Abstract
Background
Despite a higher prevalence of risk factors, atrial fibrillation (AF) is less prevalent in blacks than whites. To address this paradox, we examined racial differences in the magnitude of AF risk associated with common risk factors.
Methods
13,688 participants (mean age=63±8.4 years; 56% female; 37% black) from the REasons for Geographic And Racial Differences in Stroke study who were free of baseline AF were included. Incident AF was identified at a follow-up examination by electrocardiogram and self-reported medical history. Poisson regression was used to compute relative risk (RR) and 95% confidence intervals (CI) for the association between risk factors and incident AF in blacks and whites, separately. Age- and sex-adjusted population attributable fractions (PAF) of modifiable AF risk factors were computed.
Results
After median follow-up of 9.4 years, 997 (7.3%) incident AF cases were detected. Black race was associated with a lower risk of AF (RR=0.46, 95%CI=0.39, 0.53). Significant risk factors for AF were age, male sex, hypertension, obesity, and cardiovascular disease. A differential association was detected for smoking by race, with the association being stronger in blacks (RR=1.41, 95%CI=1.07, 1.85) compared with whites (RR=1.01, 95%CI=0.88, 1.16; p-interaction=0.030). The PAFs for hypertension (blacks=27.4%; whites=19.4%), obesity (blacks=16.9%; whites=11.8%), and smoking (blacks=17.9%; whites=2.5%) were higher for blacks than whites.
Conclusion
Modifiable risk factors are important in AF development among blacks despite a lower risk of the arrhythmia. Racial differences in the magnitude of the association of individual AF risk factors do not explain the AF paradox.
Keywords: atrial fibrillation, risk factors, race, epidemiology
INTRODUCTION
Despite a higher prevalence of well-known risk factors for atrial fibrillation (AF), this arrhythmia is less common in blacks compared with whites [1–4]. This phenomenon is known as the AF race paradox [5, 6]. One of the proposed explanations for this paradox is the differential impact of AF risk factors in blacks and whites [7]. Hence, examining racial differences in the magnitude of AF risk associated with these factors may provide insight into why blacks are protected from AF or why whites are more susceptible to develop the arrhythmia.
Prior studies attempted to address the differential impact of AF risk factors in blacks and whites [8–10]. However, these studies possibly were unable to detect racial differences, as they were limited to populations of older adults [8, 9], and women [10]. Therefore, the purpose of this analysis was to examine the association of common AF risk factors with the development of AF in blacks and whites from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
METHODS
Study Population and Design
Details of REGARDS have been published previously [11]. Briefly, REGARDS is a prospective cohort study designed to identify causes of regional and racial disparities in stroke mortality. The study over sampled blacks and persons residing in the Stroke Belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) between January 2003 and October 2007. This included participants from the stroke buckle (coastal plains of North Carolina, South Carolina and Georgia) as this region experiences a stroke mortality rate higher than other regions of the stroke belt [12]. A total of 30,239 participants aged 45 years and older were recruited from a commercially available list of residents using postal mailings and telephone data. Demographic information and medical histories were obtained using a computer-assisted telephone interview (CATI) that was conducted by trained interviewers. Additionally, a brief in-home physical examination was performed 3 to 4 weeks after the telephone interview. During the in-home visit, trained staff collected information regarding medications, blood and urine samples, and a resting electrocardiogram.
Approximately 10 years after the baseline assessment, 15,517 REGARDS participants completed a follow-up examination similar to the baseline visit. Of these, 1,048 had baseline AF and 781 with missing baseline covariates were excluded. All participants provided written informed consent and the study was approved by all participating institutional review boards.
Atrial Fibrillation
Incident AF was identified by the study electrocardiogram and also from a self-reported medical history of a physician diagnosis during the CATI surveys. The electrocardiograms were read and coded at a central reading center (Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston-Salem, NC, USA) by analysts who were blinded to other REGARDS data. Self-reported AF was defined as an affirmative response to the following question: “Has a physician or a health professional ever told you that you had atrial fibrillation?”, a question that has been shown to be a reliable predictor of incident stroke events [13].
Covariates
Age, sex, race, household income, exercise, and smoking status were self-reported. Income was dichotomized at $20,000. Exercise was classified as none versus one or more times per week. Smoking was defined as ever (e.g., current and former) or never smoker. Fasting blood samples were obtained and assayed for total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and serum glucose. Diabetes was defined as a fasting glucose ≥126 mg/dL (or a non-fasting glucose ≥200 mg/dL among those failing to fast), or self-reported diabetes medication use. The current use of aspirin, antihypertensive medications, and lipid-lowering therapies was self-reported. After the participant rested for 5 minutes in a seated position, blood pressure was measured using a sphygmomanometer. Two values were obtained following a standardized protocol and averaged. Hypertension was defined as systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg, or by the self-reported use of antihypertensive medications. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Obesity was defined as body mass index values ≥30 kg/m2. Dyslipidemia was defined as total cholesterol ≥240 mg/dL, high-density lipoprotein cholesterol ≤40 mg/dL, low-density lipoprotein cholesterol ≥160 mg/dL, or by the self-reported use of lipid lowering therapies. Using baseline electrocardiogram data, left ventricular hypertrophy (LVH) was defined by the Sokolow-Lyon Criteria [14]. Coronary heart disease (CHD) was ascertained by self-reported history of myocardial infarction, coronary artery bypass grafting, coronary angioplasty or stenting, or if evidence of prior myocardial infarction was present on the baseline electrocardiogram. Prior stroke was ascertained by participant self-reported history. Cardiovascular disease was defined as the composite of CHD and stroke.
Statistical Analyses
Baseline characteristics were compared between black and white participants. Categorical variables were reported as frequency and percentage while continuous variables were reported as mean ± standard deviation (SD). Statistical significance for categorical variables was tested using the chi-square method and the student’s t-test procedure for continuous variables.
Multivariable Poisson regression with robust variance estimates was used to compute relative risk (RR) and 95% confidence intervals (CI) for the association between common AF risk factors and incident AF in blacks and whites, separately [15]. The multivariable model included the following covariates: age, sex, income, smoking, diabetes, hypertension, obesity, exercise, dyslipidemia, LVH, and cardiovascular disease. To examine if racial differences existed between risk factors and incident AF, multiplicative interaction terms between each risk factor and race were tested.
Race-specific population attributable fractions (PAF) of modifiable AF risk factors (hypertension, diabetes, obesity, and smoking) were computed. The PAF represents the fraction of the event rate or risk in a given period that is attributable to the exposure of interest, and we hypothesized that the magnitude of the PAF for each modifiable risk factor will vary by race. Age- and sex-adjusted PAFs were computed using indirect standardization with the standardized mortality ratio using the SAS® procedure STDRATE.
Statistical significance for all comparisons including interactions was defined as p <0.05. SAS® Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
A total of 13,688 (mean age=63±8.4 years; 56% female; 37% black) participants were included in the final analysis. There were 8,611 (63%) white participants and 5,077 (37%) black participants. The baseline characteristics are shown in Table 1.
Table 1.
Baseline Characteristics (N=13,688)
| Characteristic | White (n=8,611) | Black (n=5,077) | P-value* |
|---|---|---|---|
| Age, mean (SD), years | 63 (8.5) | 63 (8.3) | <0.001 |
| Male (%) | 4,165 (48) | 1,842 (36) | <0.001 |
| Region | |||
| Stroke belt (%) | 2,919 (34) | 1,715 (34) | |
| Stroke buckle (%) | 1,928 (22) | 985 (19) | |
| Non-belt (%) | 3,764 (44) | 2,377 (47) | <0.001 |
| Income, <$20,000 (%) | 695 (8.1) | 1,036 (20) | <0.001 |
| No regular exercise (%) | 2,428 (28) | 1,709 (34) | <0.001 |
| Ever smoker (%) | 4,358 (51) | 2,592 (51) | 0.62 |
| Diabetes (%) | 1,002 (12) | 1,253 (25) | <0.001 |
| Systolic blood pressure, mean (SD), mm Hg | 124 (15) | 129 (16) | <0.001 |
| Hypertension (%) | 3,898 (45) | 3,423 (67) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 28 (5.4) | 31 (6.4) | <0.001 |
| Obesity (%) | 2,662 (31) | 2,509 (49) | <0.001 |
| Total cholesterol, mean (SD), mg/dL | 193 (38) | 194 (40) | 0.23 |
| HDL cholesterol, mean (SD), mg/dL | 52 (16) | 54 (16) | <0.001 |
| LDL cholesterol, mean (SD), mg/dL | 113 (33) | 118 (35) | <0.001 |
| Dyslipidemia (%) | 5,028 (58) | 2,659 (52) | <0.001 |
| Aspirin (%) | 3,844 (45) | 1,868 (37) | <0.001 |
| Left ventricular hypertrophy (%) | 468 (5.4) | 644 (13) | <0.001 |
| Cardiovascular disease (%) | 1,295 (15) | 729 (14) | 0.28 |
Statistical significance for categorical variables tested using the chi-square method and for continuous variables the student’s t-test was used.
HDL=high-density lipoprotein; LDL=low-density lipoprotein; SD=standard deviation.
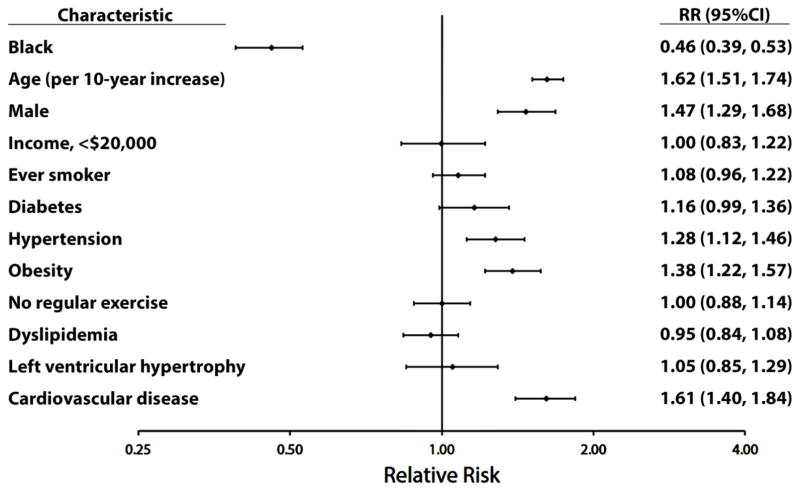
After a median follow-up of 9.4 years, 997 (7.3%) AF cases were detected. Of these, 224 (22%) were detected on the follow-up electrocardiogram and 773 (78%) were ascertained by a self-reported medical history. AF was detected more frequently in white (n=781, 9.1%) compared with black (n=216, 4.3%) participants. Black race was associated with a lower risk of AF development (RR=0.46, 95%CI=0.39, 0.53). The RRs of AF associated with common risk factors for all participants are shown in Figure 1.
Figure 1. Multivariable Relative Risk Estimates for AF Risk Factors.
AF=atrial fibrillation.
Table 2 shows the results of the multivariable RR estimates for AF risk factors stratified by race. Significant risk factors for AF were age, male sex, hypertension, obesity, and cardiovascular disease, which did not differ by race. An interaction was detected by smoking, with this variable demonstrating a positive association in blacks but not whites.
Table 2.
Multivariable Relative Risk Estimates for AF Risk Factors by Race
| Characteristics | White (n=8,611) | Black (5,077) | P-Interaction | ||
|---|---|---|---|---|---|
|
| |||||
| RR (95%CI)* | P-value | RR (95%CI)* | P-value | ||
| Age (per 10-year increase) | 1.65 (1.53, 1.79) | <0.001 | 1.52 (1.30, 1.78) | <0.001 | 0.40 |
| Male | 1.50 (1.29, 1.73) | <0.001 | 1.40 (1.05, 1.85) | 0.020 | 0.96 |
| Income, <$20,000 | 1.04 (0.82, 1.31) | 0.77 | 0.95 (0.68, 1.31) | 0.74 | 0.70 |
| Ever smoker | 1.01 (0.88, 1.16) | 0.89 | 1.41 (1.07, 1.85) | 0.016 | 0.030 |
| Diabetes | 1.21 (1.01, 1.45) | 0.037 | 1.06 (0.79, 1.43) | 0.68 | 0.59 |
| Hypertension | 1.28 (1.11, 1.48) | <0.001 | 1.24 (0.90, 1.71) | 0.19 | 0.93 |
| Obesity | 1.38 (1.20, 1.60) | <0.001 | 1.40 (1.06, 1.85) | 0.017 | 0.91 |
| No regular exercise | 0.96 (0.83, 1.11) | 0.58 | 1.14 (0.87, 1.51) | 0.35 | 0.26 |
| Dyslipidemia | 0.96 (0.83, 1.11) | 0.59 | 0.92 (0.70, 1.21) | 0.56 | 0.99 |
| Left ventricular hypertrophy | 1.02 (0.79, 1.32) | 0.89 | 1.13 (0.79, 1.61) | 0.52 | 0.78 |
| Cardiovascular disease | 1.51 (1.30, 1.77) | <0.001 | 1.97 (1.46, 2.66) | <0.001 | 0.14 |
Adjusted for age, sex, income, smoking, diabetes, hypertension, obesity, exercise, dyslipidemia, left ventricular hypertrophy, and cardiovascular disease.
AF=atrial fibrillation; CI=confidence interval; HDL=high-density lipoprotein; RR=relative risk.
We computed the age- and sex-adjusted PAFs of common AF risk factors in blacks and whites (Table 3). The PAF for diabetes was significant in whites but not blacks. In contrast, the PAFs for hypertension, obesity, and smoking were higher for blacks compared with whites.
Table 3.
Age- and Sex-Adjusted PAF of Modifiable AF Risk Factors by Race
| Characteristic | PAF (%)(95%CI)
|
|
|---|---|---|
| White | Black | |
| Diabetes | 5.2 (2.4, 7.9) | 6.2 (−1.2, 13.0) |
| Hypertension | 19.4 (14.3, 24.3) | 27.4 (15.6, 37.6) |
| Obesity | 11.8 (7.7, 15.8) | 16.9 (6.7, 25.9) |
| Smoking | 2.5 (−2.5, 7.3) | 17.9 (7.2, 27.3) |
AF=atrial fibrillation; CI=confidence interval; PAF=population attributable fraction.
DISCUSSION
The findings of the current analysis failed to explain the AF race paradox by differences in the magnitude of risk associated with common AF risk factors between blacks and whites. Despite a lower risk of AF among blacks, associations with traditional risk factors for AF were similar between these two races. A differential association was observed between smoking and AF by race, with smoking being significantly associated with AF in blacks but not whites. Additionally, the PAFs of hypertension, obesity, and smoking were higher for blacks than whites, suggesting a role for risk factor modification strategies to reduce AF burden in blacks.
Several reports have examined the race-specific impact of AF risk factors between blacks and whites [8–10]. Data from the Southern Community Cohort Study (SCCS) demonstrated that age, male sex, obesity, and CHD were associated with incident AF in blacks and whites [8]. The associations of hypertension and diabetes with AF were significant in whites but not blacks. Data from the Cardiovascular Health Study also have shown that body mass index and hypertension are predictive of incident AF after adjustment for age and sex, but diabetes was associated with AF in whites but not blacks [9]. A report from the Women’s Health Initiative (WHI) also has shown that the hazards of AF from hypertension, diabetes, obesity, heart failure and coronary artery disease are similar across race-ethnic groups of women [10]. The findings in this analysis support that common AF risk factors exist in both races, and suggest that smoking is a significant risk factor for AF in blacks but not whites. This conflicts with data from the SCCS which did not find an association between smoking and AF in blacks or whites [8]. The aforementioned reports also were limited to populations of older adults (e.g., ≥65 years) [8, 9], and women [10]. Therefore, the findings in the current analysis are more generalizable, as we included men and women aged 45 years and older.
The findings of the current study suggest that modifiable risk factors play an important role in AF development among blacks despite a lower prevalence of the arrhythmia. We observed racial differences in PAF estimates for common modifiable AF risk factors. A higher PAF estimate in blacks was observed for hypertension, obesity, and smoking, but not diabetes. Our findings are similar to results from the Multi-Ethnic Study of Atherosclerosis (MESA) which demonstrated increased PAF estimates for hypertension and current smoking among blacks compared with whites [16]. In contrast, we observed a higher PAF for obesity in blacks but this was not observed in MESA. This inconsistency may relate to the larger sample size in REGARDS yielding the power to detect a significant difference. Our results also are consistent with PAF estimates from WHI [9]. In that study, blacks had higher PAF estimates for hypertension, body mass index >25 kg/m2, diabetes, and smoking. Although we did not observe a higher PAF for diabetes, the participants in WHI were limited to women and our cohort included men and women.
Due to the higher prevalence of common AF risk factors (e.g., hypertension, diabetes, obesity) in blacks compared with whites, a higher risk of AF in blacks would be expected. However, our findings confirm that the racial differences in the rate of AF between blacks and whites are not explained by the individual risk associated with each risk factor [1–4]. Several explanations for this paradox have been proposed. Larger left atrial dimensions by echocardiography have been observed in whites compared with blacks, suggesting that whites are more susceptible to adverse atrial remodeling [17]. It also is possible that other factors, such as genetic susceptibility, vary between blacks and whites to explain this paradox [18, 19]. Additionally, blacks may be more likely to develop intermittent cases (e.g., paroxysmal) of AF, which go undetected in epidemiological studies [20]. Furthermore, poorer access to medical care possibly limits the detection of AF in blacks (e.g., less likely to self-report a prior diagnosis), as lack of access to medical care would result in fewer black participants being informed of their diagnosis [21]. It also is possible that a survival bias exists in whites in which they live longer and subsequently have more AF detected [22]. Although we offer several explanations for the AF race paradox, further research is needed to fully understand this common epidemiologic phenomenon.
Although the association between smoking and AF development remains controversial, with several reports demonstrating a positive association [23–25], and others showing no association [26], the findings of the current analysis suggest that smoking has a differential impact on AF risk by race. This finding is supported by a prior report form REGARDS which demonstrated that environmental tobacco smoke is more strongly associated with AF in blacks than whites [27]. Potentially, racial differences in susceptibility to tobacco smoke explain this finding, as black patients with chronic obstructive pulmonary disease have been reported to lose more lung function per pack-year compared with whites [26]. Additionally, lower levels of thyrotropin, a sensitive indicator of thyroid function, are associated with an increased risk for AF [28], and smoking has been associated with lower concentrations of thyrotropin [29]. This suggests that thyroid dysfunction is worsened by smoking, and racial differences in thyroid function possibly explain the increased risk of AF in blacks who smoke compared with whites. Therefore, smoking cessation strategies potentially are able to reduce the occurrence of AF in blacks.
Our results should be read in the context of certain limitations. Several baseline characteristics were self-reported and subjected our analysis to recall bias. Certain cases of AF possibly were missed due to the time-dependent nature of AF events (e.g., paroxysmal). Also, the majority of AF cases were ascertained by self-report, and access to medical care possibly influenced the likelihood of reporting a prior diagnosis. Due to the fact that blacks are less likely to be aware of their AF diagnosis than whites [30], this possibly resulted in the underdetection of incident AF in blacks. Additionally, we adjusted for several AF risk factors but acknowledge that other unmeasured factors possibly influenced our results.
In conclusion, we have demonstrated that traditional risk factors account for a similar proportion of AF risk in blacks and whites. Our data also suggest that smoking has a greater influence on AF risk in blacks compared with whites. Further studies are needed to identify other factors that explain the racial differences in AF risk.
Acknowledgments
Funding: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest in the writing of this manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all participants included in the study.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128(23):2470–7. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 5.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5(6):547–56. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 6.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204–11. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman EZ, Prineas RJ. The paradox of atrial fibrillation in African Americans. J Electrocardiol. 2014;47(6):804–8. doi: 10.1016/j.jelectrocard.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Lipworth L, Okafor H, Mumma MT, Edwards TL, Roden DM, Blot WJ, et al. Race-specific impact of atrial fibrillation risk factors in blacks and whites in the southern community cohort study. Am J Cardiol. 2012;110(11):1637–42. doi: 10.1016/j.amjcard.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the cardiovascular health study. J Am Geriatr Soc. 2013;61(2):276–80. doi: 10.1111/jgs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez F, Stefanick ML, Greenland P, Soliman EZ, Manson JE, Parikh N, et al. Racial and ethnic differences in atrial fibrillation risk factors and predictors in women: findings from the women’s health initiative. Am Heart J. 2016;176:70–7. doi: 10.1016/j.ahj.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28(5):936–40. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- 13.Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, et al. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42(10):2950–3. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC, Jr, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25(2):71–6. 6 e1. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123(4):375, e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122(20):2009–15. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnabel RB, Kerr KF, Lubitz SA, Alkylbekova EL, Marcus GM, Sinner MF, et al. Large-scale candidate gene analysis in whites and African Americans identifies IL6R polymorphism in relation to atrial fibrillation: the National Heart, Lung, and Blood Institute’s Candidate Gene Association Resource (CARe) project. Circ Cardiovasc Genet. 2011;4(5):557–64. doi: 10.1161/CIRCGENETICS.110.959197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soliman EZ. More or less irregularity in understanding an irregular rhythm: atrial fibrillation classification and racial differences. J Am Heart Assoc. 2013;2(5):e000482. doi: 10.1161/JAHA.113.000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hargraves JL, Cunningham PJ, Hughes RG. Racial and ethnic differences in access to medical care in managed care plans. Health Serv Res. 2001;36(5):853–68. [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer PA, Yoon PW, Kaufmann RB Centers for Disease C, Prevention. Introduction: CDC Health Disparities and Inequalities Report - United States, 2013. MMWR Suppl. 2013;62(3):3–5. [PubMed] [Google Scholar]
- 23.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–4. [PubMed] [Google Scholar]
- 24.Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156(6):1163–9. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011;8(8):1160–6. doi: 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(6):1110–6. doi: 10.1016/j.rmed.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.O’Neal WT, Qureshi WT, Judd SE, McClure LA, Cushman M, Howard VJ, et al. Environmental Tobacco Smoke and Atrial Fibrillation: The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Occup Environ Med. 2015;57(11):1154–8. doi: 10.1097/jom.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–52. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 29.Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2004;89(12):6077–86. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- 30.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41(4):581–7. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]