Abstract
Background
T regulatory cells attenuate development of asthma in wild-type (WT) mice with both naturally occurring (nTregs) and inducible T regulatory cells (iTregs) exhibiting suppressive activity. When transferred into CD8-deficient (CD8−/−) recipients, both cell types enhanced development of allergen-induced airway hyperresponsiveness (AHR).
Objective
To determine if the pathways leading to enhancement of lung allergic responses by transferred nTregs and iTregs differed.
Methods
nTregs (CD4+CD25+) were isolated from WT mice and iTregs were generated from WT CD4+CD25− T cells following activation in the presence of TGF-β and transferred into sensitized CD8−/− recipients prior to challenge. Development of AHR, cytokine levels, and airway inflammation were monitored.
Results
Transfer of nTregs enhanced lung allergic responses as did transfer of iTregs. While anti-IL-13 reduced nTreg-mediated enhancement, it was ineffective in iTreg-mediated enhancement; conversely, anti-IL-17 but not anti-IL-13 attenuated the enhancement by iTregs. Recovered iTregs from the lungs of CD8−/− recipients were capable of IL-17 production and expressed high levels of signature genes of the Th17 pathway, RORγt, and Il17, while reduced expression of the Treg key transcription factor Foxp3 was observed. In vitro, exogenous IL-6-induced IL-17 production in the iTregs and in vivo, conversion of transferred iTregs was dependent on recipient IL-6.
Conclusions
iTregs similar to nTregs exhibit functional plasticity and can be converted from suppressor cells to pathogenic effector cells enhancing lung allergic responses, but these effects were mediated through different pathways.
Keywords: Inducible and natural Tregs, suppression, enhancement, asthma, IL-17, IL-13
INTRODUCTION
Thymus-derived naturally occurring CD4+CD25+ T regulatory cells (nTregs), characterized by constitutive expression of the transcription factor forkhead box p3 (Foxp3), are critical in immune tolerance and homeostasis. Defects in their function and/or numbers resulted in immunological imbalance and autoimmunity as observed in patients with X-linked immune dysregulation, polyendocrinopathy, and enteropathy (IPEX) syndrome (1) and Scurfy mice (2). Other regulatory T cells have been described (3) including CD4+CD25− T cells which acquire regulatory function and phenotype by expressing Foxp3 in non-thymic tissue and in vitro following culture with TGF-β (4, 5). The immunomodulatory activities of these distinct subpopulations may be complementary in maintaining immune homeostasis and overlap, albeit with differing efficiencies, reflecting differences in developmental requirements (3), activation (6, 7), and functional stability (8–10).
In both humans and animals, allergic asthma is an inflammatory disease of the airways characterized by increases in airway hyperresponsiveness (AHR) and inflammation, type 2 cytokine skewing, goblet cell metaplasia, excessive mucus production, elevated antigen-specific IgE, and structural remodeling of the airways. Both nTregs and inducible Tregs (iTregs) have been shown to be effective regulators of lung responses following allergen sensitization and challenge (11). In part, these suppressive activities were linked to IL-10 and TGF-β released from regulatory T cells (12, 13), in both an antigen-specific (14) and antigen-nonspecific manner (15, 16). Interestingly, these suppressive activities were demonstrated following adoptive transfer into wild-type (WT) recipients (10, 16) but not in CD8-deficient (CD8−/−) recipients. In CD8−/− recipients, these same nTregs were shown capable of converting in vivo into pathogenic IL-13-producing T effector cells, enhancing the full spectrum of lung allergic responses. This enhancement was modulated by the glucocorticoid-induced TNFR-related protein (GITR)-dependent activation of JNK2 (8–10). Direct interactions between CD8+ T cells and nTregs were demonstrated (17) and shown to be necessary for expression of suppressor activity (6) and development of regulatory activities (12, 16). In the absence of CD8 (CD8−/− mice) or following antibody-mediated depletion of CD8+ T cells, the suppressive function of CD4+CD25+ T cells was attenuated, Foxp3 levels were reduced, as was the production of IL-10 and TGF-β (6, 8). In contrast, IL-6 levels in these cells were markedly increased (18). In CD8−/− mice, the loss of suppression was not terminally fixed as reconstitution (via transfer of CD8+ T cells) of CD8-deficient mice restored the regulatory function and phenotype of nTregs, suggesting reprogramming remained possible (18).
It is now evident that several subsets of T cells with similar phenotypes are capable of regulating the development of lung allergic responses. The functional fidelity of nTregs has been investigated and illustrated a plasticity that was dependent on the integration of signals from the local cytokine environment, stimulatory factors, and cell-to-cell interactions. Both loss of regulatory function and concomitant gain of effector function under certain inflammatory conditions (8, 19, 20) and loss of suppression without apparent gain of effector function following stimulation with a GITR agonist antibody (8), GITR ligand (GITRL) (9, 10), and IL-6 (18, 21, 22) have been reported for nTregs. In contrast, iTregs (CD4+CD25− T cells differentiated in the presence of TGF-β) have been less well studied in terms of their functional plasticity. In the present study, the regulatory and effector functions of nTregs and iTregs were compared. Both subsets effectively suppressed the development of lung allergic responses when transferred into sensitized and challenged WT mice. In contrast, when transferred into sensitized and challenged CD8−/− recipients, both subsets triggered the enhancement of lung allergic responses. However, unlike the IL-13-dependent enhancement demonstrated for nTregs, iTregs appeared to mediate increases in lung allergic responses through IL-17, augmenting ongoing type 2-mediated inflammatory responses.
MATERIALS AND METHODS
Animals
Pathogen-free, 6–8 week old female CD8α−/− and IL-13−/− mice were obtained from existing colonies (CD8α−/− mice (CD90.2) were provided by Dr. Philippa Marrack, verified by FACS, and IL-13−/− mice were provided by Dr. Dale Utmetsu, verified by PCR). C57BL/6 (CD90.1) and IL-17−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were maintained on an ovalbumin (OVA)-free diet. All protocols were approved by the Institutional Animal Care and Use Committee at National Jewish Health.
Sensitization and Challenge
Sensitization was carried out by intraperitoneal injection of 20 μg OVA (Sigma Aldrich, St. Louis, MO) emulsified in 2.25 mg alum hydroxide (AlumImject; Pierce, Rockford, IL) in a total volume of 100 μl on days 1 and 14. Sensitized and challenged mice, denoted OVA/OVA, and non-sensitized but challenged littermates (PBS/OVA) received aerosol challenges for 20 minutes each day on three consecutive days (days 26, 27, and 28) with 1% OVA in PBS using an ultrasonic nebulizer (Omron, Vernon Hills, IL) (21).
Measurement of Airway Responsiveness
Airway responsiveness was assessed as previously described (21). Changes in airway function to increasing concentrations of aerosolized methacholine (MCh) administered for 10 seconds (60 breaths/min, 500 μl tidal volume) were monitored. Lung resistance (RL) was continuously computed (Labview, National Instruments, Austin, TX) by fitting flow, volume, and pressure to an equation of motion. Maximum values of RL were taken and expressed as a percentage change from baseline following PBS aerosol.
Bronchoalveolar Lavage (BAL)
Immediately following measurement of AHR, lungs of each animal were lavaged once with 1 ml of Hank’s Balanced Salt Solution, 1X (HBSS, 1X) from Mediatech Inc. (Manassa, VA). Total leukocyte numbers were counted (Countess Automated Cell Counter, Invitrogen Corporation, Carlsbad, CA) and differential cell counts were performed in a blinded manner under light microscopy by counting at least 200 cells on cytocentrifuged preparations (Cytospin 2; Cytospin, Shandon Ltd., Runcorn, Cheshire, UK), stained with Leukostat (Fisher Diagnostics, Middletown, VA) and differentiated by standard hematological procedures.
Cell Preparation and Culture
CD4+CD25+ and CD4+CD25− T cells were enriched from spleens of naive C57BL/6 mice. Lymphocytes were further sorted by positive selection for CD4+CD25+ regulatory T cells using magnetic beads, resulting in a purity of >95% CD4+CD25+ cells. Following depletion of CD4+CD25+ cells, CD4+CD25− T cells in the negative fraction were re-isolated by positive selection for CD4+ T cells to >98% purity with MACS beads (Miltenyi Biotec, Bergisch-Gladbach, Germany).
Cells were washed, counted, and resuspended to a final concentration of 4x106 cells per ml in complete RPMI 1640 (Mediatech Celgro, Manassas, VA) tissue culture medium, containing heat-inactivated fetal calf serum (FCS 10%; Sigma-Aldrich), L-glutamine (5 mM), β-mercaptoethanol (2 mM), hepes buffer (15 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from Gibco, Grand Island, NY).
In Vitro Differentiation and Adoptive Transfer
Isolated CD4+CD25− T cells were cultured in X-Vivo 15 (Lonza, Wakersville, MD) supplemented with IL-2 (5 ng/ml), stimulated with bound anti-CD3 and soluble anti-CD28 (2 μg/ml) with and without TGF-β (5 ng/ml) (23), and together with or without IL-6 (5 ng/ml) for five to seven days. Differentiated cells were washed and adoptively transferred (5x105 in 50 μL of PBS) into sensitized and non-sensitized recipients prior to allergen challenge. In some experiments, intratracheal instillation of recombinant protein IL-17 (200 pg), anti-IL-13, anti-IL-17, anti-GITRL, anti-OX40, and control antibodies (100 μg) was also performed. Reagents were from eBioscience (San Diego, CA) and R&D Systems (Minneapolis, MN).
In some experiments, transferred cells (CD90.1+) were recovered by MACS bead sort following collagenase digestion of lungs from recipient mice (CD90.2+) and enriched using nylon wool columns as previously described (12).
Measurement of Cytokine Levels
Cytokine levels in the BAL fluid and supernatants of in vitro cultured cells were measured by ELISA: IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IL-21, IL-22, IFN-γ, and TGF-β from eBioscience (San Diego, CA). ELISAs were performed according to the manufacturers’ directions. The limits of detection were 4 pg/ml for IL-4, IL-5, IL-6, and IL-17; 10 pg/ml for IL-10 and IFN-γ;8 pg/ml for IL-13 and IL-22; and 6 pg/ml for TGF-β and 16 pg/ml for IL-21
FACS Analysis
Isolated cells, following preincubation with naive mouse serum in staining buffer (PBS, 2% FCS, 0.2% sodium azide), were labeled with the following conjugated antibodies purchased from BD Biosciences Pharmingen: anti-CD3 FITC, PE, PerCP, APC (17A2); anti-CD4 FITC, PE, PerCP, APC (L3T4); anti-CD25 FITC (7D4), PE (PC61); anti-GITR FITC, PE, APC; CD152 FITC, PE APC. For intracellular staining, cells were stimulated with PMA (100 ng/ml) and ionomycin (2 μg/ml, Sigma-Aldrich) in complete medium overnight and for 6 hrs in the presence of brefeldin A (10 μg/ml, Sigma-Aldrich). Cells were fixed with 4% formaldehyde in PBS, permeabilized in 0.5% saponin, and stained with anti-IL-10 PE, APC (JES5-16E3); IL-17 PE, APC (eBio17B7); IFN-γ PE, APC (XMG1.2); TGF-β PE, APC (A75-3.1) Foxp3 PE, APC (FJK-16s); and ROR-γt PE, APC (AFKJS-9) (eBioscience). Fluorochrome (FITC, PE, PerCPAPC)-labeled isotype-matched control antibodies were used for background fluorescence staining. Staining was analyzed by FACScalibur flow cytometry (BD Pharmingen,) using CellQuest Pro software. Fluorescence intensity was compared to cells stained with corresponding labeled isotype-matched control antibodies.
Real-Time PCR (RT-PCR)
Total RNA was prepared using NucleoSpin RNA II Isolation Kit (Macherey- Nagel, Duren, Germany). Complementary DNA (cDNA) was synthesized with equal amounts of RNA (0.25μg from recovered cells and 1.0μg from in vitro-differentiated cells) using QuantiTect reverse transcription kit (Qiagen, Valencia, CA). Pre-designed primers and probes for mouse Il10 (Mm00439614_m1), Il13 (Mm00434205_g1), Il17 (Mm00439618_m1), Foxp3 (Mm00475162_m1), RORγt (Mm01261022_m1), Tgfβ (Mm01227699_m1), and housekeeping gene GAPDH (Mm99999915_g1), were obtained from ThermoFisher Scientific (Marietta, OH). RT-PCR was performed using the ABI Prism 7000 Sequence Detection System. Analysis of relative gene expression was carried out using the mathematical calculation model based on delta delta Ct value, assigning expression levels of nTregs and in some experiments iTregs at 1 as described (24).
Statistical Analysis
Analysis of variance (ANOVA) was used to determine statistical significance. Comparisons for all pairs were performed by Tukey-Kramer highest significant difference test. The p values for significance were set to 0.05. Values for all measurements were expressed as the mean±standard error of the mean (SEM).
RESULTS
Inducible Tregs Regulate Development of Lung Allergic Responses in Sensitized and Challenged WT Mice
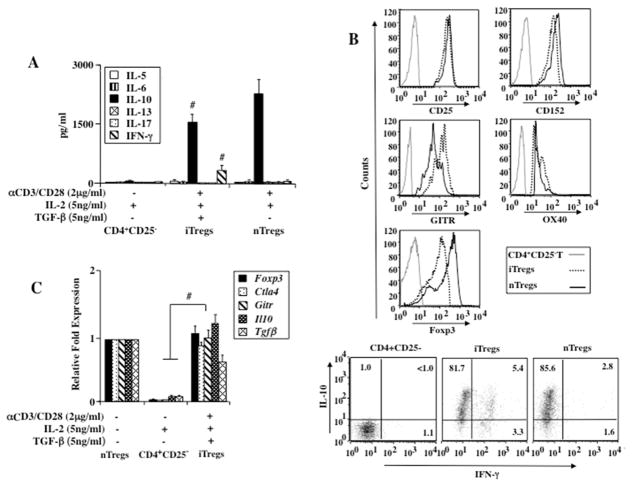
Naturally occurring T regulatory cells were shown to be potent suppressors of lung allergic responses in sensitized and challenged WT recipients (10, 16). The regulatory capacity of in vitro-derived iTregs was investigated in a similar model of experimental asthma. When compared to nTregs, iTregs generated from purified naive CD4+C25− T cells in the presence of TGF-β produced significant amounts of IL-10 and moderate amounts of IFN-γ but negligible amounts of IL-5, IL-6, IL-13, and IL-17 as detected by ELISA (Fig. 1A) and FACS (>80% IL-10+iTregs, Fig. 1B). Furthermore, similar levels of the transcription factor Foxp3 and CD25, CTLA4, GITR, and OX40 gene expression by RT-PCR and protein levels by flow cytometry were expressed in nTregs and iTregs (Figs. 1B, 1C). In contrast, none of these changes were detected in naive CD4+C25− T cells under non-polarizing (without TGF-β) conditions.
Figure 1.
Effect of nTreg, iTreg, and CD4+CD25− T cell transfer from naive donors into sensitized and challenged CD8+/+ recipients. (A) In vitro cytokine production from nTregs, iTregs, and CD4+CD25− T cells, (B) Flow cytometry of nTregs, iTregs, and CD4+CD25− T cells, (C) Gene expression levels of nTregs, iTregs, and CD4+CD25− T cells prior to transfer, #p<0.05 comparing CD4+CD25− T cells to iTregs and nTregs, (D) AHR, (E) BAL fluid inflammatory cell composition, (F) BAL fluid cytokine levels. Shown are the means±SEM from 3 independent experiments (4 mice/group, n=12). *p<0.01 comparing sensitized and challenged mice (given PBS) to mice challenged alone. †p<0.05 comparing sensitized and challenged recipients of iTregs and nTregs to recipients of PBS or CD4+CD25− T cells.
Consistent with previous studies (10, 16), sensitized and challenged WT mice given PBS or naive CD4+CD25− T cells developed significant AHR to increasing concentrations of aerosolized MCh as measured by changes in RL, eosinophilic inflammation, and increased type 2 cytokines in the bronchoalveolar lavage (BAL) fluid (Figs. 1D–1F). Transfer of nTregs or iTregs decreased AHR and eosinophilic inflammation. Levels of IL-5, IL-6, and IL-13 were lower in BAL fluid whereas increased levels of IL-10 and TGF-β were detected. Taken together, the data demonstrated that iTregs and nTregs exhibited similar gene and protein profiles and were effective regulators of lung allergic responses when transferred into sensitized and challenged WT recipients.
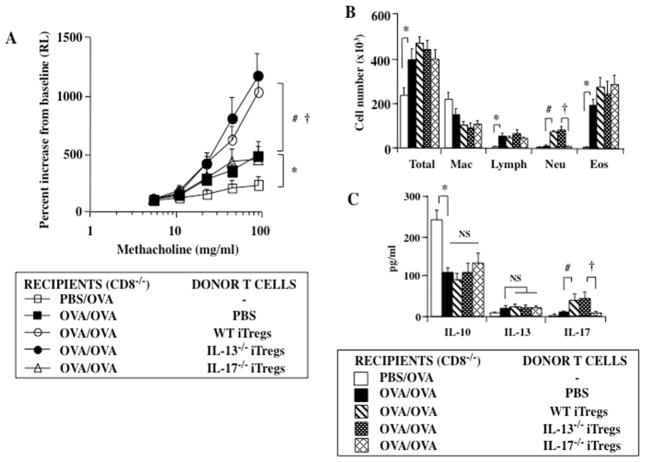
iTregs Enhance Lung Allergic Responses in Sensitized and Challenged CD8−/− Mice
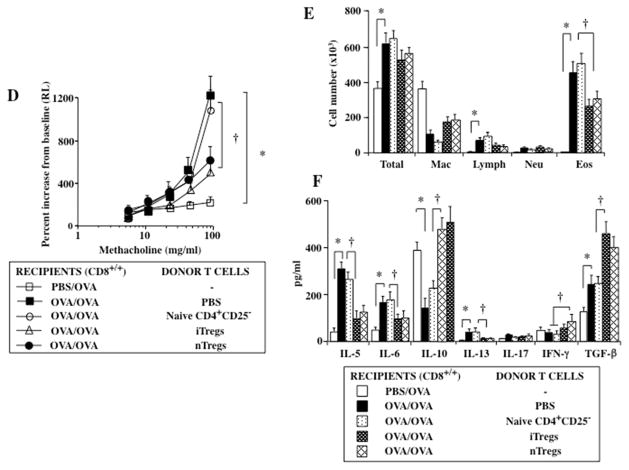
Unlike WT mice, CD8−/− mice developed limited lung allergic responses following sensitization and challenge (Figs. 2A, 2B), as previously shown (6, 8–10). In contrast to the suppression of lung allergic responses seen following transfer into sensitized and challenged WT recipients, nTregs were shown to enhance lung allergic responses, AHR, and eosinophilic inflammation when transferred into sensitized and challenged CD8−/− recipients (Figs. 2A, 2B). Associated with these changes were increases in BAL fluid levels of IL-5 and IL-13.
Figure 2.
Effect of nTreg, iTreg, and CD4+CD25− T cell transfer from naive donors into sensitized and challenged CD8−/− recipients. (A) AHR, (B) BAL fluid inflammatory cell composition, (C) BAL fluid cytokine levels. Shown are the means±SEM from 3 independent experiments (4 mice/group, n=12). *p<0.05 comparing sensitized and challenged mice (given PBS) to mice challenged alone. †p<0.05 comparing sensitized and challenged recipients of iTregs and nTregs to recipients of PBS or CD4+CD25− T cells. (D) Gene expression of recovered naive CD4, iTregs, Th17, and nTregs (compared to levels of pre-transferred iTregs set at 1) #p<0.05, (increased), †p<0.05 (decreased), Xp<0.05 comparing iTregs to Th17 cells.
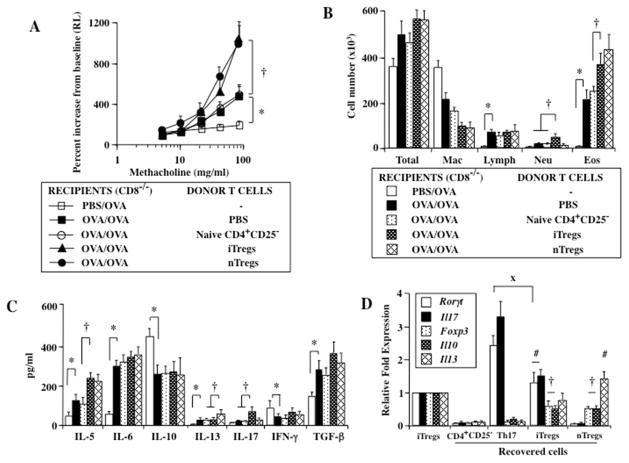
Transfer of iTregs into sensitized and challenged CD8−/− recipients, similar to nTregs, resulted in significantly increased AHR, beyond levels induced following sensitization and challenge alone (Fig. 2A). While increases in total cells, lymphocytes, and eosinophils in the BAL fluid were detected in sensitized and challenged CD8−/− mice compared to those challenged alone, further increases in eosinophil and neutrophil numbers were observed in recipients of iTregs (Fig. 2B). This was associated with increased levels of IL-5 and IL-17 in the BAL fluid of sensitized and challenged CD8−/− recipients; unlike transfer of nTregs, levels of IL-13 were not increased following transfer of iTregs (Fig. 2C).
To further elucidate the molecular basis for the conversion of transferred cells, expression of Foxp3, Il10, Tgfβ,, RORγt, Il13, and Il17 in transferred and recovered nTregs and iTregs from the lungs of sensitized and challenged recipients were analyzed by real-time PCR (RT-PCR); gene expression levels were normalized to levels seen in iTregs prior to transfer (taken as baseline and assigned a value of 1). Transferred nTregs and iTregs (CD90.1+) were recovered from the lungs of CD90.2+ recipient mice, which after selection comprised more than 98% of the cells. In parallel to the changes in lung physiology and inflammation, similar to findings in transferred and recovered nTregs, significant decreases in gene expression of Foxp3 and Il10 were detectable in iTregs. nTregs expressed negligible levels of RORγt and Il17 (Fig. 2D), whereas iTregs recovered from the lungs exhibited increased RORγt and Il17 expression (Fig. 2D). Unlike nTregs which expressed high levels of , Il13, little gene expression was detected in the iTregs. When compared to in vitro differentiated Th17 cells, expression levels of RORγt and Il17 in recovered iTregs were lower and the mRNA levels of Foxp3 and Il10 remained higher. These data demonstrated that TGF-β-induced Foxp3+ iTreg-mediated enhancement of lung allergic responses following transfer into sensitized and challenged CD8−/− recipients was associated with increased levels of IL-17 gene and protein expression, unlike the findings in nTregs.
Anti-IL-17 but not Anti-IL-13 Prevents iTreg-Mediated Enhancement of Lung Allergic Responses
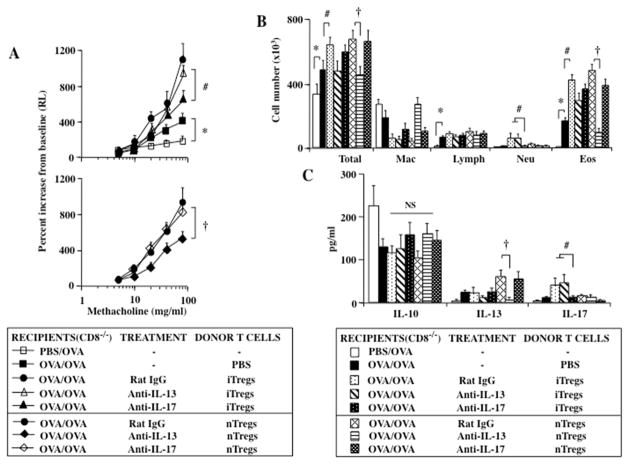
To determine if IL-17 was essential to the iTreg-mediated enhancement of lung allergic responses in CD8−/− recipients, anti-IL-13 or anti-IL-17 was administered to sensitized CD8−/− recipients just prior to the intratracheal transfer of iTregs or nTregs and allergen challenge. In contrast to recipients of iTregs treated with control antibody, administration of anti-IL-17 but not anti-IL-13 attenuated the enhancement following iTreg transfer, with significant reductions in AHR and airway neutrophilia, but little change in airway eosinophilia (Figs. 3A–C). Paralleling the changes in lung physiology, administration of anti-IL-17 resulted in a significant reduction in the amount of IL-17 detected in the BAL fluid with little change in IL-13 levels (Fig. 3C). Conversely, treatment with anti-IL-13 prevented the enhancement mediated by nTregs in association with decreased BAL levels of IL-13 and airway eosinophilia compared to responses in recipients of nTregs treated with control antibody. Neither treatment significantly altered levels of IL-4 or IL-5 (data not shown). These data demonstrated that the events associated with the functional plasticity and pathogenic conversion of iTregs and nTregs for the enhancement of lung allergic responses were clearly distinguishable; iTregs converting into Th17 cells producing IL-17 and nTregs becoming type 2 effector cells releasing IL-13.
Figure 3.
Effect of administrating anti-IL-13 or anti-IL-17 antibody on enhancement of lung allergic responses in sensitized and challenged CD8−/− recipients of iTregs and nTregs. (A) AHR, (B) BAL fluid inflammatory cell composition, (C) BAL fluid cytokine levels. Shown are the means±SEM from 3 independent experiments (4 mice/group, n=12). *p<0.05 comparing sensitized and challenged mice (given PBS) to mice challenged alone. #p<0.05 comparing recipients of iTregs given control and anti-IL-13 antibody to recipients given anti-IL-17. †p<0.05 comparing recipients of nTregs given control and anti-IL-17 antibody to recipients given anti-IL-13 antibody.
IL-13−/− but not IL-17−/− iTregs Enhance Lung Allergic Responses in Sensitized and Challenged CD8−/− Mice
The critical role of IL-17 but not IL-13 in the iTreg-mediated enhancement of lung allergic responses in sensitized and challenged CD8−/− mice was further demonstrated by transferring iTregs generated from naive CD4+CD25− T cells isolated from IL-13−/− and IL-17−/− mice. In contrast to iTregs from WT or IL-13−/− mice, iTregs from IL-17−/− mice failed to increase AHR or airway neutrophilia in sensitized and challenged CD8−/− recipients. Numbers of eosinophils in these recipients were unchanged as were levels of IL-10 and IL-13; levels of IL-17 in BAL fluid were significantly lower compared to recipients of WT and IL-13−/− iTregs (Figs. 4A–C).
Figure 4.
Effect of iTregs from WT, IL-13−/−, and IL-17−/− mice on enhancement of lung allergic responses in sensitized and challenged CD8−/− recipients. (A) AHR, (B) BAL fluid inflammatory cell composition, (C) BAL fluid cytokine levels. Shown are the means±SEM from 3 independent experiments (4 mice/group, n=12). *p<0.05 comparing sensitized and challenged mice (given PBS) to mice challenged alone. #p<0.05 comparing recipients of WT and IL13−/− iTregs to sensitized and challenged mice (given PBS). †p<0.05 comparing recipients of IL-13−/− to IL-17−/− iTregs.
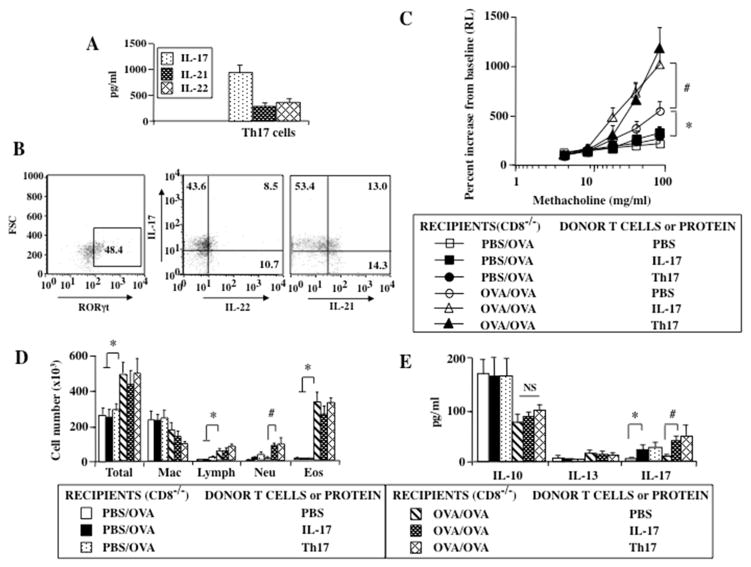
Th17 Cells or Exogenous IL-17 Enhance Lung Allergic Responses in Sensitized and Challenged CD8−/− Recipients
A role for Th17 cells and airway neutrophilia in asthma has been reported in humans and mice, although the results were somewhat inconsistent (25, 26). An association with a more severe asthma phenotype that responds poorly to treatment with corticosteroids has also been reported (27). The combination of polarizing cytokines IL-6 and TGF-β was critical for the in vitro and in vivo generation of Th17 cells (24, 28, 29) and conversion of Tregs into Th17 cells (30, 31). Although the evidence suggested that transferred iTregs had the capacity to acquire a Th17 phenotype expressing the transcription factor Rorγt and became IL-17-producing cells in the lungs of recipient mice, it was not clear whether or not the enhancement of lung allergic responses was dependent solely on Th17 cells and IL-17. To this end, the effect of administering in vitro -generated Th17 cells and exogenous IL-17 protein was assessed in sensitized and challenged and challenged alone CD8−/− mice. Purified naive CD4+CD25− T cells were differentiated into Th17 cells under polarizing conditions with IL-6 and TGF-β (28, 29, 31). In contrast to cells stimulated with TGF-β alone, increased levels of IL-17, IL-21, and IL-22 were detected in supernatants of cells cultured together with IL-6 (Fig. 5A).
Figure 5.
Effect of administrating Th17 cells or IL-17 on lung allergic responses in sensitized and challenged and challenged only CD8−/− recipients. (A) Cytokine production and (B) FACS analysis of Th17 cells, (C) AHR, (D) BAL fluid cell composition, (E) BAL fluid cytokine levels. Results shown are means±SEM from 3 independent experiments, n=12. *p<0.05 comparing sensitized and challenged mice (given PBS) to challenged alone given PBS, IL-17, or Th17 cells. #p<0.05 comparing sensitized and challenged recipients of IL-17 protein and Th17 cells to recipients of PBS.
Intratracheal administration of Th17 cells or IL-17 protein into sensitized and challenged CD8−/− recipients resulted in the enhancement of AHR, similar to findings observed following transfer of iTregs (Fig. 5B). The enhancement of AHR seen with Th17 cells or IL-17 was associated with increased airway neutrophilia and BAL IL-17 levels; airway eosinophilia was unaltered (Figs. 5C, 5D). However, Th17 cells or IL-17 alone in the absence of sensitization were insufficient to induce significant AHR, eosinophilic and neutrophilic inflammation, or alter cytokine levels (other than IL-17) in CD8−/− recipients. These data implied that the immune status and local inflammatory environment in recipient mice contributed to the enhancement of lung allergic responses. In the absence of ongoing lung inflammation, Th17 cells or IL-17 had little impact on these changes.
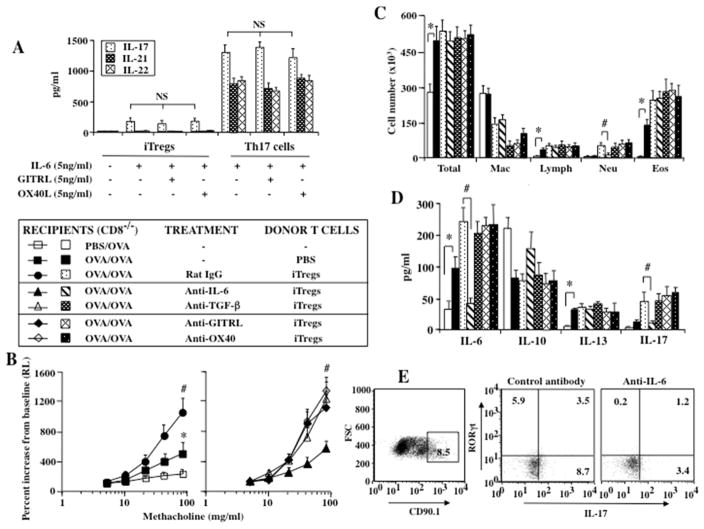
Anti-IL-6 but not Anti-TGF-β, Anti-GITR, or Anti-OX40 Prevents Conversion of iTregs in Sensitized and Challenged CD8−/− Mice
Since iTregs in the absence of exogenous IL-6 did not produce IL-17 in vitro (Fig. 6A), the detection of IL-17 in BAL fluid coupled with enhancement of lung allergic responses suggested that the recipient mice may be the source of IL-6. Further, despite expression of the surface molecules GITR and OX40, production of IL-17, IL-21, and IL-22 from iTregs detected following addition of IL-6 and Th17 cells was not influenced by ligation of GITR or OX40, unlike the alterations seen with nTregs exposed to these proteins in previous studies (8–10).
Figure 6.
Effect of administrating control IgG, anti-IL-6, anti-TGF-β, anti-GITRL, or anti-OX40 antibody on lung allergic responses in sensitized and challenged CD8−/− recipients of iTregs. (A) Effect of GITRL and OX40L on in vitro cytokine production from iTregs and Th17 cells. (B) AHR, (C) BAL fluid inflammatory cell composition, (D) BAL fluid cytokine levels, (E) Flow cytometry of recovered iTregs. Shown are the means±SEM from 3 independent experiments (4 mice/group, n=12). *p<0.05 comparing sensitized and challenged mice (given PBS) to mice challenged alone. #p<0.05 comparing recipients of iTregs given control and anti-TGF-β, anti-GITRL, and anti-OX40 antibody to recipients given anti-IL-6.
Increased levels of IL-6 following sensitization and challenge were reported in both WT and CD8−/− mice (10, 18, 24). The role of host (recipient) IL-6 and TGF-β in the in vivo development of iTregs into IL-17+ T effector cells was assessed by administrating anti-IL-6 or anti-TGF-β to recipients prior to the transfer of iTregs. The effects of anti-GITR and anti-OX40 on iTregs in vivo was also investigated. In recipients of iTregs, administration of anti-IL-6, but not anti-TGF-β, anti-GITRL, or anti-OX40, prevented the enhancement of lung allergic responses and airway neutrophilia in association with lower levels of IL-17 in the BAL fluid (Figs. 6B–6D). Since only IL-6 appeared critical to enhancement, transferred CD90.1+ cells were recovered from the lungs of CD90.2+ recipients for flow cytometry analysis of intracellular IL-17 and RORγT. Fewer cells from anti-IL-6-treated mice expressed IL-17 and RORγt compared to those that received control antibody (Fig. 6E). These findings confirmed that host-derived IL-6 was essential to the pathogenic conversion of iTregs to IL-17 production in the airways of recipient mice.
DISCUSSION
Constitutive expression of the lineage-specific “master” transcription factor Foxp3 in CD4+CD25+ nTregs confers a set of unique surface markers, development-related anergy, and capacity to produce important immunoregulatory cytokines such as IL-10 and TGF-β (3). The critical role of nTregs in the maintenance of immunological tolerance and prevention of autoimmunity is highlighted in human IPEX disease and in the Scurfy mouse (1, 2). As observed in many T cell subsets, the ability of nTregs to exhibit a suppressive phenotype does not appear terminally fixed so that under different environmental conditions, phenotypic and functional plasticity was demonstrated with changes in gene expression, surface receptor expression, and cytokine production (6, 8). As a result, the in vivo functional activity of nTregs was altered from suppression of lung allergic responses when transferred into sensitized and challenged WT recipients compared to enhancement of these responses following transfer into CD8−/− recipients. The use of CD8−/− mice to reveal the functional conversion and transcriptional reprogramming of the nTregs was in keeping with data demonstrating the critical need for CD8-nTreg interactions to maintain the suppressive phenotype. Loss of the suppressive phenotype was observed when the CD8 signal was eliminated and nTregs became producers of IL-6 (18). Enhancement of lung allergic responses was shown to be dependent on the production of IL-13 by the nTregs themselves (8–10). In the present study, we compared the events associated with nTreg conversion to that of iTregs under similar conditions. The data identified comparable suppressive activity in both cell types when transferred into WT recipients and conversion to an enhancing phenotype in CD8−/−recipients, but these outcomes were mediated through different pathways.
An experimental model of asthma with MCh-induced AHR, airway eosinophilia, and type 2 cytokine production was used to monitor the in vivo activities of nTregs and iTregs following transfer into sensitized and challenged WT and CD8−/− recipients. Similar to other studies (4, 7, 11), iTregs were effective regulators of these lung allergic responses, suppressing the development of AHR and eosinophilic inflammation in WT recipients when transferred after sensitization and prior to allergen challenge. Overall, the suppressive activity exhibited by iTregs was comparable to that seen following transfer of nTregs (6, 12, 16). In both cases, levels of IL-5, IL-6, and IL-13 in the BAL fluid were reduced while levels of IL-10, IFN-γ, and TGF-β were increased. Whereas Foxp3 expression was constitutive in CD4+CD25+ nTregs, TCR-stimulation of CD4+CD25− T cells alone was insufficient and required addition of TGF-β for induction of Foxp3+ iTregs, as shown in a dose-dependent (4) and time-dependent manner (5).
Given the similarities in functional outcomes (suppression) in WT recipients, we determined the consequences of transfer into CD8−/− recipients. Compared to WT mice, CD8−/− mice developed limited lung allergic responses following sensitization and challenge with allergen (7, 14, 18). Recipients of iTregs developed additional increases in AHR and airway eosinophilia, similar to the enhancement seen in recipients of nTregs. Unlike recipients of nTregs, recipients of iTregs also developed significant airway neutrophilia in association with increased levels of IL-17 in the BAL fluid. Since little IL-17 gene or protein expression was detected in iTregs prior to transfer and increased levels of IL-17 in BAL were only observed in recipients of iTregs, this suggested in vivo transdifferentiation and conversion of the transferred iTregs. This was supported by the findings in transferred iTregs which were recovered from the lungs of sensitized and challenged CD8−/− recipients using specific markers; in the recovered iTregs, reduced Foxp3 and Il10 mRNA levels but higher mRNA and protein levels of RORγt and Il17 were demonstrated. These findings were in contrast to transferred and recovered nTregs where lower gene expression levels of Foxp3 and Il10 and higher gene expression levels of Il13 were shown here and in previous studies (8–10, 18). Thus, it appears that the differentiation of nTregs and iTregs from a shared Foxp3+ suppressor phenotype (in CD8+/+ recipients) to divergent pathogenic effector lineages characterized by IL-13+ and IL-17+ T effector cells in CD8−/− recipients, resulted from the absence of CD8 signaling.
The requirements for IL-6, TGF-β, and the lineage-specific transcription factor RORγt are well established for the in vitro differentiation of Th17 cells (24, 28). The in vivo conversion of iTregs from suppressors to IL-17+ T effector cells in the lungs of sensitized and challenged CD8−/− recipients suggested that host (recipient)-derived IL-6 was critical. This notion was confirmed by preventing enhancement of lung allergic responses in recipients treated with anti-IL-6 antibody prior to adoptive transfer. The importance of IL-6 in the in vitro and in vivo differentiation of Th17 cells was not surprising given the pleiotropic activities of this cytokine in influencing inflammatory responses. IL-6 signaling induces phosphorylation of STAT3 that is required for inducing RORγt (31), promoting Th17 cell differentiation (24, 30), reducing TGF-β-induced expression of Foxp3 (29), and abrogating suppression of nTregs in association with decreased expression of Foxp3 (18, 21).
Conversion of CD4+CD25− T cells into iTregs was shown to critically depend on TGF-β (4, 5). Once differentiated to iTregs with TGF-β and transferred into sensitized and challenged recipients, enhancement of lung allergic responses was no longer dependent on TGF-β as treatment of recipients with anti-TGF-β did not prevent iTreg-mediated enhancement. In contrast, treatment of the recipients with anti-IL-6 prevented enhancement and Th17 differentiation following iTreg transfer. Although TGF-β contributed to the differentiation programming of both iTreg and Th17 cells, it appeared that in vivo, IL-6 from the hosts favored Th17 development and IL-17 release at the expense of iTreg development and/or maintenance (24, 28–30), and as shown here, resulted in enhancement (and not suppression) of lung allergic responses in sensitized and challenged recipients. The findings suggested that iTreg conversion to a Th17-like cell proceeded in a staged manner; the initial induction events for iTreg differentiation were dependent on TGF-β but later events were TGF-β independent and required IL-6. However, IL-6 alone was not sufficient as in the absence of TGF-β, IL-6 stimulation failed to induce the development of Th17 cells (24, 28).
The differences in transcriptional reprogramming of nTregs and iTregs were evident in the terminal events resulting in the enhancement responses. Enhancement of lung allergic responses mediated by nTregs was attenuated by treatment of recipients with anti-IL-13 while the same antibody had no effect on the events mediated by transferred iTregs. Reciprocally, anti-IL-17 had no effect on the events triggered by transferred nTregs but markedly reduced the outcomes mediated by transferred iTregs. The critical requirement for IL-17 in the enhancement of lung allergic responses was also demonstrated by the absence of such increases in recipients of IL-17-deficient iTregs. Whereas the role of IL-13 is firmly established in experimental models of asthma and in asthmatics (32–34), the contribution of Th17 cells and IL-17 remains somewhat controversial. Although Th17-dependent neutrophilia in the airways has been observed (25–27, 35), the role of neutrophils in development of altered airway function is not entirely clear. Inhibition of early airway neutrophilia had little effect on later development of AHR (36) and blocking the activity of a neutrophil chemoattractant had little benefit in asthmatics (37). In allergen challenge models in mice, the accumulation of neutrophils appeared early and transient and was later followed by the influx of eosinophils (38). Following intratracheal administration of Th17 cells or IL-17 protein into sensitized and challenged CD8−/− recipients, AHR and airway inflammation, including airway neutrophilia, were increased. Similar to findings following transfer of iTregs, the enhancement seen with Th17 cells or IL-17 was associated with increased airway neutrophilia and increased BAL IL-17 levels. However, Th17 cells or IL-17 alone were insufficient to induce any significant AHR, eosinophilic or neutrophilic inflammation, or alter cytokine levels (other than IL-17) in non-sensitized CD8−/− recipients. Thus, IL-17 and/or neutrophils likely play the role of “amplifiers”, augmenting an established allergic immune response.
There is now compelling evidence demonstrating that Foxp3+ Tregs have the potential to undergo functional reprogramming to acquire an effector/helper phenotype. The plasticity of nTregs and iTregs was influenced by extracellular stimuli and/or intracellular signaling molecules that are associated with alterations in levels of expression of Foxp3. It is likely that a complex integration of different signals dictates the final profile of subsets of T regulatory cells in the lung or other tissues. Our previous findings in nTregs suggested that these signals may be hierarchical in that signals mediated by CD8-nTreg interactions superceded those mediated through ligation of GITR, abrogating enhancement and maintaining suppressor activity (10). Inhibition or interference with the interaction/engagement of MHC I on nTregs with CD8 effectively abrogated suppression (6). In addition, interaction of CD8 and MHC I was demonstrated to be critical in the maintenance of suppressive activities in the periphery by controlling endogenous expression of IL-6 (18). Thus, an alteration in Treg function has been reported following ligation of GITR (8–10), OX40 (39), or through IL-6-IL-6 receptor interactions (18, 19, 21). In nTregs, GITR stimulation was shown to abrogate suppressive activity and blockade of signaling through GITR effectively prevented both the loss of the suppressor phenotype and conversion to pathogenic IL-13+CD4+ T effectors. Despite expression of these receptors on both nTregs and iTregs, neither signaling through GITR or OX40 appeared to influence the in vitro and in vivo conversion of iTregs to IL-17+CD4+ T effectors and production of IL-17. Similarly, stimulation of Th17 cells with ligands of GITR or OX40 did not alter the production of IL-17.
In summary, the data demonstrated an important balance in the lung between iTregs and Th17 cells following sensitization and challenge, emphasizing that the functional outcomes of nTregs and iTregs in the lung were dependent on cues in the host environment. The findings further extend the role of the pro-inflammatory cytokine IL-6 in overriding a dominant program of Treg cell function in the lung to favor Th17 differentiation. In WT (CD8+/+) recipients, both nTregs and iTregs were effective suppressors of the development of lung allergic responses, while in the absence of host CD8 signaling, these same cells acquired specific effector functions enhancing lung allergic responses as a consequence of divergent pathways. The reprogramming pathways and enhancement appeared to be distinct and cytokine-specific in that IL-13 production was GITR-dependent in nTregs while in iTregs IL-17 production was GITR-independent but IL-6-dependent. The IL-13-producing “ex-nTregs” mediated an eosinophil-predominant response in the lung whereas the IL-17-producing “ex-iTregs” elicited a neutrophil-associated enhancement. Despite these final effector differences, the fate of nTregs and iTregs following transfer shared many features, including a reduction in Foxp3 expression, loss of a specific regulatory phenotype and function, and the gain of distinct effector T cell functions. The ability to manipulate T regulatory cell function through biological manipulation has clinical implications for the treatment of immune/inflammatory disorders.
Clinical Implications.
Regulatory T cells are suppressors of development of allergic asthma, but are capable of converting into pathogenic effector cells. Maintaining suppressive activity may be essential in controlling immune/inflammatory responses.
Acknowledgments
Grant Support: This work was supported by NIH grant AI-77609. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
The assistance of Diana Nabighian in the preparation of this manuscript is gratefully acknowledged.
ABBREVIATIONS
- AHR
Airway hyperresponsiveness
- ANOVA
Analysis of variance
- BAL
Bronchoalveolar lavage
- Foxp3
Forkhead box p3
- GITR
Glucocorticoid-induced TNFR-regulated protein
- IPEX
X-linked immune dysregulation, polyendocrinopathy, and enteropathy
- iTreg
Inducible T regulatory cells
- nTreg
Naturally occurring T regulatory cells
- MCh
Methacholine
- OVA
Ovalbumin
- RL
Lung resistance
- SEM
Standard error of the mean
- WT
Wild-type
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennet CL, Christie J, Ramsdell F, Brunkow ME, Fergurson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome is caused mutations of Foxp3. Nature Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Clark LB, Appleby MW, Brunkow ME, Willkinson JE, Ziegler SF, Ramshell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. [PubMed] [Google Scholar]
- 3.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Nature. 2010;3:216–29. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. 2007;178:7667–77. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 6.Joetham A, Takeda K, Miyahara N, Matsubara S, Ohnishi H, Koya T, et al. Functional activation of naturally occurring lung CD4+CD25+ regulatory cells on lung allergic responses requires CD8 and MHC I interaction. Proc Natl Acad Sci. 2007;104:15057–62. doi: 10.1073/pnas.0706765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Ma Y, Dawicki W, Zhang X, Gordon JR. Comparison of induced versus natural regulatory T cells of the same TCR specificity for induction of tolerance to an environmental antigen. J Immunol. 2013;191:1136–43. doi: 10.4049/jimmunol.1201899. [DOI] [PubMed] [Google Scholar]
- 8.Joetham A, Matsubara S, Okamoto M, Takeda K, Miyahara N, Dakhama A, et al. Plasticity of regulatory T cells: Subversion of suppressive function and conversion to enhancement of lung allergic responses. J Immunol. 2008;180:7117–24. doi: 10.4049/jimmunol.180.11.7117. [DOI] [PubMed] [Google Scholar]
- 9.Joetham A, Ohnishi H, Okamoto M, Takeda K, Schedel M, Domenico J, et al. Loss of T regulatory cell suppression following signaling through the GITR is dependent on c-Jun N-terminal kinase activation. J Biol Chem. 2012;287:17100–8. doi: 10.1074/jbc.M111.316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joetham A, Schedel M, Takeda K, Jia Y, Ashino S, Dakhama A, et al. JNK2 regulates the functional plasticity of naturally occurring T regulatory cells and the enhancement of lung allergic responses. J Immunol. 2014;193:2238–47. doi: 10.4049/jimmunol.1400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGee HS, Agrawal DK. Naturally occurring and inducible T-regulatory cells modulating immune response in allergic asthma. Am J Respir Crit Care Med. 2009;180:211–25. doi: 10.1164/rccm.200809-1505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, et al. Naturally-occurring lung CD4+CD25+ T-cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J Immunol. 2006;178:1433–42. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 13.Presser K, Schwinge D, Wegmann M, Huber S, Schmitt S, Quaas A, et al. Coexpression of TGF-β1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J Immunol. 2008;181:7751–8. doi: 10.4049/jimmunol.181.11.7751. [DOI] [PubMed] [Google Scholar]
- 14.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOSSL pathway and inhibit allergen-induced airway hyperreactivity. Nature Med. 2002;8:1024–31. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 15.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T-cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 16.Joetham A, Takeda K, Okamoto M, Taube C, Matsuda H, Dakhama A, et al. Antigen specificity is not required for modulation of lung allergic responses by naturally occurring regulatory T cells. J Immunol. 2009;183:1821–7. doi: 10.4049/jimmunol.0900303. [DOI] [PubMed] [Google Scholar]
- 17.Arazi A, Sharabi A, Zinger H, Mozes E, Neumann AU. In vivo dynamical interactions between CD4 Tregs, CD8 Tregs and CD4+CD25− cells in mice. PlosOne. 2009;4:e8447. doi: 10.1371/journal.pone.0008447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joetham A, Okamoto M, Takeda K, Schedel M, Ohnishi H, Dakhama A, et al. CD8 regulates the endogenous production of IL-6 in naturally occurring T regulatory cells and maintains their suppressive phenotype in allergic lung disease. J Immunol. 2011;186:113–20. doi: 10.4049/jimmunol.1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan YY, Flavell RA. Regulatory T cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martines-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to generation of pathogenic memory T cells in vivo. Nature Immunol. 2009;10:1000–8. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doganci A, Elgenbrod T, Krug N, De Santis GT, Hausding M, Erpenbeck VJ, et al. The IL-6Ra chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–25. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cells regulatory functions. J Immunol. 2007;178:271–9. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 23.Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+CD25+ T regulatory cells from murine naive T cells. Nature Protocols. 2007;2:1789–94. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- 24.Betelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal development pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 26.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenzel SE, Szefler SJ, Leung DYM, Sloan SI, Rex MD, Martin RJ. Brochoscopic evaluation of severe asthma: persistent inflammation associated with high dose glucocortcoids. Am J Respir Crit Care Med. 1997;156:737–43. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 28.Kimura A, Naka T, Kishimoto T. IL-6-dependent and –independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci. 2007;104:12099–104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominitzki S, Fantini MC, Neufert C, Nikiolaev A, Galle PR, Scheller J, et al. Cutting Edge: Trans-signaling via the soluble IL-6R abrogates the induction of Foxp3 in naive CD4+CD25− T cells. J Immunol. 2007;179:2041–5. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Kitani A, Fuss I, Strober W. Cutting Edge: Regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol. 2007;178:6725–29. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 31.Basu R, Whitley SK, Bhaumik S, Zindl CL, Schoeb TR, Benveniste EN, et al. IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the Th17 cell-iTreg cell balance. N Immunol. 2015;16(3):286–95. doi: 10.1038/ni.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taube C, Duez C, Cui ZH, Takeda K, Rha YH, Park JW, et al. The role of IL-13 in established allergic airway disease. J Immunol. 2002;169:6482–9. doi: 10.4049/jimmunol.169.11.6482. [DOI] [PubMed] [Google Scholar]
- 33.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, et al. The enhancement or prevention of airway hyperresponiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol. 2005;175:1876–83. doi: 10.4049/jimmunol.175.3.1876. [DOI] [PubMed] [Google Scholar]
- 34.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: Asthma phenotype and targeted therapies. J Allergy Clin Immunol. 2012;130:829–42. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 35.Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008;39:543–50. doi: 10.1165/rcmb.2008-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taube C, Nick JA, Siegmund B, Duez C, Takeda K, Rha YH, et al. Inhibition of early airway neutrophilia does not affect development of airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;30:837–43. doi: 10.1165/rcmb.2003-0395OC. [DOI] [PubMed] [Google Scholar]
- 37.Evans DJ, Barnes PJ, Spaethe SM, van Alstyne EL, Mitchell MI, O’Connor BJ. Effect of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax. 1996;51:1178–84. doi: 10.1136/thx.51.12.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomkinson A, Cieslewicz G, Duez C, Larsen KA, Lee JJ, Gelfand EW. Temporal Association between airway hyperresponsiveness and airway eosinophilia in Ovalbumin-sensitized mice. Am J Resp Crit Care Med. 2001;163:721–30. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 39.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colobo MP. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2004;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]