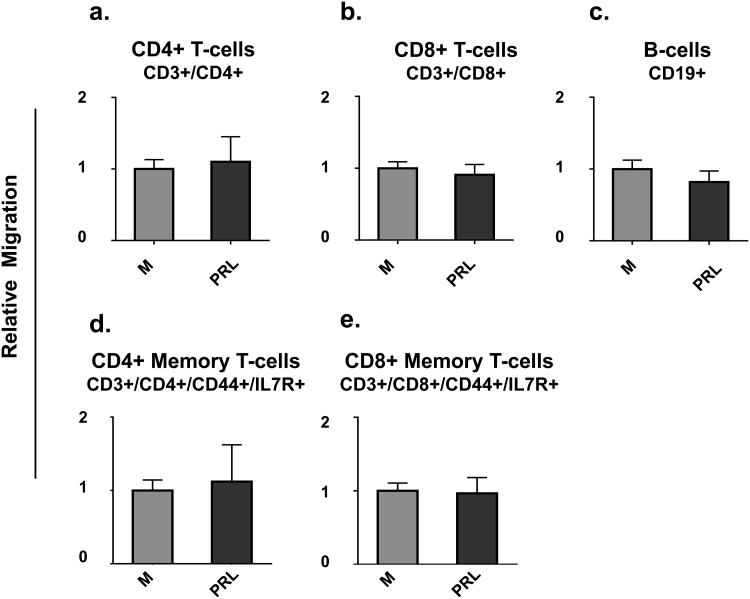
The next goal was to determine whether prolactin treatment of HC11 cells increased the chemoattractive properties of conditioned serum-free medium. Prolactin receptors are present on T cells, B cells, monocytes, macrophages, and granulocytes [49,50,51]. To determine whether prolactin (at the concentration present in conditioned media from mammary epithelial cells treated with prolactin) could independently act as a chemoattractant, non-conditioned serum-free medium with or without prolactin (100 ng/mL) was placed in the bottoms of Transwells™ and immune cell migration to the bottom of the wells determined. This dose of prolactin is within the physiological range during lactation in the mouse [52].
Under the conditions of our experimental protocol, prolactin itself at 100 ng/mL for 2h did not prove to act as a chemoattractant for B cells, CD4+ T cells, CD8+ T cells, CD4+ memory T cells, CD8+ memory T cells, macrophages, monocytes, eosinophils, or neutrophils (Fig.6 a-i). Thus, we can conclude that any observed increases in migration towards conditioned serum-free media from prolactin-treated HC11 cells were due to prolactin's effects on mammary epithelial cell production of chemokines and/or their release into the media.
Fig. 6. Prolactin does not act as a direct chemoattractant for immune cells in the time frame used.
Splenocytes were loaded into the top of Transwells™ and either serum-free medium (M) or serum-free medium containing 100 ng/mL prolactin (PRL) was placed in the bottoms of the Transwells™. Data are presented as relative migration of immune cells where the control medium (M) is set as 1. n = 9 per group. P < 0.05, ** P < 0.01, and *** P < 0.0001
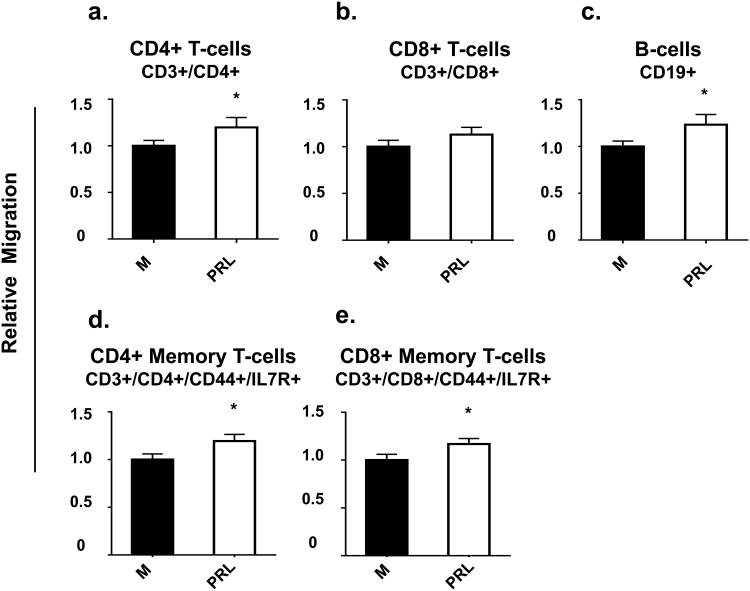
Although not a large effect, prolactin produced a consistent increase in migration of most immune cells. In comparison to conditioned serum-free medium, conditioned serum-free medium collected from HC11 cells treated with prolactin increased migration of B cells, CD4+ T cells, CD4+ memory T cells, and CD8+ memory T cells each by about 20%. However, there was no significant effect on migration of total CD8+ T cells (Fig.7a-e).
Fig. 7. Prolactin treatment of mammary epithelial cells increased migration of most immune cells.
Splenocytes were loaded into the top of Transwells™ with either mammary epithelial cell-conditioned serum-free medium (CM) or mammary epithelial cell- conditioned serum-free media from HC11 cells treated with 100 ng/mL prolactin (PRL) in the bottoms of the Transwells™. Data are presented as relative migration of immune cells with the regular conditioned medium set as 1. n = 9 per group. P < 0.05, ** P < 0.01, and *** P < 0.0001
Prolactin treatment of HC11 cells also increased migration of macrophages and monocytes towards conditioned medium by 40% and 30%, respectively (Fig.7f and 7g), and migration of neutrophils and eosinophils by 50% and 70%, respectively (Fig.7h and 7i).
Prolactin regulates mammary epithelial cell cytokine expression
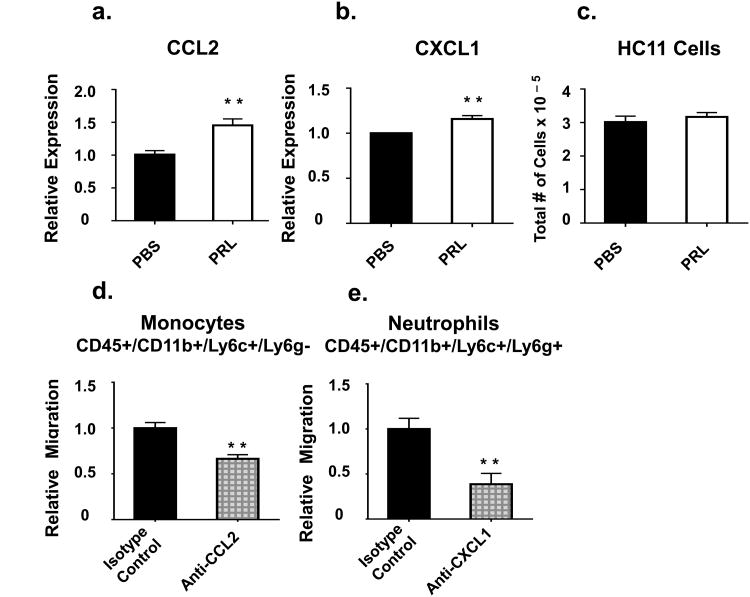
In lactating women, there are increased concentrations of both CCL2 and CXCL1 in milk as compared to the general circulation, and these molecules act as attractants for monocytes and neutrophils, respectively [53]. Prolactin increased HC11 expression of CCL2 and CXCL1 by 40% and 20%, respectively (Fig. 8a and 8b). Also determined was whether there was any effect of prolactin on cell number under the conditions used to produce the conditioned medium since an increase in cell number could have contributed to the relative amount of overall chemokine production. In these insulin and hydrocortisone-treated, confluent cells, prolactin did not affect cell number (Fig.8c).
Fig. 8. Effect of Prolactin on CCL2 and CXCL1 expression and cell number, and effect of CCL2 and CXCL1 depletion on monocyte and neutrophil migration.
Relative HC11 mRNA expression of CCL2 (A) and CXCL1 (B) normalized to GAPDH without (DPBS) or with prolactin (100ng/mL) treatment (PRL). (C) Cell numbers, as determined by hemocytometer counts, with the same treatment. Relative migration after depletion of CCL2 (D) and CXCL1 (E). Migration of each cell type in response to serum-free medium is subtracted from each condition Data are presented with control = 1. n = 9 per group. P < 0.05, ** P < 0.01, and *** P < 0.0001
CCL2 and CXCL1 depletion from conditioned serum-free medium decreased migration of monocytes/macrophages and neutrophils
To assess the roles of CCL2 and CXCL1 in the homing of monocytes and neutrophils towards mammary epithelial cells, we employed cytokine depletion experiments. When CCL2 was depleted from conditioned serum-free medium, there was a 35% reduction in monocyte migration, illustrating that CCL2 is at least in part responsible for the monocyte migration (Fig.8d).
When CXCL1 was depleted from the conditioned serum-free medium, there was a 60% reduction in neutrophil migration. The reduction in migration was not increased by two rounds of depletion (data not shown), demonstrating that CXCL1 is responsible for about 60% of neutrophil migration (Fig.8e).
Discussion
While prolactin had previously been implicated in the movement of immune cells into the mammary gland [28,39], a clear demonstration of an effect of prolactin has been lacking. Unequivocal demonstrations are complicated by two major factors, the cessation of lactation in the absence of prolactin, such as would occur in knockout animals, and the luteotrophic effects of prolactin administration in rodents. These luteotrophic effects result in elevated progesterone and pseudopregnancy [44,54] thereby making it unclear whether any observed effects of prolactin administration are due to prolactin or progesterone or both hormones. By treating animals with prolactin for a period of 28 days and examining the lymphnodes 14-16 days after the end of pseudopregnancy when there had been two weeks of normal estrous cycling, we were able to measure the effect of prolactin itself. However, we cannot totally exclude the possibility of some long-lasting effects of elevated progesterone on long-lived immune cells or cells of the mammary gland, or both. This duration of prolactin treatment also mimicked activation of prolactin receptors throughout pregnancy and the first week of lactation and hence was a long enough treatment to have developed relevant responses. Because lymphocytes are long-lived recirculating cells, analysis of lymphocyte number and phenotype in mammary-draining lymphnodes allowed us to accurately quantify the response to prolactin. Prolactin increased T lymphocyte cell flux through the mammary glands by 50% and doubled the flux of B cells. The increased flux was specific to mammary tissue, thereby demonstrating that prolactin was not just increasing general numbers of lymphocytes throughout the body nor affecting stromal cells common to all lymphnodes. Rather, this was the first indication that there was likely an indirect effect working through cells specific to the mammary gland.
Conditioned medium from partially-differentiated HC11 cells contained chemoattractants for macrophages, monocytes, all T and B cells (including the memory phenotypes) and both granulocytes. With the exception of total CD8+ cells, prolactin increased the chemoattractive properties of conditioned medium, although even among CD8+ cells, the memory subset showed an increased migration as a result of prolactin treatment. These effects were via prolactin's interaction with HC11 cells and were not due to direct chemoattractant properties of prolactin. Others have reported that direct incubation in prolactin increases migratory activity of immune cells [55], but this is not the same as chemoattraction toward prolactin, as was tested in the current experiments. Although some prolactin would have diffused from the bottom up to the top chamber in 2h, this was not sufficient in either duration or amount to directly influence migration. We can therefore conclude that luminal mammary epithelial cells are an important source of chemoattractants for many of the immune cells important to mammary development and as constituents of milk. Further, that a previously unrecognized role for prolactin in mammary gland development and lactation is through increased production of these chemoattractants.
The eosinophil is recruited into the mammary gland and is required for normal mammary ductal branching and terminal end bud formation [4]. Until now, recruitment has been attributed to effects of estrogen on epidermal growth factor receptor expression and the production of eotaxin [56]. Demonstration that prolactin directly stimulates production of a chemoattractant for eosinophils suggests either an estrogen-independent mechanism or, since estrogen elevates prolactin [57] that the effects of estrogen on recruitment of eosinophils could be via prolactin.
Macrophages and neutrophils constitute the largest proportion of cells that travel not only into mammary gland stroma but move on further into milk [11]. Since to form macrophages to go into milk one must recruit monocytes from the circulation, we focused further analysis on the monocyte-recruiting chemokine, CCL2, and the neutrophil-recruiting chemokine, CXCL1, and asked whether prolactin increased expression of CCL2 and CXCL1. The increased production of CCL2 and CXCL1 mRNA by HC11 cells in response to prolactin was similar in magnitude to the increased migration of monocytes and neutrophils observed in response to conditioned medium from cells treated with prolactin for the same duration. Furthermore, when CCL2 or CXCL1 was depleted from conditioned medium, migration of monocytes and neutrophils decreased.
In the case of CCL2, the actual degree to which migration of monocytes was dependent on this cytokine was difficult to determine since use of greater quantities of antibody-loaded beads began to cause non-specific effects with the control antibody, perhaps due to minor leeching of antibody from the beads even after multiple washes. We can therefore only say that at least 35% of the migration was due to CCL2. By contrast, a second round of depletion with anti-CXCL1, which uses a different control antibody, showed no additional inhibition of migration. We are therefore fairly confident that 60% of the migration of neutrophils is due to this cytokine, although confidence would be enhanced by direct measurement of cytokine depletion.
The concentration of CCL2 and CXCL1 is higher in milk than in the general circulation [53], suggesting that these and other chemokines designed to move cells into the milk may be secreted from the apical surface of luminal mammary epithelial cells, thereby creating a concentration gradient from milk to blood to guide migration. In addition to such a recruiting and directing function, CCL2 can be loaded onto endothelial cells and contribute to monocyte rolling and slowing in the circulation in preparation to enter tissue [58].
CD11b and CD18 form the alpha and beta subunits, respectively, of macrophage 1 antigen, an integrin important for neutrophil adhesion, migration, and phagocytosis. Our data suggest that CXCL1 and potentially other products made and secreted by mammary epithelial cells induce migration of neutrophils with greater CD11b expression. While CD18 is important for neutrophil migration across endothelial cells, CD11b is important for its migration across mammary epithelial cells [59]. Others have reported finding neutrophils with elevated CD11b in breast milk compared to neutrophils in peripheral blood and suggested that they may not contribute to immune function because the increased CD11b is an indicator that they have already been activated. [47,48,60] We would argue that rather than indicating activation, extra CD11b is an indicator of increased ability to migrate through the epithelium to enter milk.
During pregnancy and lactation, the thymus involutes [61]. T lymphocytes that move into the mammary gland are therefore predominantly derived from the periphery where cells have had the opportunity to become immunologically experienced. Passage of such cells into the milk and then the neonate's thymus changes neonatal thymic development through positive selection. In this way, the neonate develops cells responsive to antigens to which the mother has been exposed [26]. Additionally, some proportion of recruited T cells may be important to ongoing negative regulation or modulation of mammary development [3].
While under the conditions used for the current experiments, prolactin did not increase mammary epithelial cell number, prolactin is extremely important for the proliferation of mammary epithelial cells during pregnancy and lactation [62]. Therefore, in vivo, prolactin would increase both the number of cells producing chemoattractants as well as the level of expression of chemoattractants per cell.
In summary, we have shown that prolactin administration to adult female mice specifically increased immune cell flux through mammary glands and stimulated mammary epithelial cell production of chemoattractants for many immune cell types important to gland development as well as those important in milk and after passage into the neonate.
Acknowledgments
This work was supported by grants from National Institute of Child Health and Human Development # RO1-065099 and the California Breast Cancer Research Program # 171B-0053 to AMW. RD was also partially supported by Dorothy Pease and Burden fellowships. The authors thank Dr. Emma Wilson for access to the flow cytometer and Mary Y. Lorenson for provision of the prolactin and editorial assistance (both in the Division of Biomedical Sciences, University of California, Riverside).
References
- 1.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74(7):365–81. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3(3) doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plaks V, Boldajipour B, Linnemann JR, Nguyen NH, Kersten K, Wolf Y, et al. Adaptive Immune Regulation of Mammary Postnatal Organogenesis. Dev Cell. 2015;34(5):493–504. doi: 10.1016/j.devcel.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127(11):2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 5.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4(4):155–64. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilla JN, Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol. 2010;337(1):124–33. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieanrakwong D, Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Hasegawa Y, et al. Prolactin Suppression of Gonadotropin-Releasing Hormone Initiation of Mammary Gland Involution in Female Rats. Endocrinology. 2016;157(7):2750–8. doi: 10.1210/en.2016-1180. [DOI] [PubMed] [Google Scholar]
- 8.Van Leeuwenhoek A. Arcana Naturae Detecta Delphis Batavorum. Apud Henricum a Krooneveld. 1685 Epistola 106. [Google Scholar]
- 9.Simon C, Balzer K, Welsch U, Strutte HJ. Cytology of human milk. Schweiz Med Wochenschr. 1970;100(38):1603–10. [PubMed] [Google Scholar]
- 10.Breborowicz D, Szlapka Z, Breborowicz H. Morphological features of colostrum and human milk cells. Pol Tyg Lek. 1971;26(6):201–3. [PubMed] [Google Scholar]
- 11.Crago SS, Prince SJ, Pretlow TG, McGhee JR, Mestecky J. Human colostral cells. I. Separation and characterization. Clin Exp Immunol. 1979;38(3):585–97. [PMC free article] [PubMed] [Google Scholar]
- 12.Wirt DP, Adkins LT, Palkowetz KH, Schmalstieg FC, Goldman AS. Activated and memory T lymphocytes in human milk. Cytometry. 1992;13(3):282–90. doi: 10.1002/cyto.990130310. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JE, Harvey BA, Soothill JF. Phagocytosis and killing of bacteria and yeast by human milk cells after opsonisation in aqueous phase of milk. Br Med J. 1978;1(6125):1443–5. doi: 10.1136/bmj.1.6125.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings NP, Neifert MR, Pabst MJ, Johnston RB., Jr Oxidative metabolic response and microbicidal activity of human milk macrophages: effect of lipopolysaccharide and muramyl dipeptide. Infect Immun. 1985;49(2):435–9. doi: 10.1128/iai.49.2.435-439.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speer CP, Schatz R, Gahr M. Function of breast milk macrophages. Monatsschr Kinderheilkd. 1985;133(11):913–7. [PubMed] [Google Scholar]
- 16.Speer CP, Gahr M, Pabst MJ. Phagocytosis-associated oxidative metabolism in human milk macrophages. Acta Paediatr Scand. 1986;75(3):444–51. doi: 10.1111/j.1651-2227.1986.tb10228.x. [DOI] [PubMed] [Google Scholar]
- 17.Pittard WB, 3rd, Polmar SH, Fanaroff AA. The breastmilk macrophage: a potential vehicle for immunoglobulin transport. J Reticuloendothel Soc. 1977;22(6):597–603. [PubMed] [Google Scholar]
- 18.Sheldrake RF, Husband AJ. Intestinal uptake of intact maternal lymphocytes by neonatal rats and lambs. Res Vet Sci. 1985;39(1):10–5. [PubMed] [Google Scholar]
- 19.Seelig LL, Jr, Head JR. Uptake of lymphocytes fed to suckling rats. An autoradiographic study of the transit of labeled cells through the neonatal gastric mucosa. J Reprod Immunol. 1987;10(4):285–97. doi: 10.1016/0165-0378(87)90031-3. [DOI] [PubMed] [Google Scholar]
- 20.Tuboly S, Bernath S, Glavits R, Kovacs A, Megyeri Z. Intestinal absorption of colostral lymphocytes in newborn lambs and their role in the development of immune status. Acta Vet Hung. 1995;43(1):105–15. [PubMed] [Google Scholar]
- 21.Tuboly S, Bernath S, Glavits R, Medveczky I. Intestinal absorption of colostral lymphoid cells in newborn piglets. Vet Immunol Immunopathol. 1988;20(1):75–85. doi: 10.1016/0165-2427(88)90027-x. [DOI] [PubMed] [Google Scholar]
- 22.Jain L, Vidyasagar D, Xanthou M, Ghai V, Shimada S, Blend M. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child. 1989;64(7 Spec No):930–3. doi: 10.1136/adc.64.7_spec_no.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101(4):570–80. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvola M, Gustafsson E, Svensson L, Jansson L, Holmdahl R, Heyman B, et al. Immunoglobulin-secreting cells of maternal origin can be detected in B cell-deficient mice. Biol Reprod. 2000;63(6):1817–24. doi: 10.1095/biolreprod63.6.1817. [DOI] [PubMed] [Google Scholar]
- 25.Ma LJ, Guzmán EA, DeGuzman A, Muller HK, Walker AM, Owen LB. Local cytokine levels associated with delayed-type hypersensitivity responses: modulation by gender, ovariectomy, and estrogen replacement. 2007 doi: 10.1677/JOE-06-0024. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh MK, Nguyen V, Muller HK, Walker AM. Maternal Milk T Cells Drive Development of Transgenerational Th1 Immunity in Offspring Thymus. J Immunol. 2016 doi: 10.4049/jimmunol.1502483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabbaj S, Ghosh MK, Edwards BH, Leeth R, Decker WD, Goepfert PA, et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol. 2005;174(5):2951–6. doi: 10.4049/jimmunol.174.5.2951. [DOI] [PubMed] [Google Scholar]
- 28.Weisz-Carrington P, Roux ME, McWilliams M, Phillips-Quagliata JM, Lamm ME. Hormonal induction of the secretory immune system in the mammary gland. Proc Natl Acad Sci U S A. 1978;75(6):2928–32. doi: 10.1073/pnas.75.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanneau GM, Hibrand-Saint Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J Histochem Cytochem. 1999;47(12):1581–92. doi: 10.1177/002215549904701210. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Soto H, Oldham ER, Buchanan ME, Homey B, Catron D, et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) J Biol Chem. 2000;275(29):22313–23. doi: 10.1074/jbc.M001461200. [DOI] [PubMed] [Google Scholar]
- 31.Morteau O, Gerard C, Lu B, Ghiran S, Rits M, Fujiwara Y, et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol. 2008;181(9):6309–15. doi: 10.4049/jimmunol.181.9.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200(6):805–9. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low EN, Zagieboyloa L, Martinoa B, Wilson E. IgA ASC Accumulation to the Lactating Mammary Gland is Dependent on VCAM-1 and alpha4 Integrins. Mol Immunol. 2010;47(7-8):1608–12. doi: 10.1016/j.molimm.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- 35.Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ. Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13(1):13–28. doi: 10.1007/s10911-008-9069-5. [DOI] [PubMed] [Google Scholar]
- 36.Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, et al. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210(1):96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006;17(3):110–6. doi: 10.1016/j.tem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Wongdee K, Charoenphandhu N. Regulation of epithelial calcium transport by prolactin: from fish to mammals. Gen Comp Endocrinol. 2013;181:235–40. doi: 10.1016/j.ygcen.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Mackern-Oberti JP, Valdez SR, Vargas-Roig LM, Jahn GA. Impaired mammary gland T cell population during early lactation in hypoprolactinemic lactation-deficient rats. Reproduction. 2013;146(3):233–42. doi: 10.1530/REP-12-0387. [DOI] [PubMed] [Google Scholar]
- 40.Chen TJ, Kuo CB, Tsai KF, Liu JW, Chen DY, Walker AM. Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinology. 1998;139(2):609–16. doi: 10.1210/endo.139.2.5758. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when DU145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61(16):6098–104. [PubMed] [Google Scholar]
- 42.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11(2):167–78. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 44.Gunnet JW, Freeman ME. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev. 1983;4(1):44–61. doi: 10.1210/edrv-4-1-44. [DOI] [PubMed] [Google Scholar]
- 45.Simon SI, Kim MH. A day (or 5) in a neutrophil's life. Blood. 2010;116(4):511–2. doi: 10.1182/blood-2010-05-283184. [DOI] [PubMed] [Google Scholar]
- 46.Merlo GR, Graus-Porta D, Cella N, Marte BM, Taverna D, Hynes NE. Growth, differentiation and survival of HC11 mammary epithelial cells: diverse effects of receptor tyrosine kinase-activating peptide growth factors. Eur J Cell Biol. 1996;70(2):97–105. [PubMed] [Google Scholar]
- 47.Keeney SE, Schmalstieg FC, Palkowetz KH, Rudloff HE, Le BM, Goldman AS. Activated neutrophils and neutrophil activators in human milk: increased expression of CD11b and decreased expression of L-selectin. J Leukoc Biol. 1993;54(2):97–104. doi: 10.1002/jlb.54.2.97. [DOI] [PubMed] [Google Scholar]
- 48.Goldman AS, Chheda S, Garofalo R. Evolution of immunologic functions of the mammary gland and the postnatal development of immunity. Pediatr Res. 1998;43(2):155–62. doi: 10.1203/00006450-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrini I, Lebrun JJ, Ali S, Kelly PA. Expression of prolactin and its receptor in human lymphoid cells. Mol Endocrinol. 1992;6(7):1023–31. doi: 10.1210/mend.6.7.1508218. [DOI] [PubMed] [Google Scholar]
- 50.Gala RR, Shevach EM. Identification by analytical flow cytometry of prolactin receptors on immunocompetent cell populations in the mouse. Endocrinology. 1993;133(4):1617–23. doi: 10.1210/endo.133.4.8404602. [DOI] [PubMed] [Google Scholar]
- 51.Dogusan Z, Hooghe R, Verdood P, Hooghe-Peters EL. Cytokine-like effects of prolactin in human mononuclear and polymorphonuclear leukocytes. J Neuroimmunol. 2001;120(1-2):58–66. doi: 10.1016/s0165-5728(01)00420-9. [DOI] [PubMed] [Google Scholar]
- 52.Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH, FSH and prolactin in aged female C57BL/6 mice. J Reprod Fertil. 1980;58(2):377–86. doi: 10.1530/jrf.0.0580377. [DOI] [PubMed] [Google Scholar]
- 53.Kverka M, Burianova J, Lodinova-Zadnikova R, Kocourkova I, Cinova J, Tuckova L, et al. Cytokine profiling in human colostrum and milk by protein array. Clin Chem. 2007;53(5):955–62. doi: 10.1373/clinchem.2006.077107. [DOI] [PubMed] [Google Scholar]
- 54.Jakubowski M, Terkel J. Female reproductive function and sexually dimorphic prolactin secretion in rats with lesions in the medial preoptic-anterior hypothalamic continuum. Neuroendocrinology. 1986;43(6):696–705. doi: 10.1159/000124607. [DOI] [PubMed] [Google Scholar]
- 55.Savino W, Mendes-da-Cruz DA, Lepletier A, Dardenne M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol. 2016;12(2):77–89. doi: 10.1038/nrendo.2015.168. [DOI] [PubMed] [Google Scholar]
- 56.Aupperlee MD, Zhao Y, Tan YS, Leipprandt JR, Bennett J, Haslam SZ, et al. Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland. Endocrinology. 2014;155(6):2301–13. doi: 10.1210/en.2013-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shull JD, Gorski J. The hormonal regulation of prolactin gene expression: an examination of mechanisms controlling prolactin synthesis and the possible relationship of estrogen to these mechanisms. Vitam Horm. 1986;43:197–249. doi: 10.1016/s0083-6729(08)60421-5. [DOI] [PubMed] [Google Scholar]
- 58.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, et al. Inflammatory Chemokine Transport and Presentation in HEV: A Remote Control Mechanism for Monocyte Recruitment to Lymph Nodes in Inflamed Tissues. J Exp Med. 2001;194:1361–74. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smits E, Burvenich C, Guidry AJ, Massart-Leen A. Adhesion receptor CD11b/CD18 contributes to neutrophil diapedesis across the bovine blood-milk barrier. Vet Immunol Immunopathol. 2000;73(3-4):255–65. doi: 10.1016/s0165-2427(00)00157-4. [DOI] [PubMed] [Google Scholar]
- 60.Michie CA, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw. 1998;9(2):123–9. [PubMed] [Google Scholar]
- 61.Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol. 2012;42(5):1073–9. doi: 10.1002/eji.201142305. [DOI] [PubMed] [Google Scholar]
- 62.Horseman ND, Gregerson KA. Prolactin actions. J Mol Endocrinol. 2014;52(1):R95–106. doi: 10.1530/JME-13-0220. [DOI] [PubMed] [Google Scholar]