Abstract
Glaucomatous visual field progression has both personal and societal costs and therefore has a serious impact on quality of life. At the present time, intraocular pressure (IOP) is considered to be the most important modifiable risk factor for glaucoma onset and progression. Reduction of IOP has been repeatedly demonstrated to be an effective intervention across the spectrum of glaucoma, regardless of subtype or disease stage. In the setting of approval of IOP-lowering therapies, it is expected that effects on IOP will translate into benefits in long-term patient-reported outcomes. Nonetheless, the effect of these medications on IOP and their associated risks can be consistently and objectively measured. This helps to explain why regulatory approval of new therapies in glaucoma has historically used IOP as the outcome variable. Although all approved treatments for glaucoma involve IOP reduction, patients frequently continue to progress despite treatment. It would therefore be beneficial to develop treatments that preserve visual function through mechanisms other than lowering IOP. The United States Food and Drug Administration (FDA) has stated that they will accept a clinically meaningful definition of visual field progression using Glaucoma Change Probability criteria. Nonetheless, these criteria do not take into account the time (and hence, the speed) needed to reach significant change. In this paper we provide an analysis based on the existing literature to support the hypothesis that decreasing the rate of visual field progression by 30% in a trial lasting 12–18 months is clinically meaningful. We demonstrate that a 30% decrease in rate of visual field progression can be reliably projected to have a significant effect on health-related quality of life, as defined by validated instruments designed to measure that endpoint.
Keywords: Glaucoma, Intraocular pressure, Neuroprotection, Perimetry, Progression, Clinical trials
1. Introduction
Glaucoma is characterized by progressive, irreversible damage to the optic nerve resulting in serious vision loss and blindness. This gradual progression of vision loss has both personal and societal costs and therefore has a serious impact on quality of life (QOL). The purpose of this document is to provide the scientific basis and methodological approach for measuring visual field progression in prospective clinical trials and relating it to a clinically meaningful decline in visual function.
At the present time, intraocular pressure (IOP) is considered to be the most important modifiable risk factor for glaucoma onset and progression. Reduction of IOP has been repeatedly demonstrated to be an effective intervention across the spectrum of glaucoma, regardless of subtype or disease stage (AGIS, 2000; CNTGS, 1998b; Gordon et al., 2002; Heijl et al., 2002; Lichter et al., 2001; Miglior et al., 2005). It is therefore not surprising that numerous medications to reduce IOP have been approved by regulatory agencies worldwide. In the setting of approval of IOP-lowering therapies, it is expected that effects on IOP will translate into benefits in long-term patient-reported outcomes. Aside from a recent randomized clinical trial (RCT) in the United Kingdom (Garway-Heath et al., 2015) the direct link between a specific pharmaceutical product to lower IOP and the prevention or delay of visual field progression has not been established nor are these medications specifically approved for such a functional outcome. Nonetheless, the effect of these medications on IOP and their associated risks can be consistently and objectively measured. This helps to explain why regulatory approval of new therapies in glaucoma has historically used IOP as the outcome variable.
Although all approved treatments for glaucoma involve IOP reduction, patients frequently continue to progress despite treatment. Both RCTs and clinical practice demonstrate that a substantial number of patients progress despite significant IOP-lowering therapy, and the risk of blindness over long periods of time is considerable. It would therefore be tremendously beneficial to develop treatments that preserve visual function through mechanisms other than lowering IOP.
In this paper we provide an analysis based on the existing literature to support the hypothesis that decreasing the rate of visual field progression by 30% in a trial lasting 12–18 months is clinically meaningful. We use three independent and mutually supportive methods to support this hypothesis:
We demonstrate that a 30% decrease in rate of visual field progression can be reliably projected to have a significant effect on health-related quality of life (HRQOL), as defined by validated instruments designed to measure that endpoint. This line of reasoning is based on population-based studies that assess visual fields and HRQOL.
The United States Food and Drug Administration (FDA) has stated that they will accept a clinically meaningful definition of visual field progression with Glaucoma Change Probability (GCP) criteria: “Visual field changes may be acceptable as a clinically relevant primary endpoint provided a between-group difference in field progression is demonstrated. The progression of visual field loss will be suspected if five or more reproducible points, or visual field locations, have significant changes from baseline beyond the 5% probability levels for the GCP analysis” (Weinreb and Kaufman, 2009). Nonetheless, these criteria do not take into account the time (and hence, the speed) needed to reach significant change. We demonstrate that this event-based outcome corresponds to a rate (slope) of visual field progression equal to or faster than −0.5 dB/yr for at least five abnormal test locations at baseline. This line of reasoning is supported by data from clinical trials that assessed both event-based and trend-based outcomes.
We demonstrate that a 30% decrease in progression rate with a trend-based analysis of visual field data is equivalent to that seen with a 2–3 mm Hg decrease in IOP. This line of reasoning is supported by data from non-regulatory clinical trials and other key studies that investigated the relationship between IOP and visual field change. That level of IOP decrease is considered clinically meaningful in patients with glaucoma who are progressing while on IOP-lowering therapy.
This paper presents the background for these arguments and reviews the critical literature related to the epidemiology of glaucoma, effects on QOL, measurement of progression, and detection of the effects of therapeutic intervention. Wherever possible, we use data derived from large-scale population-based and/or randomized studies to avoid bias. We use this reasoning to conclude that a therapy which results in a 30% decrease in visual field progression rate over 12–18 months in patients with glaucoma is clinically meaningful, and therefore would be valuable for treating glaucoma patients who are progressing despite IOP-lowering therapy.
2. Glaucoma – change in population demographics and socioeconomic burden
2.1. Glaucoma is a leading cause of blindness in the United States and worldwide
2.1.1. Background
The glaucomas are a group of chronic eye diseases that damage the optic nerve and for which there are currently no clinically-proven methods to reverse damage. Patients with glaucoma present to eye health professionals with varying degrees of severity. In its early stages, glaucoma damage is relatively asymptomatic for four reasons: (1) patients are frequently unaware of damage in the peripheral visual field; (2) the pace of progression is often slow (but continuous); (3) there is tremendous redundancy in the sensory system, including the ability of the visual cortex to fill-in loss of visual field; and (4) the binocular nature of vision means that one eye may compensate for early losses in the other. As progression occurs, the patient may unconsciously compensate for a steadily worsening visual field, contrast sensitivity, and even color vision.
However, at the same time that the disease is progressing, there is visual dysfunction from visual field damage and contrast sensitivity loss which is reflected in slow but relentless development of problems with everyday life (e.g., driving, reading, and risk of falls). Eventually, there is vision-related disability, loss of visual acuity (VA) and legal blindness from severely constricted visual fields, poor VA, or both.
Major risk factors for developing glaucoma include higher IOP, greater cup-to-disc ratio, decreased central corneal thickness (CCT), older age, pseudoexfoliation syndrome, genetic factors, and ocular perfusion pressure (AGIS, 2002; De Moraes et al., 2012b; Gordon et al., 2002; Leske et al., 2007; Lichter et al., 2001; Miglior et al., 2005; Musch et al., 2009). These and other risk factors also increase the chance of progressive disease severity.
2.1.2. Glaucoma is a common condition which increases in prevalence as the population ages
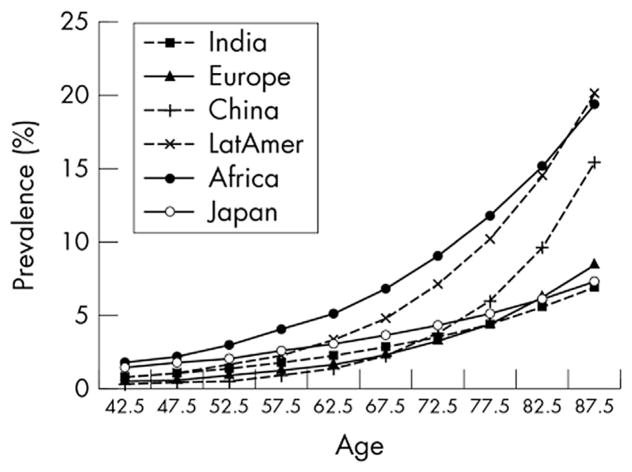
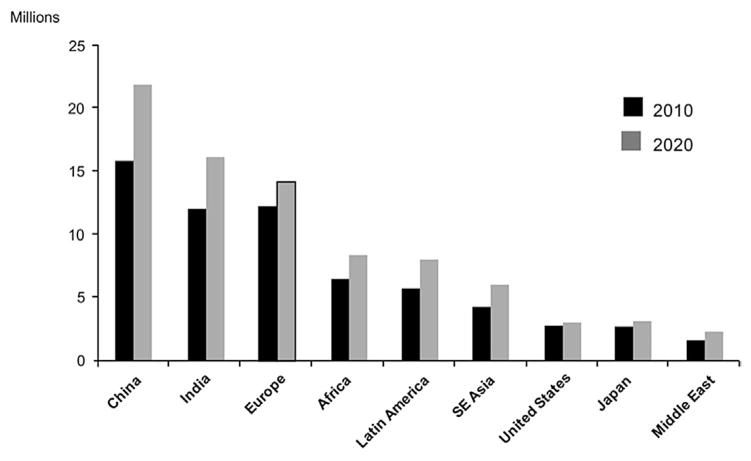
In the year 2000, glaucoma affected nearly 68 million persons worldwide and caused bilateral blindness in almost 7 million persons(Quigley, 1996). A 2006 review of worldwide glaucoma prevalence models estimated that by 2010 glaucoma would be the second leading overall cause of blindness in the world and leading cause of irreversible blindness, affecting 60.5 million people with prevalence varying worldwide (Quigley and Broman, 2006) (Fig. 1). Open-angle glaucoma (OAG) was estimated to affect 2.22 million people in the United States in 2002 (Fig. 2) (Friedman et al., 2004). Over 8.4 million people were estimated to be bilaterally blind from primary glaucoma in 2010, rising to 11.1 million by 2020.
Fig. 1. Estimated prevalence of glaucoma in 2010 based on prevalence model data.
Source: Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. Mar 2006;90(3):262–267.
Fig. 2. Estimated prevalence of glaucoma – 2010 and 2020.
Source: Adapted from Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. Mar 2006;90(3):262–267.
Previous estimates based on blindness prevalence surveys (Resnikoff et al., 2004) suggested that 12% of world blindness (4.4 million people) was caused by glaucoma. Blindness estimates differ because of methodological issues with prevalence surveys frequently assigning the most “treatable” disease as the primary cause of blindness. As such, cataract is often assumed to be more treatable than glaucoma, which leads to an underestimation of glaucoma blindness. Moreover, these numbers probably underestimated the true glaucoma prevalence because they were based on population-based studies that defined OAG without regard to IOP level and required both disc and field tests showing abnormal results to define glaucoma, as opposed to other definitions that rely mostly on the presence of glaucomatous optic neuropathy (GON). Wolfs et al. evaluated this conservative definition for OAG (Wolfs et al., 2000), and determined that it was likely to specify those with definite disease. In addition, failure to test the visual field can miss up to one third of those with the disease (Tielsch et al., 1991). Disc examination alone is not adequately specific, and studies that use “expert” subjective assessment of disc and field may lack reproducibility (Tielsch et al., 1988; Varma et al., 1992).
Glaucoma is most commonly a disease of the elderly, and its prevalence is likely to continue to increase throughout the world as life expectancy rises. Life expectancy has increased considerably during the last 50 years (by 10 years in the United States (Peters et al., 2013b)) and is expected to increase further. As life expectancy increases, not only will glaucoma prevalence increase, but glaucoma patients will be exposed to the disease for a longer period of time, further increasing the lifetime risk of blindness from glaucoma.
At present, treatment of OAG is directed at IOP lowering, which continues to be the only proven and treatable risk factor for the disease. There are several modalities of treatment for lowering IOP, including medicinal therapy, laser surgery, and incisional surgery. However, lowering of IOP does not halt all cases of progression (AAO, 2010; De Moraes et al., 2012b; Drance et al., 2001; Gordon et al., 2002; Leske et al., 2007; Musch et al., 2009). In some individuals with progression, it is not practical to sufficiently lower the IOP. In other individuals, factors other than IOP alone, or in combination with IOP, may be damaging the optic nerve.
2.1.3. Population-based studies demonstrate high rates of blindness due to glaucoma
2.1.3.1. Study 1: Malmo, Sweden
Population-based studies have provided important information regarding the burdens of glaucoma as a main cause of blindness. Based upon the World Health Organization (WHO) criteria for low vision (0.05 [20/400] ≤ VA < 0.3 [20/60] and/or 10° ≤ central visual field <20°) and blindness (VA < 0.05 [20/400] and/or central visual field <10°), in a study performed in Malmo, Sweden, investigators defined the following four categories of low vision and blindness with glaucoma as the main cause: (1) unilateral low vision: patients with low vision in one eye; (2) bilateral low vision: patients with low vision in the best eye; (3) unilateral blindness: patients blind in one eye; (4) bilateral blindness: patients with both eyes blind, mainly caused by glaucoma in at least one eye (Peters et al., 2013b). The date of the glaucoma diagnosis was set to the date of the first reliable visual field showing a glaucomatous defect. The time for low vision or blindness was the first visit when the standard automated perimetry (SAP) result was centrally constricted to less than 20° or 10°, respectively, or when VA was permanently reduced to below 0.3 (20/60) or 0.05 (20/400), respectively.
In this study of lifetime risk for blindness, a large proportion of patients (42.2%) were blind from glaucoma in at least one eye at the last hospital or Habilitation and Assistive Technology Service visit, and 16.4% were bilaterally blind from glaucoma. The cumulative risk for unilateral and bilateral blindness from glaucoma was considerable and many blind patients were blind for more than three years. Numbers of patients with low vision and blindness from glaucoma at the last visit are shown in Table 1.
Table 1.
Number of patients with low vision and blindness from glaucoma at last visit where (1) visual field data was available at diagnosis; (2) where visual field data was available from follow-up; and (3) all included patients (Peters et al., 2013b).
| All patients (n = 592) n (%) |
Follow-up Only group (n = 169) n (%) |
Data at diagnosis group (n = 423) n (%) |
|
|---|---|---|---|
| Unilateral low vision | |||
| OAG | 52 (8.8) | 13 (7.7) | 39 (9.2) |
| Bilateral low vision | |||
| OAG + OAG | 7 (1.2) | 2 (1.2) | 5 (1.2) |
| OAG + other cause | 5 (0.9) | 1 (0.6) | 4 (0.9) |
| In total: | 12 (2.0) | 3 (1.8) | 9 (2.1) |
| Unilateral blindness | |||
| OAG | 153 (25.8) | 51 (30.2) | 102 (24.1) |
| Bilateral blindness | |||
| OAG + OAG | 67 (11.3) | 22 (13.0) | 45 (10.6) |
| OAG + other cause | 30 (5.1) | 10 (5.9) | 20 (4.7) |
| In total | 97 (16.4) | 32 (18.9) | 65 (15.4) |
OAG = open-angle glaucoma.
The Data at Diagnosis group represents patients with visual field data available at the time of diagnosis. The Follow-up Only group represents patients diagnosed outside and later referred to the Skåne University Hospital, and for whom the first visual field data were available after the time of diagnosis.
Source: Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. Oct 2013; 156(4): 724–730.
Other reasons for unilateral blindness were age-related macular degeneration (AMD) (26 patients), a combination of cataract and other disease (10 patients), and other causes (32 patients). Seventeen patients were bilaterally blind because of reasons other than glaucoma (16 from AMD, 1 patient from other reason). A combination of causes for blindness was found in one eye of seven blind patients. In patients who developed blindness attributable to glaucoma, the median time with bilateral blindness was two years (range,1–13, mean 3.0 ± 3.1). Patients who became bilaterally blind from glaucoma did so at a median age of 86 years (range, 66–98; mean 85.7 ± 6.1). Thirteen patients (13.5% of blind patients and 2.2% of all patients) became blind before the age of 80 years (Peters et al., 2013a, b).
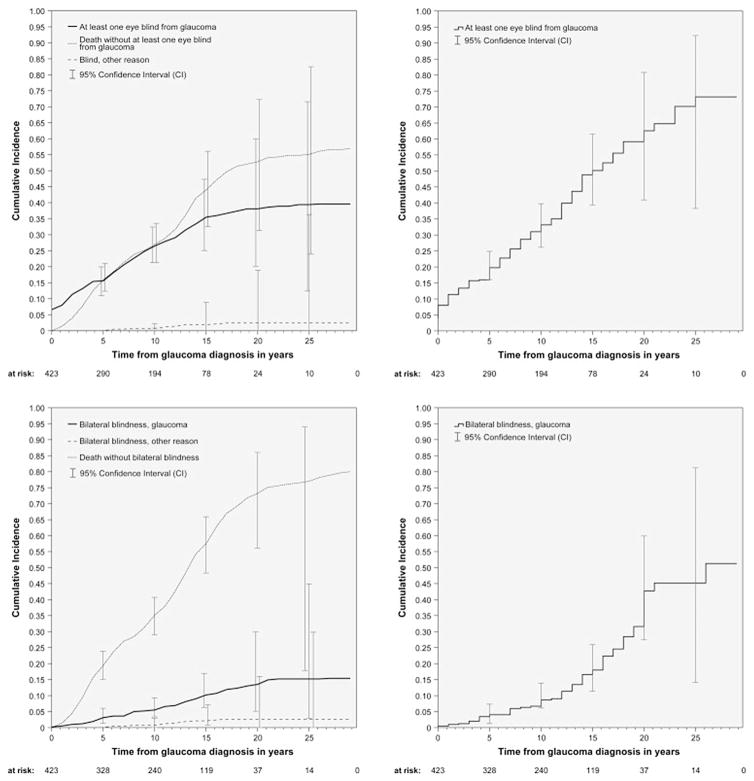
The median duration with diagnosed glaucoma was 12 years (range, 1–29; mean 11.2 ± 6.6), with 74.7% (316 of 423 patients) of patients having their glaucoma diagnosis for more than six years. The cumulative incidence for blindness in at least one eye and bilateral blindness from glaucoma was 26.5% and 5.5%, respectively, at 10 years and 38.1% and 13.5%, respectively, at 20 years after diagnosis (Fig. 3, top left and bottom left). The corresponding cumulative incidence for blindness caused by other reason was 0.7% and 0.7%, respectively, at 10 years and 2.4% and 2.6%, respectively, at 20 years. The Kaplan-Meier estimates for blindness in at least one eye caused by glaucoma were 33.1% at 10 years and 73.2% at 20 years (Fig. 3, top right) and 8.6% at 10 years and 42.7% at 20 years for bilateral blindness from glaucoma (Fig. 3, bottom right) (Peters et al., 2013a, b).
Fig. 3. Cumulative incidence rates for unilateral and bilateral blindness caused by glaucoma.
Source: Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. Oct 2013;156(4):724–730.
2.1.3.2. Study 2: Olmsted County, Minnesota
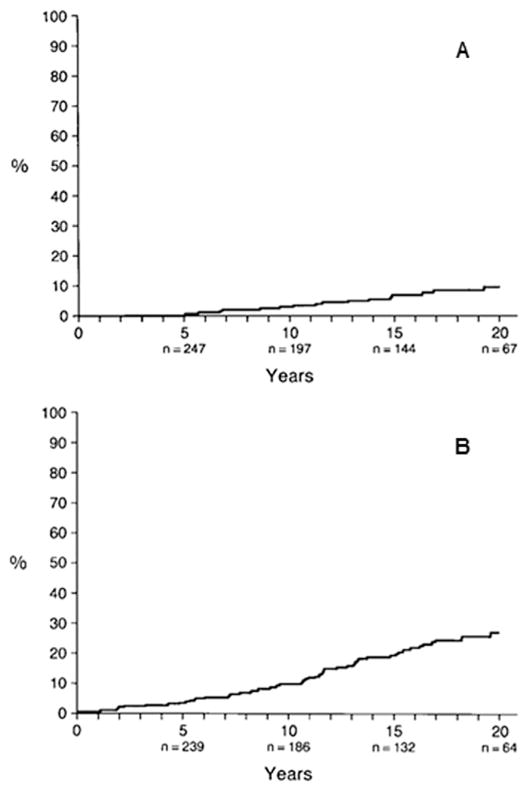
A study conducted in Olmsted County, Minnesota included 295 residents newly diagnosed with and treated for OAG between 1965 and 1980, with a mean follow-up of 15 years (standard deviation ± 8 years) (Hattenhauer et al., 1998). Legal blindness, defined as a best-corrected VA of 20/200 or worse, and/or visual field constricted to 20° or less in its widest diameter with the Goldmann III4e test object or its equivalent on SAP, secondary to glaucomatous loss, was measured. The mean age at diagnosis of OAG was 66 years (±14 years), and the median age was 68 years. Of the 295 patients, 29 (10%) were blind in at least one eye at diagnosis. Five of the 29 patients were bilaterally blind and 24 were unilaterally blind. The cumulative probability of blindness from classic glaucoma (defined as two or more of the following three characteristics: increased IOP, visual field defects, or glaucomatous optic nerves), treated ocular hypertension, and unsupported glaucoma (glaucoma without documented clinical features) at 20 years was estimated to be 9% (95% confidence interval (CI), 5%–14%) for both eyes (Table 2, Fig. 4A) and 27% (95% CI, 20%–33%) in one eye (Table 3, Fig. 4B).
Table 2.
Kaplan-Meier cumulative probability of glaucoma-related blindness at 20 years in both eyes.
| Blindness by visual acuity and/or visual field (%) | Blindness by visual acuity alone (%) | Blindness by visual field alone (%) | |
|---|---|---|---|
| All glaucomaa (n = 295) | 9 | 5 | 8 |
| Treated ocular hypertension (n = 191) | 4 | 2 | 4 |
| Classic glaucoma (n = 100) | 22 | 16 | 16 |
Includes treated ocular hypertension, classic glaucoma, and unsupported glaucoma.
Source: Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. Nov 1998; 105(11):2099–2104.
Fig. 4. Kaplan-Meier cumulative probability of glaucoma-related blindness in both eyes (A) and at least one eye (B).
Source: Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. Nov 1998;105(11):2099–2104.
Table 3.
Kaplan-Meier cumulative probability of glaucoma-related blindness at 20 years in at least one eye.
| Blindness by visual acuity and/or visual field (%) | Blindness by visual acuity alone (%) | Blindness by visual field alone (%) | |
|---|---|---|---|
| All glaucomaa (n = 295) | 27 | 11 | 23 |
| Treated ocular hypertension (n = 191) | 14 | 5 | 11 |
| Classic glaucoma (n = 100) | 54 | 27 | 50 |
Includes treated ocular hypertension, classic glaucoma, and unsupported glaucoma.
Source: Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. Nov 1998; 105(11):2099–2104.
Analyzed solely by VA criteria, there was a 5% probability of bilateral blindness at 20 years (95% CI, 2%–9%). The probability of bilateral blindness based solely on visual field criteria was 8% probability at 20 years (95% CI, 4%–13%). Because some patients were diagnosed blind by both VA and visual field criteria, the sum of VA and visual field cumulative probabilities for blindness exceeded 9%. The majority of patients (270 of 295) in the study were diagnosed with primary OAG. The cumulative probability of blindness for this subgroup of patients with OAG was also evaluated. The probability of blindness in both eyes and in at least one eye was calculated to be 9% (95% CI, 4%–11%) and 26% (95% CI, 19%–33%) at 20 years, respectively. There were 21 patients diagnosed with bilateral glaucoma who were unilaterally blind at diagnosis. For the endpoint of blindness in both eyes, there was no significant difference between genders (P = 0.41), but there was a significant effect of older age (P = 0.02). Similarly, in blindness affecting at least one eye, there was no significant difference between genders (P = 0.44) but a significant effect of older age on the risk of becoming blind (P < 0.001).
Blindness occurred more often by visual field criteria than by VA criteria. It was not uncommon for patients to have double arcuate defects but still have 20/20 VA. Because of this, establishing the degree of functional visual impairment from a disease such as OAG can be difficult. Perhaps the most important factor in assessing visual compromise is that of functional visual constraints in the context of activities of daily living (Hattenhauer et al., 1998).
2.1.3.3. Study 3: United Kingdom
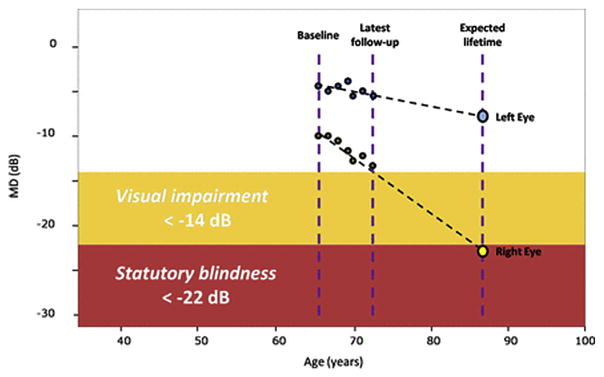
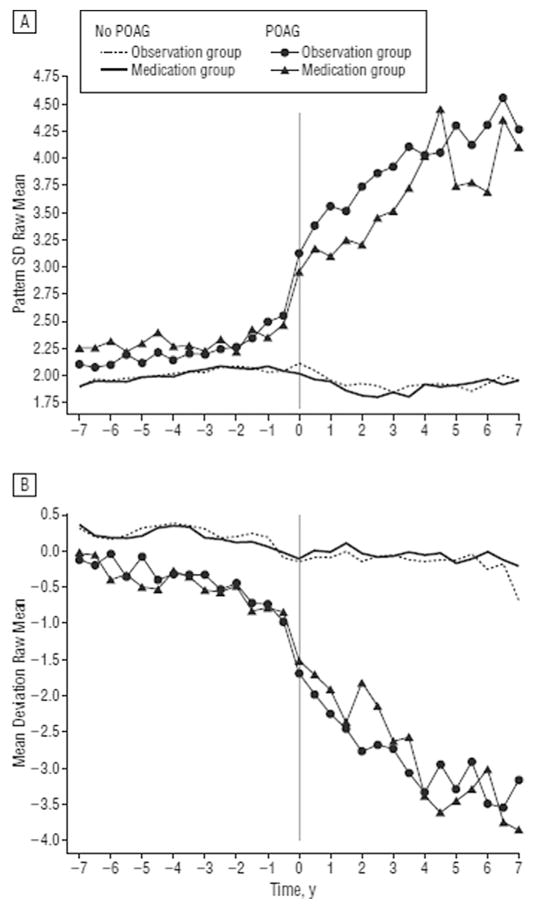
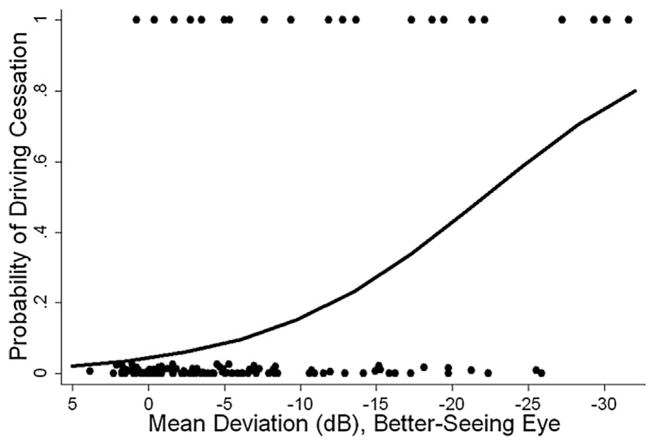
In the United Kingdom, Saunders et al. evaluated the proportion of patients in glaucoma clinics progressing at rates that would result in visual disability within their expected lifetime (Saunders et al., 2014). Based on criteria defined by the US Social Security Administration (SSA) with SAP, a mean deviation (MD) of −14 dB or worse in the better eye was deemed ‘visual impairment’ and a MD of −22 dB or worse in the better eye corresponded to the visual field definition of ‘statutory blindness’ (Fig. 5) (Administration; Saunders et al., 2014).
Fig. 5. Visual field series from the left and right eyes of a patient demonstrate a linear rate of loss in each eye (dB/year).
Source: Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. Jan 2014;55(1):102–109.
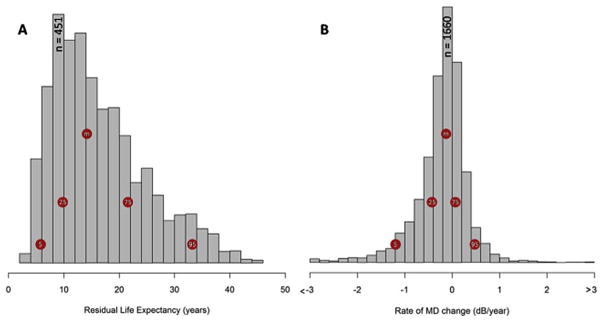
Fig. 6A and B demonstrate the distribution of patient eye follow-up times, patient residual life expectancy, and progression rates in all eyes, respectively. It is apparent from Fig. 6B that the majority (74%–95%) of eyes had a decrease in MD approximating ± 0.5 dB/yr. A smaller proportion of patient eyes progressed at a rate worse than −1.0 dB/yr (6.9%–8.2%) and 3.0% (2.7%–3.4%) of eyes progressed faster than −1.5 dB/yr. Of the 3359 patients with a visual field series from both eyes (Table 4), 5.2% progressed to statutory blindness (both eyes progressing to a MD worse than −22.0 dB) with a further 10.4% progressing to visual impairment (both eyes progressing to an MD level of worse than −14.0 dB) in their expected residual lifetime (Fig. 7).
Fig. 6. Distribution of patient eye follow-up times, patient residual life expectancies, and progression rates in all eyes.
Source: Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. Jan 2014;55(1):102–109.
Table 4.
The proportion of patients likely to suffer VF impairment in the course of their lifetime.
| Visual impairment at death | % No impairment (95% CIa) | % Visual impairment (95% CIa) | % Statutory blindness (95% CIa) |
|---|---|---|---|
| Including patients with a visual field series for each eye only, n = 3359 | 84.4 (83.2–85.6) | 10.4 (9.4–11.4) | 5.2 (4.5–6.0) |
| All patients best-case scenario, n = 3790 | 84.9 (83.7–86.1) | 10.0 (9.0–11.0) | 5.1 (4.3–5.8) |
| All patients worst-case scenario, n = 3790 | 81.5 (80.2–82.8) | 11.5 (10.4–12.5) | 7.1 (6.2–7.9) |
95% CIs were calculated with the normal approximation of a binomial distribution.
Source: Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. Jan 2014; 55(1): 102–109.
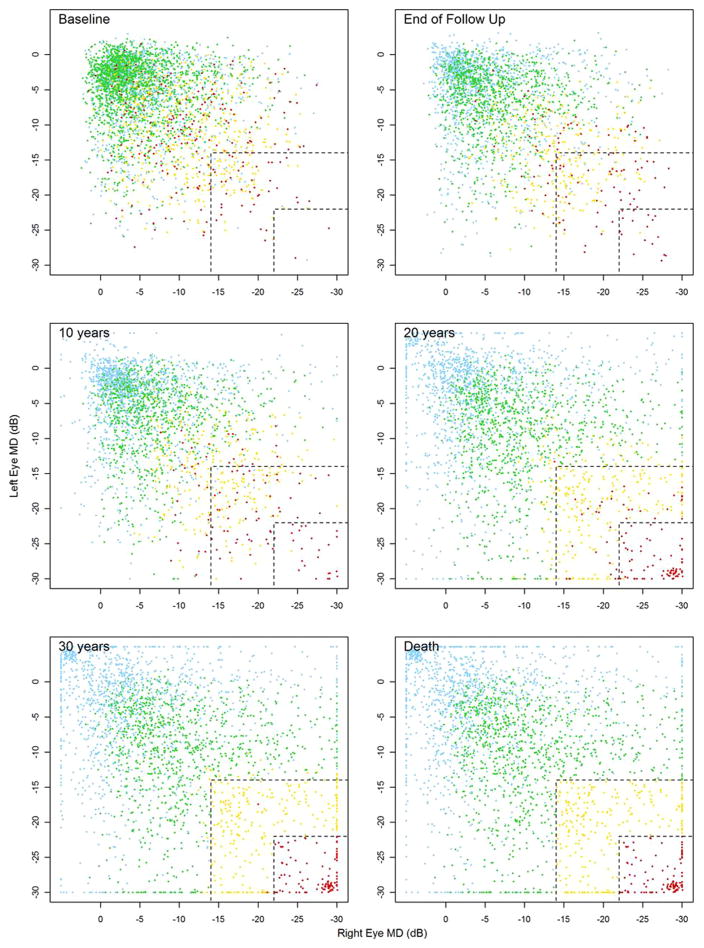
Fig. 7. A series of scatterplots showing MD in left (y-axis) and right (x-axis) eyes at baseline, at the end of follow-up, through extrapolating current rates of MD deterioration, after 10, 20, and 30 years of follow-up and at the end of expected lifetime. Both eyes in the plot had to fulfill the original inclusion criteria. The patients are colored according to their visual disability status at expected time of death. Blue represents a patient where at least one of the eyes has a positive slope over time, green represents progression, but no significant impairment by the end of the patient’s lifetime, yellow represents degradation to visual impairment (−14 dB or worse in both eyes), and red corresponds to statutory blindness in both eyes (below–22 dB). It is worth noting that most of the red symbols are not found in the top left corner of the baseline plot where both eyes are at an early stage of glaucoma.
Source: Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. Jan 2014;55(1):102–109
When fewer than three VF’s were available, the data were analyzed either as a the “best-case scenario” (0 dB/yr progression) or “worst-case scenario” (−1.5 dB/yr progression). The best-case scenario produced similar results to those considering eyes with two series, but under the “worst-case scenario” the number of patients at risk of statutory blindness increased to 7.1%, with a further 11.5% at risk of visual impairment (Table 4). When just patients with series in both eyes tested were considered, 159 of the 175 patients (90.9% CI, 86.6%–95.1%) who reached statutory blindness had an MD worse than −6 dB in at least one eye at baseline. This MD level is equivalent to what is considered to be at least a “moderate defect” for one criterion of the Hodapp-Parrish-Anderson index (Hodapp et al., 1993). Patients who were predicted to progress to statutory blindness were approximately 70% more likely to have moderate damage (MD worse than −6 dB) in at least one eye at baseline than patients not predicted to progress to this stage (positive likelihood ratio, 1.7; 95% CI, 1.6–1.8). Put differently, 1.1% (CI, 0.6%–1.6%) of the patients who were had early visual field defects, with an MD better than −6 dB in both eyes (44% of the study population), progressed to statutory blindness. Strikingly, almost 60% (52.0%–66.4%) of patients progressing to statutory blindness had one eye with an MD already worse than −14 dB in at least one eye at baseline.
The main conclusion of this study is therefore that late diagnosis of glaucoma is the most important risk factor for visual impairment and blindness (Saunders et al., 2014).
2.1.4. Glaucoma treatment decreases progression but has risks
Compelling evidence provided by the studies described above supports the notion that a method of assessing risk of progression from ocular hypertension to loss of functional vision for individual patients is needed. Weinreb et al. described the first attempt to reach that aim with the available data and several assumptions regarding disease progression to model the risk of blindness in patients with ocular hypertension (Weinreb et al., 2004). Overall, their calculations suggest that treatment may reduce the risk of progressing from untreated ocular hypertension to blindness by an estimated range of 1.2%–8.1% over 15 years (range of differences in risk of progression between untreated and treated patients according to different models).
How can these calculations affect our decision-making in managing the ocular hypertension patient? One approach to this problem is to determine the number of patients that need to be treated to prevent unilateral blindness in one patient (“number-needed-to-treat” [NNT]). The NNT is calculated with the following formula: 1/(difference in absolute risk between no treatment and treatment groups). Based on their estimates, between 12 and 83 patients with ocular hypertension will require treatment to prevent one patient from progressing to unilateral blindness over a 15-year period (Table 5). Similar models are warranted for patients with established disease at different severity levels.
Table 5.
Estimated risk of progression from ocular hypertension to unilateral blindness in treated patients and number-needed to treat to prevent blindness in one patient over 15 years.
| Data set used in calculation | OHTS (Kass et al., 2002) + St. Lucia (AGIS VF criteria) (Wilson et al., 2002) | OHTS (Kass et al., 2002) + Olmsted data (Hattenhauer et al., 1998) for Glaucoma to unilateral blindness | Olmsteddata (Hattenhauer et al., 1998) for ocular hypertension to unilateral blindness |
|---|---|---|---|
| Risk of progression, untreated | 1.5% | 2.6% | 10.5% |
| Risk of progression, treateda (0.23 the risk of untreated) | 0.3% | 0.6% | 2.4% |
| Difference between risks | 1.2% | 2.0% | 8.1% |
| Number needed to treat (1/difference) | 83 | 50 | 12 |
AGIS = Advanced Glaucoma Intervention Study; EMGT = Early Manifest Glaucoma Trial; OHTS = Ocular Hypertension Treatment Study; VF = visual field.
Treatment benefits found in OHTS (Gordon et al., 2002) and EMGT (Heijl et al., 2002).
Source: Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol.. Sep 2004; 138(3): 458–467.
While treatment effectively slows progression, adverse effects of therapy can themselves have effects on HRQOL and visual function.
3. Measurement of visual field progression
3.1. Background
Visual field analysis (perimetry) is a critical part of detecting and following functional loss in patients with glaucoma because VA is usually not affected until late in the disease. Although historically manual methods of perimetry such as tangent screen and Goldmann visual field analysis were used, in the modern era most cooperative patients are followed with automated perimetry with Humphrey, Octopus, or similar devices.
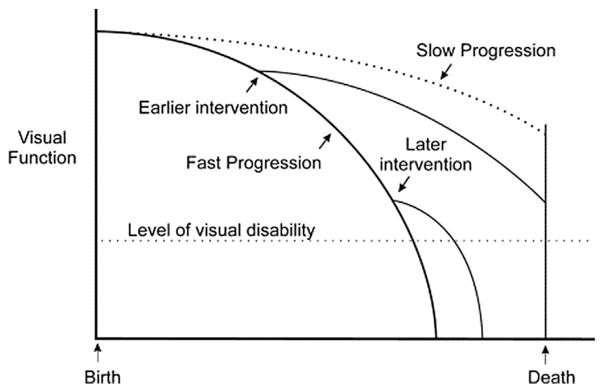
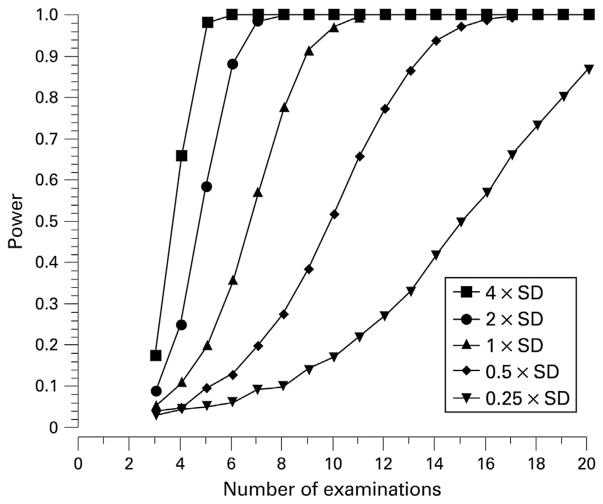
Because glaucoma is a progressive disease, it is critically important to accurately detect and measure visual field progression in individual patients. In recent years, attention has been focused on developing methods to detect early glaucoma damage and progression. However, knowing the rate of disease progression with trend-based analysis in an individual is fundamental to the long-term goal of preservation of vision in patients with glaucoma. The ability to differentiate fast progressors from slow progressors would direct appropriately aggressive treatment to those who are at highest risk for visual disability (Fig. 8).
Fig. 8. Effect of timing of intervention on rate of progression.
Source: Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol.. Feb 2008;145(2):191–192.
Tools to measure the rate of functional glaucomatous damage are critically important to patient care and have been a focus of research for more than 30 years. As described in the Introduction, one of our goals with this paper is to show that glaucoma visual field outcomes measured with rates of MD deterioration (dB/year) can be shown to be clinically meaningful even when the length of follow-up is as short as 12–18 months.
There are several issues that can make it difficult to measure visual field progression. As glaucoma becomes advanced, a diffuse (non-localized) component of visual field loss can manifest. In fact, diffuse loss can be present even in early disease as the only sign of visual field damage (Chauhan et al., 1997). Any index that is selectively sensitive to localized visual field defects may underestimate the amount of damage present in advanced disease. The progression of glaucoma may not be linear and would depend on the instrument being used to assess progression. The long-term behavior of a glaucoma progression index or similar indices in a large group of patients will help to determine the most appropriate model to use. A regression analysis of any visual field index requires a sufficiently large number of fields. The results of such analyses with fewer than eight measurements, for example, may become less reliable and more poorly predictive. The signal-to-noise ratio problem of visual field measurements can be reduced by averaging repeated measurements; the noise is reduced and the signal becomes more easily detectable (Casas-Llera et al., 2009). There are, of course, practical limitations to how many visual fields to which a patient can be subjected.
The rate of glaucoma progression varies across individuals. Since some persons tend to have a low rate of progression, not all of those affected will become visually impaired during their lifetimes. If patients with a near-zero risk of visual impairment could be identified, a source of excess treatment and excess monitoring—-with all of its side effects and costs—could be identified. The occurrence of future visual impairment (depicted as a certain amount of visual field loss) can be predicted from the current visual field loss of a patient together with his or her rate of progression (Chauhan et al., 2008; Holmin and Krakau, 1982).
This seemingly attractive concept has at least two major limitations. First, a reliable measurement of the rate of progression is required. Such a measurement, however, takes at least 5 years if typical clinic-based intervals for visual field measurements are used(Jansonius, 2010). Note that in clinical trials, significantly more visual fields spaced more frequently are used to assess progression rates in a shorter time. In the clinic, however, reliable information on visual field progression will not be available at the time of the initial decision-making, and may never become available in the aging patient. Hence, initial decision-making is necessarily based on general knowledge of rates of progression as found in observational clinical studies and trials.
Second, the remaining number of years of life has to be known. This number is usually approximated by the difference between the current age and the median life expectancy at birth, the latter being in between 80 and 85 years of age in the western world. This approximation, however, is unsuitable for estimating life expectancy in the elderly, as the median age-of-dying becomes higher with age. Moreover, it does not take into account variability in survival. As a consequence, many glaucoma patients are diagnosed, monitored, and treated at an age considerably beyond the age corresponding to their median life expectancy at birth. To make a proper estimate of the life expectancy of glaucoma patients, life expectancy should be adjusted for the age already reached, a concept known as residual life expectancy. More importantly, an estimate of the upper limit of life expectation should be taken into account rather than the median residual life expectancy, in order to deal with variability in survival (Wesselink et al., 2011). It is therefore helpful to communicate with the patient’s primary health care provider regarding medical conditions, particularly comorbidities associated with the leading causes of mortality.
3.2. Measuring glaucomatous visual field damage
Glaucoma management aims to preserve the patient’s vision, and by extension, prevent vision-related disability. Tests of vision, such as perimetry, are therefore of considerable clinical importance. Visual field testing aims to locate damaged areas in a patient’s field of vision with automated algorithms that systematically measure the patient’s ability to identify different intensities of light stimuli and hence determine their threshold of contrast sensitivity in the measured “island of vision”.
The most commonly used measurement unit is the decibel (dB), which is 10 times the log of the reciprocal of the light stimulus contrast, as measured in lamberts (L) (i.e., dB = 10 * log10 [1/L]), where 0 dB is defined as the greatest contrast stimulus that the instrument can present, and so varies between manufacturers. The results at each test location can be summarized by global perimetric indices, such as the MD, visual field index (VFI), and pattern standard deviation (PSD) in the Humphrey perimeter (Carl Zeiss, Meditec, Inc.). The visual field MD is calculated by averaging the age-corrected threshold sensitivities after adjusting for each test location’s variability and eccentricity. The weighting procedure allots greater weight to points with smaller inter-test variability, which mainly comprises the most central test locations. The VFI employs pointwise deviations from age-corrected sensitivities that fall outside normal limits (P < 5%) which are calculated as percentages (%) to produce a weighted average also based on eccentricity. The PSD is the standard deviation of the values in the pattern deviation (PD) plot. This plot is derived from the total deviation (TD) plot, which depicts age-corrected threshold sensitivities for all tested locations. The PD plot values are calculated after subtracting the value of the 85th percentile of highest sensitivity deviation from all the values in the TD plot. This procedure minimizes the effect of causes of diffuse sensitivity loss due to cataract and other types of media opacity. The Octopus perimeter (Haag Streit, GmbH) provides global indices that are analogous to the MD and the PSD, namely the mean sensitivity (MS) and loss variance (LV), respectively.
3.3. Effects of visual field severity and population aging on measurements of glaucoma progression
The interpretation of results from standard automated perimetry (SAP) is challenging because visual field measurements are variable, as revealed by psychophysical experiments with frequency-of-seeing procedures (Chauhan et al., 1993; Spry et al., 2001) and test-retest clinical studies (Heijl et al., 1989; Piltz and Starita, 1990). Variability of SAP measurements requires frequent monitoring and/or a long period of time to accurately detect true disease progression (Gardiner and Crabb, 2002; Spry et al., 2002).
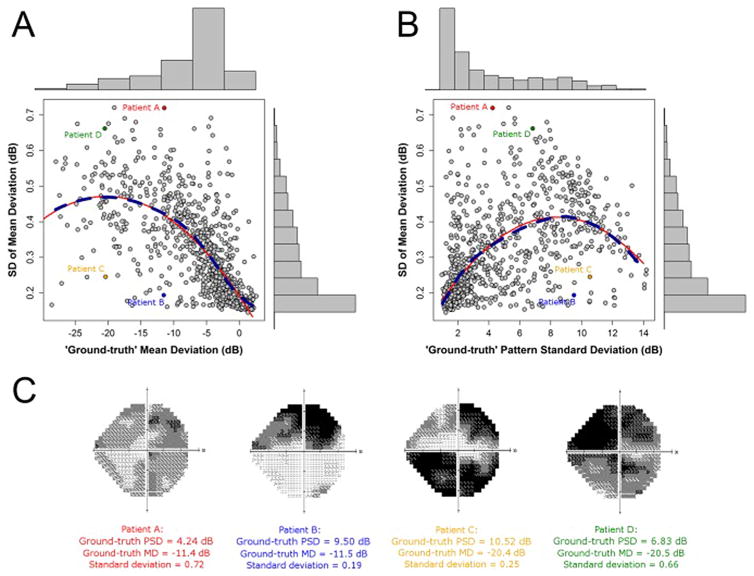
Studies that investigated the relationship between MD variability, the levels of MD and PSD, and the pattern of point-wise visual field damage showed that MD variability is a function of the absolute level of MD and PSD (Administration; Hodapp et al., 1993; Saunders et al., 2014). The dashed dark blue lines in Fig. 9 indicate the locally weighted polynomial regression, which gives an indication of how variability changes, on average, with the change in level of MD. The red lines, on the other hand, illustrate the results of fitting a second order model with a quadratic predictor (Russell et al., 2013).
Fig. 9. Variability in Mean Deviation.
Source: Russell RA, Garway-Heath DF, Crabb DP. New insights into measurement variability in glaucomatous visual fields from computer modelling. PloS ONE. 2013;8(12):e83595.
Fig. 9A suggests that variability tends to increase as the level of MD worsens, with some evidence that variability peaks around −20 dB. Fig. 9B suggests that variability tends to increase as the level of PSD increases, with some evidence that variability peaks around 8 dB of the PSD. For visual field loss associated with early glaucoma, where a significant amount of MD loss is defined as approximately −2 dB, variability is half that observed when MD is equal to −10 dB, which corresponds to the visual field loss associated with more severe glaucoma.
It has now been well-established that most visual functions decline with age both for the fovea and the periphery (Strasburger et al., 2011), and it is common practice to take this into account when assessing function in pathologic eyes, particularly in cases of glaucomatous loss. However, the relative rates of decline of different functions are unclear. For example, it has been suggested that processes mediated by magnocellular pathways, which have been reported as being responsible for the frequency-doubling illusion, are more affected by age than those mediated by parvocellular pathways (Steinman et al., 1994). For achromatic SAP, Heijl et al. estimated the rate of age-related loss as being 0.6–0.7 dB per decade and greater peripherally than centrally (Heijl et al., 1987). Wild et al. estimated loss as being 0.7 dB per decade for SAP and 1.96 dB per decade for blue-on-yellow short wavelength automated perimetry (SWAP) (Wild et al., 1998). Johnson et al. found that the reduction in sensitivity of short wavelength-sensitive cone pathways (such as those stimulated in SWAP) resulting from aging was greater than that for longer wavelength-sensitive cone pathways (Johnson et al., 1988). For frequency doubling technology (FDT) perimetry, Adams et al. estimated loss resulting from aging as being approximately linear at 0.6 dB per decade up to the age of 70 and greater after that age (Adams et al., 1999). Demirel et al. reported an age-related decline in detection acuity perimetry (DAP) of 0.04–0.05 logarithm of the minimum angle of resolution (logMAR) depending on eccentricity and of 0.03 logMAR for resolution acuity perimetry (RAP) (Demirel et al., 2012). Care must be taken when comparing these results directly because of the different scales on which results are measured with each test and the differing dynamic ranges of the instruments (Gardiner et al., 2006).
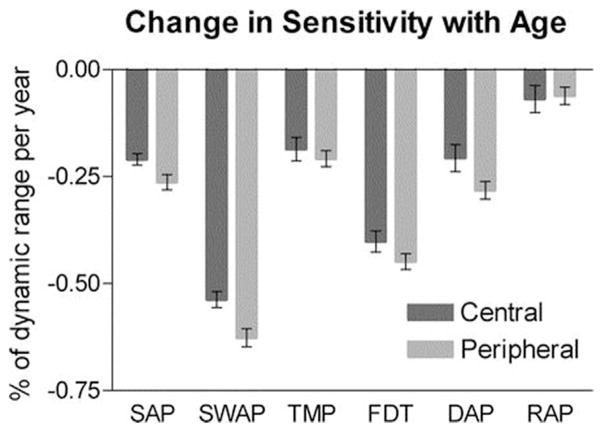
Vision function tests provide a tool to quantify progression as a function of age. Fig. 10 shows the slope of a linear regression of sensitivity against age averaged over all locations in the central and peripheral regions. The error bars represent the 95% CI for the mean. The aging effect is seen to be greatest for SWAP and FDT, least for RAP, and approximately equal for the other tests. Aging had a slightly greater effect peripherally than centrally, and this was statistically significant for SAP, SWAP, and FDT (Gardiner et al., 2006). Therefore, aging affects subject performance in visual field tests, namely achromatic SAP (more commonly used to define glaucoma and its severity), leading to worse threshold sensitivities resulting in greater variability, as described above.
Fig. 10. Slope of a linear regression of sensitivity against age averaged over all locations in the central and peripheral regions.
Source: Gardiner SK, Johnson CA, Spry PG. Normal age-related sensitivity loss for a variety of visual functions throughout the visual field. Optom Vis Sci. Jul 2006;83(7):438–443.
3.4. Effects of IOP lowering on visual field progression
With regard to the previously mentioned issues relating IOP and visual field deterioration, RCTs in glaucoma have provided invaluable information regarding the role of IOP, treatment modalities, and risk factors for disease onset and progression (Table 6 and Table 7). The discussion below will show how the findings of RCTs with regard to IOP treatment effects and rates of MD progression can be considered with respect to trend analysis.
Table 6.
Major glaucoma studies – objectives and results.
| Study | Aim | Result |
|---|---|---|
| Ocular Hypertension Treatment Study (Gordon et al., 2002; Kass et al., 2002) | Efficacy and safety of topical ocular medications in preventing or delaying the development of POAG in individuals with raised IOP (1636 patients) | With mean IOP-lowering of 22.5%, the probability of developing glaucomatous change (optic disc or field change) was 4.4% in the medication group and 9.5% in the observation group at 60 months. Baseline age, vertical cup disc ratio, visual field abnormalities, and IOP were good predictors of progression. Corneal thickness was a powerful predictor of progression |
| Glaucoma Laser Trial (GLT, 1995) | Efficacy and safety of argon laser trabeculoplasty or medicine as initial treatment in POAG (271 patients) | Eyes treated with laser trabeculoplasty had slightly reduced IOP (1.2 mm Hg) and improved visual field (0.6 dB) after median follow-up of 7 years |
| Collaborative Initial Glaucoma Treatment Study (Lichter et al., 2001) | Effects of randomizing patients to either initial medical or surgical treatment (607 patients) | Surgery lowered the IOP more than medical treatment (average during follow-up 14–15 mm Hg vs 17–18 mm Hg), but with no statistical difference in visual field progression over 5 years |
| Early Manifest Glaucoma Treatment Study (Heijl et al., 2002; Leske et al., 2003) | Effects of treatment with a topical β blocker and laser trabeculoplasty versus observation in patients with newly detected POAG (255 patients) | Progression was less frequent in the treatment group (45% vs 62%) with median follow-up of 6 years, Other important predictors of glaucoma progression included lens exfoliation, bilateral glaucoma, IOP >21 mm Hg, more advanced visual field loss, disc hemorrhages, and age ≥ 68 years |
| Collaborative Normal Tension Glaucoma Study (CNTGS, 1998a) | Effect of pressure lowering (30%) on optic nerve damage and field loss in normal tension glaucoma (140 patients) | Only 12% of treated patients progressed (optic disc and visual field progression) compared with 35% in the untreated group |
| Advanced Glaucoma Intervention Study (AGIS, 2000) | Effect of treatment sequences of laser trabeculoplasty and trabeculectomy (surgery) in advanced glaucoma (776 eyes of 581 patients) | Outcome depended on race. In patients who had laser trabeculoplasty first, black patients were at a lower risk than white patients of failure. In patients who received surgery first, black patients were at a higher risk of first failure than white patients. Patients with lower IOP had less progression |
| United Kingdom Glaucoma Treatment Study (Garway-Heath et al., 2015) | Effect of treatment on vision preservation in patients given latanoprost (258 patients) compared with those given placebo (258 patients) | Visual field preservation was significantly longer in the latanoprost group than in the placebo group: adjusted hazard ratio (HR) 0.44 (95% CI 0.28–0.69) |
POAG = primary open-angle glaucoma. IOP = intraocular pressure.
Source: Modified from Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. May 22 2004; 363(9422):1711–1720.
Table 7.
Major progression studies and outcomes in treated and untreated patients.
| Author(s) (Study) | No. of patients and studly designa | Disease and criteriaa | Definition of failure/end point | Mean study length | Progression of treated patients | Progression of untreated patients | Comments |
|---|---|---|---|---|---|---|---|
| Kass et al. (Kass et al., 2002) (OHTS) | 1636; prospective, randomized to no treatment or medication (20% reduction in IOP; ≤24) | Ocular hypertension: IOP 24–32 in one eye and 21–32 in the other eye; no detectable glaucomatous damage | Reproducible VF abnormalities or disc deterioration | 72 months (median) | At 60 months, 4.4% cumulative probability of developing OAG | At 60 months, 9.5% cumulative probability of developing OAG | |
| Hattenhauer et al. (Hattenhauer et al., 1998) and Oliver et al. (Oliver et al., 2002) (Olmsted) | 295; retrospective | 3 groups treated pts; (1) 114 OAG (2 of 3 criteria: IOP ≥21, ON or VF defects); (2) 177 ocular hypertension (treated IOP ≥21); (3) 4 undocumented OAG | Legal blindness | 15 years ± 8 years | 9% overall probability of bilateral blindness at 20 years (4% for ocular hypertension; 22% for OAG); 27% probability of unilateral blindness | No untreated patient | Various VF evaluation techniques; IOP variability while on therapy a risk factor for progression |
| Wilson et al. (Wilson et al., 2002) (St. Lucia) | 205; follow-up; untreated pts | OAG and suspects with either elevated IOP, abnormal optic s and/or VFs | VF loss | 10 years | 55% of 146 right eyes and 52% of 141 left eyes showed VF progression; probability of blindness in 10 years = 16% | All study participants were black | |
| AGIS Investigators (AGIS, 2002) | 789 eyes of 591 pts; prospective randomized to 1 of 2 surgical sequences, ATT or TAT | Advanced POAG (some field loss and inadequately controlled with maximal medical therapy); one of 9 combinations of IOP; VF, and ON defect | Sustained reduction of VF or VA; these outcomes were specifically defined in the methods | 8 to 13 years; 10.8 years (median) | 30% of eyes had sustained VF loss (10-year probability: ATT = 0.39, TAT = 0.33); 30% had VA loss (10-year probability: ATT = 0.32, TAT = 0.41) | No untreated | At least one intervention in all eyes |
| Lichter et al. (Lichter et al., 2001) (CIGTS) | 607; prospective randomized to medical therapy | Newly diagnosed OAG and 1 of 3 combinations of qualifying criteria: IOP, VF, and disc findings | Primary outcome was VF loss; secondary outcomes were VA, IOP, cataract | 60 months | Medical therapy: substantial VF loss in 10.7%; VA loss in 3.9% | No untreated | |
| or trabeculectomy | Surgical therapy: VF loss in 13.5%; VA loss in 7.2% | ||||||
| Heijl et al. (Heijl et al., 2002) (EMGT) | 255; prospective, randomized; laser trabeculectomy plus betaxolol or no treatment | Early OAG; visual defects; median IOP = 20 | VF and ON outcomes | 6 years (median); 51–102 months | 58/129 (45%) progressed; progression delayed compared with controls | 78/126 (62%) progressed | |
| Garway-Heath et al. (UKGTS) | 516; prospective randomized to latanoprost or placebo. | Newly diagnosed OAG; disc, IOP, and VF | Repeatable VF loss | 12–24 months | 35/231 (15%) at 24 months | 59/230 (25%) at 24 months | Treatment differences were significant at 12 and 18 months |
AGIS = Advanced Glaucoma Intervention Study; ATT = argon laser trabeculoplasty (ALT)-trabeculectomy-trabeculectomy; CIGTS = Collaborative Initial Glaucoma Treatment Study; EMGT = Early Manifest Glaucoma Trial; IOP = intraocular pressure; OAG = open-angle glaucoma; OHTS = Ocular Hypertension Treatment Study; ON = optic nerve; POAG = primary open-angle glaucoma; pts = patients; TAT = trabeculectomy-ALT-trabeculectomy; VA = visual acuity; VF = visual field.
Units of measure for IOP are mm Hg.
Source: Modified from Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. Sep 2004; 138(3): 458–467.
3.5. Randomized clinical trials and glaucoma progression
3.5.1. Early Manifest Glaucoma Trial (EMGT)
The Early Manifest Glaucoma Trial (EMGT) randomized patients with newly diagnosed glaucoma to treatment and observation arms, confirming the important effect of baseline IOP levels and IOP-lowering on progression (Leske et al., 2007). In addition, lower CCT, presence of exfoliation syndrome, and older age were related to progression. The updated analyses of the trial also suggest a potential role for vascular factors on progression, given the positive associations with low ocular systolic perfusion pressure, low systolic blood pressure, and cardiovascular disease history.
The effects of EMGT treatment were maintained over time and continued to suggest an approximate halving of risk (and an 18-month delay in progression) even after longer follow-up. Of note, 59% of treated versus 76% of untreated patients progressed based on SAP after a median of eight years of follow-up, despite an average IOP reduction of 25% in the treatment group (Leske et al., 2007). The hazard ratio (HR) for baseline IOP was 1.77, revealing that for each mm Hg higher baseline IOP the risk of progression increased by 77% on average. For follow-up IOP, each 1 mm Hg lower pressure decreased the risk by 10%.
These findings demonstrate the value of baseline IOP as a risk factor, as well as the effect of IOP-lowering treatment. When examining the role of follow-up IOP in patients with higher vs. lower baseline IOP, a similar magnitude of effects was observed. These results confirm the importance of IOP lowering in patient management, regardless of the IOP levels at baseline. In addition, progression was considerably and significantly faster in older than in younger patients (P = 0.002).
The frequency of disc hemorrhages at follow-up was also a significant risk factor for progression among all patients which, along with the blood pressure findings, suggests that vascular abnormalities may be an additional variable leading to continued progression independent of IOP reduction.
Given the high proportion of progressing patients despite therapy (almost 60%), there is an unmet need for additional modalities of glaucoma therapy, such as those targeting IOP-independent factors. When assessing the rate of visual field loss with the MD, the median and interquartile rates of visual function loss in the observation group of the EMGT were −0.40 (1.05) dB/year. Thus, inter-patient variability was large. Mean rates were considerably higher than medians: −1.08 dB/year. In the treated group, the median MD rate was reduced by approximately 50% (Heijl et al., 2002, 2009). This can be translated into an average decrease of median MD rate of progression from −0.40 to −0.20 dB/year in treated patients. The mean change in visual field loss from baseline to the end of follow-up, as expressed by the MD, was a worsening by 2.24 dB in the treatment group and 3.90 dB in the observation group. A separate analysis has shown that the amount of visual field deterioration needed to reach EMGT-defined visual field progression (3 locations) is associated with a worsening in MD by 2.26 dB (Heijl et al., 2002). For 5 locations, the MD worsening should be approximately 3.7 dB.
3.5.1.1. Effect of IOP lowering on progression
Since the average IOP reduction was 25% (20.6–15.45 mm Hg), a 4.5 mm Hg treatment effect was associated with a 50% reduction in rates of progression. Assuming a linear relationship, this translates to a treatment effect of 2–3 mm Hg to decelerate the rate of MD progression by 30% (Heijl et al., 2009) (see also the 3 points in the Introduction). The EMGT investigators also showed that the median change in number of significant test locations (defined at P < 0.5% in the pattern deviation map) was 0.06 per month in the observation arm (Heijl et al., 2002). This translates into a median change of 5 significant test locations in approximately 6.9 years. More importantly, the study showed that visual function as measured by SAP affected vision-targeted QOL up to six years after study enrollment, as measured with HRQOL questionnaires throughout the trial (Hyman et al., 2005).
Not all test locations in the 24-2 visual field progress similarly. In fact, they progress at highly variable rates depending on multiple factors, including eccentricity (Fig. 10) and depth of the defect (e.g.: floor effect; see also Section 3.1 below). Nonetheless, if the MD rate of progression is given as X dB/yr, a simplifying assumption is that on average each test location progresses at X dB/yr. Therefore, based upon the EMGT data described above, a clinically meaningful definition of visual field progression according to the FDA would translate into approximately 3.7 dB/6.9 years = 0.53 dB/yr in terms of pointwise rates of progression.
3.5.2. Collaborative Initial Glaucoma Treatment Study (CIGTS)
The Collaborative Initial Glaucoma Treatment Study (CIGTS) compared visual field outcomes between patients randomized to filtering surgery (trabeculectomy) versus medical therapy (Musch et al., 2009).
Patients who underwent trabeculectomy maintained the same average visual field scores over the 5-year follow-up. The results showed that initial surgery resulted in a 0.36 unit worse visual field score than initial medical treatment (P = 0.03); however, when the influence of cataract extraction on visual field data was included in the model, the difference decreased to 0.28 units and was only marginally significant (P = 0.07) (Lichter et al., 2001). The rate of significant visual field loss, defined as a 3-unit increase in visual field score, at five years was similar in both treatment groups. Almost 11% of medically treated and 14% of surgically treated patients had significant visual field progression during their follow-up.
3.5.2.1. Translating IOP lowering into effect on progression
Given the use of a visual field score system that prevents the determination of rates of MD change, a comparison between IOP-treatment effects and changes in rate of progression cannot be objectively calculated.
Predictors of significant visual field loss in both groups were time in study, increasing age, diabetes, nonwhite race, and cataract development (Musch et al., 2009). Regarding HRQOL parameters, patients assigned to trabeculectomy suffered, on average, a 3-letter loss of vision on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart after initial treatment, whereas medically treated eyes had stable VA during the first year. Vision decreased in both groups after the first year and average VA was similar in both treatment arms at the 4-year follow-up. Over the 5-year follow-up period, 3.9% of medically treated patients developed a 15-letter or greater loss in VA compared with 7.2% of patients who were surgically treated. Major predictors of 15 + letters of visual loss were initial surgical treatment, cataract development, nonwhite race, diabetes, and worse baseline vision.
The local eye symptoms subscale of the symptom and health problem checklist asked questions about eye irritation, foreign body sensation, eye pain, red eye, tearing, skin sensitivity, and ptosis. At the 12-month visit, patients in the trabeculectomy group reported significantly worse scores in all categories. At 5 years, scores were still higher in all categories in patients who underwent trabeculectomy but were only statistically significant for eye irritation and ptosis. The visual function subscale asked about blurred vision, difficulty with bright lights, visual problems with steps, and visual distortion. Surgically treated patients reported more difficulty with almost every symptom at every time point. The differences were found to be statistically significantly different with blurred vision, difficulty with bright lights, and visual problems with steps at the 12-month time point.
3.5.3. Ocular Hypertension Treatment Study (OHTS)
The Ocular Hypertension Treatment Study (OHTS) compared the effects of IOP-lowering therapy on the risk of OAG development among subjects with statistically high IOP despite normal visual field results and optic nerve head evaluation (Gordon et al., 2002).
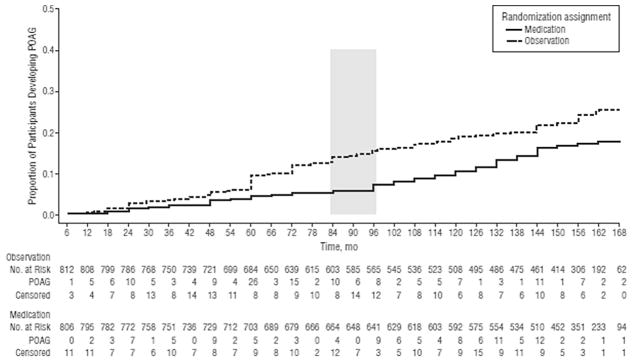
The cumulative proportion of participants in the original observation group who developed POAG at 13 years was 0.22 (95% CI, 0.19–0.25), versus 0.16 (95% CI, 0.13–0.19) in the original medication group (P = 0 0.009). Among participants at the highest third of baseline risk of developing OAG, the cumulative proportion who developed POAG was 0.40 (95% CI, 0.33–0.46) in the original observation group and 0.28 (95% CI, 0.22–0.34) in the original medication group (Figs.11 and 12) (Kass et al., 2010). Other issues of risk calculation in the OHTS are discussed in detail in a separate publication (Gordon et al., 2007).
Fig. 11. Survival plot of the cumulative probability of developing primary open-angle glaucoma (POAG) in the Ocular Hypertension Treatment Study over the entire course of the study (February 1994 to March 2009) by randomized group. The number of participants at risk was those who had not developed POAG at the beginning of each 6-month period. Participants who did not develop POAG and withdrew before the end of the study were censored from their last completed visit. Participants who did not develop POAG and died were censored at their date of death. The shaded column indicates initiation of medication in the original observation group.
Source: Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment Study. Arch Ophthalmol. Mar 2010;128(3):276–287.
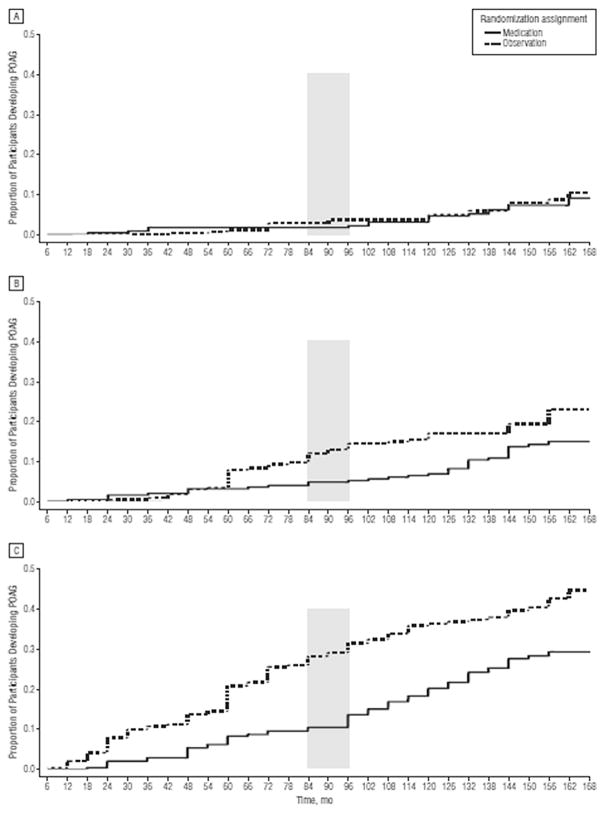
Fig. 12. Survival plot of the cumulative probability of developing primary open-angle glaucoma (POAG) in the Ocular Hypertension Treatment Study during the entire course of the study by randomized group for participants with the lowest tertile (<6.0) (A), middle tertile (6.0%–13%) (B), and highest tertile (>13%) (C) of baseline predicted 5-year risk of POAG. Participants who did not develop POAG and withdrew before the end of the study were censored from the interval of their last completed visit. Participants who did not develop POAG and died were censored at their date of death. The shaded column indicates initiation of medication in the original observation group.
Source: Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment Study. Arch Ophthalmol. Mar 2010;128(3):276–287.
These findings suggest that individuals at high risk of developing OAG would benefit from more frequent examinations and early preventive treatment. When looking at the rates of visual field change, the average MD progression rate was −0.08 ± 0.20 dB/yr (±SD) during the trial. Eyes that converted to OAG (n = 359) had significantly worse MD progression rates (−0.26 ± 0.36 dB/yr) than non-POAG eyes (n = 2250; −0.05 ± 0.14 dB/yr; P < 0.001, Fig. 13). Eyes that reached POAG endpoints based on only visual field change (n = 74; −0.29 ± 0.31 dB/yr) or only optic disc change (n = 158; −0.12 ± 0.19 dB/yr) had significantly worse MD progression rates than non-POAG eyes (both P < 0.001). Eyes that reached POAG endpoints for both visual field and optic disc change (n = 127) deteriorated more rapidly (−0.42 ± 0.46 dB/yr) than eyes showing only VF change (P = 0.017) or only optic disc change (P < 0.001) (Demirel et al., 2012).
Fig. 13. Survival plot of the cumulative probability of developing primary open-angle glaucoma (POAG) in the Ocular Hypertension Treatment Study during the entire course of the study by randomized group for participants with the lowest tertile (<6.0) (A), middle tertile (6.0%–13%) (B), and highest tertile (>13%) (C) of baseline predicted 5-year risk of POAG. Participants who did not develop POAG and withdrew before the end of the study were censored from the interval of their last completed visit. Participants who did not develop POAG and died were censored at their date of death. The shaded column indicates initiation of medication in the original observation group.
Source: Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment Study. Arch Ophthalmol. Mar 2010;128(3):276–287.
3.5.4. Advanced Glaucoma Intervention Study (AGIS)
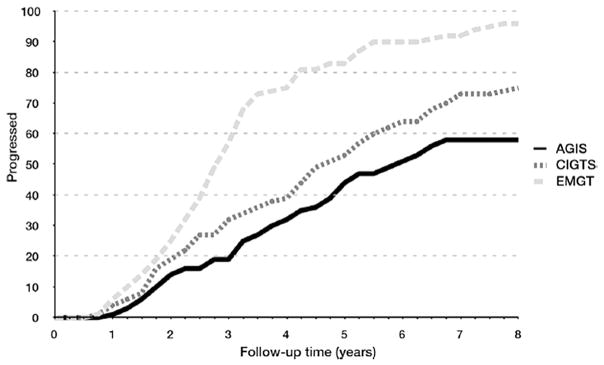
The Advanced Glaucoma Intervention Study (AGIS) investigated the efficacy of two treatment modalities in patients with severe disease.
Besides the key findings described in numerous reports (AGIS, 2000; Nouri-Mahdavi et al., 2004a, 2004b), the AGIS investigators presented for the first time an analysis of rates of visual field change (trend-based analysis) in a cohort from a RCT. In one of their reports, a total of 161 eyes (of 161 patients) were evaluated. In 64 (40%) eyes, the visual field became worse after 8 years, according to their trend-based progression criteria looking at pointwise rates.
The following variables were found to be significantly different between the progressing and non-progressing groups on univariate analysis: sum of pointwise slopes of sensitivity change during the first 4 years (P < 0.001), age (P < 0.001), and disease severity measured with the AGIS scores (P = 0.001). Neither average IOP nor IOP fluctuation during the first 4 years of follow-up was significantly different between the progressing and nonprogressing eyes. On multivariate analysis, the following two variables were associated with fast visual field progression: a more negative sum of slopes during the first 4 years (P < 0.001) and older age (P = 0.049) (Nouri-Mahdavi et al., 2004a, 2004b).
This finding depicts how measured rates of visual field change in a given period can be predictive of future functional outcomes. Eyes progressing at faster rates are more likely to reach progression endpoints during further follow-up. Hence, assessment of patients previously progressing at faster rates (or at high risk of progression) may be helpful in designing studies investigating IOP- and non-IOP lowering therapies within relatively short periods of follow-up.
3.5.4.1. Effect of IOP lowering on progression
Since the difference in MD rates of progression between treatment arms was not published, one cannot estimate the relationship between IOP-lowering effects and changes in rates of MD progression in the AGIS.
3.5.5. Low-pressure Glaucoma Treatment Study (LoGTS)
The Low-pressure Glaucoma Treatment Study (LoGTS) was a multicenter, double-masked, prospective RCT that aimed to investigate visual field outcomes in low-pressure glaucoma patients treated with either a topical beta-adrenergic antagonist (timolol maleate 0.5%) or alpha 2-adrenergic agonist (brimonidine tartrate 0.2%) (De Moraes et al., 2012b).
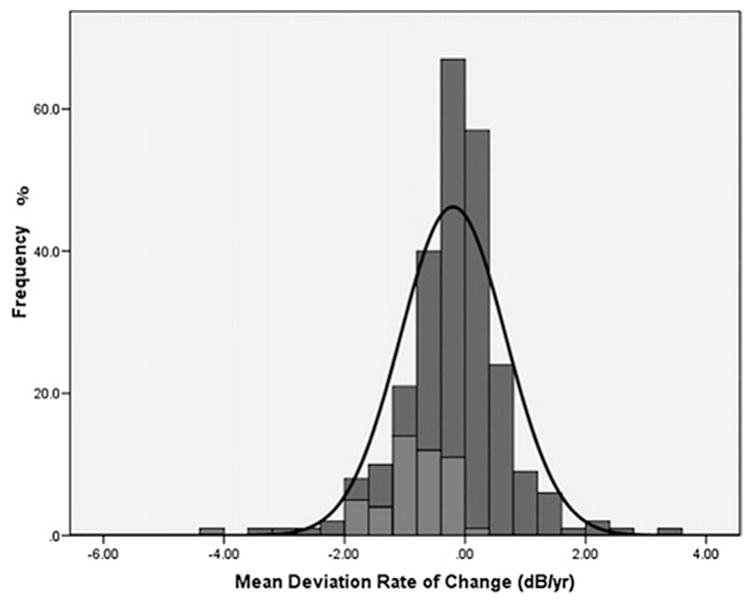
Of the 127 participants, 69 (54%) were randomized to timolol and 58 (46%) to brimonidine (P = 0.20). Forty-eight eyes (48/253; 19%) of 40 patients (40/127; 31%) met the predefined trend analysis pointwise linear regression (PLR) progression criteria (31 patients randomized to timolol; 9 patients randomized to brimonidine, P < 0.01).
As expected, the rate of MD change (dB/yr) was significantly faster in eyes that met the progression criteria than in those that did not (−0.87 ± 0.7 versus −0.04 ± 0.8 dB/yr, P < 0.01, Fig. 14). In the multivariate analysis (Table 8), older age (HR = 1.41/decade older, 95% CI, 1.05 to 1.90, P = 0.022), use of medications for systemic hypertension (HR = 2.53, 95% CI, 1.32 to 4.87, P = 0.005), and lower MOPP during follow-up (HR = 1.21/mm Hg, 95% CI, 1.12 to 1.31, P < 0.001) were associated with increased risk of progression, whereas randomization to brimonidine was significantly associated with decreased risk of progression (HR = 0.26, 95% CI, 0.12–0.55, P < 0.001) (De Moraes et al., 2012b).
Fig. 14. Low-pressure Glaucoma Treatment Study: Comparison of mean deviation (MD) rates of change (dB/yr) between progressing (light gray) and nonprogressing (dark grey) eyes based on the pointwise linear regression criteria. The black curve corresponds to Gaussian curves based on the estimates from study patients.
Source: De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. Oct 2012;154(4):702–711.
Table 8.
Low-pressure Glaucoma Treatment Study: Cox Proportional Hazards Multivariate Model with a Backward Elimination Approach based on likelihood ratios.a
| Parameter | Hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Randomization (brimonidine) | 0.26 | 0.12 to 0.55 | <0.001 |
| Age (per decade older) | 1.41 | 1.05 to 1.90 | 0.022 |
| Use of systemic antihypertensives | 2.53 | 1.32 to 4.87 | 0.005 |
| Mean ocular perfusion pressure during follow-up (per mm Hg lower) | 1.21 | 1.12 to 1.31 | <0.001 |
Variables were entered in the model if P < 0.05 and removed if P > 0.10 in the saturated multivariate model.
Source: adapted from De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol.. Oct 2012; 154(4): 702–711.
3.5.5.1. Effect of IOP lowering on progression
Since there was no difference in IOP reduction between the two treatment arms, the IOP-treatment effect needed to achieve a minimum 30% decrease in rate of MD progression cannot be estimated from the LoGTS data. However, the data from this trial can be used to see whether trend-based and event-based analysis methods correlated with each other, because the trial had three outcome measures, one event-based and two trend-based. The (trend-based) MD rate of progression for patients reaching the (event-based) Glaucoma Change Probability (GCP) progression endpoint (N = 43) was −0.87 dB/yr [SD = 0.6] and for those who did not (N = 210) was −0.62 dB/yr [SD = 0.8]. This difference was statistically significant (P < 0.001) (De Moraes et al., 2012b).
The correlation between the two fundamentally different progression criteria provides additional support for the use of trend-based outcomes with rates of MD progression as an alternative significant outcome for clinical trials to the more conventional event-based outcomes.
3.5.6. United Kingdom Glaucoma Treatment Study (UKGTS)
Although numerous clinical trials demonstrated that IOP-lowering (with different agents or procedures) significantly lower the risk of glaucoma progression, no placebo-controlled trials have assessed whether a specific agent can effectively preserve visual function. Moreover, the length of these trials was relatively long (typically 5 years). In the UKGTS, the investigators, for the first time, tested the hypothesis that a specific anti-glaucoma medication (latanoprost) can preserve visual function measured in a shorter period of time (2 years) when compared to placebo (Garway-Heath et al., 2015).
Patients with newly diagnosed open-angle glaucoma (258 in each group) were randomized to receive either latanoprost 0·005% or placebo eye drops and were initially planned to be followed for 24 months. Visual field progression was defined if at least three visual field locations deteriorated more than baseline at the 5% levels in two consecutive reliable visual fields and at least three visual field locations (note necessarily the same previous three locations) also deteriorated more than baseline at the 5% levels in the two subsequent consecutive reliable visual fields with 24-2 SITA testing.
At 24 months, mean IOP reduction was 3.8 mm Hg in the latanoprost group and 0.9 mm Hg in the placebo group. Visual field preservation was significantly longer in the latanoprost group than in the placebo group (HR: 0.44, 95% CI 0.28–0.69; P = 0.0003). Strikingly, statistically significant difference between treatment groups were already evident at 12 months (HR: 0.47; 0.23–0.95; P = 0.035) and 18 months (HR: 0.43; 0.26–0.71; P = 0.001). It is important to note, however, that effect sizes between a treatment group and placebo are expected to be larger than when two active agents are compared. Hence, we cannot make any conclusions regarding what would be the minimum length of follow-up to detect significant differences if instead of placebo, the investigators used another drug, laser, or incisional surgery.
3.5.6.1. Effect of IOP lowering on progression
A 3.8 mm Hg IOP reduction with latanoprost translated into a 56% risk reduction in 24 months, 57% in 18 months, and 53% in 12 months. The median change in visual field MD between baseline examination and the time at which progression was confirmed was −1.6 dB. No data are yet available on differences in rates of MD change or number of progression visual field locations between treated and untreated patients.
3.6. Large cohort studies and glaucoma progression
Besides the RCTs, other cohort studies also showed sustained visual field progression even in populations treated with different IOP-lowering modalities.
3.6.1. Canadian Glaucoma Study (CGS)
In the non-randomized Canadian Glaucoma Study (CGS), patients reaching an endpoint based on event-based analysis underwent 20% or greater reduction in IOP (Chauhan et al., 2010). Trend analysis with rates of MD change was used to assess the effects of treatment and potential risk factors for progression in that sample.
Patients who reached 0, 1, or 2 event-based progression endpoints had a median of 18, 23, and 25 examinations, respectively. The median MD rate in progressing patients prior to the first endpoint was significantly worse compared with those with no progression (−0.35 and 0.05 dB/yr, respectively).
Increasing age was associated with a worse MD rate, but female gender and mean follow-up IOP were not. Interestingly, the investigators found that anticardiolipin antibody level was associated with a significantly worse MD rate compared with a normal anticardiolipin antibody level (−0.57 and −0.03 dB/yr, respectively), once again suggesting the role of systemic risk factors (in addition to IOP) on rates of progression (Chauhan et al., 2010).
3.6.1.1. Effect of IOP lowering on progression
All CGS subjects entered the study with a minimum IOP reduction from untreated values of at least 30%. Of note, after the first progression endpoint, the median IOP decreased from 18.0 to 14.8 mm Hg (20% in individual patients), resulting in a significant MD rate change from −0.36 to −0.11 dB/yr (70% slower). For those patients who reached a second progression endpoint, the IOP was lowered by 18% on average, and the median MD rate of change decreased from −1.07 to −0.83 dB/yr (18% slower).
3.6.2. New York Glaucoma Progression Study (NY-GAPS)
In the first report of the New York Glaucoma Progression Study (NY-GAPS), 205 patients with treated, established glaucoma were followed for an average of 6.5 years and performed approximately 12 visual fields (De Moraes et al., 2009). Patients were divided into 3 groups: initial superior defect (group A; n = 79; MD, −3.4 [1.9] dB), initial inferior defect (group B; n = 61; MD, −3.4 [1.8] dB), and both hemifields affected (group C; n = 65; MD, −4.2 [1.5] dB).
Group C progressed faster than did groups A and B (P < 0.02). Multivariate analysis showed significant effect of higher baseline IOP, thinner corneas, and initial damage to both hemifields of faster rates of progression with PLR. In another report comparing patients with high- and low-pressure glaucoma, patients with high-pressure were significantly older (mean ± SD: 72.6 ± 9.4 years vs. 62.7 ± 12.8 years, P < 0.01), had higher mean IOPs (16.5 ± 3.2 mm Hg vs. 13.3 ± 2.0 mm Hg, P < 0.01) and greater CCT (544.0 ± 35.7 μm vs. 533.9 ± 35.9 μm; P = 0.01). During a similar period, high-tension glaucoma patients progressed globally almost twice as rapidly as did those with statistically normal pressures (−0.64 ± 0.7 dB/yr vs. −0.35 ± 0.3 dB/yr, P < 0.01), which became non-significant after adjustment for differences in age, mean IOP, and CCT.
In a multivariate model, variables significantly associated with progression were higher mean IOP (odds ratio [OR], 1.09, P = 0.03) and lower CCT (OR per microns thinner: 1.37, P = 0.03). Progression within the paracentral VF was more common in the NTG group (75% vs. 57.3%, P = 0.04).
The most important factor associated with paracentral progression among eyes that reached a progression outcome was the diagnosis of low-pressure glaucoma. Baseline central visual loss (within the central four points on the 24-2 VF) occurred more frequently in low-pressure glaucoma patients (58.9% versus 31.8% of eyes, P < 0.01) (Ahrlich et al., 2010). In a larger cohort with 587 eyes of 587 patients, the univariate model of risk of progression based on PLR criteria revealed that an increased risk of fast visual progression was associated with, older age (OR, 1.19 per decade; P = 0.01), baseline diagnosis of exfoliation syndrome (OR, 1.79; P = 0.01), lower CCT (OR, 1.38 per 40 μm thinner; P < 0.01), a detected disc hemorrhage (OR, 2.31; P < 0.01), presence of beta-zone parapapillary atrophy (OR, 2.17; P < 0.01), and all IOP parameters (mean follow-up, peak, and fluctuation; P < 0.01). In the multivariable model, peak IOP (OR, 1.13; P < 0.01), lower CCT (OR, 1.45 per 40 μm thinner; P < 0.01), a detected disc hemorrhage (OR, 2.59; P < 0.01), and presence of beta-zone parapapillary atrophy (OR, 2.38; P < 0.01) were associated with visual field progression (De Moraes et al., 2012b).
3.6.2.1. Effect of IOP lowering on progression
The similarity of the results of this study with those of RCT’s that used event-based progression endpoints (e.g., OHTS, EMGT) also support the contention that trend-based analysis with MD rates of progression can be used to measure clinically significant treatment effects that are consistent with FDA statements.
Moreover, progressing eyes and stable eyes had a mean (SD) global rate of VF change of −1.0 (0.8) dB/yr and −0.20 (0.4) dB/yr, respectively (P < 0.01) and the difference in mean IOP values between progressing and non-progressing eyes was 2.6 mm Hg.
4. Nature of glaucomatous visual field progression
4.1. Progression usually occurs in already abnormal areas
In addition to knowing the rate of visual field progression in glaucoma, it is important to know the pattern of progression. This knowledge can shed light on where to look for progression and whether intervention may be more beneficial. Glaucomatous visual field progression occurs most often in already abnormal areas, and is reflected by deepening of the defect and extension to contiguous areas.
Mikelberg and Drance studied 42 eyes of 42 patients with glaucoma to determine the pattern of progression of their visual field defects (Mikelberg and Drance, 1984). In 33 eyes (79%) the scotomas became denser with progression. Enlargement occurred in 22 eyes (52%) and 21 eyes (50%) developed new scotomas. Increased density of the scotomas was the only manifestation of change in ten eyes (24%), three eyes (7%) showed enlargement only, and six eyes (14%) showed only new scotomas. Seventeen eyes (57%) with single hemifield involvement maintained a defective single hemifield throughout the follow-up period.
In another study, Mikelberg et al. (Mikelberg et al., 1986) investigated 45 eyes of 45 patients with chronic OAG to evaluate the rate of progression of scotomata by analyzing only areas with pre-existing visual field damage. “Scotoma mass” was calculated and regressed over time to obtain the rate of visual field change. Twenty-two eyes (49%) showed a linear progression, nine (20%) showed a curvilinear progression, three (7%) showed episodic progression, and 11 (24%) showed no significant progression.
We performed a similar analysis with the NY-GAPS database by investigating the rate of progression of abnormal locations at baseline as compare with the global MD rate (De Moraes et al., 2011). POAG patients were ranked based on their MD rates of progression into two groups: 1) rates faster or equal to −1.0 dB/yr (N = 60 patients) and 2) rates between −0.5 (included) and −1.0 dB/yr (N = 86 patients). Table 9 shows for each group the average baseline MD and PSD, their average and SD rate of MD change in the study period, and number of visual field tests analyzed. Table 10 shows the main outcome variables for the analyses of both groups. Note that when comparing the global MD rate of progression and rate of progression of abnormal points at baseline (“scotoma mass”), about 90% of the global progression is driven by the abnormal points. This observation combined with what we discussed with regard to MD, visual field locations, and HRQOL scores, heightens the fact that searching for treatment effects at abnormal visual field locations over time could provide more patient-important information than when abnormal locations are diluted by normal visual field areas.
Table 9.
Primary open-angle glaucoma patients from the New York Glaucoma Progression Study (NY-GAPS) were divided into 2 groups based on their rates of mean deviation (MD) progression. The table shows for each group the average baseline MD and pattern standard deviation (PSD), their average and standard deviation rate of MD change in the study period, and number of visual field tests analyzed.
| −0.5 to −1.0 dB/yr (N = 86 eyes/patients) | Faster than −1.0 dB/yr (N = 60 eyes/patients) | Both Groups Combined (N = 146 eyes/patients) | |
|---|---|---|---|
| Baseline MD (SD) | −6.64 (4.75) dB | −6.68 (4.22) dB | −6.66 (4.52) dB |
| Baseline PSD (SD) | 5.93 (2.95) dB | 5.57 (3.30) dB | 5.78 (3.03) dB |
| Average MD rate of progression (SD) | −0.70 (0.14) dB/yr | −1.55 (0.65) dB/yr | −1.05 (0.60) dB/yr |
| Average number of fields analyzed (SD) | 12.79 (4.02) | 12.05 (3.24) | 12.48 (3.72) |
MD = mean deviation; SD = standard deviation; PSD = pattern standard deviation.
Source: De Moraes CG, Juthani VJ, Liebmann JM, Teng CC, Tello C, Susanna R Jr., Ritch R. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011; 129(5): 562–8.
Table 10.
The same group of patients described in Table 9. The ‘average of the average’ rate of progression was calculated by summing each eye’s mean rate of progression of abnormal points and dividing by the total N in that group. The ‘SD of the average’ corresponds to the SD of the mean slopes of progression of abnormal points of all N eyes. Similarly, the ‘average SD’ was calculated by summing each eye’s SD of rates of progression of abnormal points and dividing by the total N in that group.
| −0.5 to −1.0 dB/yr (N = 86 eyes/patients) | Faster than −1.0 dB/yr (N = 60 eyes/patients) | Both groups combined (N = 146 eyes/patients) | |
|---|---|---|---|
| ‘Average of average’ rates of progression | −0.837 dB/yr | −1.669 dB/yr | −1.179 dB/yr |
| ‘SD of the average’ rates of progression | 0.322 | 0.674 | 0.644 |
| ‘Average SD’ of rates of progression | 0.791 | 1.028 | 0.888 |
SD = standard deviation.
Source: De Moraes CG, Juthani VJ, Liebmann JM, Teng CC, Tello C, Susanna R Jr., Ritch R. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011; 129(5): 562–8.
In another study investigating patterns of progression, the majority of progressed eyes showed a deepening of an existing scotoma; 11% by clinical criteria and 12% by GCP criteria (Boden et al., 2004). The next most common finding was a combination of expansion and deepening of an existing scotoma. The authors concluded that glaucomatous visual fields progress in the area of the visual field where baseline testing showed an existing scotoma and that follow-up testing might be improved by concentrating on already defective locations and with sparser test patterns or screening algorithms in normal areas of the visual field.
More recently, investigators of the Diagnostic Innovations in Glaucoma Study (DIGS) (Pascual et al., 2007) determined the spatial characteristics of glaucomatous visual field progression in persons with glaucomatous-appearing optic neuropathy (GON) followed with serial Full Threshold 24-2 SAP examinations (Humphrey Field Analyzer II; Carl Zeiss Meditec, Inc., Dublin, CA) of 200 patients with GON confirmed on two occasions by optic disc stereophotograph review. The proportion of patients exhibiting pattern deviation (PD) plot progression was determined at each of 52 locations for patients with a baseline abnormal result (P < 5% or worse) in one or more of 52 PD locations in either the first or second baseline test for a total of 2704 location pairings for each possible level of negative PD change. Progression was defined as any worsening of PD plot value in the follow-up test relative to the average PD plot value in the baseline tests. Monte Carlo simulation was used to determine the significance of the observed patterns of PD plot progression. They found that changes in PDs were dependent on their location relative to abnormal PD locations in the first test. Of those patients with an abnormality at a location at baseline (mean, 0.23 ± 0.07 dB), the proportion of patients changing by −2 dB or more ranged between 0.09 and 0.55 (mean, 0.29 ± 0.06) across locations. For changes of −6 dB or more, the proportions ranged between 0.00 and 0.26 (mean, 0.08 ± 0.04) of patients. The proportional probabilities are consistent with a map of the retinal nerve fiber layer bundles. They concluded that visual field progression occurs in retinotopically constrained patterns, consistent with changes along nerve fiber bundles.
One important factor to be considered when searching for progression among abnormal points at baseline is the “floor effect” that occurs at very depressed visual field locations. Once a threshold of abnormality is reached, progression may continue even though the available methods to measure it will falsely evaluate these points as stable. This effect is one of the reasons why RCTs have shown contradictory results regarding the role of baseline visual field damage on progression. In the OHTS, in which all patients had normal visual fields at baseline, worse global visual field indices – namely the PSD – was an independent predictor of conversion to POAG in the multivariable analysis (Gordon et al., 2002). In the EMGT, a baseline MD worse than −4 dB also increased the risk of progression by 46% (Leske et al., 2007). On the other hand, the AGIS showed that patients with better baseline MD values were at increased risk of progression (Nouri-Mahdavi et al., 2004a), most likely because the majority of patients had severe visual fields at baseline which precluded the detection of change due to the “floor effect.” For that reason, in clinical trials that aim to test the effectiveness of therapy in perimetrically abnormal locations, it is worthwhile to exclude from the analyses patients whose visual field results reveal very severe MD values (e.g., worse than −15 dB) or test locations with absolute threshold sensitivities worse than 19 dB. In addition, test-retest variability increases as threshold sensitivities worsen. This leads to widening of the 95% CIs of normality for test-retest repeatability, which can be used to define significant progression with event-based analysis.
This topic is discussed later with respect to detection and measurement of visual field progression.
4.2. Glaucomatous visual field progression often occurs linearly, sometimes accelerating later in the course of the disease
There is an ongoing discussion in the glaucoma literature on whether visual field progression measured with trend-analysis dB values (which are a logarithmic scale) occurs in a linear or nonlinear fashion over time. The studies described below suggest that despite some evidence from statistical modeling that progression is non-linear in some patients and some areas of the visual field, a linear approximation generally provides a good measure of goodness-of-fit and has a reasonable ability to predict future outcomes by extrapolation of linear trends. Moreover, the simplicity of linear models and their ease of interpretation by clinicians make this approach a strong one for measuring rates of change and treatment effects among patients with a sufficient number of visual field tests. In particular, an analysis by Gardiner et al., based upon data from the OHTS, showed that when performing linear regression of MD data over time, shorter series of length (between 6 and 9 visual fields) were able to measure treatment effects whereas longer series concealed such effects (Gardiner et al., 2013). With longer follow-up and greater number of visual field tests, it becomes more difficult to rely on linear trends as progression can occur in a stepwise or other non-linear pattern, particularly if one considers changes in treatment that are often implemented over time and interim risk factors for acceleration of progression (e.g. disc hemorrhages).
The use of a linear rate of progression of MD over time may not reflect the true nature of glaucomatous deterioration given that there is some evidence to show patients tend to progress more quickly at older ages, although it is unknown whether this is a result of older age or more advanced visual field deterioration, as discussed elsewhere. Nevertheless, linear regression of MDs is commonly used in clinical practice. The Glaucoma Progression Analysis software in the Humphrey Field Analyzer (HFA), for instance, presents this as “one method of tracking rate of progression” (Saunders et al., 2014). Furthermore, studies suggest that linear rates of progression for summary measures are adequate (Bengtsson et al., 2009), and it is important to note that a linear decline in decibels represents an exponential decay in retinal sensitivity. Although loss of sensitivity could occur at greater than an exponential rate, no research to date has suggested that another type of model should be used to measure the rate of decay of MD. In addition, previous work has shown that a linear model of visual field progression tends to provide robust estimates of future measurements (Bryan et al., 2013; McNaught et al., 1995). Loss of visual field sensitivity can occur at linear and non-linear patterns. Investigators have tested the performance of different in both fitting longitudinal data and predicting outcomes. Chen et al. compared the ability of pointwise linear, exponential, and logistic functions, and combinations of functions, to model the longitudinal behavior of visual field series and predict future visual field loss in patients with glaucoma. They found that while the logistic model best fit glaucomatous visual field behavior over a long time period, the exponential model provided the best average predictions (Chen et al., 2014). Azarbod et al. investigated a pointwise exponential regression model to calculate average rates of faster and slower deteriorating visual field components, as well the entire visual field. They found that this model was a robust indicator of rates across a wide range of disease severity and could predict future global indices accurately (Azarbod et al., 2012). Finally, Otarola et al. modeled the process of glaucomatous visual field decay over the entire perimetric range from normal to perimetric blindness and found that a pointwise sigmoid regression had a better ability to fit perimetric decay (Otarola et al., 2016). These study results support the concept that the measured behavior of glaucomatous visual field loss within the entire range of damage is probably non-linear and that its course of deterioration may change with the course of disease. Despite these compelling data, linear models still showed an overall good fit in most studies and given their ease of use, we believe they have practical usefulness in endpoint determination in clinical trials, particularly with shorter series of fields.
However, it is important to be aware that this MD regression does not imply a constant rate of sensitivity loss; a loss of −1 dB implies much more damage going from −5 dB to −6 dB than from −25 to −26 dB, as a result of the logarithmic scaling used for the measurement. It is further noteworthy that the “future” forecasts based on current linear rates of visual field loss may make the estimates of future prognosis in the patients studied overly pessimistic, as treatment is usually intensified if a patient is in danger of progressing to visual disability(Heijl, 2013). On the other hand, the modeling takes no account of concomitant eye disease, which ultimately might precipitate levels of lifetime visual disability that are worse than those predicted.
4.3. Central versus peripheral glaucomatous visual field damage
Over 30% of the RGCs are within the macular region (Hood et al., 2014), which is often defined as the central 8° of the visual field. Moreover, given the cortical magnification of images from the retina, this small area of the field corresponds to up to 60% of visual cortex (V1). While it is clear that early, even initial, glaucomatous damage can involve these RGCs and the macula (Hood et al., 2013, 2014), the nature of this damage is incompletely understood.
Hood et al. have investigated the prevalence and nature of central vision loss in early glaucoma with 10-2 visual fields. In this observational cohort study, 100 eyes from 74 patients with glaucomatous optic neuropathy and a 24-2 visual field with MD better than −6 dB at baseline were prospectively studied and tested with a 10-2 test (Traynis et al., 2014). Reliable visual fields hemifields were classified as abnormal based on a cluster criterion, and abnormal 10-2 VFs were categorized based on the pattern of abnormal points: arcuate-like, widespread, or other. In addition, at each point of the 10-2 visual field, the total deviation values were averaged across eyes and the number of abnormal points with total deviation values below a specific criterion level was calculated. The results revealed that there were as many abnormal 10-2 hemifields (53%) as abnormal 24-2 hemifields (59%) in this sample of patients with early glaucoma who had their central field scrutinized regardless of the results of conventional 24-2 perimetry. Of the eyes with normal 24-2 hemifields, 16% were classified as abnormal when the 10-2 test was used. Of the abnormal 10-2 hemifields, 68%, 8%, and 25% were arcuate-like, widespread, and other, respectively. The average total deviation values and number of abnormal points revealed superior visual field defects that were deeper and closer to fixation than those in the inferior hemifield. Their conclusion was that the 10-2 visual field tests was abnormal in nearly as many hemifields as was the 24-2 visual field, including some with normal 24-2 visual field, suggesting that the 24-2 test is not optimal for detecting early damage of the macula. In fact, although the macular region contains about 30% of RGCs, only 4/54 (7%) of the points tested in conventional 24-2 perimetry are concentrated in the macular region.
Not only is this pattern of central defect often overlooked, but some consider that abnormal central points suggest a different pathogenesis of glaucomatous damage compared to peripheral points in the nasal field. Park et al. (Park et al., 2011) compared clinical characteristics between patients with initial paracentral defects with those with peripheral nasal defects. Maximum untreated IOP was significantly lower (21.6 ± 4.5 vs. 28.3 ± 9.6 mm Hg; P < 0.001), and frequency of disc hemorrhage detection was significantly higher (44% vs. 17%; P = 0.001) in patients with paracentral loss than in patients with a peripheral, nasal loss. Systemic risk factors (hypotension, migraine, Raynaud’s phenomenon, and sleep apnea) was also significantly higher (16%, 23%, 24%, and 9% vs. 0%, 4%, 9%, and 0%; P = 0.001, 0.002, 0.025, and 0.030, respectively) in patients with paracentral loss. There were no significant differences in age, gender, family history of glaucoma, refractive error, CCT, and disc area between the two groups (all P > 0.1). Mean deviation was similar between the two groups (P = 0.346), but the PSD was significantly greater in the paracentral group (P = 0.043), suggesting that despite occupying a small proportion of the visual field printout, these defects are deeper and hence may have greater impact in vision-related quality of life (QOL) than those that start in the periphery, as discussed above.
5. Detection of visual field progression
There is currently no gold-standard to define visual field progression in glaucoma. For decision making and tailoring therapy, most clinicians either rely on subjective evaluation of a sequence of visual fields or utilize the machine assessment of statistically significant progression calculated by proprietary software with automated algorithms. Software such as Glaucoma Progression Analysis (GPA, Carl Zeiss Meditec, Inc.) have greatly improved inter-observer agreement when defining progression compared to subjective, expert judgement (Tanna et al., 2012). Given this lack of consensus, each of the major RCTs defined visual field outcomes in a different way. Even though the overall main conclusions regarding IOP-treatment effects on visual field progression are consistent among these trials, including most of the recognized risk factors for progression, the agreement among these different visual field progression criteria is not optimal. Some studies have assessed the rates of agreement/disagreement among these trials as means to compare their sensitivities and specificities with different arbitrary reference criteria. An inherent increase in sensitivity at the expense of lower specificity (and vice-versa) occurs in all cases. As we emphasize in this article, dichotomizing “progressing” vs. “stable” is an arbitrary – although often useful – decision. However, in reality, the progression rate is a continuous scale and there are no truly ‘stable’ visual fields (particularly when one considers the effects of aging). Decisions regarding the best method to use in clinical practice happen on an individual basis and are dictated mainly by experience/familiarity with a particular method or by weighing the impact of false-positive/false-negative results on the QOL of patients with particular stages and therapeutic possibilities.
5.1. Detection and measurement of clinically significant glaucomatous visual field progression
In normal individuals, visual field measures are not perfectly repeatable and individual test locations exhibit both short- and long-term sensitivity variations. This physiologic variability is greatly increased in glaucoma and confounds detection of real progressive loss in visual function. Distinguishing progressive glaucomatous visual field loss from test variability therefore represents a complex task. Methods to detection of glaucomatous visual field progression may be grouped into four main categories: 1) clinical judgment, 2) defect classification systems, 3) trend-based analyses, and 4) event-based analyses (Spry and Johnson, 2002). All of these methods demonstrate distinct benefits and drawbacks, making each useful in specific circumstances, although no single method appears universally ideal. At the present time, the best method of detection of progression may be to rely upon confirmation of change at successive examinations and also by correlation of visual field changes with other clinical observations(Spry and Johnson, 2002). Nonetheless, even when structural information is available for comparisons, one needs to be cautious regarding the poor correlation between structure (e.g. OCT retinal nerve fiber layer) and function (e.g. visual field MD) in early and late stages. Here we focus our discussion on trend- and event-based analyses.
5.1.1. Trend-based analysis
Trend analyses evaluate test parameters sequentially to determine temporal patterns that may exist within the data. Such analyses are of value because they are capable of determining long-term characteristics with use of information from all visual field examinations performed on a patient, and therefore have the potential to discriminate subtle progressive loss from considerable degrees of test variability. It is possible to employ this method for the detection of progression on any continuous variable that quantifies perimetric information by plotting it against time and finding the line of best fit though the data.
Trend-based analysis offers some advantages over event-based visual field progression analysis, despite requiring longer series of visual fields. All visual field information over time is used with a trend-based analysis, while an event-based analysis only uses the baseline(s) and most recent tests, and not the ones in between. In addition, since they typically provide a continuous outcome variable (rate of change) instead of a binary outcome, trend-based methods have much greater statistical power to distinguish between groups in a clinical trial. Finally, the rate of change can be interpreted in conjunction with information about the life expectancy, which cannot be done when employing event-based analyses. When using regression to evaluate trends within visual field data, it is important to consider both the slope of the line (rate of change of the variable on time) and the corresponding confidence interval (CI), which can be set at different levels depending on the desired trade-off between sensitivity and specificity (e.g. 95 or 99%).
To date, linear regression has been reported on global indices, mean sensitivity within sectors of the visual field, and for individual test locations (pointwise) (Wu et al., 1987). HFA, linear regression analysis is currently available for routine clinical use in two forms. The first of these is part of STATPAC whereby use of the “Change Analysis” option performs a linear regression of MD. The change analysis procedure graphically plots MD for sequential tests and gives a regression line slope, the corresponding 95% CI and a P value if the regression line slope is significantly different from zero. The advantage of using MD is that it is calculated with reference to age-matched normative data and, therefore, should not show any deterioration as a function of normal aging. Any negative slope may be considered as progressive visual field loss. However, a major disadvantage of this approach is that MD quantifies overall visual field loss, and, therefore, any subtle localized glaucomatous progression may be masked, for example, by increasing cataract. Also, in a similar manner to data reduction by defect classification systems, use of any global index may diminish information from local defects and reports from clinical investigations have demonstrated that MD (or equivalent) is relatively insensitive to progressive glaucomatous visual field loss (Chauhan et al., 1990). Therefore, as for lack of change for defect classification systems it is critical to recognize that a non-significant MD regression line slope does not rule out small degrees of visual field change. For the Octopus perimeters, a similar analysis is available from PeriTrend, part of the PeriData software package (PeriData, GmbH, Huerth, Germany).
Linear regression analysis of pointwise threshold data is also commercially available for the HFA in the PROGRESSOR software package (Medisoft Inc., Leeds, UK). This analysis procedure is run on a personal computer that accepts HFA data from disc or by serial connection to the perimeter. PROGRESSOR calculates the relationship between threshold sensitivity and test date for each test location and displays the data spatially so that degree of change at each location is visible and significance of any trend is clearly discernible from color coding (Fig. 15). The advantage of this procedure is that important spatial relationships between locations that appear to be progressive are preserved rather than being lost by data reduction.
Fig. 15.
PROGRESSOR output. Top: summary statistics depicting the global rate of progression, the number of progressing visual field points, and their average rate of progression. Bottom: Visual field representation showing the location of progressing points. Note that while the left eye progressed significantly, the right eye was more stable.
However, there are disadvantages to pointwise linear regression, including assumptions about test point independence and loss of specificity. A number of studies have shown that pointwise regression detects more cases of progression than global indices do, suggesting that it has a higher degree of sensitivity, whereas global indices may have greater specificity (Katz et al., 1997; Smith et al., 1996). This is actually a basic statistical concept, given that as one goes from global indices to local ones, variability increases and makes detection of significant change more difficult, even though the probability of detecting change increases by the simple fact that more locations are being tested. Use of intermediate-sized visual field areas, e.g., Glaucoma Hemifield Test (GHT) zones, have been suggested as a compromise (Katz et al., 1997; Nouri-Mahdavi et al., 2007). Because no gold standard exists to define progressive loss, it is impossible to identify which of these techniques has the highest discriminatory power for the detection of real change. A practical disadvantage of PROGRESSOR is that downloading data to a separate computer and subsequent program operation requires additional time and resources. Pointwise linear regression is available for the Octopus perimeters in PeriData’s Global Analysis of Topographical Trends as well.
The length of follow-up required to detect progression with linear regression is influenced by a number of factors, including examination frequency, underlying rate, type of progression, the specific variable being evaluated, the degree of variability, and position of the visual fields within the time series (Spry and Johnson, 2002). Practically, for pointwise linear regression, it has been suggested that a minimum of 7 or 8 visual fields are required to achieve reasonable levels of sensitivity and specificity (Holmin and Krakau, 1982; Katz et al., 1997; Spry et al., 2000a).
A further important consideration with this technique is the outcome measure used to signify progressive loss. Some studies have defined progressive loss as any significant negative slope, irrespective of gradient, with a P value equivalent to 0.001 for a single test location (Katz et al., 1997; Smith et al., 1996). Other studies have employed a criterion for slope magnitude in addition to a significance criterion. For example, Noureddin et al. (Noureddin et al., 1991) required individual test locations to have significant (P < 0.05) slopes of −2.4 dB/year to be considered progressive, whereas a number of other investigations employed the criterion of significant slopes worse than −1.0 dB/year (P < 0.05) (Viswanathan et al., 1997a, 1997b). Therefore, it appears that there is no consensus as to the pointwise trend analysis outcome for determining progressive visual field loss.
It has been suggested that the designated criteria described above are not based on physiological sensitivity loss with age and are in fact many times more than expected from cross-sectional studies of the perimetric-aging effect (Wild et al., 1997). However, it should be noted that at present no longitudinal data exist that demonstrate the effect of normal aging within the same individuals over time. It seems reasonable to suggest that the lack of consensus may be due to differing perspectives. Some investigators may wish to investigate any degree of progressive sensitivity loss, however small in magnitude, and so may select liberal criteria that demonstrate statistically significant negative trends, whereas other investigators may wish to identify what they may consider clinically significant or meaningful rates of loss.
5.1.2. Event-based analysis
Event analyses are valuable because they attempt to identify single events of significant change relative to a reference examination. Event analyses can be relatively simple, and can look for statistically significant differences between one examination and another, such as used within the DELTA program of the Octopus perimeter (Fankhauser and Jenni, 1981). This particular method employs paired t-tests to determine whether significant differences are present between one test result and another. More recently, however, refinements of event analyses have attempted to account for test–retest variability. By quantifying the test–retest variability found in a group of stable glaucoma patients (usually a group of glaucoma patients tested repeatedly over a short period of time), this analysis approach identifies occasions when the amount of change in a given variable between one test and another exceeds the amount expected due to scatter of threshold measurements. Test–retest analyses appropriate for this approach have been described for SAP (Heijl et al., 1989) and other perimetric tests and strategies (Chauhan and Johnson, 1999; Spry et al., 2000b).
A well-known example of this approach to event analysis is the GCP, which is available on STATPAC 2 of the HFA. The GCP examines changes on a point-by-point basis, thereby providing information upon spatial relationships between progressive test locations. The GCP calculates the difference in pointwise total deviation at each test location between an examination result and that found for the corresponding location at baseline, the baseline comprising an average of two automatically selected examination results of the same test program. If the difference at an individual test location falls outside either tail of the test–retest distribution for stable glaucoma patients (5th or 95th percentiles), the test location will be marked as progressing or improving. Care should be taken when using GCP as the automatic selection of the two test results used to average for baseline are the first two sequential tests stored for the particular patient in the instrument’s internal database. This is a drawback because these test results may not be representative, for example, they may include early learning examinations. Automatic selection can, however, be overridden by the operator. GCP results are printed sequentially for successive examination results, with progressive test locations denoted by closed triangles, improving locations with open triangles and unchanged locations with a small dot.
GCP has since been largely replaced by Guided Progression Analysis (GPA). The GPA is based on the pattern deviation plots, as opposed to the total deviation plots used by the GCP. In addition, the GPA runs on SITA tests, but also accepts Full-Threshold tests (the GCP does not), which is convenient for patients whose follow-up started with full-threshold perimetry. Nonetheless, the transition from full-threshold testing to SITA testing is usually accompanied by a slight increase in threshold sensitivity (approximately 1 dB) which is probably due to reduced test time (Wild et al., 1999). Not taking this into consideration can obscure the detection of progression in patients who transition between the two strategies.
A major advantage of GCP/GPA is that it may theoretically identify test locations that appear progressive with as few as three test results. There are a number of disadvantages with this approach, the first being that the ability to detect progression is dependent upon the degree of change exceeding test–retest variability. Since this variability is known to be already high for damaged locations this analysis method may be insensitive to small progressive changes. Another major disadvantage is that the probabilistic nature of GCP/GPA will also negatively affect specificity because each test location will worsen or improve on 5% of occasions by chance. In order to overcome this problem and maintain specificity, some investigators have required one or more retests of those test locations that appear to be progressive. For example, FDA has suggested a requirement for progression at 5 or more locations in repeated examinations to establish clinical meaningfulness(Weinreb and Kaufman, 2009). Although the use of a confirmation criterion improves specificity, such criteria are not empirically based, and the impact of varying confirmation criteria upon sensitivity has not been reported.
5.2. Comparisons of methods for detecting visual field progression
Three major glaucoma trials, all employing the same Humphrey visual field tests, specified different criteria to define visual field progression. One study compared the performance of these criteria with a reference standard of unanimous classifications by three independent glaucoma experts in a sample of patients with manifest glaucoma from the EMGT (Heijl et al., 2008). The visual field series of one eye of each of 245 EMGT patients were classified by three independent glaucoma specialists as definitely progressing, definitely non-progressing, or neither. Field series that were classified in the first two categories by all three experts met the reference standards for the progressing and non-progressing groups and were analyzed according to the progression criteria of the AGIS, CIGTS, and the EMGT. Sensitivity, specificity, time to progression, and sustainability were calculated. In their results, 77 field series were definitely progressing, and 95 series were definitely non-progressing. Among progressing eyes, 45 (58%) of 77 were identified with AGIS criteria, 58 (75%) of 77 were identified with CIGTS criteria, and 74 (96%) of 77 were identified with EMGT criteria; all comparisons of sensitivities were significant simultaneous (P < 0.001) and pairwise (P < 0.01). The specificity for EMGT criteria was 89%, lower (P < 0.05) than that of AGIS (98%) and CIGTS (99%) criteria. Median time to progression was considerably shorter with EMGT criteria (33 months; 95% CI, 30–36 months) than with AGIS (66 months; 95% CI, 57–78 months) and CIGTS (55 months; 95% CI, 48–66 months) criteria.
Sustainability increased with time after first progression averaging 79%, 84%, and 81%, respectively, for AGIS, CIGTS, and EMGT criteria during the first year after the first progression and 95%, 100%, and 93% during the fourth year after progression. While sustainability was high for all three sets of criteria, it was significantly higher for CIGTS criteria (Fig. 16). The authors concluded that the EMGT criteria identified progression earlier and more often than AGIS and CIGTS criteria and that specificity was good for all criteria but was better with AGIS and CIGTS than with EMGT criteria.
Fig. 16. Survival curves showing time to progression for the three sets of criteria (EMGT, AGIS, CIGTS studies).
Source: Heijl A, Bengtsson B, Chauhan BC, et al. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology. Sep 2008;115(9):1557–1565.
Another study included a trend-based method to define progression compared with other available criteria. The investigators compared the performance of pointwise linear regression (a trend-based analysis), GCP analysis, and the AGIS method in predicting visual field progression in glaucoma with the visual field data from AGIS (Nouri-Mahdavi et al., 2007). Data from 156 patients with eight or more years of follow-up were included and the prediction of outcomes at eight years was used to evaluate the performance of each method. The authors found that visual field progression at eight years was detected in 35%, 31%, and 22% of patients by pointwise linear regression, GCP analysis, and the AGIS method, respectively. The trend-based analysis and GCP analysis had the highest pairwise concordance (kappa = 0.58 [SD, 0.07]). The false prediction rates at four and eight years varied between 1% (point-wise linear regression) and 3% (GCP) when two confirmations were required at four years to define progression, and ranged from 4% to 11% if only one confirmation was required. GCP analysis predicted final (eight year) outcomes at four years better than did trend analysis (sensitivity of 45% vs. 19% for 2 confirmation tests and 63%–32% for 1 confirmation test; P = 0.001). Overall, all algorithms had low false prediction rates. Importantly, algorithms did not perform differently as a function of baseline damage, which is an important confounder given worse test-retest variability and the floor effect seen as disease worsens. Although the trend-based method and GCP analysis showed the highest agreement, they did not agree well regarding spatial distribution of worsening test locations.
In the OHTS, trend analysis techniques were used to measure rates of visual field change from normal baseline fields to conversion to POAG based on visual field tests and optic nerve head assessment. First, the investigators determined the number of visual field tests before and after intervention needed to detect significant IOP-lowering treatment effects (Gardiner et al., 2013). Series of visual fields (mean 24 per eye) completed at 6-month intervals from participants randomized initially to observation were split into subseries before and after the initiation of treatment (the “split-point”). The progression (defined as MD rate of change) was derived with the subseries of fields before and after the initiation of treatment. The investigators studied the different values of the “window length” (W), which was the length of the number of tests. With shortened subseries with W = 7 tests, the progression rate slowed by 0.14 dB/yr upon initiation of treatment (P < 0.001), and the proportion of eyes showing “rapid deterioration” (progression rate faster than −0.5 dB/yr with P < 5%) decreased from 11.8% to 6.5% (P < 0.001). With the entire sequence of tests, no significant change in progression rate was detected (P = 0.796) and there was no change in the proportion of eyes progressing (P = 0.084). Window lengths 6 ≤W ≤9 produced similar benefits. In addition to confirming the OHTS previous results, specifically that event analysis revealed a beneficial treatment effect in this dataset, the authors concluded that this effect was not detected by linear trend analysis if applied to an entire series, but was detected when using shorter subseries of length between six and nine fields before and after treatment initiation.
Translating these results to the present analyses, this study showed that, while an event-based analysis requires a mean follow-up period of five years to demonstrate treatment-effects in the OHTS, a trend-based analysis allows the detection of change in rates of MD progression by using as few as six tests (three years) after the intervention. It is worth noting that OHTS patients had normal visual fields at baseline and, as discussed previously, their fields tended to progress more slowly than if pre-existing abnormal points had been used to measure rates of progression. In patients with baseline visual field damage, we have discussed that trend analysis can detect treatment-effects with as few as five fields in two years in the NY-GAPS population (Folgar et al., 2010). In a clinical trial where subjects are enriched for progression, even less time is necessary, if the number of fields is maintained.
In another study applying trend analysis in the OHTS visual field database, the MD progression rate of the six visual field tests prior to the initiation of treatment was significantly different from the MD progression rate of the six visual field tests after treatment initiation (mean ± SD, −0.23 ± 0.6 vs. −0.06 ± 0.5 dB/year, respectively, P < 0.001) (De Moraes et al., 2012a). Similarly, with pointwise linear regression criteria, the number of progressing locations per eye decreased significantly after the treatment point, from 2.1 ± 6.0 to 1.0 ± 4.0 locations, respectively (P < 0.001). Among eyes that did not reach a POAG endpoint during follow-up (641 eyes of 380 participants), the MD progression rate prior to the treatment point was significantly different from the MD progression rate after the treatment point (−0.17 ± 0.6 vs. −0.01 ± 0.5 dB/year respectively, P < 0.001). Similarly, with pointwise linear regression criteria, the number of progressing locations per eye over the sequence of six visual field tests decreased significantly, from 1.5 ± 5.1 locations before treatment to 0.6 ± 2.7 locations after treatment initiation (P < 0.001), which shows that trend analysis can detect changes due to treatment even among eyes experiencing slow progression. For the subset of eyes that reached a POAG endpoint (n = 139 eyes from 109 participants), there was a significant improvement in the MD progression rate after the treatment point (−0.5 ± 0.8 vs. −0.3 ± 0.7 dB/year, respectively, P < 0.01). Similarly, with the PLR definition, the number of progressing locations per eye decreased significantly after the treatment point (4.9 ± 8.5 vs. 2.7 ± 7.2, respectively, P < 0.01).
5.3. Signal-to-noise ratio in perimetric progression: the importance of frequent testing
Trend-analysis has been proven to have high sensitivity and specificity for detecting and measuring progression as long as a substantial number of examinations is available (Nouri-Mahdavi et al., 2007). However, certain issues should be taken into account in order to obtain an accurate determination of rates of visual field progression. Chauhan et al. (Chauhan et al., 2008) suggested that the effect of test-retest repeatability be taken into account for determining rates of visual field progression in glaucoma. The objective of their perspective was to provide practical recommendations for measuring clinically relevant rates of glaucomatous visual field progression to help identify patients at risk for visual impairment. They focus on the frequency of examinations required for detecting various amounts and rates of visual field change.
In routine clinical glaucoma practice, the frequency of visual field examinations varies significantly and usually falls substantially below recommendations for maintaining minimum practice standards. An unacceptable number of glaucoma patients become visually impaired or even blind while under care, as previously noted. Several factors, including over-reliance on IOP to measure treatment adequacy, could contribute to this finding. Having sufficient visual field data and analysis methods may help the ophthalmologist deliver more targeted care resulting in better functional outcomes. While criteria for progression have been clearly defined in clinical trials, the identical criteria may not be applicable to clinical practice. The lack of a definition for clinically significant progression and its practical application may be a disincentive to performing frequent examinations. Consequently, clinical decisions could be made with inadequate information, or serious progression could be missed because of an insufficient number of examinations. The guidelines for glaucoma published by the National Institute of Clinical Excellence (NICE) in the UK highlighted the lack of evidence about how patients with glaucoma should be monitored over time, while the European Glaucoma Society (EGS) recommends that all newly-diagnosed glaucoma patients should be tested three times per year during the first 2 years in order to establish the rate of visual field loss at an early stage(EGS, 2008; NICE, 2009).
The statistical power to detect various rates of MD change (expressed as a multiple of the SD) for given numbers of examinations is shown in Fig. 17. Detecting a very rapid rate of change (four times the SD of MD variability) with 80% power requires between four and five examinations. In other words, such a magnitude of change will be detected four times out of five with between four and five examinations. On the other hand, detecting a small rate of change (1/4 of the SD of MD variability) requires 19 examinations for the same power. In practical terms, these data can be expressed as the time period required to detect MD deterioration of −0.5 dB/year, −1.0 dB/year and −2.0 dB/year with 80% power with one, two and three examinations per year (Table 11).
Fig. 17. Statistical power to detect various rates of MD change (expressed as a multiple of the SD) for given numbers of examinations.
Source: Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. Apr 2008;92(4):569–573.
Table 11.
Time period (years) required to detect various rates of MD change with 80% power in visual fields with low, moderate, and high degrees of variability with one (a), two (b), and three (c) examinations per year.
| (a) 1 examination/year Progression rate (dB/year) | Variability
|
||
|---|---|---|---|
| Low | Moderate | High | |
| −0.25 | 13 | 19 | 30 |
| −0.5 | 9 | 13 | 19 |
| −1.0 | 6 | 9 | 13 |
| −2.0 | 5 | 6 | 9 |
|
| |||
| (b) 2 examination/year Progression rate (dB/year) | Variability
|
||
| Low | Moderate | High | |
|
|
|
||
| −0.25 | 6.5 | 8.5 | 15 |
| −0.5 | 4.5 | 6.5 | 8.5 |
| −1.0 | 3 | 4.5 | 6.5 |
| −2.0 | 2.5 | 3 | 4.5 |
|
| |||
| (c) 3 examination/year Progression rate (dB/year) | Variability
|
||
| Low | Moderate | High | |
|
|
|
||
| −0.25 | 4.3 | 6.3 | 10 |
| −0.5 | 3 | 4.3 | 6.3 |
| −1.0 | 2 | 3 | 4.3 |
| −2.0 | 1.7 | 2 | 3 |
Source: Chauhan BC, Garway-Heat DF, Goni FJ et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. Apr 2008;92(4): 569–573
These data clearly show that detecting rates of visual field change with certainty generally requires many visual fields; however, larger rates of change require fewer examinations. In a patient with rapid progression (−2 dB/year) and low variability, the time required to detect change with 80% power is 5 years if examinations are performed once a year, 2.5 years with two examinations per year and as little as 1.7 years if examinations are performed three times per year. In a more typical scenario with moderate field progression (−0.5 dB/year) and moderate variability, the respective times to detection are 13, 6.5, and 4.3 years for one, two and three examinations per year.
These findings from simulation explain why clinical trial designs to detect and measure progression rates require large numbers of visual field tests.
Visual field examinations generate a considerable amount of data, hence the use of available software programs is recommended to perform objective and automated analyses. All reliable examinations should be used in the analyses to measure progression accurately. Decision-making based on examination of grey-scale plots should be avoided.
Clinically important “signals” in the visual field are often small compared with the variability between successive tests (“noise”). This applies to the detection of abnormalities with single examinations as well as to the measurement of change over time with serial examinations(Artes and Chauhan, 2009). In summary, with typical variability, at least three years of every six month tests are needed to detect sight-threatening rates of visual field progression (Chauhan et al., 2008).
As mentioned previously, test-retest variability is dependent on severity of damage, either pointwise or globally. In addition, the depth of visual field defect may affect the ability of patients to accurately see stimuli during perimetry testing. Studies have shown that RGC responses saturate at high contrast, implying that the probability of detecting perimetric stimuli may not increase beyond this contrast.
Gardiner et al. investigated the lower limit of the effective dynamic range of SAP beyond which reliable measures of sensitivity cannot be obtained (Gardiner et al., 2014). They studied 33 patients with moderate to severe glaucoma (i.e., MD on their last clinic visit averaged −10.47 dB, range −19.75 dB to −3.38 dB). They found that 73 of the 132 locations tested had perimetric sensitivity ≤18 dB. The frequency of seeing (FOS) curves were constructed at four non-adjacent visual field locations by applying the method of constant stimuli (MOCS), with 35 stimulus presentations at each of seven contrasts (Fig. 18). The four locations were chosen a priori, and included at least two locations with glaucomatous damage but sensitivity no worse than 8 dB. Cumulative Gaussian curves were fit to the FOS data, first assuming a 5% false-negative rate, and second allowing the asymptotic maximum response probability to float as a free parameter. The correlation between perimetric sensitivity (mean of last two clinic visits) and MOCS sensitivity (from the experiment) were assessed at 0.5 dB intervals for all locations within bins with a perimetric sensitivity within ±4 dB of each selected value.
Fig. 18. Response probabilities for a sample study subject at 4 tested locations (at positions as labeled in degrees). The dashed line indicates the frequency-of-seeing (FOS) curve as fitted using the primary analysis, in which the maximum response probability would be 95% if contrast could be made sufficiently high (assuming a 5% false-negative rate). The dotted line indicates the FOS curve fit.
Source: Gardiner SK, Swanson WH, Goren D, Mansberger SL, Demirel S. Assessment of the Reliability of Standard Automated Perimetry in Regions of Glaucomatous Damage. Ophthalmology. 2014 Jul;121(7):1359–69.
When pooling all locations with perimetric sensitivity ≤15 dB, the relationship with MOCS sensitivity had an R2 < 0.1% (P > 0.05), whereas for locations >19 dB the relationship was >0.1% (P < 0.05). At 57 of the 81 locations (70%) with perimetric sensitivity ≤19 dB, the fitted asymptotic maximum response probability was below 80%, supporting the hypothesis of response saturation. At 30 of these locations the asymptotic maximum was below 50%, therefore, contrast sensitivity (50% response rate) was undefined. The authors concluded that sensitivities estimated to be below approximately 15–19 dB during clinical perimetry are mostly random and hence unreliable. They should be interpreted with caution both clinically and in data analyses.
As a practical recommendation, pointwise visual field deterioration below this threshold should not be considered reliable unless a substantial amount of dB loss is seen (e.g. worsening to <0 dB). In other words, pointwise perimetric changes for points that have reached a floor of 15–19 dB of absolute threshold sensitivity should be considered unreliable – or that noise outweighs the signal – and the correlation with clinical signs and structural measurements should provide more reliable conclusions. In this case, clinicians and researchers should focus on looking for evidence of perimetric change at remaining, particularly adjacent locations above 15–19 dB.
From the perspective of planning a clinical trial, assessment of treatment effects on rates of visual field progression should not include eyes with a large number of test locations with absolute threshold sensitivities below that cut-off value. Since this can be challenging and result in small numbers of eligible participants, one could alternatively censor the progression data of locations worse than 15–19 dB. In fact, Gardiner et al. recently tested whether censoring these field locations (below 15–19 dB) affected the overall performance to detect visual field progression compared to conventional non-censoring approaches. They found that the proportion of eyes flagged as progressing was not decreased by censoring unreliable sensitivities. They concluded that restricting the range of contrast used in clinical perimetry may be possible without hampering the ability to monitor glaucomatous visual field progression (Gardiner et al., 2016). For short-term trials, censoring abnormal points at baseline with sensitivities below that threshold should be considered if one aims to reliably test the effect of treatment modalities on rates of visual field progression of abnormal points. Therefore, for patients whose visual field results are above that threshold (i.e., MD better than −14 dB or test points better than 15–19 dB), treatment effects are more likely to be accurately detected. This is one of the key premises of the present proposal.
In clinical practice, one method to overcome the effect of variability in severe glaucoma is to increase the stimulus size used for automated perimetry. The Goldmann size III is the most widely used stimulus size in automated perimetry and is also used for legal definitions of blindness. The III4e consists of a 4 mm2 target of with a luminance of 318 cd/m2 (1000 apostilbs). Perimetric contrast sensitivity is known to increase with stimulus size in both normal and diseased eyes. It has been proposed that use of a size V4e (64 mm2) stimulus reduces variability, allowing reliable visual field testing to be performed later into the disease process (Wall et al., 1997, 2009, 2010).
One reason for this is that test-retest variability is lowest in areas with abnormal sensitivity with a size V stimulus (Wall et al., 1997). Also, size V stimuli have a greater effective dynamic range than size III and have about twice as many discriminable steps. Regarding the dynamic range, the number of steps from normal to blind in SAP is determined by the test-retest variability and the stimulus brightness range. SAP size III has 4 discriminable steps for progression with a floor around 15–19 dB, below which the reliability of responses becomes compromised, as previously discussed. Investigators who compared the dynamic ranges of different tests and stimulus sizes found that SAP size V has as many as 8 discriminable steps for progression and a floor around 4–8 dB (Wall et al., 2010). In other words, once patients progress to low sensitivities with conventional SAP size III, switching to size V could help monitoring changes for a longer period with sustained reliability.
In another study the investigators found no evidence that use of a size V stimulus significantly decreased the lower limit of the reliable stimulus range beyond 15–19 dB (Gardiner et al., 2015); however, using a size V stimulus resulted in a higher sensitivity at the same location. For instance, a test location that reached a sensitivity value of 15 dB with size III may reveal a sensitivity of 20 dB when tested with a size V stimulus. This higher sensitivity means that a location will not reach the lower limit of reliable testing until later in the disease process, resulting in more reliable and less variable estimates of sensitivity at damaged visual field locations.
Therefore, both studies agree that switching to size V may help monitor progression of points with low sensitivity. Ideally, algorithms should automatically make this modification during testing when an area of very depressed sensitivities is identified (or based on previous tests). One alternative approach could be to alternate stimulus sizes between test days. A limitation is that progression analyses can only be done by comparing tests with the same stimulus size. Thus, the number of tests available for automated progression analysis would decrease, and the frequency of follow-up would need to be increased.
5.4. Detection of clinically significant visual field progression is best done with subjects who have mild to moderate glaucoma
A key point to keep in mind is that visual field sensitivity and its rates are typically measured in dB, which is a logarithmic scale. Hence, changing from a MD of 0.0 to −5.0 dB represents much greater loss in absolute sensitivity than changing from −10.0 to −15.0 dB. This also reflects different number of RGCs lost at each of these scenarios.
Medeiros et al. evaluated the relationship between change in estimated RGC counts and change in measures of functional and structural damage in glaucoma, from cross-sectional data (Medeiros et al., 2012). A non-linear relationship was observed between SAP MD and RGC counts (Fig. 19). The same amount of RGC loss corresponded to largely different amounts of MD change depending on the stage of the disease. For spectral-domain optical coherence tomography (SDOCT) average retinal nerve fiber layer (RNFL) thickness, a linear relationship was seen with RGC counts throughout most of the spectrum of disease, but reaching a plateau in advanced glaucoma. Changes in RGC counts for eyes with early damage corresponded to small changes in MD, but to relatively larger changes in RNFL thickness. For eyes with advanced disease, changes in RGC counts produced relatively larger changes in MD but only small or no changes in average RNFL thickness (Table 12). These results help support the notion of “floor effect” discussed above, and how measurement of progression becomes more difficult as disease worsens.
Fig. 19. Analysis of the relationship between visual field parameters and estimated RGC counts. (A) Relationship between MD and estimated RGC counts. (B) First derivatives of the curve shown on A plotted against estimated RGC counts. The derivatives indicate the amount of change in MD per 10,000 RGCs at different levels of RGC counts.
Source: Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. Oct 2012;53(11):6939–6946.
Table 12.
Change in SAP MD index corresponding to different amounts of change in estimated RGC counts at different stages of the disease.
| Stage of disease
|
Change in MD, dB, for a change of:
|
|||
|---|---|---|---|---|
| MD, dB | Estimated RGC count | 10,000 RGCs | 35,000 RGCs | 100,000 RGCs |
| 0.4a | 1,020,000 | 0.04 | 0.11 | 0.33 |
| −2 | 710,000 | 0.15 | 0.56 | 1.79 |
| −5 | 560,000 | 0.25 | 0.94 | 2.98 |
| −10 | 403,000 | 0.39 | 1.34 | 3.99 |
| −15 | 281,000 | 0.47 | 1.78 | 5.78 |
| −20 | 193,000 | 0.64 | 2.35 | 7.02 |
| −25 | 121,000 | 0.71 | 2.53 | 7.25 |
Average mean deviation (MD) of the healthy eyes included in the study.
Source: Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. Oct 2012; 53(11): 6939–6946.
The relationship between severity of visual field loss at presentation and rate of visual field loss progression in glaucoma was studied (Rao et al., 2011). The authors included 512 eyes of 310 POAG patients who had >5 VFs between 1989 and 2008. Based on the baseline MD, they classified the visual field loss as mild (MD > −6 dB), moderate (MD ≤ −6 to ≥ −12 dB) or severe (MD < −12 dB). GPA software with the VFI loss per year was used to assess the rates of progression. The rates of progression increased (worsened) by 0.02% per year for every dB worsening of baseline MD (P = 0.02) and for every year of increasing age (P = 0.001). Association between MD and rates of progression showed a significant positive relationship in mild (β = 0.18; P = 0.001) and a significant negative relationship in severe (β = −0.16; P < 0.001) visual field loss. The association between MD and rate of progression was not significant in moderate visual field loss (β = −0.05; P = 0.61). In addition, as discussed previously, Gardiner et al. showed that for visual field in locations with perimetric sensitivity ≤15–19 dB, the fitted asymptotic maximum response probability was below 80%, supporting the hypothesis of response saturation. This suggests that sensitivities estimated to be below approximately 15–19 dB during clinical perimetry are mostly random, and unrelated to true functional status (Gardiner et al., 2014). They should be interpreted with caution both clinically and in data analyses.
Fig. 20 schematically illustrates the relationship between severity of visual field at baseline and rates of progression in linear (1/Lambert, 1/L) and non-linear (dB) scales. The relationship between these two scales is: 1/L = 10(dB/10). In the top panel, the slopes of 3 hypothetical patients with mild (−1 dB), moderate (−6 dB), and severe (−12 dB) baseline MD are depicted. All 3 patients are progressing at −1.0 dB/year based upon ordinary linear regression over 10 years. In the lower panel, the corresponding slopes after converting to linear scale are shown along with the best-fit equations and goodness-of-fit (R-squared values). Note that despite progressing at the same rate in dB/year, their slopes in 1/L/year are slower in more severe cases (mild: −0.06; moderate: −0.02; severe: −0.005 1/L/year). According to the Hood and Kardon structure vs. function model, visual field sensitivity measured in linear scale (1/L) has a linear relationship with retinal nerve fiber layer (RNFL) thickness measured with OCT (Hood and Kardon, 2007). Therefore, the rates of progression measured with 1/L/year also show a linear relationship with the rates of RNFL loss/year in glaucoma, suggesting that 1/L/year is more directly correlated with the rate of ganglion cell loss than dB/yr. In other words, conventional trend analysis of visual fields in dB/year overestimates the true (structural) rate of progression in moderate to severe stages. This is consistent with the conclusions reported by Medeiros et al. described above (Medeiros et al., 2012). Of course the examples below are hypothetical and assume a perfectly linear rate of progression of −1.0 dB/year at all stages, which is unlikely to be seen in a real-world scenario. In particular, it does not take into account the increased variability as disease progresses. In fact, more variability in severe cases would lead to less reliable estimates of the slope and likely result in even flatter (slower) slopes. Nonetheless, this extreme example illustrates the importance of taking into account the baseline level of visual damage when defining fast vs. slow progressors.
Fig. 20.
Relationship between baseline visual field damage and rates of progression using linear (1/Lambert) and non-linear (dB) scales.
Given the combination of a “floor effect,” the use of a non-linear scale of sensitivity, increased test-retest variability as visual fields progress, and the minimal utility of analyzing points with very low sensitivities, it is advisable that clinical trials employing trend-based progression endeavor to select populations with mild to moderate visual field damage. Additionally, the analysis of rates of progression of abnormal points should be censored or evaluated more carefully once sensitivities reach values below 15–19 dB.
5.5. One can reliably extrapolate visual field progression over a shorter time to a longer time
In a study that used statistical modeling techniques to identify models that both describe glaucomatous sensitivity decay and allow predictions of future field status, initially normal fellow eyes of untreated patients with confirmed normal tension glaucoma were evaluated (McNaught et al., 1995). All had in excess of 15 Humphrey fields with a mean follow-up of 5.7 years. From this cohort, individual field locations were selected for analysis if they demonstrated unequivocal deterioration at the final two visual fields. Forty-seven locations from five eyes satisfied this criterion and were analyzed with curve-fitting software which automatically applies 221 different models to sensitivity (y) against time of follow-up (x). Curve-fitting was then repeated on the first five fields, followed by projection to the date of the final field to generate a predicted threshold which was compared to the actual threshold. Competing models were therefore assessed on their performance at adequately fitting the data (R2) and their potential to predict future field status. The investigators found that models that provide the best fit to the data were all complex polynomial expressions (median R2 = 0.93). Other simple expressions fitted fewer locations and exhibited lower R2 values. However, accuracy in predicting future deterioration was superior with these less complex models. In this group, a linear expression demonstrated an adequate fit to the majority of the data and generated the most accurate predictions of future field status. Therefore, a linear model of the pointwise sensitivity values against time of follow-up can provide a framework for detecting and forecasting glaucomatous field progression and while allowing for the estimation of the clinically important rate of sensitivity loss.
Given that classic regression with least square estimates is based on certain assumptions that conflict with visual field data, another study investigated different regression models and their assumptions in order to determine pointwise visual field progression in glaucoma and to better predict future visual field loss for personalized clinical glaucoma management (Bryan et al., 2013). Standard automated visual fields of 130 patients with primary glaucoma with a minimum of six years of follow-up were included and sensitivity estimates at each visual field location were regressed on time with classical linear and exponential regression models, as well as with different variants of these models that take into account censoring and allow for robust fits. These models were compared for the best fit and for their predictive ability. The prediction was evaluated at six measurements ahead (approximately three years) with varying numbers of measurements.
The results showed that, for fitting the data, the classical uncensored linear regression model had the lowest root mean square error and 95th percentile of the absolute errors. These errors were reduced in all models when increasing the number of measurements used for the prediction of future measurements, with the classical uncensored linear regression model having the lowest values for these errors irrespective of how many measurements were included. Although all models performed similarly and despite violation of its assumptions, the classical uncensored linear regression model appeared to provide the best fit for the data. In addition, this model appeared to perform the best when predicting future visual fields.
A tactic to dealing with the increase in perimetric variability in progressing disease is to take into account this non-stationary variance when performing trend-based analysis. Commonly used ordinary linear regression models assume normally-distributed errors and homoscedasticity (i.e.: variance around the regression line is the same throughout time). An alternative approach incorporates non-stationary variability modeled with non-Gaussian distributions, and has been shown to detect visual field progression significantly earlier than conventional methods, particularly in short time series, at matched false-positive rates (Zhu et al., 2014). This inferential statistical model, termed ‘Analysis with Non-Stationary Weibull Error Regression and Spatial Enhancement’ (ANSWERS), incorporates non-stationary variability modeled as a mixture of Weibull distributions, and takes into account the spatial correlation of measurements with a Bayesian framework. At a false-positive rate of 5%, ANSWERS detected progression in one sample in 5 years, while MD regression with ordinary least squares regression took 7 years (Zhu et al., 2014). These Weibull distributions were calculated from a defined hospital based cohort, so caution should be taken over the generalizability to other datasets. In that sense, PLR is more generalizable and can be applied to any dataset because it does not depend on a prior distribution for its calculation.
Assuming visual field progression is linear, one would expect that if previous information on rates of progression is available and assuming no further changes in risk factors, it is plausible to predict future visual field outcomes by extrapolation of the slopes measured previously. Bengtsson et al. (Bengtsson et al., 2009) investigated how well short-term progression rates could predict long-term visual field outcomes in patients with glaucoma. The authors calculated visual field rates of progression with linear regression analysis of the VFI for 100 consecutive patients with glaucoma having 10 or more Swedish Interactive Thresholding Algorithm (SITA) SAP tests. Final VFI was predicted on the basis of linear extrapolation of the slope defined by the initial five visual field test results. Final VFI also was estimated with linear regression of all qualifying examination results for each patient. Primary outcome measures were the absolute difference and the correlation between predicted and estimated final VFI values. The results revealed that median VFI progression rate was −1.1% per year both for the initial five test results and also for the complete series. Seventy percent of patients had a predicted final VFI within ±10% of the estimated final VFI, and the two VFI calculations had a correlation coefficient of 0.84.
The authors concluded that linear extrapolation based on five initial visual field test results could be a reliable predictor of future field loss in most patients. As a consequence, patients in whom linear regression analysis suggests dangerously rapid rates of visual field progression may be candidates for significant intensification of therapy. A similar analysis has been performed with MD values instead of the VFI. Azarbod et al. compared the performance of extrapolation of linear trends between the MD and VFI in forecasting final visual field outcomes. The area under the receiver operating characteristics (ROC) curve was 0.75 for the MD vs. 0.69 for the VFI (Azarbod et al., 2012). More recently, De Moraes et al. showed a strong positive correlation between 10 and 2 MD slopes of the first 5 visual fields and the entire sequence of tests. They found a correlation coefficient of 0.86 at P < 0.001, confirming that the extrapolation of linear trends of MD slopes with an initial sequence of tests provides realistic estimates of rates of progression during the entire evaluation period and final MD values (De Moraes et al., 2014).
5.6. Abnormal visual field points are where the disease worsens, and so are the best way to assess effects of an intervention
Analysis of localized points of progression may be a more sensitive and specific means of assessing active, progressive glaucomatous damage. Global rates of visual field progression include those points that remain stable throughout the visual field series. Global rates of progression in dB/yr are thus confounded by points without reproducible defects, secondary to unreliable test taking, and by points with permanent defects at baseline whose dB signal may fluctuate across a series of visual fields despite stable functional loss. By identifying and analyzing the localized points of progression (i.e., points located on the advancing edge of an arcuate scotoma), one can gauge the true effectiveness of any type of glaucoma therapy more sensitively and specifically. Additionally, monitoring locations adjacent to these scotomas can add invaluable information as it allows detection not only deepening of pre-existing defects, but also their expansion. As previously described, this kind of visual field analysis likely represents the most direct and definitive method available for objective assessment of functional glaucomatous damage and its impact in QOL.
6. Results of standard automated perimetry correlates with health-related quality of life (HRQOL)
6.1. Background
A “patient-reported outcome” is defined by the National Institutes of Health as “a characteristic or variable that reflects how a patient feels, functions, or survives” (NIH, 2000), that is, all outcomes leading to important changes for patient life. These include clinical events, functional status, pain, and QOL. On the other hand, “surrogate outcomes” are defined as “a characteristic intended to substitute for a clinical endpoint” (NIH, 2000; Pino et al., 2012). These are typically biochemical markers, physiological parameters, or subclinical endpoints that were not generally perceived directly by patients, but were nevertheless associated with patient-important outcomes. For example, high blood pressure does not reflect how a patient feels, functions, or survives but is known to be associated with increased risk of stroke. A recent study aimed to characterize pivotal efficacy trials for newly approved novel therapeutic agents between 2005 and 2012, showed that although patient-reported clinical outcomes and scales were used in many pivotal trials, trials with surrogate endpoints as their primary outcome formed the exclusive basis of approval for nearly half of the approved indications (Downing et al., 2014).
Traditionally, the success or failure of medical therapy has sometimes been judged by how well it meets an objective biomedical measure that is not meaningful to the patient. Over the past years, a consensus has evolved that preferentially values the perception of the patient as the central determinant in monitoring the outcomes of medical intervention. This stems from the recognition that patients themselves are not interested in improvements in a biomedical criterion, but rather in how treatment affects their quality of life. Meeting an objective treatment goal, such as lowering total serum cholesterol levels, remains an important therapeutic concept; however, one must consider that the means by which a treatment achieves this goal affects the perception of well-being and the ability to function effectively as an independent “whole person.” Although physicians have traditionally considered themselves genuinely interested in the well-being of patients in ways that extend beyond the assessment of objective data, the instruments (e.g., questionnaires, interview techniques) that permit valid, reliable, responsive, easily analyzed, and generalizable determinations of general health status have more recently been developed.
The World Health Organization defined quality of life as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns. It is a broad ranging concept affected in a complex way by the person’s physical health, psychological state, level of independence, social relationships, and their relationship to salient features of their environment” (WHO, 1993). The term “quality of life,” or the subjective perception of well-being and wholeness, was initially applied by behavioral researchers who attempted to evaluate the effects of social programs(Andrews and Withey, 1976; Flanagan, 1982). This concept of quality of life was later utilized in the assessment of chronically ill patients, including those with mental illness, cancer, and cardiovascular disease, and in the elderly, including glaucoma patients (Flanagan, 1982; Gutierrez et al., 1997; Parrish, 1996).
6.2. Relationships between different visual field indices and vision-related quality of life
The field test version of the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ), a targeted multidimensional survey, was designed to represent the perspective of the patient with respect to visual disabilities and their impact on daily functioning. The field test version of the NEI-VFQ consists of a generic core of 51 items that are relevant to the majority of visually impaired adults, irrespective of the cause of the visual disability. The NEI-VFQ employs 12 different scales including: (1) general health, 2 items; (2) general vision, 2 items; (3) visual pain, 2 items; (4) near activities, 7 items; (5) distance activities, 7 items; (6) vision-specific social functioning, 4 items; (7) mental health, 8 items; (8) expectations, 3 items; (9) role difficulties, 5 items; (10) dependency, 5 items; (11) driving, 4 items; and (12) color vision, 1 item. A single question regarding peripheral vision that is not part of a scale is included.
Glaucoma impacts patient HRQOL in multiple ways, including driving, walking, and reading (Gutierrez et al., 1997; Haymes et al., 2007; Nelson et al., 2003). The psychological burden increases as vision decreases, along with a growing fear of blindness, social withdrawal from impaired vision, and depression. HRQOL, a concept that reflects a person’s overall well-being, focuses on dimensions of physical functioning, social functioning, mental health, and general health perceptions. While the components of a good HRQOL may differ among individuals, maintaining enough visual ability to successfully manage daily activities and lead a productive life is a high priority to all.
Parrish demonstrated significant correlations between NEI-VFQ scores and visual field mean defect as defined by the MD and other monocular/binocular instruments (Parrish, 1996) Since the early stage of glaucoma tends to be asymptomatic, it is not surprising that visual or symptomatic impairment was not detected in early stages.
A separate study tested whether another global visual field index, the PSD, also correlates with instruments measuring HRQOL (Parrish, 1996). Seventy-seven patients with glaucoma were consecutively selected to complete two forced-choice questionnaires each. Both questionnaires were evaluated among all of the considered patients and the results were compared. The questionnaire that performed best was used to test the QOL in three different subgroups based on the mean deviation of the worse eye. The overall (all patients) MD was −6.5 ± 6.8 dB. A significant (P < 0.0001) correlation was found between the instruments’ scores and MD (r = 0.79), as well as the PSD (r = −0.68).
Alternatively, scores of disease severity used to measure progression in RCTs were compared with HRQOL instruments. In one study with the AGIS scoring system, the AGIS scores were calculated for each eye based on the main deviation plot from the automated perimetry visual fields (Gutierrez et al., 1997). These scores represent the number and depth of depressed visual field sites found in less than 5% of normal values and an index for quantifying visual field defects for the entire eye. AGIS scores can range from 0 (no defects) to 20 (near or complete visual field loss). These scores are calculated from points awarded to three areas: the upper field, the lower field, and the nasal area. A maximum of nine points can be awarded to the upper and lower fields, while the nasal area can be awarded a maximum of two points. Greater visual field defects in the better eye were significantly associated with poorer NEI-VFQ (correlation coefficients of −0.40 and −0.36 for better and worse eyes, respectively; P < 0.001 for both). These findings were most dramatic for patients with the most severe visual field loss in the better eye. Compared to a generic HRQOL measures, vision-targeted questionnaires were more sensitive to differences between glaucoma and normal reference participants. These findings indicate that self-reports of vision-targeted HRQOL are sensitive to visual field loss and may be useful in tandem with the clinical examination to fully understand outcomes of treatment for glaucoma.
In the CIGTS, 607 patients received standardized examinations of visual field at enrollment. In addition, they completed a telephone-administered, HRQOL questionnaire which included the Visual Activities Questionnaire (VAQ) and a symptom and health problem checklist (Mills et al., 2001). The VAQ total and subscale scores, particularly the peripheral vision subscale, correlated weakly but significantly with global visual field scores (MD and PSD). Symptoms attributed to glaucoma also correlated weakly but significantly to visual field scores.
To assess the relationship between additional objective and subjective measures of visual function and QOL, a study was conducted with three methods of assessing visual loss caused by glaucoma: standard clinical tests of vision, self-reported quality of life, and the ability to perform activities of daily living. The study enrolled 192 glaucoma patients with a full range of glaucomatous visual loss that were selected from the Glaucoma Service of Wills Eye Institute (Richman et al., 2010). Subjects were evaluated clinically by VA, contrast sensitivity, visual field, stereopsis, the Disc Damage Likelihood Scale, and IOP. Subjects were evaluated subjectively by the 25-item NEI-VFQ-25 and objectively by a performance-based measure of visual function, the Assessment of Disability Related to Vision (ADREV). The investigators observed that clinical tests had higher correlations with ADREV than with the NEI-VFQ-25. Interestingly, there was a moderate, but not strong, connection between how patients rated their own visual ability with how they performed when objectively tested.
Patients who have faster rates of MD progression are more likely to reach scores of visual impairment and HRQOL sooner, and as a consequence, live more years with that disability than those with slower rates of MD progression.
6.3. Visual field indices, activities of daily living, and comorbidities
6.3.1. Psychiatric conditions
Psychiatric conditions may also concur with visual disability from glaucoma. In a study aimed to determine the prevalence of depression and its association with visual field impairment, QOL, objective assessment of visual function, and glaucoma severity in elderly patients with glaucoma, the prevalence of depression increased with glaucoma severity, reaching statistical significance in patients aged 70–79 years (P < 0.02) (Skalicky and Goldberg, 2008). Summary and sub-factor scores of HRQOL instruments reflected decreased QOL with increased glaucoma severity. Assessment of function related to vision scores indicated worsening visual function with increasing glaucoma severity. On multivariate regression analysis, depression status was correlated with age and one of the tested summary scores (Glaucoma Quality of Life – 15). The authors concluded that depression is more common in patients with increasing glaucoma severity (mainly in those aged 70–79 years). In addition, among patients with glaucoma or ocular hypertension, age and HRQOL summary scores are independent risk factors for depression.
In another study that assessed depression and mood indicators in subjects newly diagnosed with chronic OAG from the CIGTS, participants responded at baseline to QOL telephone interviews with different instruments. The authors correlated the responses to these instruments with VA and CIGTS visual field scores. They found correlations (r = 0.24 to 0.38, all P values ≤0.001) between patients’ perception of their vision and each item the instruments tested. The strongest correlation between a clinical measure and an item from an instrument was between worse visual field and worry about the possibility of blindness (P = 0.005). The odds ratio of reporting mood indicators and symptoms of depression increased with patients’ perceptions of worsening visual function (Jampel et al., 2007).
6.3.2. Ocular surface disease
Ocular surface disease (OSD), another condition associated with glaucoma therapy, can have an effect on HRQOL. A study was conducted in 124 participants (patients with mild, moderate, or severe OAG and controls [glaucoma suspects]) not receiving glaucoma treatment to investigate the relationship between ocular surface disease and glaucoma-related QOL, glaucoma severity, and treatment (Skalicky et al., 2012). Severity was stratified according to binocular visual field MD loss. OSD scores and the number of patients with OSD increased with increasing glaucoma severity (P < 0.001 and P < 0.005). HRQOL scores reflected decreased indices with increasing glaucoma severity (P < 0.001). These trends were maintained after sub-stratification for age and sex. On univariate regression, OSD was significantly correlated with HRQOL summary scores, glaucoma severity, multiple topical glaucoma medications, worse eye MD and PSD, use of topical beta blockers, topical carbonic anhydrase inhibitors, daily dose of benzalkonium chloride (BAK), and glaucoma filtration surgery. On multivariate regression, HRQOL summary score (OR, 4.14, 95% CI, 2.59–6.63, P < 0.001) and a daily dose of BAK greater than 3 (OR, 2.47, 95% CI, 1.17–5.21, P = 0.018) were predictive of OSD score.
6.3.3. Driving performance
Given the increased prevalence of glaucoma with age and the reality that the ability to drive is important for better QOL for many older adults, driving performance is often included as one of the sub-scales of HRQOL scores. A study aimed to determine if glaucoma and glaucomatous visual field loss are associated with driving cessation, limitations, and deference to another driver in older adults, 81 glaucoma subjects and 58 glaucoma suspect controls between age 60 and 80 years reported if they had ceased driving, limited their driving in various ways, or preferred another individual to drive (van Landingham et al., 2013). Twenty-three percent of glaucoma patients and 6.9% of suspects had ceased driving (P = 0.01). Glaucoma subjects also had more driving limitations than suspects (P = 0.007). In multivariable models, driving cessation was more likely for glaucoma subjects as compared to suspects (OR 4.0; 95% CI, 1.1–14.7; P = 0.03). The odds of driving cessation doubled with each 5 dB decrement in the better-eye visual field MD (OR, 2.0; 95% CI, 1.4–2.9; P < 0.001; Fig. 21). Glaucoma subjects were also more likely than suspects to report a greater number of driving limitations (OR 4.7; 95% CI, 1.3–16.8; P = 0.02). The likelihood of reporting more limitations increased with the worse visual field MD (OR 1.6 per 5 dB decrement in the better-eye VF MD; 95% CI, 1.1–2.4; P = 0.02).
Fig. 21. Modeled probability of not driving as a function of better-eye visual field loss in glaucoma patients. In addition to better-eye mean deviation, the multivariable logistic regression model includes age, gender, unemployment, cognition, comorbidities, and depressive symptoms.
Source: van Landingham SW, Hochberg C, Massof RW, Chan E, Friedman DS, Ramulu PY. Driving patterns in older adults with glaucoma. BMC Ophthalmol. 2013;13:4.
6.3.4. Falls
One of the main concerns of the impact of glaucomatous vision loss is with regard to falls and fear of falling among the elderly, especially given the future rise in the number of senior patients living with the disease.
To determine if visual field loss resulting from glaucoma is associated with greater fear of falling, investigators designed a prospective, observational study which recruited 143 subjects (83 glaucoma subjects with bilateral visual field loss and 60 control subjects with good VA and no significant visual field loss) from a patient pool that was being followed up for suspicion of glaucoma (Ramulu et al., 2012). After completion of a standard questionnaire to assess fear of falling, the extent of fear of falling was assessed with Rasch analysis. Subject ability to perform tasks without fear of falling was expressed in logits, with lower scores implying less ability and greater fear of falling. They observed that glaucoma subjects had greater visual field loss than control subjects (median better-eye MD of −8.0 dB vs. +0.2 dB; P < 0.001), however, no statistically significant differences were seen with regard to age, race, gender, employment status, the presence of other adults in the home, body mass index (BMI), grip strength, cognitive ability, mood, or comorbid illness (P ≥ 0.1 for all). In multivariate models, glaucoma subjects reported greater fear of falling as compared with controls (β = −1.20 logits; 95% CI, −1.87 to −0.53; P = 0.001), and fear of falling increased with visual field MD (β = −0.52 logits per 5-dB decrement in the better eye VF MD; 95% CI, −0.72 to −0.33; P < 0.001; Fig. 22).
Fig. 22. Fear of falling levels by severity of visual field loss. Lower fear of falling scores indicate greater fear of falling, evidenced by fear with easier tasks. The relationship between fear of falling scores and better-eye mean deviation is plotted as a linear relationship using bivariate regression.
Source: Ramulu PY, van Landingham SW, Massof RW, Chan ES, Ferrucci L, Friedman DS. Fear of falling and visual field loss from glaucoma. Ophthalmology. Jul 2012;119(7):1352–1358.
Other variables predicting greater fear of falling included female gender (β = −0.55 logits; 95% CI, −1.03 to −0.06; P = 0.03), higher BMI (β = −0.07 logits per 1-unit increase in BMI; 95% CI, −0.13 to −0.01; P = 0.02), living with another adult (β = −1.16 logits; 95% CI, −0.34 to −1.99 logits; P = 0.006), and greater comorbid illness (β = −0.53 logits/1 additional illness; 95% CI, −0.74 to −0.32; P < 0.001). The investigators concluded that bilateral visual field loss resulting from glaucoma is associated with greater fear of falling, with an impact that exceeds numerous other risk factors. Therefore, given the physical and psychological repercussions associated with fear of falling, significant QOL improvements may be achievable in patients with visual field loss by screening for, and developing interventions to minimize, fear of falling.
6.4. When does significant functional impairment begin?
In the Los Angeles Latino Eye Survey (LALES) (McKean-Cowdin et al., 2007), the relationship between the NEI-VFQ-25 and MD of the better- or worse-seeing eye was investigated. A monotonic trend was observed between visual field loss and most NEI-VFQ-25 subscale scores, such that as visual field loss worsened, the QOL scores worsened. Interestingly, coefficients for NEI-VFQ were slightly diminished after adjusting for knowledge of glaucoma history and treatment status. Coefficients based on data from the better seeing eyes were statistically significant for 6 of 12 NEI-VFQ subscales and the NEI-VFQ composite score. Persons with visual field loss had the greatest difficulty with driving activities and dependency. A three dB difference in visual field loss was associated with an approximately 5-point difference in the NEI-VFQ driving subscale.
Although the visual field MD is a robust method to objectively measure field of vision and which has been consistently shown to correlate with HRQOL, it is important to emphasize that different visual field locations have different relative importance when determining one’s ability to perform tasks.
One study aimed to create a vision-related QOL prediction system to identify visual field test points associated with decreased vision-related QOL in patients with glaucoma (Murata et al., 2013). In general, QOL-important visual field test points were concentrated along the horizontal meridian. Particular areas of the visual field were important for different tasks: peripheral superior and inferior areas in the left hemifield for the ‘letters and sentences’ task; peripheral, mid-peripheral and para-central inferior regions for the ‘walking’ task; the peripheral superior region for the ‘going out’ task; and a broad scattered area across the visual field for the ‘dining’ task. In a similar study focused on driving, the upper temporal visual field in the better eye was the most strongly correlated (r = 0.509) (Sawada et al., 2014). For role limitation and peripheral vision, the lower peripheral visual field in the better eye had the highest correlation coefficients at 0.459 and 0.425, respectively.
6.5. Socioeconomic burdens associated with significant functional impairment due to glaucoma
In addition to negatively affecting HRQOL, the financial burden of glaucoma increases as disease severity increases (Fig. 23).
Fig. 23. Financial burden of glaucoma with disease severity.
Source: Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. May 2009;116(5):823–832.
A study in the US (Rein et al., 2009) found a 4-fold increase in direct ophthalmology-related costs as severity increased from asymptomatic ocular hypertension/earliest glaucoma (stage 0) through advanced glaucoma (stage 3) to end-stage glaucoma/blindness (stage 5): average direct costs per patient per year were US$623, $1915, and $2511, respectively. The majority of costs were medication-related at all severity stages. Meaningful effort has been made to estimate the cost-effectiveness to identify and to treat glaucoma and ocular hypertension (McKean-Cowdin et al., 2007; Murata et al., 2013; Rein et al., 2009).
A study that applied a computer model of 20 million people aged 50 years or older in the US simulated routine ophthalmologic care and resulting glaucoma diagnoses and medical treatment (Rein et al., 2009). The authors tested the incremental cost-effectiveness of current patterns of ophthalmologic assessments that can detect glaucoma (“routine” care) followed by the schedule of treatment recommended by the American Academy of Ophthalmology’s (AAO’s) Preferred Practice Patterns (PPPs) for diagnosed patients compared with no assessment and no treatment. Compared with no treatment and when including diagnostic assessment costs, the incremental cost-effectiveness of routine assessment and treatment was $46,000 per quality-adjusted life years (QALYs) gained, assuming conservative treatment efficacy, and $28,000 per QALY gained, assuming optimistic treatment efficacy. The cost-effectiveness was most sensitive to the treatment costs and the value of QALY losses assigned to visual field loss (Fig. 24).
Fig. 24. Sensitivity of cost-effectiveness ratio to changes in major model parameters. The cost-effectiveness of routine diagnosis and subsequent treatment compared with no treatment given (A) the efficacy seen in the EMGT and (B) the efficacy seen in the CIGTS. CIGTS = Collaborative Initial Glaucoma Treatment Study; EMGT = Early Manifest Glaucoma Trial; QALY = quality adjusted life year.
Source: Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. May 2009;116(5):823–832.
The results of the study above can also be interpreted in terms of rates of visual field MD progression. For simulation purposes, the investigators defined incident glaucoma as a minimum visual field MD of −4 dB in one (75% of patients) or both (25% of patients) eyes, with the split between monocular and binocular impairment based on results from the Barbados Eye Study (Stewart et al., 2008). This conservative estimate of glaucoma incidence starting at −4 dB was consistent not only with the epidemiologic estimates used to estimate incidence but also with a visual field loss large enough to have an associated statistically and clinically meaningful decline in scores on the NEI-VFQ (McKean-Cowdin et al., 2007). The authors assumed that, without treatment, patients with incident glaucoma progressed at −0.8 dB/yr, on average. Based on data from studies described above, treated patients progress at rates ranging from −0.3 to −0.5 dB/yr, on average (Chauhan et al., 2010; De Moraes et al., 2011; De Moraes et al., 2012b). Compared with no treatment and when including diagnostic assessment costs, the incremental cost-effectiveness of routine assessment and treatment translates to $46,000 per QALY gained, assuming a 50% deceleration in rate of MD progression, and to approximately $28,000 per QALY when decelerating the rate by 30%.
In another study, Stewart et al. (Stewart et al., 2008) developed a model to evaluate the long-term cost-effectiveness of treating ocular hypertension in the US to prevent progression to glaucoma. While treating all patients with ocular hypertension did not prove to be cost-effective, treating those with risk factors identified by the OHTS did seem to prevent the onset of glaucoma cost-effectively. Similarly, Kymes et al. (Kymes et al., 2006) concluded that treating individuals with an IOP of 24 mm Hg and who had ≥2% annual risk of developing glaucoma met cost-effectiveness standards accepted in most developed countries.
7. Patient-relevant effects of therapy on progression
7.1. Clinical meaningful effects of therapy
Currently, clinical drug trials are focused on IOP measurements and/or visual field tests (a functional outcome measure) for the assessment of clinical IOP-lowering efficacy and changes in optic nerve function. In some studies, stereoscopic optic disc photography (a structural outcome measure) has been used as a secondary endpoint. The FDA considers changes in visual function to be outcome measures in clinical trials that serve as the basis for approving neuroprotective glaucoma therapies, provided those outcomes are proven predictive of function that is clinically relevant to a patient.
The purpose of the NEI/FDA CDER Glaucoma Clinical Trial Design and Endpoints Symposium (Weinreb and Kaufman, 2009) was to provide a forum for discussing outcome measures—based on the newer technologies—for evaluating neuroprotective products for glaucoma in clinical trials to facilitate bringing safe and efficacious glaucoma pharmacotherapies to the U.S. market. Important issues that surround endpoints in clinical trials of glaucoma drugs include establishing and validating new parameters for glaucoma diagnosis and detection of progression, assessing the degree of structural and functional involvement, ascertaining correlations between structural and functional loss, predicting which level or kind of glaucomatous vision loss eventually affects a patient’s QOL, and validation of equipment and technology for assessing involvement.
There is no agreement about what constitutes a clinically significant change in visual function in patients with glaucoma. There is no universally established approach for estimating future clinical progression of glaucoma. Predictive models are in their early stages; however, these models may ultimately pinpoint future glaucomatous optic nerve damage based on current locations of visual field deficits and past rate of progression. Adding variables such as age, IOP, and CCT may improve the predictive models.
The estimation of progression rates in glaucoma is important given that knowing progression rates will help direct treatment to the patients in a timely manner to prevent significant vision loss. Estimates of progression are extremely difficult because of variability within each patient (sometimes referred to as long-term fluctuation) and among patients.
Trend analysis or event analysis may shed some light on progression rates in glaucoma. In trend analysis, the rate of change of the visual field is measured and the statistical significance of that rate is calculated. In event analysis, the first few visual fields in a series are used as a baseline and then compared to subsequent visual fields to determine whether change has occurred. An advantage of trend analysis is that it provides information about rates of change, but it requires several reliable tests to determine a statistical trend. Event analysis generally requires fewer tests but gives relatively little information about rates. Furthermore, event-based methods require confirmatory test results to achieve adequate specificity, and there is no consensus about which event to use as a standard.
Perimetry frequently detects changes in visual function before patients become aware that their vision is affected. This makes perimetry an important tool for diagnosing and quantitating the progression of disease as well as for monitoring the ability of treatments to prevent additional ganglion cell dysfunction or loss. As visual field testing is subjective, there generally is considerable variability in functional measures. Advantages of SAP include standardization of the testing procedure from test to test and among different locations. With SAP, there is the ability to compare test results to normative data, integrate data from multiple study centers, and perform statistical analyses on groups of patients or even individuals.
For new drug approvals, regulatory agencies differentiate between a glaucoma indication and an IOP-lowering indication. In other words, an IOP-lowering drug does not have to show an effect on the disease process. A critical feature in considering new IOP-lowering (or any other) drugs is the benefit-to-risk ratio. The benefit is determined by efficacy in clinical trials. The calculated assessment of risk is based on evidence from the clinical studies. The current benchmark for proposed IOP-reducing agents is typically equivalency to one of four approved products: timolol maleate ophthalmic solution, latanoprost ophthalmic solution, bimatoprost ophthalmic solution, and travoprost ophthalmic solution, the last three of which are prostaglandin analogues. From this, it becomes evident that a gap will exist once treatment modalities focused on IOP-independent pathways become available, as one cannot directly compare treatment effects with the above standards for equivalency (Weinreb and Kaufman, 2009). Rather, how these new drugs affect visual outcomes is possibly the only available means to demonstrate equivalency for new drug proposals.
Therefore, future studies testing treatment modalities focused on IOP-independent pathways (e.g. neuroprotective agents) should be designed to compare functional outcomes. Since RCTs that enroll glaucoma patients randomized to placebo alone are likely to face ethical issues, the design of these trials will require comparison between arm(s) treated with at least one IOP-lowering medication and one arm where the IOP-independent drug is added to IOP lowering. The UKGTS was the most recent clinical trial in glaucoma patients to include a placebo group. Nonetheless, the investigators minimized ethical issues by including patients at very early stages of the disease, for whom treatment is not routinely given in some countries, and offering treatment once their interim analyses showed significant benefits of treatment. As discussed previously regarding the UKGTS, in which significant differences between arms were seen as early as at 12 months of follow-up, it is important to note that effect sizes between a treatment group and placebo are expected to be larger - and significant differences are likely to be seen sooner - than when two active agents are compared. Therefore, some of the strategies to improve the viability of future RCTs testing neuroprotective agents, without resorting to larger sample sizes and longer follow-up periods, are to (i) select patients at high-risk risk of progression at baseline (for example, based on risk calculators) (De Moraes et al., 2012c; Gordon et al., 2007) (ii) and employing trend-based visual field progression criteria, particularly those that employ methods to overcome the increase of variability with progression (Zhu et al., 2014) and that censor severe defects (Gardiner et al., 2016).
Quigley also addressed the challenges of designing RCTs for glaucoma neuroprotection and provided additional recommendations to improve their design (Quigley, 2012). For instance, some of the suggested approaches to improve the speed or accuracy with which visual field progression can be detected included the recruitment of patients with a minimum standard of reliability indices and employing newer trend-based analyses of progression. Based upon a number of assumptions, he estimated that in order to detect a 20% reduction in the rate of progression (from −0.54 to −0.38 dB/yr) in eyes with small visual field variability (SD = 0.52 and 0.36, respectively), approximately 125 eyes in each group would be needed (with a two-sided test) in 2 years with fields performed every 3 months. Of course, with large treatment effects (e.g. 50%), this number would be even smaller (38 in each group). As we have discussed, such a large treatment effect is not a reasonable assumption if both arms are treated with IOP-lowering medications, and thus 30% is more realistic.
From the perspective of regulatory approval, the levels of functional change indicated by an endpoint would have to represent clinical findings that most clinicians would agree to be clinically meaningful. Different endpoints may be pursued in glaucoma trials but the trial sponsor would have to justify the clinical relevance. The FDA stated that, for the indication for the treatment of glaucoma, as opposed to IOP reduction, a product under evaluation by the FDA would have to demonstrate, as a functional endpoint, an effect on progression of the disease (e.g., currently, visual field progression). Visual field changes may be acceptable as a clinically relevant primary endpoint provided an acceptable between-group difference in field progression is demonstrated (Weinreb and Kaufman, 2009). Again, the purpose of these definitions is to translate visual field outcomes to estimates of QOL.
7.2. Decreasing the rate of visual field progression by 30% translates to a meaningful difference in quality of life
The World Health Organization defines QOL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns (WHO, 1993). It is a broad-ranging concept affected in a complex way by the person’s physical health, psychological state, level of independence, social relationships, and their relationship to salient features of their environment”.
The FDA, in draft guidance calls HRQOL “a multidomain concept that represents the patient’s overall perception of the impact of an illness and its treatment” (Banerjee et al., 2013). In ophthalmology, HRQOL instruments are used to assess self-reported general and visual functioning of patients. Although many different instruments have been used for measuring QOL in patients with glaucoma, the NEI-VFQ-25 is the most widely used. A shortcoming of the NEI-VFQ-25 may be that there is little information about its inter-interviewer reliability or its ability to detect change in glaucoma. As described previously, a 3–4 dB difference in visual field loss is associated with a clinically meaningful 5-point difference in the NEI-VFQ driving subscale and significant changes in the composite scores (McKean-Cowdin et al., 2007). Loss in HRQOL scores for glaucoma participants is already present in people with mild visual field loss and continues to worsen through moderate to severe disease.
QOL measures provide very useful supportive information, and the FDA encourages their use in clinical trials(Weinreb and Kaufman, 2009). Whether these measures end up in the product label depends on proper administration of the instrument, proper validation, and adequate statistical power to show differences between study groups. The FDA has drafted a document (Study Endpoints and Label Development [SEALD])1 describing the requirements for internal and external validation. The NEI-VFQ-25 has not been adequately externally validated nor has it met all criteria for validating instruments. Despite that fact, the results of clinical trials and other studies discussed in this document suggest a good correlation between these scores and visual field progression, the latter defined with either event- or trend-based approaches.
Lisboa et al. tested the hypothesis that for two patients with similar degrees of integrated binocular visual field (BVF) loss, the patient with a history of faster disease progression is more likely to report worse vision-related quality of life (VRQOL) than the patient with slowly progressing damage (Lisboa et al., 2013). A total of 796 eyes of 398 patients with diagnosed or suspected glaucoma followed up for a mean (SD) of 7.3 (2.0) years were recruited from the DIGS and the African Descent and Glaucoma Evaluation Study (ADAGES). The VFRQOL was evaluated with the NEI VFQ-25 which had been completed for all patients at the last follow-up visit. Integrated BVFs were calculated from the monocular fields of each patient. Linear regression of the visual field MD values was used to evaluate rates of BVF change during the follow-up period. Logistic regression models were used to investigate the association between abnormal VRQOL and rates of BVF change, while adjusting for potentially confounding socioeconomic and demographic variables.
Their results revealed that 32 patients (8.0%) had abnormal VRQOL as determined by the results of the NEI VFQ-25. In concordance with what we described above with regard to different populations, patients with abnormal VRQOL had significantly faster rates of BVF change than those with normal VRQOL (−0.18 vs −0.06 dB/yr; P < 0.001). Rates of BVF change were significantly associated with abnormality in VRQOL (OR, 1.31 per 0.1 dB/yr faster; P = 0.04), after adjustment for confounding variables. This corresponds to a 30% worsening in VFRQOL scores for each 0.1 dB/yr faster rate of visual field progression. The study concluded that patients with faster rates of BVF change were at higher risk of reporting abnormal VRQO and thus the assessment of rates of BVF change may provide useful information in determining risk of functional impairment in glaucoma.
An alternative way of looking at the effects of decreasing rate of visual field progression is with respect to progression to blindness. For example: a 60-year-old patient starting from an average baseline MD of −10 dB in the better seeing eye, with a global MD rate of progression of −1.5 dB/year, would reach legal blindness (−22 dB) in eight years. This means that this patient would be legally blind by the age of 68 years. With a median residual life expectancy of 25 years, this represents 15 years of life being blind. After treatment, however, with a mean rate of −0.5 dB/year, the time to become legally blind would increase to 24 years, translating to a legally blind age of 84 years, which is beyond the patient’s median residual life expectancy. Currently available modalities of glaucoma treatment, which are exclusively focused on IOP reduction, do not totally halt progression, but significantly improve the patient’s visually functional lifespan. The same would be true for any treatment that decreases the rate of progression. A 30% reduction in progression rate would increase the number of blindness-free years from x to x/(1–0.3) = 1.42x. In other words, a 30% decrease in progression rate translates to 42% more years free of blindness.
7.3. Decreasing the rate of visual field progression by 30% translates to an effect equivalent to what FDA has suggested for clinical meaningfulness with event-based methods
As discussed previously, the faster the MD rate of progression, particularly the rate of more rapidly progressing test locations, the more sensitive to treatment effects trend-based analysis becomes, allowing earlier detection of change with a smaller number of fields. Note that eyes in the OHTS that started with normal visual fields (MD ~ 0.0 dB) and converted to POAG based on event analysis progressed at −0.5 dB/yr on average. Also, approximately five test locations progressed significantly in order to deem the last visual field abnormal and, as previously discussed, these rapidly progressing points are most likely the ones with worse sensitivities at baseline (despite still being within the 95% limits of normality at baseline).
FDA has stated that “significant visual field changes may be acceptable as a clinically relevant primary endpoint provided a between-group difference in field progression is demonstrated. The progression of visual field loss will be suspected if five or more reproducible points, or visual field locations, have significant changes from baseline beyond the 5% probability levels for the GCP analysis.” One important point not addressed by these criteria is how long it should take to reach that endpoint. In other words, the implications of meeting the above criteria in 2 years are far more severe than if it happened over 10 years. This is why we believe employing trend analysis (dB/year) is more clinically relevant.
The event-based criteria used in the OHTS were different from the one described above, even though GCP was still employed. Nevertheless, this comparison with trend-based outcomes suggests that a global MD rate of progression of −0.5 dB/yr or faster leads to similar event-based outcomes recommended by the FDA. The fact that, in the OHTS, visual field locations were statistically normal and thus progress more slowly, in eyes with five abnormal points at baseline the “scotoma mass” should progress even faster than the average −0.5 dB/yr seen in the OHTS, and hence there is a greater likelihood that these five or more test locations will significantly change from baseline beyond a 5% probability level.
With regard to vision-related QOL, OHTS patients who reached a POAG endpoint would take approximately 28 years to become severely visually impaired if they were never treated, which is within the lifespan of the average OHTS patient (mean age at study entry, 56 years). As discussed previously, the extrapolation of linear trends has good accuracy in predicting future visual field outcomes. Therefore, the commencement of treatment in those patients triples the projected time to advanced visual impairment. These estimates, of course, are based on mean values, and one should take into consideration the large variability in individual patient behavior in this and other populations.
With regard to patients with baseline visual field damage with significantly abnormal points, the EMGT provides compelling support to the assumptions discussed in the previous paragraphs. In a study analyzing EMGT patients in the untreated arm, investigators were able to better understand the natural history of glaucoma. The mean rates of visual function loss were −1.08 dB/year overall and −1.31 in high-tension (HTG), −0.36 in normal-tension (NTG), and −3.13 dB/year in pseudoexfoliative glaucoma (PEXG). By an average of six years of the study, 68% of patients had progressed overall with event-based criteria (GCP-GPA), 74% of those with HTG, 56% of those with NTG, and 93% of those with PEXG (P = 0.012) (Heijl et al., 2009).
These data demonstrate a direct relationship between MD rates of change (trend analysis) and proportion of eyes reaching a GPA endpoint (event analysis). Rates of MD progression equal to or faster than −0.5 dB/yr on average corresponds to clinically significant endpoints with GCP criteria ranging from 78% to 100% based on the correlations above.
In another analysis of the EMGT data, the investigators provided estimates of the amount of visual field worsening required to reach the EMGT definition of definite perimetric progression (Heijl et al., 2003). In the 148 eyes that reached definite progression, the authors first determined changes between baseline and the time of definite EMGT progression, both for MD and for the number of highly significantly (P < 0.5%) depressed test points in pattern deviation probability maps. They found that in eyes reaching progression, the mean change in MD from baseline was −1.93 dB (~−0.40 dB/year) and the mean change in number of significant points was +4.85 (in 5 years). They concluded that a loss of about −2 dB in MD and an increase in about five highly significant points expresses in more conventional units the GCP-GPA event-based criteria used in the trial.
Therefore, it is plausible that a trend-based visual field progression criterion with rates faster than −0.5 dB/yr would parallel the current FDA endpoint recommendations, with the advantage of requiring shorter follow-up time, allowing long-term extrapolations, and objective measurement of a continuous variable to assess treatment effects. Moreover, use of abnormal points makes these estimates more powerful because the class of drugs to be studied (i.e., neuroprotection) is theoretically more likely to have an effect on abnormal points getting worse than on normal points becoming abnormal (the latter requiring much longer follow-up to detect significant change).
7.4. Effects of IOP-Lowering on rates of visual field progression determined by trend analysis
As mentioned previously, data from RCTs can be used to assess the effects of IOP lowering on decrease in progression rates of visual fields. For example, in the EMGT, the average IOP reduction was 25% (20.6–15.45 mm Hg), and this 4.5 mm Hg treatment effect was associated with a 50% reduction in MD rates of progression. This is also consistent with a treatment effect of 3 mm Hg decreasing the rate of MD progression by 30% (Heijl et al., 2009).
In the NY-GAPS, the investigators aimed to determine the efficacy of glaucoma surgery in decreasing localized (i.e., previously abnormal points) and global (MD) rates of visual field progression (Folgar et al., 2010). For patients who underwent successful glaucoma surgery (not requiring further surgical intervention and postoperative mean IOP < 18 mm Hg), the mean ± SD IOP decreased from 19.0 ± 3.9 mm Hg before surgery to 11.3 ± 3.7 mm Hg after surgery (40% reduction; P < 0.01). Mean global progression rates decreased from −1.48 ± 1.4 dB/year before surgery to −0.43 ± 0.8 dB/year after surgery (70% reduction; P = 0.01). Twelve eyes (42.8%) had at least one significantly progressing point before surgery, whereas only two (7.1%) had at least one progressing point after surgery, which was sustained for at least two years.
These findings suggest that treatment has a significant effect on visual field locations which were previously abnormal and which had been progressing at fast rates. By using trend analysis, the authors suggest two new concepts regarding visual field progression in IOP-treated eyes. First, effectiveness of IOP-lowering therapies can be measured directly with a continuous variable (dB/yr), which can be reassessed at any time point and hence be readjusted as needed. Second, a greater treatment effect on functional loss was observed at points with significant and faster progression prior to intervention. Not only was there a global reduction in the velocity of visual field deterioration, but there was an additional, positive, differential effect on the most rapidly progressing points.
In the same study, linear regression analysis comparing the absolute IOP-lowering (mm Hg) and the decay in global rate of MD progression (dB/year) was positive and significant (R2 = 0.20, P = 0.01). Each 1 mm Hg of IOP reduction after surgery resulted in 0.1 dB/year decrement in the global rate of progression. This means that a 30% deceleration in MD rates of progression in a population of glaucoma patients treated with IOP-lowering medication, which in the NY-GAPS corresponds to slowing the rate from an average of −0.35 to −0.24 dB/yr, corresponds to an IOP-lowering effect of at least 1 mm Hg. For high-risk, rapidly progressing patients (~−1.0 dB/yr) a 30% deceleration in the rates of MD progression translates to at least 3 mm Hg of treatment-effect.
8. Summary and future directions
This review presents data and analysis to support the assertion that a 30% reduction in the rate of visual field deterioration, when demonstrated over a 12–18 month study, would be clinically meaningful. The following was presented, based on published studies in the peer-reviewed literature, with particular emphasis on multicenter RCTs and large cohort studies:
In Section 1, we showed that glaucoma is common, increasing in prevalence, and results in remarkably high rates of blindness and disability, despite modern IOP-lowering therapies. In addition, medical and surgical IOP-lowering therapies can be associated with mild, moderate, or severe adverse effects. Together, these show that therapy of progressive glaucoma remains an unmet need, and we show how IOP-independent therapies could assist in preventing visual loss.
In Section 2, we discussed how visual field loss and progression are measured, and explained how increases in variability from aging or severe field loss can affect measurements. We then analyzed the major glaucoma RCTs and two large cohort studies, and calculated the relation between amount of IOP lowering in these studies with decrease in visual field progression (when possible).
In Section 3, we focused on specific aspects of glaucomatous visual field progression, and showed how deterioration preferentially occurs in areas that are already abnormal. This issue is relevant to a strategy for clinical trial design, where the endpoints are based on progression in abnormal areas of the visual field (as opposed to analysis of the entire field). We then reviewed the data supporting a linear progression in visual field deterioration. This is relevant to clinical trials based on short- and medium-term observation of patient groups, because it allows estimation of effects over the longer term.
In Section 4, we discussed different strategies for detecting and quantifying visual field progression, and showed how a trend-based strategy has advantages for measuring deterioration. We then discussed the number of tests needed under various scenarios, and applied the use of linear regression-based prediction to show that a short-term measurement of progression with a trend approach predicts the long-term progression. Finally, we showed why abnormal points should be used for measuring the effect of an intervention in decreasing progression.
In Section 5, we showed that visual field progression strongly correlates with HRQOL, including both standardized scales and meaningful events such as falls or losing the ability to drive. We also demonstrated the correlation of visual field progression with negative socioeconomic impact.
In Section 6, we discussed the importance of a clinically meaningful effect of a therapy on patients and regulatory approval. We then used the analyses derived in the previous sections as the basis for three different approaches to show that decreasing the rate of visual field progression by 30% is clinically meaningful. First, we described how a 30% slowing of visual field deterioration translates to a meaningful difference in QOL, including increasing the number of years until blindness is reached by about 40%. Second, we established that a 30% decrease in progression rate is essentially equivalent to an event-based system at 5 abnormal points for assessing progression rate, the latter which is already acceptable to the FDA with respect to clinical meaningfulness. Third, we showed how the effect of decreasing visual field progression by 30% is equivalent to about 3 mm Hg of IOP lowering, which would be clinically relevant to patients who are already progressing despite maximal medical therapy.
In summary, we have shown that (1) a 30% decrease in visual field deterioration with trend-based analysis is clinically meaningful with respect to QOL and matches FDA statements for event-based analyses; (2) such a decrease measured over a short period of time predicts a decrease over a longer period of time based on studies showing visual field progression is often linear; and (3) focusing on abnormal areas of the visual field in the analysis is clinically relevant and improves the ability to detect clinically significant effects. Given that glaucoma is highly prevalent and continues to blind or disable people at high rates, a therapy that could slow glaucomatous progression by 30% would be clinically meaningful to patients.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers: R01 EY025253 (CGDM); R21 EY025074 and P30 EY016665 (LAL)]; unrestricted departmental grant from Research to Prevent Blindness, Inc. New York, NY (CGDM, JML); unrestricted departmental grant from Research to Prevent Blindness, Inc. New York, NY (LAL); LAL is Canada Research Chair in Translational Visual Science.
Footnotes
The guidance document is available at the FDA Web site (http://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm349031.htm www.fda.gov/cder/guidance/5460dft.htm).
Disclosures
CGDM: None; JML: Consultant; Allergan Inc., Alcon Inc., Bausch & Lomb, Inc, Quark, Inc., Aerie, Inc., LAL: Consultant on neuroprotection research to Aerie, GlaxoSmithKline, Inotek, Quark, and Regenera. Support for a previous version of this manuscript was provided by Merz Pharma.
References
- AAO. Preferred Practice Pattern® Guidelines. Primary Open-Angle Glaucoma. American Academy of Ophthalmology; San Francisco, CA: 2010. [Google Scholar]
- Adams CW, Bullimore MA, Wall M, Fingeret M, Johnson CA. Normal aging effects for frequency doubling technology perimetry. Optom Vis Sci off Publ Am Acad Optom. 1999;76:582–587. doi: 10.1097/00006324-199908000-00027. [DOI] [PubMed] [Google Scholar]
- Administration, U.S.S. Disability evaluation under Social Security. [Google Scholar]
- AGIS. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- AGIS. The advanced glaucoma intervention study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol. 2002;134:499–512. doi: 10.1016/s0002-9394(02)01659-8. [DOI] [PubMed] [Google Scholar]
- Ahrlich KG, De Moraes CG, Teng CC, Prata TS, Tello C, Ritch R, Liebmann JM. Visual field progression differences between normal-tension and exfoliative high-tension glaucoma. Investig Ophthalmol Vis Sci. 2010;51:1458–1463. doi: 10.1167/iovs.09-3806. [DOI] [PubMed] [Google Scholar]
- Andrews FM, Withey SB. Social Indicators of Well-Being: America’s Perceptions of Life Quality. Plenum; New York: 1976. [Google Scholar]
- Artes PH, Chauhan BC. Signal/noise analysis to compare tests for measuring visual field loss and its progression. Investig Ophthalmol Vis Sci. 2009;50:4700–4708. doi: 10.1167/iovs.09-3601. [DOI] [PubMed] [Google Scholar]
- Azarbod P, Mock D, Bitrian E, Afifi AA, Yu F, Nouri-Mahdavi K, Coleman AL, Caprioli J. Validation of point-wise exponential regression to measure the decay rates of glaucomatous visual fields. Investig Ophthalmol Vis Sci. 2012;53:5403–5409. doi: 10.1167/iovs.12-9930. [DOI] [PubMed] [Google Scholar]
- Banerjee AK, Okun S, Edwards IR, Wicks P, Smith MY, Mayall SJ, Flamion B, Cleeland C, Basch E. Patient-reported outcome measures in safety event reporting: PROSPER consortium guidance. Drug Saf Int J Med Toxicol drug Exp. 2013;36:1129–1149. doi: 10.1007/s40264-013-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B, Patella VM, Heijl A. Prediction of glaucomatous visual field loss by extrapolation of linear trends. Arch Ophthalmol. 2009;127:1610–1615. doi: 10.1001/archophthalmol.2009.297. [DOI] [PubMed] [Google Scholar]
- Boden C, Blumenthal EZ, Pascual J, McEwan G, Weinreb RN, Medeiros F, Sample PA. Patterns of glaucomatous visual field progression identified by three progression criteria. Am J Ophthalmol. 2004;138:1029–1036. doi: 10.1016/j.ajo.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Bryan SR, Vermeer KA, Eilers PH, Lemij HG, Lesaffre EM. Robust and censored modeling and prediction of progression in glaucomatous visual fields. Investig Ophthalmol Vis Sci. 2013;54:6694–6700. doi: 10.1167/iovs.12-11185. [DOI] [PubMed] [Google Scholar]
- Casas-Llera P, Rebolleda G, Munoz-Negrete FJ, Arnalich-Montiel F, Perez-Lopez M, Fernandez-Buenaga R. Visual field index rate and event-based glaucoma progression analysis: comparison in a glaucoma population. Br J Ophthalmol. 2009;93:1576–1579. doi: 10.1136/bjo.2009.158097. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, Drance SM, Douglas GR. The use of visual field indices in detecting changes in the visual field in glaucoma. Investig Ophthalmol Vis Sci. 1990;31:512–520. [PubMed] [Google Scholar]
- Chauhan BC, Garway-Heath DF, Goni FJ, Rossetti L, Bengtsson B, Viswanathan AC, Heijl A. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Johnson CA. Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Investig Ophthalmol Vis Sci. 1999;40:648–656. [PubMed] [Google Scholar]
- Chauhan BC, LeBlanc RP, Shaw AM, Chan AB, McCormick TA. Repeatable diffuse visual field loss in open-angle glaucoma. Ophthalmology. 1997;104:532–538. doi: 10.1016/s0161-6420(97)30279-6. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, Mikelberg FS, Artes PH, Balazsi AG, LeBlanc RP, Lesk MR, Nicolela MT, Trope GE. Canadian glaucoma study: 3. Impact of risk factors and intraocular pressure reduction on the rates of visual field change. Arch Ophthalmol. 2010;128:1249–1255. doi: 10.1001/archophthalmol.2010.196. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, Tompkins JD, LeBlanc RP, McCormick TA. Characteristics of frequency-of-seeing curves in normal subjects, patients with suspected glaucoma, and patients with glaucoma. Investig Ophthalmol Vis Sci. 1993;34:3534–3540. [PubMed] [Google Scholar]
- Chen A, Nouri-Mahdavi K, Otarola FJ, Yu F, Afifi AA, Caprioli J. Models of glaucomatous visual field loss. Investig Ophthalmol Vis Sci. 2014;55:7881–7887. doi: 10.1167/iovs.14-15435. [DOI] [PubMed] [Google Scholar]
- CNTGS. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998a;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- CNTGS. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998b;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Demirel S, Gardiner SK, Liebmann JM, Cioffi GA, Ritch R, Gordon MO, Kass MA. Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Investig Ophthalmol Vis Sci. 2012a;53:1704–1709. doi: 10.1167/iovs.11-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CG, Juthani VJ, Liebmann JM, Teng CC, Tello C, Susanna R, Jr, Ritch R. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129:562–568. doi: 10.1001/archophthalmol.2011.72. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2012b;154:702–711. doi: 10.1016/j.ajo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Prata TS, Tello C, Ritch R, Liebmann JM. Glaucoma with early visual field loss affecting both hemifields and the risk of disease progression. Arch Ophthalmol. 2009;127:1129–1134. doi: 10.1001/archophthalmol.2009.165. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Sehi M, Greenfield DS, Chung YS, Ritch R, Liebmann JM. A validated risk calculator to assess risk and rate of visual field progression in treated glaucoma patients. Investig Ophthalmol Vis Sci. 2012c;53:2702–2707. doi: 10.1167/iovs.11-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CG, Song C, Liebmann JM, Simonson JL, Furlanetto RL, Ritch R. Defining 10-2 visual field progression criteria: exploratory and confirmatory factor analysis using pointwise linear regression. Ophthalmology. 2014;121:741–749. doi: 10.1016/j.ophtha.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Demirel S, De Moraes CG, Gardiner SK, Liebmann JM, Cioffi GA, Ritch R, Gordon MO, Kass MA. The rate of visual field change in the ocular hypertension treatment study. Investig Ophthalmol Vis Sci. 2012;53:224–227. doi: 10.1167/iovs.10-7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005–2012. JAMA J Am Med Assoc. 2014;311:368–377. doi: 10.1001/jama.2013.282034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- EGS. Terminology and Guidelines for Glaucoma. European Glaucoma Society; Savona, Italy: 2008. [Google Scholar]
- Fankhauser F, Jenni A. Programs SARGON and DELTA: two new principles for the automated analysis of the visual field. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie Albrecht von Graefe’s archiv Clin Exp Ophthalmol. 1981;216:41–48. doi: 10.1007/BF00407775. [DOI] [PubMed] [Google Scholar]
- Flanagan JC. Measurement of quality of life: current state of the art. Archiv Phys Med rehabilit. 1982;63:56–59. [PubMed] [Google Scholar]
- Folgar FA, de Moraes CG, Prata TS, Teng CC, Tello C, Ritch R, Liebmann JM. Glaucoma surgery decreases the rates of localized and global visual field progression. Am J Ophthalmol. 2010;149:258–264. e252. doi: 10.1016/j.ajo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Wolfs RC, O’Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Crabb DP. Frequency of testing for detecting visual field progression. Br J Ophthalmol. 2002;86:560–564. doi: 10.1136/bjo.86.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Demirel S, De Moraes CG, Liebmann JM, Cioffi GA, Ritch R, Gordon MO, Kass MA. Series length used during trend analysis affects sensitivity to changes in progression rate in the ocular hypertension treatment study. Investig Ophthalmol Vis Sci. 2013;54:1252–1259. doi: 10.1167/iovs.12-10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Demirel S, Goren D, Mansberger SL, Swanson WH. The effect of stimulus size on the reliable stimulus range of perimetry. Transl Vis Sci Technol. 2015;4:10. doi: 10.1167/tvst.4.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Johnson CA, Spry PG. Normal age-related sensitivity loss for a variety of visual functions throughout the visual field. Optom Vis Sci off Publ Am Acad Optom. 2006;83:438–443. doi: 10.1097/01.opx.0000225108.13284.fc. [DOI] [PubMed] [Google Scholar]
- Gardiner SK, Swanson WH, Demirel S. The effect of limiting the range of perimetric sensitivities on pointwise assessment of visual field progression in glaucoma. Investig Ophthalmol Vis Sci. 2016;57:288–294. doi: 10.1167/iovs.15-18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Swanson WH, Goren D, Mansberger SL, Demirel S. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology. 2014;121(7):1359–1369. doi: 10.1016/j.ophtha.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, Suzuki K, White ET, Wormald RP, Xing W, Zeyen TG. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet (Lond, Engl. 2015;385:1295–1304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- GLT. The glaucoma laser trial (GLT) and glaucoma laser trial follow-up study: 7. Results Am J Ophthalmol. 1995;120:718–731. doi: 10.1016/s0002-9394(14)72725-4. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, DKH, EJH, CAJ, JLK, JPM, PRKMR, WMAK The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Torri V, Miglior S, Beiser JA, Floriani I, Miller JP, Gao F, Adamsons I, Poli D, D’Agostino RB, Kass MA. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–19. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez P, Wilson MR, Johnson C, Gordon M, Cioffi GA, Ritch R, Sherwood M, Meng K, Mangione CM. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115:777–784. doi: 10.1001/archopht.1997.01100150779014. [DOI] [PubMed] [Google Scholar]
- Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, Butterfield LC, Gray DT. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- Haymes SA, Leblanc RP, Nicolela MT, Chiasson LA, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Investig Ophthalmol Vis Sci. 2007;48:1149–1155. doi: 10.1167/iovs.06-0886. [DOI] [PubMed] [Google Scholar]
- Heijl A. The times they are a-changin’: time to change glaucoma management. Acta Ophthalmol. 2013;91:92–99. doi: 10.1111/j.1755-3768.2011.02328.x. [DOI] [PubMed] [Google Scholar]
- Heijl A, Bengtsson B, Chauhan BC, Lieberman MF, Cunliffe I, Hyman L, Leske MC. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology. 2008;115:1557–1565. doi: 10.1016/j.ophtha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Heijl A, Bengtsson B, Hyman L, Leske MC. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hussein M. Measuring visual field progression in the early manifest glaucoma trial. Acta Ophthalmol Scand. 2003;81:286–293. doi: 10.1034/j.1600-0420.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Early manifest glaucoma trial. G Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108:130–135. doi: 10.1016/0002-9394(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- Hodapp E, Parrish RK, Anderson DR. Clinical Decisions in Glaucoma. The CV Mosby Co; St. Louis: 1993. [Google Scholar]
- Holmin C, Krakau CE. Regression analysis of the central visual field in chronic glaucoma cases. A follow-up study using automatic perimetry. Acta Ophthalmol (Copenh) 1982;60:267–274. doi: 10.1111/j.1755-3768.1982.tb08381.x. [DOI] [PubMed] [Google Scholar]
- Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Slobodnick A, Raza AS, de Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Investig Ophthalmol Vis Sci. 2014;55:632–649. doi: 10.1167/iovs.13-13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman LG, Komaroff E, Heijl A, Bengtsson B, Leske MC. Treatment and vision-related quality of life in the early manifest glaucoma trial. Ophthalmology. 2005;112:1505–1513. doi: 10.1016/j.ophtha.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Jampel HD, Frick KD, Janz NK, Wren PA, Musch DC, Rimal R, Lichter PR. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144:238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- Jansonius NM. On the accuracy of measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2010;94:1404–1405. doi: 10.1136/bjo.2009.164897. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Adams AJ, Twelker JD, Quigg JM. Age-related changes in the central visual field for short-wavelength-sensitive pathways. J Opt Soc Am A, Opt image Sci. 1988;5:2131–2139. doi: 10.1364/josaa.5.002131. [DOI] [PubMed] [Google Scholar]
- Kass MA, Gordon MO, Gao F, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JK, Miller JP, Parrish RK, Wilson MR Ocular Hypertension Treatment Study G. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010;128:276–287. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(829):701–713. doi: 10.1001/archopht.120.6.701. discussion, 730. [DOI] [PubMed] [Google Scholar]
- Katz J, Gilbert D, Quigley HA, Sommer A. Estimating progression of visual field loss in glaucoma. Ophthalmology. 1997;104:1017–1025. doi: 10.1016/s0161-6420(97)30192-4. [DOI] [PubMed] [Google Scholar]
- Kymes SM, Kass MA, Anderson DR, Miller JP, Gordon MO. Management of ocular hypertension: a cost-effectiveness approach from the ocular hypertension treatment study. Am J Ophthalmol. 2006;141:997–1008. doi: 10.1016/j.ajo.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Early manifest glaucoma trial. G Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, Mills RP. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- Lisboa R, Chun YS, Zangwill LM, Weinreb RN, Rosen PN, Liebmann JM, Girkin CA, Medeiros FA. Association between rates of binocular visual field loss and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol. 2013;131:486–494. doi: 10.1001/jamaophthalmol.2013.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught AI, Crabb DP, Fitzke FW, Hitchings RA. Modelling series of visual fields to detect progression in normal-tension glaucoma. Graefe’s archiv Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und Exp Ophthalmol. 1995;233:750–755. doi: 10.1007/BF00184085. [DOI] [PubMed] [Google Scholar]
- Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Investig Ophthalmol Vis Sci. 2012;53:6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European glaucoma prevention study. Ophthalmology. 2005;112:366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Mikelberg FS, Drance SM. The mode of progression of visual field defects in glaucoma. Am J Ophthalmol. 1984;98:443–445. doi: 10.1016/0002-9394(84)90128-4. [DOI] [PubMed] [Google Scholar]
- Mikelberg FS, Schulzer M, Drance SM, Lau W. The rate of progression of scotomas in glaucoma. Am J Ophthalmol. 1986;101:1–6. doi: 10.1016/0002-9394(86)90457-5. [DOI] [PubMed] [Google Scholar]
- Mills RP, Janz NK, Wren PA, Guire KE. Correlation of visual field with quality-of-life measures at diagnosis in the collaborative initial glaucoma treatment study (CIGTS) J glaucoma. 2001;10:192–198. doi: 10.1097/00061198-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Murata H, Hirasawa H, Aoyama Y, Sugisaki K, Araie M, Mayama C, Aihara M, Asaoka R. Identifying areas of the visual field important for quality of life in patients with glaucoma. PloS one. 2013;8:e58695. doi: 10.1371/journal.pone.0058695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P, Aspinall P, Papasouliotis O, Worton B, O’Brien C. Quality of life in glaucoma and its relationship with visual function. J glaucoma. 2003;12:139–150. doi: 10.1097/00061198-200304000-00009. [DOI] [PubMed] [Google Scholar]
- NICE. CG85 Glaucoma. 1. National Institute of Health and Clinical Excellence; London, UK: 2009. Published Clinical guidelines. [Google Scholar]
- NIH. Biomarkers and Surrogate Endpoints. Elsevier; Amsterdam: 2000. [Google Scholar]
- Noureddin BN, Poinoosawmy D, Fietzke FW, Hitchings RA. Regression analysis of visual field progression in low tension glaucoma. Br J Ophthalmol. 1991;75:493–495. doi: 10.1136/bjo.75.8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004a;111:1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Hoffman D, Gaasterland D, Caprioli J. Prediction of visual field progression in glaucoma. Investig Ophthalmol Vis Sci. 2004b;45:4346–4351. doi: 10.1167/iovs.04-0204. [DOI] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Hoffman D, Ralli M, Caprioli J. Comparison of methods to predict visual field progression in glaucoma. Arch Ophthalmol. 2007;125:1176–1181. doi: 10.1001/archopht.125.9.1176. [DOI] [PubMed] [Google Scholar]
- Oliver JE, Hattenhauer MG, Herman D, Hodge DO, Kennedy R, Fang-Yen M, Johnson DH. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol. 2002;133:764–772. doi: 10.1016/s0002-9394(02)01403-4. [DOI] [PubMed] [Google Scholar]
- Otarola F, Chen A, Morales E, Yu F, Afifi A, Caprioli J. Course of glaucomatous visual field loss across the entire perimetric range. JAMA Ophthalmol. 2016 Mar 10; doi: 10.1001/jamaophthalmol.2016.0118. http://dx.doi.org/10.1001/jamaophthalmol.2016.0118 [Epub ahead of print] [DOI] [PubMed]
- Park SC, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology. 2011;118:1782–1789. doi: 10.1016/j.ophtha.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Parrish RK., 2nd Visual impairment, visual functioning, and quality of life assessments in patients with glaucoma. Trans Am Ophthalmol Soc. 1996;94:919–1028. [PMC free article] [PubMed] [Google Scholar]
- Pascual JP, Schiefer U, Paetzold J, Zangwill LM, Tavares IM, Weinreb RN, Sample PA. Spatial characteristics of visual field progression determined by Monte Carlo simulation: diagnostic innovations in glaucoma study. Investig Ophthalmol Vis Sci. 2007;48:1642–1650. doi: 10.1167/iovs.06-0966. [DOI] [PubMed] [Google Scholar]
- Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2013a;92(5):421–425. doi: 10.1111/aos.12203. [DOI] [PubMed] [Google Scholar]
- Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. 2013b;156:724–730. doi: 10.1016/j.ajo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Piltz JR, Starita RJ. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1990;109:109–111. [PubMed] [Google Scholar]
- Pino C, Boutron I, Ravaud P. Outcomes in registered, ongoing randomized controlled trials of patient education. PloS one. 2012;7:e42934. doi: 10.1371/journal.pone.0042934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol. 2012;23:144–154. doi: 10.1097/ICU.0b013e32834ff490. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu PY, van Landingham SW, Massof RW, Chan ES, Ferrucci L, Friedman DS. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012;119:1352–1358. doi: 10.1016/j.ophtha.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HL, Kumar AU, Babu JG, Senthil S, Garudadri CS. Relationship between severity of visual field loss at presentation and rate of visual field progression in glaucoma. Ophthalmology. 2011;118:249–253. doi: 10.1016/j.ophtha.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Rein DB, Wittenborn JS, Lee PP, Wirth KE, Sorensen SW, Hoerger TJ, Saaddine JB. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116:823–832. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- Richman J, Lorenzana LL, Lankaranian D, Dugar J, Mayer J, Wizov SS, Spaeth GL. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010;128:1576–1582. doi: 10.1001/archophthalmol.2010.275. [DOI] [PubMed] [Google Scholar]
- Russell RA, Garway-Heath DF, Crabb DP. New insights into measurement variability in glaucomatous visual fields from computer modelling. PloS one. 2013;8:e83595. doi: 10.1371/journal.pone.0083595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Investig Ophthalmol Vis Sci. 2014;55:102–109. doi: 10.1167/iovs.13-13006. [DOI] [PubMed] [Google Scholar]
- Sawada H, Yoshino T, Fukuchi T, Abe H. Assessment of the vision-specific quality of life using clustered visual field in glaucoma patients. J glaucoma. 2014;23:81–87. doi: 10.1097/IJG.0b013e318265bbdc. [DOI] [PubMed] [Google Scholar]
- Skalicky S, Goldberg I. Depression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric depression scale-15, assessment of function related to vision, and the glaucoma quality of life-15. J glaucoma. 2008;17:546–551. doi: 10.1097/IJG.0b013e318163bdd1. [DOI] [PubMed] [Google Scholar]
- Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9. e2. doi: 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Investig Ophthalmol Vis Sci. 1996;37:1419–1428. [PubMed] [Google Scholar]
- Spry PG, Bates AB, Johnson CA, Chauhan BC. Simulation of longitudinal threshold visual field data. Investig Ophthalmol Vis Sci. 2000a;41:2192–2200. [PubMed] [Google Scholar]
- Spry PG, Henson DB, Sparrow JM, North RV. Quantitative comparison of static perimetric strategies in early glaucoma: test-retest variability. J glaucoma. 2000b;9:247–253. doi: 10.1097/00061198-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Spry PG, Johnson CA. Identification of progressive glaucomatous visual field loss. Surv Ophthalmol. 2002;47:158–173. doi: 10.1016/s0039-6257(01)00299-5. [DOI] [PubMed] [Google Scholar]
- Spry PG, Johnson CA, Bates AB, Turpin A, Chauhan BC. Spatial and temporal processing of threshold data for detection of progressive glaucomatous visual field loss. Arch Ophthalmol. 2002;120:173–180. doi: 10.1001/archopht.120.2.173. [DOI] [PubMed] [Google Scholar]
- Spry PG, Johnson CA, McKendrick AM, Turpin A. Variability components of standard automated perimetry and frequency-doubling technology perimetry. Investig Ophthalmol Vis Sci. 2001;42:1404–1410. [PubMed] [Google Scholar]
- Steinman SB, Steinman BA, Trick GL, Lehmkuhle S. A sensory explanation for visual attention deficits in the elderly. Optom Vis Sci off Publ Am Acad Optom. 1994;71:743–749. doi: 10.1097/00006324-199412000-00004. [DOI] [PubMed] [Google Scholar]
- Stewart WC, Stewart JA, Nasser QJ, Mychaskiw MA. Cost-effectiveness of treating ocular hypertension. Ophthalmology. 2008;115:94–98. doi: 10.1016/j.ophtha.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Strasburger H, Rentschler I, Juttner M. Peripheral vision and pattern recognition: a review. J Vis. 2011;11:13. doi: 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna AP, Budenz DL, Bandi J, Feuer WJ, Feldman RM, Herndon LW, Rhee DJ, Whiteside-de Vos J, Huang J, Anderson DR. Glaucoma progression analysis software compared with expert consensus opinion in the detection of visual field progression in glaucoma. Ophthalmology. 2012;119:468–473. doi: 10.1016/j.ophtha.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Quigley HA, Miller NR, Sommer A. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology. 1988;95:350–356. doi: 10.1016/s0161-6420(88)33177-5. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, Javitt J, Sommer A. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102–1110. doi: 10.1093/oxfordjournals.aje.a116013. [DOI] [PubMed] [Google Scholar]
- Traynis I, De Moraes CG, Raza AS, Liebmann JM, Ritch R, Hood DC. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol. 2014;132:291–297. doi: 10.1001/jamaophthalmol.2013.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Landingham SW, Hochberg C, Massof RW, Chan E, Friedman DS, Ramulu PY. Driving patterns in older adults with glaucoma. BMC Ophthalmol. 2013;13:4. doi: 10.1186/1471-2415-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Steinmann WC, Scott IU. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology. 1992;99:215–221. doi: 10.1016/s0161-6420(92)31990-6. [DOI] [PubMed] [Google Scholar]
- Viswanathan AC, Fitzke FW, Hitchings RA. Early detection of visual field progression in glaucoma: a comparison of PROGRESSOR and STATPAC 2. Br J Ophthalmol. 1997a;81:1037–1042. doi: 10.1136/bjo.81.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan AC, Hitchings RA, Fitzke FW. How often do patients need visual field tests? Graefe’s archiv Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und Exp Ophthalmol. 1997b;235:563–568. doi: 10.1007/BF00947085. [DOI] [PubMed] [Google Scholar]
- Wall M, Kutzko KE, Chauhan BC. Variability in patients with glaucomatous visual field damage is reduced using size V stimuli. Investig Ophthalmol Vis Sci. 1997;38:426–435. [PubMed] [Google Scholar]
- Wall M, Woodward KR, Doyle CK, Artes PH. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Investig Ophthalmol Vis Sci. 2009;50:974–979. doi: 10.1167/iovs.08-1789. [DOI] [PubMed] [Google Scholar]
- Wall M, Woodward KR, Doyle CK, Zamba G. The effective dynamic ranges of standard automated perimetry sizes III and V and motion and matrix perimetry. Arch Ophthalmol. 2010;128:570–576. doi: 10.1001/archophthalmol.2010.71. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Friedman DS, Fechtner RD, Cioffi GA, Coleman AL, Girkin CA, Liebmann JM, Singh K, Wilson MR, Wilson R, Kannel WB. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–467. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Kaufman PL. The glaucoma research community and FDA look to the future: a report from the NEI/FDA CDER Glaucoma clinical trial design and endpoints symposium. Investig Ophthalmol Vis Sci. 2009;50:1497–1505. doi: 10.1167/iovs.08-2843. [DOI] [PubMed] [Google Scholar]
- Wesselink C, Stoutenbeek R, Jansonius NM. Incorporating life expectancy in glaucoma care. Eye (Lond) 2011;25:1575–1580. doi: 10.1038/eye.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Study protocol for the world health organization project to develop a quality of life assessment instrument (WHOQOL) Qual Life Res. 1993;2:153–159. [PubMed] [Google Scholar]
- Wild JM, Cubbidge RP, Pacey IE, Robinson R. Statistical aspects of the normal visual field in short-wavelength automated perimetry. Investig Ophthalmol Vis Sci. 1998;39:54–63. [PubMed] [Google Scholar]
- Wild JM, Hutchings N, Hussey MK, Flanagan JG, Trope GE. Pointwise univariate linear regression of perimetric sensitivity against follow-up time in glaucoma. Ophthalmology. 1997;104:808–815. doi: 10.1016/s0161-6420(97)30229-2. [DOI] [PubMed] [Google Scholar]
- Wild JM, Pacey IE, O’Neill EC, Cunliffe IA. The SITA perimetric threshold algorithms in glaucoma. Investig Ophthalmol Vis Sci. 1999;40:1998–2009. [PubMed] [Google Scholar]
- Wilson MR, Kosoko O, Cowan CL, Jr, Sample PA, Johnson CA, Haynatzki G, Enger C, Crandall D. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies Am J Ophthalmol. 2002;134:399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- Wolfs RC, Borger PH, Ramrattan RS, Klaver CC, Hulsman CA, Hofman A, Vingerling JR, Hitchings RA, de Jong PT. Changing views on open-angle glaucoma: definitions and prevalences–The Rotterdam Study. Investig Ophthalmol Vis Sci. 2000;41:3309–3321. [PubMed] [Google Scholar]
- Wu DC, Schwartz B, Nagin P. Trend analyses of automated visual fields. In: Greve EL, Heijl A, editors. Seventh International Visual Field Symposium; Amsterdam. September 1986; Netherlands: Springer; 1987. pp. 175–189. [Google Scholar]
- Zhu H, Russell RA, Saunders LJ, Ceccon S, Garway-Heath DF, Crabb DP. Detecting changes in retinal function: analysis with non-stationary Weibull error regression and spatial enhancement (ANSWERS) PloS one. 2014;9:e85654. doi: 10.1371/journal.pone.0085654. [DOI] [PMC free article] [PubMed] [Google Scholar]