Abstract
Objective
DSM-5 revised diagnostic criteria for anorexia nervosa (AN) by eliminating the amenorrhea requirement, liberalizing weight and psychological criteria, and adding the formal diagnosis of “atypical AN” for individuals with AN psychological symptoms without low weight. We sought to determine whether bone density (BMD) is impaired in women diagnosed with AN using the new, more liberal DSM-5 criteria.
Method
Cross-sectional study of 168 women, 18–45y: 1) AN by DSM-IV (DSM-IV)(n=37), 2) AN by DSM-5 but not DSM-IV criteria (DSM-5)(n=33), 3) atypical AN (ATYPICAL)(n=77), 4) healthy comparison group (HC)(n=21). Measurements included dual energy x-ray absorptiometry, Eating Disorder Examination-Questionnaire, Eating Disorder Inventory-2, Hamilton Depression and Anxiety Rating Scales.
Results
BMD Z-score <−1.0 was present in 78% of DSM-IV, 82% of DSM-5, and 69% of ATYPICAL. Mean Z-scores were comparably low in DSM-IV and DSM-5, intermediate in ATYPICAL, and highest in HC. Lack of prior low weight or amenorrhea was, but history of overweight/obesity was not, protective against bone loss. Mean lean mass and percent fat mass were significantly lower in all AN groups than HC. DSM-IV, DSM-5 and ATYPICAL had comparable psychopathology.
Discussion
Despite liberalizing diagnostic criteria, many women diagnosed with AN and atypical AN using DSM-5 criteria have low BMD. Presence or history of low weight and/or amenorrhea remain important indications for DXA. Loss of lean mass, in addition to fat mass, is present in all AN groups, and may contribute to low BMD. The deleterious effect of eating disorders on BMD extends beyond those with current low weight and amenorrhea.
Keywords: anorexia nervosa, bone density, osteoporosis
Anorexia nervosa (AN) is an eating disorder historically characterized by persistent food restriction, low body weight, and difficulty recognizing the seriousness of the illness (1). A large body of literature highlights important psychological co-morbidities and medical sequelae of AN as diagnosed by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), including comorbid psychopathology, endocrine complications such as amenorrhea and bone loss (2), and high mortality (3). DSM-5 recently supplanted DSM-IV for the diagnosis of psychiatric disorders and introduced multiple changes broadening the definition of AN, including removing a specific low-weight guideline, changing weight phobia to be explicitly or implicitly present (instead of requiring explicit endorsement), and eliminating the amenorrhea criterion (4). Furthermore, the formal diagnosis of “atypical AN” was created within Other Specified Feeding or Eating Disorder (OSFED) for individuals who meet AN psychological criteria but are not low weight—previously, atypical AN was not named but rather described as a numbered example of Eating Disorder Not Otherwise Specified (EDNOS) in DSM-IV (4). Bone loss, body composition, and psychiatric characteristics have been well-documented in DSM-IV AN, but much less is known about clinical features across the AN spectrum of eating disorders in DSM-5.
Data suggest that the prevalence of DSM-5 AN is significantly greater than that of DSM-IV AN (5–7). Furthermore, atypical AN may be even more common than DSM-5 AN (8). Given that DSM-5 AN and atypical AN capture a broader clinical group than DSM-IV AN, the extent to which clinical features—and therefore clinical interventions— that are well-characterized for DSM-IV AN will generalize to DSM-5 AN and atypical AN remains unknown. No prior study has compared bone mineral density (BMD) and/or body composition across DSM-IV AN, DSM-5 AN, atypical AN, and a healthy comparison group. If low BMD is prevalent in AN diagnosed by DSM-5 criteria despite less stringent weight and amenorrhea criteria, mental health practitioners treating AN spectrum disorders may consider referring patients for BMD screening. In addition, recent data suggest similar levels of eating disorder and general psychopathology between women with DSM-IV AN and DSM-5 AN (9–11), and between women with DSM-5 AN and atypical AN (8,12), but no study has directly compared the three groups.
METHODS
This study was approved by the Partners Human Research Committee, and we obtained written informed consent from all participants.
We studied 168 premenopausal women, ages 18–45 years, who were consecutively recruited for three different NIH trials between 5/2004 and 7/2015 and met the following diagnostic criteria:
DSM-IV AN (N=37): <85% ideal body weight (IBW) (1959 Metropolitan Life Insurance Tables (13) with medium frame size) (Criterion A) and with amenorrhea (lack of menses for ≥3 months) or taking estrogen/progestin therapy (Criterion D) (1).
DSM-5 AN (N=33): Body mass index (BMI) <18.5 kg/m2 (a suggested guideline in DSM-5 (4)), but not meeting the more stringent DSM-IV criteria (because weight was ≥85% IBW or eumenorrhea was present).
ATYPICAL AN (N=77): BMI ≥18.5 kg/m2, but <25 kg/m2. No such participants would meet full criteria for either DSM-IV or DSM-5 AN.
Healthy comparison group (N=21): ≥90% IBW with BMI <25 kg/m2. No history of amenorrhea or disordered eating.
For an AN spectrum diagnosis, criterion B (weight phobia) and C (body image disturbance, undue influence of weight or shape on self-evaluation, or lack of recognition of illness severity) were assessed via Structured Clinical Interview for DSM-IV (SCID-IV) in 81/147 participants, and were inferred based on prior assessment in the rest. AN participants reported age, highest and lowest past weight (reported in 140/147 participants), duration of illness, estrogen/progestin use, date of last menstrual period, history of amenorrhea (reported in 137/147 participants), and treatment history. For analysis of the association between current menstrual status and BMD, women who were currently amenorrheic (n=52), eumenorrheic (n=50), or on oral contraceptive pills (OCPs) (n=35) were included; women taking Depo-Provera, transdermal estrogen, or an intra-uterine device (n=8) were excluded, as were those without current menstrual data (n=2). Physical activity was quantified using the Paffenbarger scale (14).
Healthy women were recruited from the general population through newspaper advertisements, posters, website announcements, and emails. Additional exclusion criteria for healthy women included abnormal thyroid function tests, conditions or medications known to affect bone metabolism, major psychiatric diagnosis (e.g. depression or anxiety syndromes, schizophrenia, bipolar disorder), chronic arthritis or pain syndromes, pregnancy or breastfeeding, diabetes mellitus, active substance abuse, or hemoglobin <10 g/dL.
We assessed BMD and body composition by dual-energy X-ray absorptiometry (DXA) (Hologic 4500, Hologic, Inc., Waltham, MA, USA) (precision of 0.01 g/cm2 at the spine and 3% for fat mass (15)). Postero-anterior (PA) spine BMD, measured from lumbar vertebra 1 through lumbar vertebra 4, was available for 166/168 participants. Lateral spine BMD, also measured from lumbar vertebra 1 through lumbar vertebra 4, was available for 105/168 participants. Total hip BMD, with a region of interest at the proximal femur, was available for 119/168 participants. Total radius BMD, with a region of interest comprised of the distal 1/3, mid-distal, and ultra-distal radius, was available for 114/168 participants. We measured eating-disorder psychopathology using the Eating Disorder Examination-Questionnaire (EDE-Q) (16) and the Eating Disorder Inventory (EDI-2) (17). Co-morbid psychopathology was assessed by the Hamilton Depression Rating Scale (HAM-D) (18) and Hamilton Anxiety Rating Scale (HAM-A) (19). Clinical characteristics, BMD, and body composition for subgroups of DSM-IV and DSM-5 AN participants have previously been reported (20–25), but data for those with ATYPICAL AN have not been previously published.
Statistical Powering
With 20 participants per group, the probability was 98% that the study would detect a difference at a two-sided 0.05 significance level if the true difference in PA spine Z-scores is 1.0, based on a SD of 0.78 (26). This difference is relevant because the difference in PA spine BMD T-scores in low-weight amenorrheic women with AN (−1.9 ± 0.1) and low-weight eumenorrheic women with AN (−0.9 ± 0.1) was 1.0 in Miller et al. (26).
Data Analysis
JMP Statistical Discovery Software, version 11 Professional (SAS Institute, Inc., Cary, NC) was used. Variables were assessed for normality using the Shapiro-Wilk test, and if non-normal, were log-transformed. We compared continuous variables across the four groups (i.e., DSM-IV AN, DSM-5 AN, ATYPICAL AN, and a healthy comparison group) using Tukey-Kramer to adjust for multiple comparisons. We compared the frequency of categorical variables across the three AN groups using Fisher’s Exact Test; pairwise comparisons were only performed when the overall p-value was ≤0.05 to correct for multiple comparisons. We divided the ATYPICAL AN group according to lowest lifetime IBW (i.e. <85% or ≥85%) or history of amenorrhea (present or absent) because prior low weight and/or amenorrhea may impact current BMD. Three-way comparisons were performed with Fisher’s Least Significant Difference testing. Additional corrections for multiple comparisons are not indicated when this method is used for three-group comparisons (27). Significance was defined as a two-tailed p-value ≤0.05. Data are reported as mean±SEM and n (%).
RESULTS
Clinical Characteristics (Table 1)
Table 1.
Clinical characteristics of DSM-IV AN, DSM-5 AN, atypical AN, and healthy controls
| DSM-IV AN (n=37) | DSM-5 AN (n=33)* | Atypical AN (n=77) | Healthy controls (n=21) | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age (y) | 25.9 ± 6.4 | 24.9 ± 5.8 | 25.7 ± 6.0 | 27.0 ± 7.1 |
| BMI (kg/m2) | 17.2 ± 0.7a | 17.5 ± 0.9a | 19.3 ± 0.7b | 22.3 ± 1.4c |
| % IBW | 80.5 ± 3.2a | 82.6 ± 4.4a | 90.8 ± 3.5b | 104.9 ± 7.5c |
| Highest Past BMI (kg/m2) | 22.3 ± 3.3ab | 21.4 ± 4.1a | 23.2 ± 3.0b | 24.0 ± 2.2b |
| Lowest Past BMI (kg/m2) | 15.0 ± 1.6a | 15.5 ± 1.7a | 16.6 ± 1.7b | 21.1 ± 2.1c |
| Anorexia Nervosa Subtype (% binge/purge type) | 32.4 | 27.3 | 32.4 | n/a |
| Duration of Illness (clinician diagnosis, years) | 6.3 ± 4.4 | 6.1 ± 5.8 | 5.3 ± 5.9 | n/a |
| Estrogen/Progestin use (oral or transdermal, %) | 27.0 | 18.2 | 29.3 | n/a |
| Amenorrheic, not on OCPs (%) | 70.3a | 18.2b | 27.8b | n/a |
| Duration of current amenorrhea (months)** | 53.1 ± 61.6a | 5.2 ± 10.2b | 14.9 ± 22.3c | n/a |
| History of amenorrhea (%) | 91.7 | 80.0 | 83.1 | n/a |
| Current Psychiatric Medication Use (%) | 54.1 | 53.1 | 64.9 | n/a |
| History of Eating Disorder Inpatient Treatment (%) | 50.0 | 45.5 | 59.7 | n/a |
| Eating Disorder Psychopathology | ||||
| EDE-Q Restraint Subscale | 3.2 ± 1.5a | 3.2 ± 1.6a | 3.0 ± 1.6a | 0.4 ± 0.6b |
| EDE-Q Eating Concern Subscale | 3.0 ± 1.5a | 2.6 ± 1.5a | 2.3 ± 1.4a | 0.2 ± 0.3b |
| EDE-Q Shape Concern Subscale | 3.7 ± 1.7a | 3.6 ± 1.7a | 3.8 ± 1.6a | 0.8 ± 0.7b |
| EDE-Q Weight Concern Subscale | 3.1 ± 1.5a | 3.2 ± 1.8a | 3.3 ± 1.6a | 0.5 ± 0.5b |
| Number of Purging Episodes per Week | 0.8 ± 1.7 | 1.9 ± 5.1 | 0.3 ± 0.6 | n/a |
| Number of Bingeing Episodes per Week | 1.2 ± 1.5 | 1.6 ± 2.3 | 1.3 ± 1.7 | n/a |
| EDI-2 Drive for Thinness | 10.9 ± 6.8a | 10.1 ± 6.6a | 11.7 ± 6.6a | 1.1 ± 1.4b |
| EDI-2 Bulimia | 2.7 ± 4.2 | 2.2 ± 4.2 | 1.4 ± 2.6 | 0.4 ± 0.8 |
| EDI-2 Body Dissatisfaction | 11.8 ± 7.4a | 11.4 ± 7.8a | 12.6 ± 8.3a | 3.9 ± 4.0b |
| Paffenbarger Vigorous Activity/Week (hours) | 3.9 ± 5.7 | 5.3 ± 6.2 | 4.6 ± 4.9 | 4.8 ± 4.1 |
| Compensatory Exercise in the Past 3 Months (%) | 35.1 | 42.4 | 46.5 | n/a |
| Compensatory Exercise in the Past 28 days (days) | 5.9 ± 8.9 | 5.6 ± 8.1 | 4.9 ± 6.3 | n/a |
| Associated Psychopathology | ||||
| HAM-D | 16.6 ± 7.3a | 14.8 ± 5.3a | 16.5 ± 7.1a | 1.9 ± 2.3b |
| HAM-A | 13.6 ± 7.2a | 13.5 ± 6.1a | 14.8 ± 7.9a | 1.6 ± 2.2b |
This group excludes participants who met DSM-IV AN criteria;
Last menstrual period in women not receiving estrogen/progestin;
Estradiol levels for women not receiving estrogen;
Groups with different superscripts differ significantly at p ≤0.05
Note: n/a= not applicable; BMI = Body mass index; IBW = Ideal body weight; OCP = oral contraceptive; EDE-Q = Eating Disorder Examination-Questionnaire; EDI-2 = Eating Disorder Inventory-2; HAM-D = Hamilton Depression Rating Scale; HAM-A = Hamilton Anxiety Rating Scale
Although currently normal weight, the majority of women with ATYPICAL AN (n=62/75, 83%) were low weight (BMI <18.5 kg/m2) in the past, and therefore would have met DSM-5 AN criteria in the past. A smaller majority of women with ATYPICAL AN (n=46/70, 66%) would have met DSM-IV AN criteria in the past given a history of both low weight (IBW <85%) and amenorrhea. As anticipated based on diagnostic criteria, the prevalence of current amenorrhea was significantly greater in DSM-IV AN (p<0.0001) (women who were not amenorrheic were on OCPs as per DSM-IV criteria (1)). A similar percentage of participants (approximately 85%) in all three AN groups had a history of amenorrhea in the past.
Bone Mineral Density
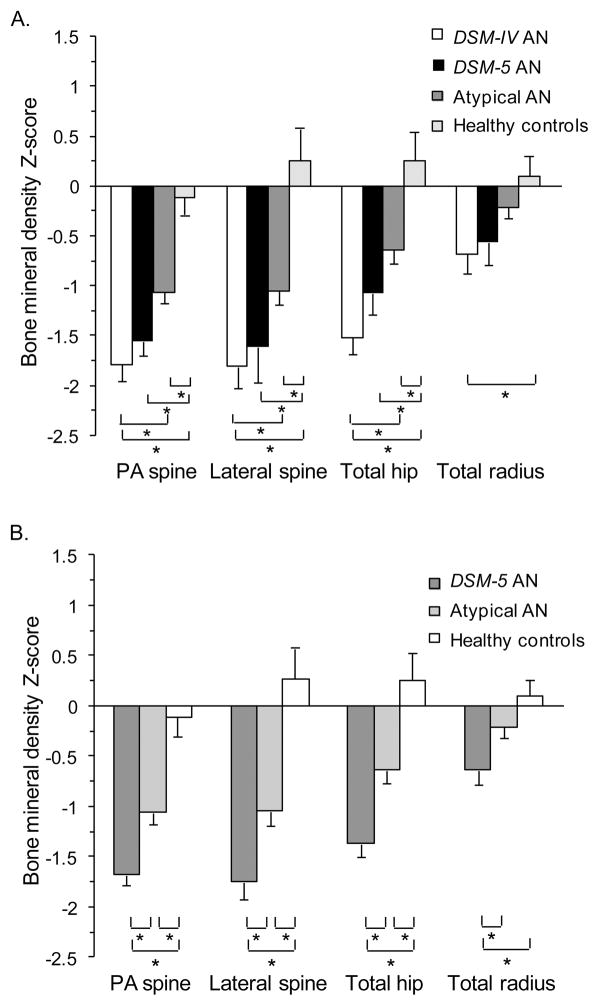
DSM-5 AN had mean Z-scores at the PA spine, lateral spine, and total hip that were significantly lower than healthy women (p<0.001), and similarly low compared to DSM-IV AN (Figure 1A). ATYPICAL AN had significantly higher mean Z-scores at the PA spine, total hip, and total radius compared to DSM-IV AN (p<0.05), but still had significantly lower mean Z-scores at those three sites compared to healthy women (p<0.005) (Figure 1A). A BMD Z-score <−1.0 (28) at any site was present in 78% (n=29/37) of DSM-IV AN, 82% (n=27/33) of DSM-5 AN, and 69% (n=53/77) of ATYPICAL AN (p=0.28). A BMD Z-score <−2.0 (28) at any site was present in 49% (n=18/37) of DSM-IV AN, 39% (n=13/33) of DSM-5 AN, and 24% (n=18/77) of ATYPICAL AN (p<0.0001).
Figure 1.
Mean bone mineral density (BMD) Z-scores of women with anorexia nervosa (AN) spectrum disorders compared to a healthy comparison group. A. Mean BMD Z-scores of four groups: 1) DSM-IV AN, 2) DSM-5 AN who did not meet DSM-IV criteria, 3) atypical AN, and 4) healthy women. B. Mean BMD Z-scores of three groups according to current clinical practice: 1) DSM-5 AN whether or not they would have met the more stringent DSM-IV criteria (in contrast to Figure 1A which focused on women who met DSM-5, but would not have met the more stringent DSM-IV), 2) atypical AN, and 3) healthy women. Mean ± SEM. * p ≤0.05.
In order to determine the prevalence of low BMD in AN as defined in current clinical practice, we performed a separate analysis that categorized participants according to current DSM (i.e., DSM-5) criteria: 1) DSM-5 AN whether or not they would have met the more stringent DSM-IV criteria (in contrast to the previous analyses that focused on women who met DSM-5, but would not have met the more stringent DSM-IV), 2) ATYPICAL AN, and 3) healthy comparison group (Figure 1B). Mean Z-scores were lowest in the DSM-5 AN group, intermediate in the ATYPICAL AN group, and highest in healthy women (p ≤0.0005). A BMD Z-score <−1.0 at any site was present in 80% (n=56/70) of DSM-5 AN, and 69% (n=53/77) of ATYPICAL AN (p=0.14). A BMD Z-score <−2.0 at any site was present in 44% (n=31/70) of DSM-5 AN, and 25% (n=19/77) of ATYPICAL AN (p=0.01).
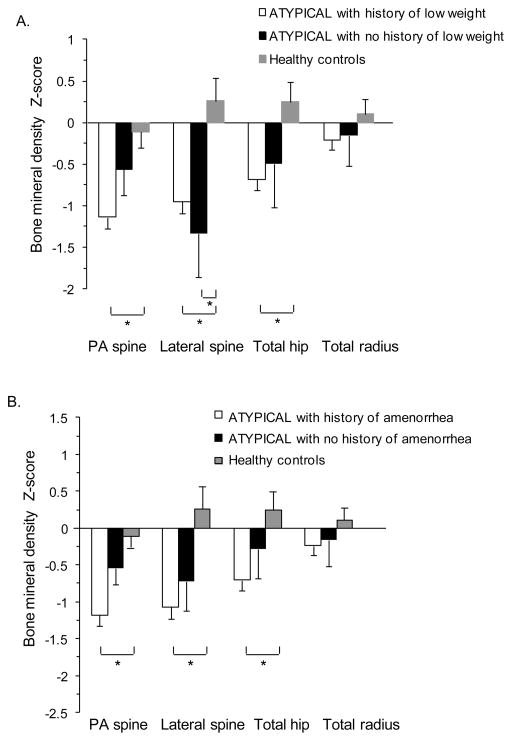
Across the groups, there was a negative linear relationship between lowest lifetime BMI and BMD Z-scores at the PA spine (R=0.37, p<0.0001), lateral spine (R=0.31, p=0.002), total hip (R=0.42, p<0.0001), and total radius (R=0.32, p=0.0008). After adjusting for current BMI, however, the relationship only remained significant at the total radius (p=0.03). To determine whether a history of low weight is a predictor of current BMD specifically in women with ATYPICAL AN, we analyzed mean BMD Z-scores in three groups: 1) ATYPICAL AN with prior low weight (defined as BMI <18.5kg/m2), 2) ATYPICAL AN without a history of low weight, and 3) healthy comparison group (Figure 2A). ATYPICAL AN with prior low weight (n=62/75, 83%) had lower mean Z-scores at the PA spine, lateral spine, and total hip (p<0.05), and ATYPICAL AN without a history of low weight (n=13/75, 17%) had similar mean Z-scores at the PA spine and total hip, compared to healthy women. A BMD Z-score <−1.0 was present in 76% (n=47/62) of ATYPICAL AN with prior low weight vs 31% (n=4/13) of ATYPICAL AN without a history of low weight (p=0.002). A BMD Z-score <−2.0 was present in 24% (n=15/62) of ATYPICAL AN with prior low weight vs 23% (n=3/13) of ATYPICAL AN without a history of low weight (p=0.91). Of the women with ATYPICAL AN without a history of low weight but a BMD Z-score <−1.0, 100% (n=4/4) had a history of amenorrhea. When low weight is defined as IBW <85%, the data differed in the following way: ATYPICAL AN without a history of low weight had lower mean Z-scores at the PA spine compared to healthy women (p<0.05).
Figure 2.
Mean bone mineral density (BMD) Z-scores of women with atypical anorexia nervosa (AN) compared to a healthy comparison group. A. Mean BMD Z-scores of three groups: 1) atypical AN with prior low weight (defined as BMI <18.5kg/m2), 2) atypical AN without a history of low weight, and 3) healthy women. B. Mean BMD Z-scores of three groups: 1) atypical AN with a history of amenorrhea, 2) atypical AN without a history of amenorrhea, and 3) healthy women. Mean ± SEM. * p ≤0.05.
Across all anorexia nervosa groups, those with a history of overweight/obesity (n=27/147, 18%) had significantly lower mean Z-scores at the PA spine, lateral spine, and total hip compared to healthy women (p<0.001). Across all anorexia nervosa groups, those with a history of overweight/obesity had similar mean Z-scores at the lateral spine, total hip, and total radius, and significantly higher mean PA spine Z-scores (p<0.05), compared to those without a history of overweight/obesity (n=120/147, 82%). To determine whether a history of overweight/obesity was protective for BMD specifically in women with ATYPICAL AN, we compared ATYPICAL AN with a history of overweight/obesity (n=18/74, 24%) to healthy women. Women with ATYPICAL AN and a history of overweight/obesity had significantly lower mean Z-scores at the lateral spine and total hip, and a trend towards lower mean Z-scores at the PA spine (p<0.10), compared to healthy women (p<0.05). ATYPICAL AN with a history of overweight/obesity had similar mean Z-scores at the lateral spine, total hip, and wrist, but significantly higher mean PA spine Z-scores (p<0.05), compared to ATYPICAL AN without a history of overweight/obesity. The majority of women with ATYPICAL AN and a history of overweight/obesity also had a history of low weight (n=13/18, 72%). Our cohort did not include a large enough sample of participants with ATYPICAL AN and a history of overweight/obesity but without a history of low weight to perform statistical analysis to determine whether such women, in the setting of rapid weight loss, have evidence of bone loss.
Regarding the impact of amenorrhea on the anorexia nervosa group as a whole (all participants with anorexia nervosa and atypical anorexia nervosa combined, n=147), both women with a history of amenorrhea (n=116/137, 85%) and women without a history of amenorrhea (n=21/137, 15%) had significantly lower mean BMD Z-scores at the PA spine, lateral spine, and total hip compared to healthy women (p<0.05). There was no difference in mean BMD Z-scores at the PA spine, lateral spine, total hip, or total radius between women with a history of amenorrhea and women without a history of amenorrhea. Women with current amenorrhea (n=52/137, 38%), current spontaneous menses (n=50/137, 36%), and current OCP use (n=35/137, 26%) all had significantly lower mean BMD Z-scores at the PA spine, lateral spine, and total hip compared to healthy women (p<0.001). Women with current spontaneous menses had significantly higher mean BMD Z-scores at the PA and lateral spine compared to women with current amenorrhea (p≤0.05), but similar mean BMD Z-scores at the total hip.
To determine whether a history of amenorrhea is a predictor of BMD in ATYPICAL AN, we compared mean BMD Z-scores in three groups: 1) ATYPICAL AN with a history of amenorrhea, 2) ATYPICAL AN without a history of amenorrhea, and 3) healthy comparison group (Figure 2B). ATYPICAL AN with a history of amenorrhea (n=59/71, 83%) had significantly lower mean Z-scores at the PA spine, lateral spine, and total hip compared to healthy women (p<0.001). In contrast, ATYPICAL AN with no history of amenorrhea (n=12/71, 17%) had mean Z-scores that were not statistically different than healthy women, although the mean Z-score was <0 at all sites. A BMD Z-score <−1.0 was present in 73% (n=43/59) of ATYPICAL AN with a history of amenorrhea vs 50% (n=6/12) of ATYPICAL AN without a history of amenorrhea (p=0.17). A BMD Z-score <−2.0 was present in 31% (n= 18/59) of ATYPICAL AN with a history of amenorrhea vs 8% (n= 1/12) of ATYPICAL AN without a history of amenorrhea (p=0.17). Of the women with ATYPICAL AN without a history of amenorrhea but a BMD Z-score <−1.0, 100% (n=6/6) had a history of low weight.
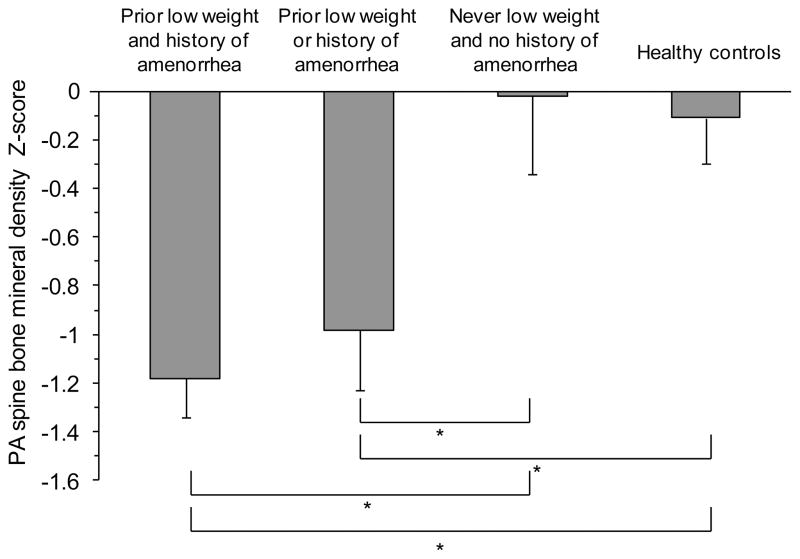
To determine the combined effect of prior weight and menstrual status in ATYPICAL AN, we compared mean PA spine BMD Z-scores in four groups: 1) ATYPICAL AN with prior low weight (BMI <18.5 kg/m2) AND history of amenorrhea, 2) ATYPICAL AN with prior low weight OR history of amenorrhea, 3) ATYPICAL AN with no history of low weight AND no history of amenorrhea, and 4) healthy comparison group (Figure 3). ATYPICAL AN with no history of low weight AND no history of amenorrhea (n=5/76, 7%) had a mean PA spine Z-score that was similar to healthy women. In contrast, both ATYPICAL AN with a history of low weight or amenorrhea (n=14/76, 18%) and ATYPICAL AN with a history of low weight and amenorrhea (n=50/76, 66%) had mean PA spine Z-scores that were significantly lower than healthy women (p<0.02). Sufficient numbers of other BMD sites were not available for comparison.
Figure 3.
Mean postero-anterior (PA) spine bone mineral density (BMD) Z-scores of four groups: 1) atypical AN with prior low weight (defined as BMI <18.5kg/m2) and history of amenorrhea, 2) atypical AN with prior low weight or history of amenorrhea, 3) atypical AN with no history of low weight or amenorrhea, and 4) healthy women. Mean ± SEM. * p ≤0.05.
Body Composition
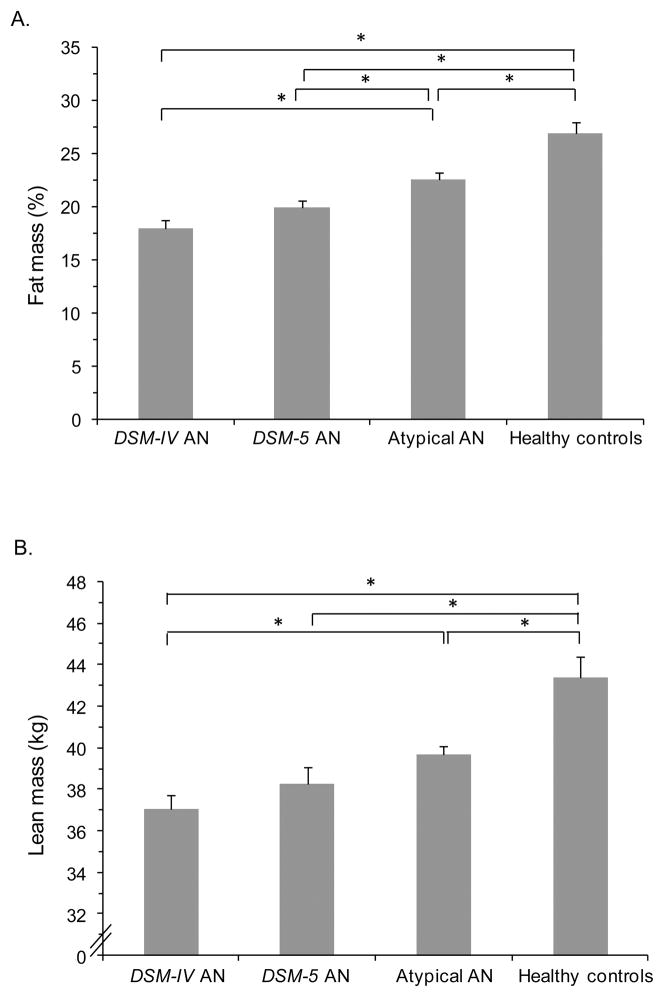
We compared body composition variables in four groups: 1) DSM-IV AN, 2) DSM-5 AN who did not meet the stricter DSM-IV criteria, 3) ATYPICAL AN, and 4) healthy comparison group (Figure 4). Mean lean mass and percent fat mass in DSM-5 AN were similar to DSM-IV AN, and significantly lower than healthy women (p<0.0001), with intermediate quantities in ATYPICAL AN (p ≤0.01). Although mean percent fat mass differed between DSM-5 and ATYPICAL AN (p<0.05), mean lean mass did not. Across the three AN groups, amenorrheic women had significantly lower mean percent fat mass compared to eumenorrheic women (p<0.0005). Across the groups, there was a positive linear relationship between lean mass and mean BMD Z-scores at the PA spine (R=0.34, p<0.0001), lateral spine (R=0.35, p=0.003), total hip (R=0.40, p<0.0001), and total radius (R=0.29, p=0.002). After adjusting for BMI, the relationship between lean mass and total hip and total radius Z-scores remained significant (p<0.05), and the relationship between lean mass and PA spine Z-score trended toward significance (p<0.10).
Figure 4.
Body composition measured by dual-energy x-ray absorptiometry (DXA) of women with anorexia nervosa (AN) spectrum disorders compared to a healthy comparison group. A. Mean percent fat mass in four groups: 1) DSM-IV AN, 2) DSM-5 AN who did not meet DSM-IV criteria, 3) atypical AN, and 4) healthy women. B. Mean lean mass in four groups: 1) DSM-IV AN, 2) DSM-5 AN who did not meet DSM-IV criteria, 3) atypical AN, and 4) healthy women. Mean ± SEM. * p ≤0.05.
Psychopathology
There was no difference in eating disorder, depression or anxiety scores across the three AN groups (Table 1). All AN groups had increased psychopathology relative to healthy women.
DISCUSSION
Our data suggest that despite broadening AN diagnostic criteria to include higher weight and eumenorrheic women, DSM-5 continues to capture a group of women across the AN spectrum with commensurately severe BMD impairment and psychopathology. Individuals with DSM-5 AN who do not meet the more stringent DSM-IV criteria have equally low BMD Z-scores as those with DSM-IV AN. Moreover, normal-weight women with atypical AN have BMD between women with DSM-IV AN and healthy women. However, absence of a history of low weight and amenorrhea may be relatively protective against low BMD in women with an anorexia nervosa spectrum eating disorder. In addition, both individuals with DSM-5 AN and atypical AN have significantly lower mean lean mass and percent fat mass than healthy women, suggesting that both fat and muscle mass decline with weight loss. This is the first report of direct statistical comparisons across DSM-IV AN, DSM-5 AN, atypical AN, and a healthy comparison group regarding BMD, body composition, and psychopathology, and suggests that women diagnosed with DSM-5 AN, and normal-weight women with atypical AN, should be considered for BMD screening, especially those with past low weight and/or amenorrhea. Mental health practitioners play a key role in assessing AN spectrum illnesses and as such need to be aware of these comorbidities.
It is well-known that women who meet DSM-IV AN criteria have low BMD; this study adds that women with DSM-5 AN not previously captured by the more stringent DSM-IV criteria and those with atypical AN also have significantly impaired BMD. Previous studies using DSM-IV AN criteria reported that >50% of women with AN have osteopenia, >30% have osteoporosis, and <15% have normal BMD at all skeletal sites (29,30). Women diagnosed with DSM-IV AN may have a seven-fold greater risk of fracture than expected for sex and age (31). A recent meta-analysis of a combined group of women diagnosed with AN using DSM-III, DSM-IV, or DSM-5 criteria reported a prevalence of osteoporosis of 22%, a prevalence of osteopenia of 46%, and a significantly increased risk of fracture compared to healthy women (32). Our study demonstrates that women who meet DSM-5 AN criteria, but who would not have met the stricter DSM-IV criteria, have mean BMD Z-scores that are similarly low to those with DSM-IV AN. Normal-weight women diagnosed with atypical AN, especially those with a history of low weight or amenorrhea, also have evidence of significant bone loss compared to healthy women. We previously demonstrated that in individuals with DSM-IV AN, amenorrhea negatively impacts BMD independent of BMI, especially at the PA spine (26,29), and that regaining of menses is a stronger predictor of bone mass recovery than weight gain (26). Our study expands these findings to the broader AN spectrum, and demonstrates that women with atypical AN and a history of low weight and/or amenorrhea have a significantly lower mean PA spine BMD Z-score compared to healthy women despite not being currently low weight. It is notable that a current higher weight does not fully protect against low BMD associated with an AN spectrum eating disorder in the setting of prior low weight and/or amenorrhea. Similarly, a history of overweight/obesity does not protect against low BMD in women with an AN spectrum eating disorder. Therefore, a history of prior low weight and/or amenorrhea remain important indications for DXA in women with DSM-5 AN and atypical AN. Given the small number of women in this study with atypical AN and no history of low weight nor amenorrhea, it is unclear whether all such women should be screened for bone loss; this is an area for future research.
We also demonstrate significant differences in body composition across DSM-IV AN, atypical AN, and healthy women, as well as between DSM-5 AN and healthy women. Mean percent fat mass was similarly low in women with DSM-IV and DSM-5 AN, higher in those with atypical AN, and highest in healthy women, with a similar pattern for lean mass except there was no difference between DSM-5 AN and atypical AN. DSM-5 is now capturing two new groups of women, DSM-5 AN and atypical AN, who share a characteristic feature with DSM-IV AN—low percent body fat. As BMI decreases from a healthy comparison group to atypical AN and DSM-IV AN, there is also concurrent loss of lean mass—not just fat mass—suggesting that loss of muscle mass is an unavoidable consequence of pathological food restriction, even though weight loss occurs in the setting of body image disturbance and “fat phobia.” Loss of lean mass may also contribute to low BMD.
Our data suggest that women with DSM-5 AN or atypical AN exhibit similarly severe eating-disorder-specific and associated psychopathology in comparison to women with DSM-IV AN, including on depression and anxiety rating scales. This is despite broadening diagnostic criteria for AN spectrum eating disorders in DSM-5 to include weight phobia as explicitly or implicitly present, liberalizing the weight criterion, and removing the amenorrhea requirement, which may all be markers of the severity of psychiatric disease. These data confirm that expanding the DSM-5 criteria for AN spectrum eating disorders did not dilute psychopathology severity.
Limitations of the study include its cross-sectional design, such that causality cannot be determined. Research participants may not be representative of women with anorexia nervosa and atypical anorexia nervosa in community samples. For example, most women with atypical AN in this study had a history of low weight whereas there may be significant numbers of women who present in the community with atypical anorexia nervosa who have never been low weight or who have lost weight from an overweight or obese state. We attempted to address this by separately evaluating the minority of women with atypical AN and no history of low weight, and we demonstrated that this subset of women had similar mean PA spine and total hip BMD Z-scores compared to healthy women. In addition, most women (94%, n=66/70) with low-weight AN had BMI between 16 and 18.5 kg/m2; the small number of participants with BMI <16 kg/m2 did not permit comparisons of BMI-severity groups as newly defined by DSM-5, but is typical of individuals participating in outpatient eating-disorder research. Other limitations include that not all participants had BMD measured at every site, which limited our ability to evaluate BMD Z-scores in smaller subgroups, serum was not available for hormone determination, and historical information about prior low weight and menstrual status was collected restrospectively via survey, and may not be entirely reliable.
In conclusion, impairments in BMD and psychopathology are prevalent and severe in women with DSM-5 AN and atypical AN despite broadening diagnostic criteria of AN spectrum eating disorders to include individuals who do not meet the strict weight and amenorrhea criteria of DSM-IV. Women with DSM-5 AN, who would not have been diagnosed as having AN by the more stringent DSM-IV criteria, have equally low BMD Z-scores as DSM-IV AN. In addition, more than two-thirds of normal-weight women with atypical AN have BMD Z-scores <−1.0 at one or more skeletal sites. It is notable that a higher weight does not fully protect against low BMD associated with an AN spectrum eating disorder in the setting of prior low weight and/or amenorrhea. This information is highly salient to psychiatry clinical practice because it demonstrates that despite the loosening of classification criteria, DSM-5 captures two new groups of women with significant psychopathology and low body fat – low-weight women with DSM-5 AN and normal-weight women with atypical AN who have a history of low weight and/or amenorrhea – who should be considered for BMD screening. Moreover, recognition that lean mass—not just fat mass—is lost with pathological food restriction may enhance motivation for change in women who fear that they will only gain weight as fat during weight restoration. In conclusion, BMD impairment and comorbid psychopathology in women with AN spectrum eating disorders extend beyond those with current low weight and amenorrhea as historically defined by DSM-IV.
Acknowledgments
Funding/support: This study was funded in part by the National Institutes of Health (Bethesda, MD, USA) with the following grants: T32 DK007028, K24 HL092902, R01 MH083657, R01 DK052625, M01 RR01066, UL1 RR025758, 8UL1 TR000170, 1UL 1TR001102
Footnotes
Disclosure of Conflicts: Nothing to declare
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: American Psychiatric Publishing; 2000. [Google Scholar]
- 2.Miller KK. Endocrine effects of anorexia nervosa. Endocrinol Metab Clin North Am. 2013 Sep;42(3):515–28. doi: 10.1016/j.ecl.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keshaviah A, Edkins K, Hastings ER, Krishna M, Franko DL, Herzog DB, et al. Re-examining premature mortality in anorexia nervosa: a meta-analysis redux. Compr Psychiatry. 2014 Nov;55(8):1773–84. doi: 10.1016/j.comppsych.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Mancuso SG, Newton JR, Bosanac P, Rossell SL, Nesci JB, Castle DJ. Classification of eating disorders: comparison of relative prevalence rates using DSM-IV and DSM-5 criteria. Br J Psychiatry. 2015 Jun;206(6):519–20. doi: 10.1192/bjp.bp.113.143461. [DOI] [PubMed] [Google Scholar]
- 6.Thomas JJ, Eddy KT, Murray HB, Tromp MDP, Hartmann AS, Stone MT, et al. The impact of revised DSM-5 criteria on the relative distribution and inter-rater reliability of eating disorder diagnoses in a residential treatment setting. Psychiatry Res. 2015 Sep 30;229(1–2):517–23. doi: 10.1016/j.psychres.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Smink FRE, van Hoeken D, Oldehinkel AJ, Hoek HW. Prevalence and severity of DSM-5 eating disorders in a community cohort of adolescents. Int J Eat Disord. 2014 Sep;47(6):610–9. doi: 10.1002/eat.22316. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Marti CN, Rohde P. Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. J Abnorm Psychol. 2013 May;122(2):445–57. doi: 10.1037/a0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birgegård A, Norring C, Clinton D. DSM-IV versus DSM-5: implementation of proposed DSM-5 criteria in a large naturalistic database. Int J Eat Disord. 2012 Apr;45(3):353–61. doi: 10.1002/eat.20968. [DOI] [PubMed] [Google Scholar]
- 10.Keel PK, Brown TA, Holm-Denoma J, Bodell LP. Comparison of DSM-IV versus proposed DSM-5 diagnostic criteria for eating disorders: reduction of eating disorder not otherwise specified and validity. Int J Eat Disord. 2011 Sep;44(6):553–60. doi: 10.1002/eat.20892. [DOI] [PubMed] [Google Scholar]
- 11.Nakai Y, Fukushima M, Taniguchi A, Nin K, Teramukai S. Comparison of DSM-IV versus proposed DSM-5 diagnostic criteria for eating disorders in a Japanese sample. Eur Eat Disord Rev. 2013 Jan;21(1):8–14. doi: 10.1002/erv.2203. [DOI] [PubMed] [Google Scholar]
- 12.Ekeroth K, Clinton D, Norring C, Birgegård A. Clinical characteristics and distinctiveness of DSM-5 eating disorder diagnoses: findings from a large naturalistic clinical database. J Eat Disord. 2013;1:31. doi: 10.1186/2050-2974-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metropolitan Life Foundation. New weight standards for men and women. Statistical Bulletin Metropolitan Life Insurance Company. 1959;40:1–4. [Google Scholar]
- 14.Paffenbarger RS, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993 Jan;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Mazess R, Barden H, Bisek J, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 16.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994 Dec;16(4):363–70. [PubMed] [Google Scholar]
- 17.Garner DM. Eating Disorder Inventory-2 Professional Manual. Odessa, FL: Psychological Assessmet Resources; 1991. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Lawson E, Donoho D, Miller K, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–6. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson E, Eddy K, Donoho D, Misra M, Miller K, Meenaghan E, et al. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011;164:253–61. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson E, Donoho D, Blum J, Meenaghan E, Misra M, Herzog D, et al. Decreased nocturnal oxytocin levels in anorexa nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011;72:1546–51. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson E, Miller K, Blum J, Meenaghan E, Misra M, Eddy K, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf) 2012;76:520–5. doi: 10.1111/j.1365-2265.2011.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011 Jul;96(7):2081–8. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddy KT, Lawson EA, Meade C, Meenaghan E, Horton SE, Misra M, et al. Appetite regulatory hormones in women with anorexia nervosa: binge-eating/purging versus restricting type. J Clin Psychiatry. 2015 Jan;76(1):19–24. doi: 10.4088/JCP.13m08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, et al. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004 Sep;89(9):4434–8. doi: 10.1210/jc.2004-0720. [DOI] [PubMed] [Google Scholar]
- 27.Meier U. A note on the power of Fisher’s least significant difference procedure. Pharm Stat. 2006;5:253–63. doi: 10.1002/pst.210. [DOI] [PubMed] [Google Scholar]
- 28.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000 Nov 21;133(10):790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller K, Grinspoon S, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nerovsa. Arch Intern Med. 2005;165:561–6. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 31.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991 Mar 6;265(9):1133–8. [PubMed] [Google Scholar]
- 32.Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatrica Scandinavica. 2016 Jan; doi: 10.1111/acps.12556. n/a – n/a. [DOI] [PubMed] [Google Scholar]