Abstract
This study was undertaken to scrutinize efficacy of salicylic acid (SA) and/or sodium nitroprusside [SNP, source of nitric oxide (NO)] to mitigate injury symptoms of saline stress in Pisum sativum L. Exposure to sodium chloride (NaCl) was found to be injurious to germinating P. sativum L. (var. Shubhra IM-9101) and a direct correlation between severity of toxicity and NaCl-concentrations could be discernible. Both SA and NO serves as signal molecules in plant stress responses, and play crucial roles in key regulatory pathways of growth, development and metabolism. The limiting effects of salinity on radicle length and biomass accumulation were considerably released by SA and/or SNP and among which their combined application was found to be the most promising. Supplemented SA and/or SNP, particularly their cocktail, resulted in a substantial decline in reactive oxygen species accumulation, which later caused reduced accumulations of malondialdehyde, 4-hydroxy-2-nonenal and protein carbonyl, in NaCl subjected germinating P. sativum L. seeds. SA and/or SNP had significant inducing effects on activities of superoxide dismutase, catalase, guaiacol peroxidase and ascorbate peroxidase. Additionally, exogenous SA and/or SNP led to the higher proline, sugar and glycinebetaine contents, than that of the control. On the basis of accumulated results, it could be concluded that the cocktail of SA and SNP may be efficiently used to overcome the adverse signatures of salinity stress.
Keywords: Nitric oxide, Oxidative stress, Pisum sativum L., Reactive oxygen species, Salicylic acid, Salinity
Introduction
In the natural environment, plants often experience abiotic stresses like salinity, drought, high or low temperatures, flood, etc., causing serious threats to crop productivity (Keshavkant et al. 2012). Among these, salinity is one of the most brutal environmental constrains limiting productivity of crops and is caused by inappropriate existence of salt in both soil and irrigation water (Agami 2013). Total area of salt affected soils, which includes both saline and sodic soils, was estimated to be 831 million ha and is approximately 6% of world’s total land area. In addition, around 1532 million ha of land was reported to be affected by secondary salinity (FAO 2005). Such unfavourable soil of low fertility is generally unsuitable for crop production, culminating unacceptable yield reduction (Khan et al. 2014). Because of the increased need of food production and day-by-day rise in salt dominated soil area throughout the world, researches on plant responses to salinity have rapidly expanded recently.
Majority of crop plants are sensitive to salt even below 30 mM (Keshavkant et al. 2012). Salinity inhibits growth and development in variety of plants in two distinct phases. Initially (Phase 1), growth phenomenon is affected adversely because of osmotic stress imposed cellular responses, and in the subsequent phase (Phase 2) growth is significantly reduced due to cytotoxic effects of accumulated salt. At high levels of salinity, the prominent cause of crop sensitivity may possibly be due to the ionic cytotoxicity via substitution of K+ with Na+ and non-covalent interaction of both Na+ and Cl− with cellular amino acids (Agami 2013). The metabolic inequities due to osmotic stress, ionic toxicity and nutritional deficiency, may lead to overproduction of reactive oxygen species (ROS), thus results oxidative stress in cells/organs (Khan et al. 2014). Various forms of ROS were confirmed to be generated under abiotic stress conditions including salinity, which incorporates superoxide anion (O2˙−), hydroxyl radical and a non-radical molecule hydrogen peroxide (H2O2) (Chandrakar et al. 2016a). These potentially toxic molecules can firmly be shown to provoke peroxidation of membrane lipid moieties thereby releasing cytotoxic by-products like malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE), oxidation of both nucleic acids and proteins, hence, resulting in DNA fragmentation and protein carbonyl (PCO) formation, inhibition in the activities of key enzymes, escalation of cellular apoptotic pathway and ultimately death of the cells/tissues. The end or intermediate products of both lipid–protein oxidation reactions, in particular, MDA, 4-HNE and PCO are severely harmful and genotoxic in nature, therefore, if accumulated in their excess inside the cell, in any circumstances, impose degenerative changes and death even (Parkhey et al. 2012, 2014a).
As ROS generation under unfavourable conditions is a spontaneous and unavoidable cellular process, therefore, to look after these cytotoxic molecules plants have evolved detoxification mechanisms that include both enzymic and non-enzymic systems (Chandra et al. 2015). Enzymes like superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POX) and ascorbate peroxidase (APX) constitute the enzymic components of the detoxification system while, the non-enzymic part includes ascorbic acid, glutathione, α-tocopherol, sugar, carotenoids, etc. (Yadu et al. 2016). The very first step taken towards removal/detoxification of cellular ROS is by SOD. It is one of the metalloenzymes that leads the conversion of two O2˙− into H2O2 and O2 (Chandra and Keshavkant 2016). This initial step of ROS detoxification is later followed by a second step that involves the restriction of H2O2 accumulation by involving members of ascorbate–glutathione cycle, namely, CAT, POX and APX that degrades it into water and oxygen (Sarkar et al. 2013). In addition to antioxidants, certain ion transporters and osmolytes are also employed to resist against sodium chloride (NaCl)-induced stress (Khan et al. 2014). The roles of non-enzymic antioxidants and osmolytes like carotenoids, α-tocopherol, proline and glycinebetaine against NaCl-stress is evident from a number of observations (Maimaiti et al. 2014; Naliwajski and Sklodowska 2014). The above compounds are chiefly osmoprotectants and can protect macromolecules and cellular membranes during abiotic stresses (Chandrakar et al. 2016a). Their exogenous application and/or accumulation in enough quantities have been amply reported to reduce the osmotic potential of cell and thereby restore the potential gradient between the inside and outside of the cell/plant (Khan et al. 2014). The coordinated operations of above discussed defence mechanisms are able to maintain a suitable level of ROS that are essentially required for cell signalling processes (Agami 2013). The degree of resistance of cells/plants against saline environment is often related to both quantitative and qualitative changes in above antioxidants. In line, a number of salt-resistant engineered plants over-expressing antioxidant components have already been reported (Campo et al. 2013).
In the recent past, a number of strategies have been floated by distinguished researchers to overcome the degree of cellular injury exerted by salt and also to improve salinity tolerance in plants (Khan et al. 2014). In regard, treatments of some of the compounds viz; signalling molecules, plant hormones, osmoprotectants, antioxidants, polyamines and trace elements have popularly been shown to confer salt stress tolerance to plants (Hayat et al. 2012; Agami 2013). Among those, the salicylic acid (SA) and nitric oxide (NO) are recognized to improve salt stress tolerance and regulate plant growth and productivity efficiently (Kazemi et al. 2010; Hayat et al. 2012; Pirasteh-Anosheh et al. 2014).
Like few other growth regulators, SA, a phenolic compound, plays prominent and diversified functions in plant protection under NaCl-stress (Hayat et al. 2012). Available literature testifies that exogenous application of SA ameliorates the injurious effects of salt by modulating the activities of important antioxidants (Ismail 2013). It was shown to reduce the extent of lipid peroxidation reaction and associated membrane perturbation, and also to improve rate of seed germination and ion uptake/transport (Hayat et al. 2012; Pirasteh-Anosheh et al. 2014). Exogenously applied SA was shown to minimize the Na+, Cl− and ROS contents, which were increased during salinity stress (Hayat et al. 2012). In addition, foliar application of SA alleviated the damaging effects of various abiotic stresses, which were chiefly ascribed due to the enhanced synthesis or accumulation of free proline and cellular proteins (Pirasteh-Anosheh et al. 2014; Chandrakar et al. 2016b).
Nitric oxide, an important signalling molecule, plays crucial roles in a variety of plant responses like germination, growth, iron absorption and adaptive mechanisms against environmental stresses (Lamattina et al. 2003; Kazemi et al. 2010). It has already been established that exogenous addition of one of the NO donor sodium nitroprusside (SNP) reduces adverse effects of salt exposure (Lamattina et al. 2003). NO has also been shown to play a role in cyto-protection by reducing both level and toxicity of free radicals (Guo et al. 2009). Being a signalling molecule, it is involved in two respiratory electron transport pathways of mitochondria and regulates ROS synthesis via enhancing antioxidants in abiotically stressed plants. Exogenous application of NO conferred increased salt resistance in Cucumis sativus L. by increasing the activity of SOD and minimizing ROS (Shi et al. 2007).
The aim of the present study was to investigate the mechanism(s) involved in SA and/or SNP mediated salt stress resistance in Pisum sativum L. Therefore, to elucidate the roles of SA and SNP in the mitigation of salinity induced oxidative damage, we have evaluated (1) the deleterious effects of salinity in the growth of P. sativum L., (2) cell death, (3) levels of oxidative stress markers like O2˙−, H2O2, MDA, 4-HNE and PCO, (4) anatomy and histochemical localization of both O2˙− and H2O2, (5) quantitative changes in protein, sugar, proline and glycinebetaine, (6) assay and gel electrophoresis of antioxidant enzymes (SOD, CAT, POX and APX) in order to explore the extent of damage caused by NaCl-stress, and (7) amelioration of NaCl-induced injury symptoms by exogenous application of SA and/or SNP (source of NO).
Materials and methods
Seed germination, salinity treatment and growth analysis
The P. sativum L. (var. Shubhra IM-9101) seeds were procured from the local market. Healthy and uninfected seeds were sorted out and washed initially with 0.1% (v/v) sodium hypochlorite solution for 2–5 min following rigorous washing with MilliQ water (MW) (Millipore, Gradient A-10, USA). Healthy seeds were then placed for germination over two layers of wet filter paper towels, pre-soaked with MW, in germination boxes of 26 × 16 × 3 cm size (Chandrakar et al. 2016a). These boxes were kept in darkness at room temperature (26–28 °C) until the emergence of radicles (1 mm long).
Seeds bearing 1 mm long radicle were now subjected to series of NaCl solutions viz. 0 (control), 100 and 400 mM, either with or without 0.15 mM SA, 0.1 mM SNP and combination of SA (0.15 mM) + SNP (0.1 mM). Concentration of both SA and SNP used for treatments were selected on the basis of screening experiments. In a preliminary investigation, various concentrations of SA (0.125, 0.15 and 0.175 mM) and SNP (0.075, 0.1 and 0.15 mM) were used to rescue the detrimental effects of salt in P. sativum L. Looking to the growth of radicles, 0.15 mM SA and 0.1 mM SNP were finalized for current study, where germinating seeds revealed increments in the radicle length by 33 and 54% respectively, under 400 mM NaCl, on 7th day of investigation. While, two other concentrations of both SA (0.125 and 0.175 mM) and SNP (0.075 and 0.15 mM) revealed comparatively less (18–25%) increments in radicle length, under similar set of experimental conditions. Experimental boxes were then incubated in darkness at room temperature so as to allow the seeds/radicles to grow further. On 7th day of sample incubation in each of the pre-defined solutions, the radicles were removed carefully. A part of the excised radicles were utilized for biomass determination and remains were stored in plastic vials after grinding with liquid nitrogen (LN2). All the evaluations were done in five replicates and repeated twice.
Change in radicle length was assessed using a meter scale and to monitor dry weight (DW), five sets of ten radicles each were oven dried at 103 °C for 17 h and weighed electronically (Parkhey et al. 2014b).
Monitoring of cell death using Evans blue staining
Cell death was evaluated by Evans blue staining, following the method of Suzuki et al. (1999). Evans blue is a non-permeating exclusion dye which leaks through ruptured membranes and stains the content of dead cells. Ten radicles were incubated with 0.25% (w/v) aqueous Evans blue solution, for 30 min. After this, radicles were drained and rinsed thoroughly with MW. Then, cross sections of root tips were done using a sharp razor and mounted over a clean glass slide, and was observed under Binocular Digital CCD Compound Light Microscope (Labomed, India) carefully.
Extraction of Evans blue dye was performed by homogenizing 10 mm long tips of ten stained (Evans blue) radicles with 1 ml of 1% (w/v) sodium dodecyl sulfate to release the trapped Evans blue from the cells. The homogenates were centrifuged at 14,000 rpm for 10 min and then absorbance of the supernatants were measured at 600 nm using an UV–Vis spectrophotometer (Lambda 25, Perkin Elmer, USA).
Measurement and localization of reactive oxygen species
Superoxide anion content was measured following Schopfer et al. (2001). Both control and treated radicles were imbibed in 20 mM potassium phosphate buffer of pH 6, consisting 2,3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (500 µM, sodium salt) in the dark at 26 °C on a shaker. Absorbance of the soaking media was read at 470 nm. Content of O2˙− was calculated using an extinction coefficient of 2.16 × 104 M−1 cm−1 and expressed as µmol min−1 g−1 fresh weight (FW).
To estimate H2O2 level, weighed (0.2 g) quantities of LN2 crushed tissue powder were extracted with 2 ml of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 12,000 rpm for 15 min, at room temperature (Velikova et al. 2000). An aliquot (0.75 ml) of supernatant was added to equal volumes of 10 mM potassium phosphate buffer (pH 7) and potassium iodide (1 M). The absorbance of the reaction mixture was read at 390 nm and content of H2O2 was calculated using an extinction coefficient of 0.28 µM−1 cm−1. Amount of H2O2 was expressed as µmol g−1 FW.
Sites of O2˙− and H2O2 production in P. sativum L. radicles were localized by nitroblue tetrazolium (NBT) and 3,3-diaminobenzidine (DAB) staining respectively (Roach et al. 2010; Chaudhuri et al. 2013). Cross sections of root tips were done using a sharp razor and mounted over a clean glass slide, and was observed under Binocular Digital CCD Compound Light Microscope (Labomed, India) carefully.
Assessment of malondialdehyde
An end product of lipid peroxidation reaction MDA was measured in terms of thiobarbituric acid (TBA) reactive metabolites, following Velikova et al. (2000). The 0.2 g LN2 grinded tissue powder was mixed rigorously with 3 ml of 20% (w/v) TCA comprising 0.5% (w/v) TBA. The mixture was heated (95 °C) in a water bath for 30 min, chilled in a freezer (15 min) and then centrifuged (10,000 rpm for 15 min). Absorbance of the complex was measured at 540 nm subtracting the non-specific absorbance recorded at 600 nm. Content of MDA was calculated using extinction coefficient of 0.155 M−1 cm−1 and denoted as nmol g−1 FW.
Determination of 4-hydroxy-2-nonenal
The 4-HNE, an important biomarker of lipid oxidation, was determined by the method of Ray et al. (2007). Weighed (0.2 g) amount of LN2 crushed radicle was homogenized with 2 ml of cold 0.2 M borate buffer (pH 7.4) and then 1.5 ml 10% (w/v) TCA was added to it. The homogenate was rolled at 12,000 rpm for 15 min. The 1 ml of (1%, w/v) 2, 4-dinitrophenylhydrazine (DNPH) was added into supernatant (1 ml) and was allowed to stand at room temperature for 2 h. Now, the mixture was extracted with 0.5 ml hexane and then evaporated under LN2. After cooling at room temperature, 2 ml methanol was added to it and absorbance was read at 350 nm by taking methanol as a blank. Content of 4-HNE was calculated following extinction coefficient 13,750 M−1 cm−1 and was expressed as mmol g−1 FW.
Extraction and estimation of protein and enzymes
To extract protein and enzymes, the 0.5 g LN2 crushed fine tissue powder was homogenized with 10 ml of cold potassium phosphate buffer (10 mM, pH 7.2) containing 1 mM ethylenediaminetetracetic acid, 2 mM dithiothreitol and 0.2% (v/v) Triton X-100 in a pre-chilled pestle and mortar. The homogenates were then centrifuged at 14,000 rpm for 20 min at 4 °C. The supernatant thus obtained was used as source of both protein and enzymes.
Protein was assayed following Bradford (1976). Bovine serum albumin was used to prepare standard curve and protein content was expressed as μg g−1 FW.
Determination of protein carbonyls
Contents of PCO were determined by their reaction with DNPH (Levine et al. 1994). To 500 µl of isolated protein, 200 µl each of Triton X-100 (0.03%, v/v) and streptomycin sulphate (1%, w/v) were added and then incubated for 20 min at room temperature. After centrifugation (11,000 rpm, 15 min, 4 °C), 200 µl supernatant was taken out and to which 300 µl DNPH (10 mM, prepared in 2 M HCl) was added and allowed to stand for 1 h at room temperature. In the control tube, only 2 M HCl was added. Afterwards, proteins available in the mixture were precipitated with 10% (w/v) TCA and were washed with ethanol:ethyl acetate (1:1, v:v) cocktail. Washed pellets were then dissolved in 1 ml of 6 M guanidine hydrochloride (prepared in 20 mM phosphate buffer of pH 2.3) and its absorbance was measured at 370 nm. Amount of PCO was derived following extinction coefficient of 22,000 M−1 cm−1 and denoted as mmol g−1 FW.
Assay of antioxidant enzymes
Following Marklund and Marklund (1974), the activity of SOD (EC 1.15.1.1) was determined by estimating the percent inhibition of pyrogallol auto-oxidation by the enzyme at 420 nm. Enzyme activity was expressed as Units of SOD min−1 g−1 FW.
CAT (EC 1.11.1.6) was assayed spectrophotometrically by the protocol of Chance and Maehly (1955) and its activity was calculated using an extinction coefficient 39.4 mM−1 cm−1 and expressed as nmol min−1 g−1 FW.
Activity of POX (EC 1.11.1.7) was measured after Chance and Maehly (1955) by estimating oxidation of guaiacol in presence of H2O2. POX activity was expressed as µmol min−1 g−1 FW.
APX (EC 1.11.1.11) was assayed according to Nakano and Asada (1981) by monitoring the rate of ascorbate oxidation at 290 nm. Enzyme activity was calculated following extinction coefficient of 2.8 mM−1 cm−1 and expressed as µmol min−1 g−1 FW.
Gel electrophoresis
The isoenzymes of SOD, CAT, POX and APX were resolved on polyacrylamide gels under non-denaturing conditions. The stacking and separating gels contained 4.5 and 10% (w/v) polyacrylamide, respectively. Proteins were electrophoretically separated at 4 °C applying 20 mA current for 2 h.
For the detection of SOD isoenzymes, the gels were incubated in the dark for 20 min in the 2.45 mM NBT solution, and were then immersed in 36 mM dipotassium hydrogen phosphate (pH 7.8) containing 28 µM riboflavin and 28 mM N,N,N,N-tetramethylethylenediamine (TEMED), until the gel turns blue except the region showing SOD activity (Beauchamp and Fridovich 1971).
Isozymes of CAT were stained following the method of Woodbury et al. (1971). The gels were incubated in 0.03% (v/v) H2O2 solution for 10 min. The gels were rinsed quickly in MW and stained in a solution containing 1% (w/v) each of potassium ferricyanide and ferric chloride. As soon as a green colour began to appear, gels were washed with MW.
Isozymes of POX were revealed following Srivastava and Huystee (1977). The gels were equilibrated with 100 mM potassium phosphate buffer (pH 6.5) for 15 min and then in 12.5 mM guaiacol solution containing benzidine (1.7 mM) and H2O2 (12 mM). After gentle shaking, brown coloured bands appeared against a clear background.
Detection of APX isoenzyme was based on the method of Mittler and Zilinskas (1993). The gels were pre-run for 30 min to allow the ascorbate (present in the carrier buffer) to enter the gel prior to the application of the samples. Following electrophoretic separation, the gels were equilibrated in the 50 mM sodium phosphate buffer (pH 7.0) with 2 mM ascorbate for 30 min. Equilibration buffer was changed in every 10 min. Afterwards, the gels were incubated with 50 mM sodium phosphate buffer (pH 7) containing 4 mM ascorbate and 4 mM H2O2 for 20 min. Finally, gels were washed twice with sodium phosphate buffer (50 mM, pH 7) and stained in 50 mM sodium phosphate buffer (pH 7.8) containing 28 mM TEMED and 2.45 mM NBT. The reaction was continued for 10–15 min and stopped by a brief wash in MW. The APX activity was observed as an achromatic band on a purple-blue background (usually within 3–5 min).
Determination of osmolytes
Content of sugar was determined following Spiro (1966). Standard curve was prepared using d-glucose and amount was expressed as mg g−1 FW.
In the radicle tissues, free proline content was determined applying the protocol of Bates et al. (1973) and expressed as mg g−1 FW. The 0.2 g LN2 grinded tissue was extracted with 3% (v/v) sulfo-salicylic acid (10 ml) and centrifuged at 10,000 rpm for 15 min at room temperature. The supernatant (2 ml) was mixed thoroughly with 2 ml of freshly prepared acid ninhydrin reagent and 2 ml of glacial acetic acid. The mixture was now incubated at 100 °C for 60 min, cooled in an ice bath and then 4 ml toluene was added to it and shaken rigorously. The chromophore containing toluene was aspirated out carefully and its absorbance was read at 520 nm against toluene. A standard curve of proline was prepared.
Content of glycinebetaine was measured in terms of betaine–peridotite complex following the method of Grieve and Grattan (1983). Weighed (0.5 g) amount of LN2 extracted tissue powder was homogenized with 10 ml MW and was incubated for 24 h at 25 °C. The homogenate was filtered and the filtrate was mixed with 2 N sulphuric acid in the ratio of 1:1 (v:v). This mixture (0.5 ml) was taken in a vial and kept in an ice tub (1 h). To this, 0.2 ml cold potassium tri-iodide reagent was added and kept at 4 °C for 16 h, centrifuged at 14,000 rpm for 15 min at 0 °C. Absorbance of the supernatant was read at 365 nm. A standard curve of glycinebetaine was prepared and its content was expressed as µmol g−1 FW.
Statistical analysis
Collected data were analysed statistically by analysis of variance using SPSS (Ver 16.0) for windows. Relationships between tested parameters were examined using Pearson’s correlation coefficient test. Values presented are mean ± SE of five replicates. Significance difference was tested at P < 0.05.
Results
Growth traits
Growth indices of P. sativum L. radicles were negatively influenced by NaCl treatment. The 100 mM of NaCl slightly reduced the length (16%) and DW (9%) of the growing radicles, as compared to MW grown controls (Table 1). The growth reduction was substantially high (length: 87%, DW: 35%) at 400 mM salt supply (Table 1). On the other hand, addition of SA, SNP or SA + SNP, as seed soaking in absence of salt stimulated the growth traits that were remarkably (P < 0.05) higher than the controls. Moreover, SA, SNP or SA + SNP also uplifted the growth of the P. sativum L. grown under NaCl and the data obtained were statistically significant (P < 0.05) than those of grown under salt alone (Table 1). However, the response expressed by combination of SA + SNP was most effective than that generated by SNP or SA alone.
Table 1.
Comparison of recovery abilities of salicylic acid and/or sodium nitroprusside in terms of growth and reactive oxygen species in P. sativum L. under different salinity regimes
| Treatments | Radicle length (mm) | Dry weight (g) | Superoxide radical (µmol min−1 g−1 FW) | Hydrogen peroxide (µmol g−1 FW) |
|---|---|---|---|---|
| C | 116.66c ± 1.25 | 2.62c ± 0.03 | 11.66ef ± 1.44 | 0.57g ± 0.01 |
| 100 mM NaCl | 97.9e ± 2.50 | 2.39e ± 0.01 | 20d ± 2.5 | 0.68e ± 0.01 |
| 400 mM NaCl | 14.33i ± 1.38 | 1.69i ± 0.01 | 47.5a ± 2.5 | 1.42a ± 0.02 |
| SA | 117.66c ± 0.76 | 2.66c ± 0.01 | 9.16fgh ± 2.88 | 0.53gh ± 0.02 |
| SA + 100 mM NaCl | 111.7d ± 0.81 | 2.42e ± 0.02 | 14.16e ± 1.44 | 0.64ef ± 0.07 |
| SA + 400 mM NaCl | 19.16h ± 0.80 | 1.76h ± 0.02 | 40b ± 2.5 | 1.15b ± 0.009 |
| SNP | 119.9b ± 0.36 | 2.75b ± 0.03 | 6.25hi ± 1.25 | 0.51h ± 0.01 |
| SNP + 100 mM NaCl | 116.56c ± 1.60 | 2.52d ± 0.02 | 10.25fg ± 0.90 | 0.63f ± 0.01 |
| SNP + 400 mM NaCl | 22.1g ± 0.36 | 1.84g ± 0.02 | 25.83c ± 3.81 | 1.05c ± 0.007 |
| SA + SNP | 125.83a ± 1.04 | 2.92a ± 0.03 | 4.41i ± 0.62 | 0.46i ± 0.01 |
| SA + SNP + 100 mM NaCl | 119.9b ± 0.36 | 2.71b ± 0.03 | 7.66ghi ± 1.01 | 0.56g ± 0.008 |
| SA + SNP + 400 mM NaCl | 25.73f ± 1.10 | 1.97f ± 0.02 | 19.16d ± 1.44 | 0.97d ± 0.01 |
Data are means of five replicates ± SE. Different letters indicate significant difference between treatments at P < 0.05
Reactive oxygen species
In view to unravel the depth of salt-induced damage, levels of both O2˙− and H2O2 were measured in NaCl subjected radicles of P. sativum L. Increases in NaCl concentration from 100 to 400 mM significantly (P < 0.05) increased ROS levels. The percentages of increase were 171–407% (O2˙−) and 191–249% (H2O2), compared to MW subjected controls (Table 1). Accumulated data revealed that exposure of P. sativum L. radicle to SA, SNP or SA + SNP reduced the accumulation of ROS as compared with the corresponding salt level. Least level of ROS was recorded by SA + SNP treatment under stress-free environment as compared to the other treatments (Table 1). However, SA + SNP-imposed a more promising impact than the SNP or SA alone.
To confirm the regulatory role of SA or SNP against salt stress, histochemical staining of O2˙− and H2O2 with NBT and DAB respectively, were also performed. Radicles of P. sativum L. treated with NaCl alone were extensively stained by NBT and DAB both, while those treated with SA + SNP + NaCl were slightly stained (Figs. 1, 2). Moreover, in this analysis exogenous addition of SA revealed comparatively less staining in salt treated P. sativum L. radicles than those treated with SNP, suggesting SA to be more potent towards salt stress mitigation.
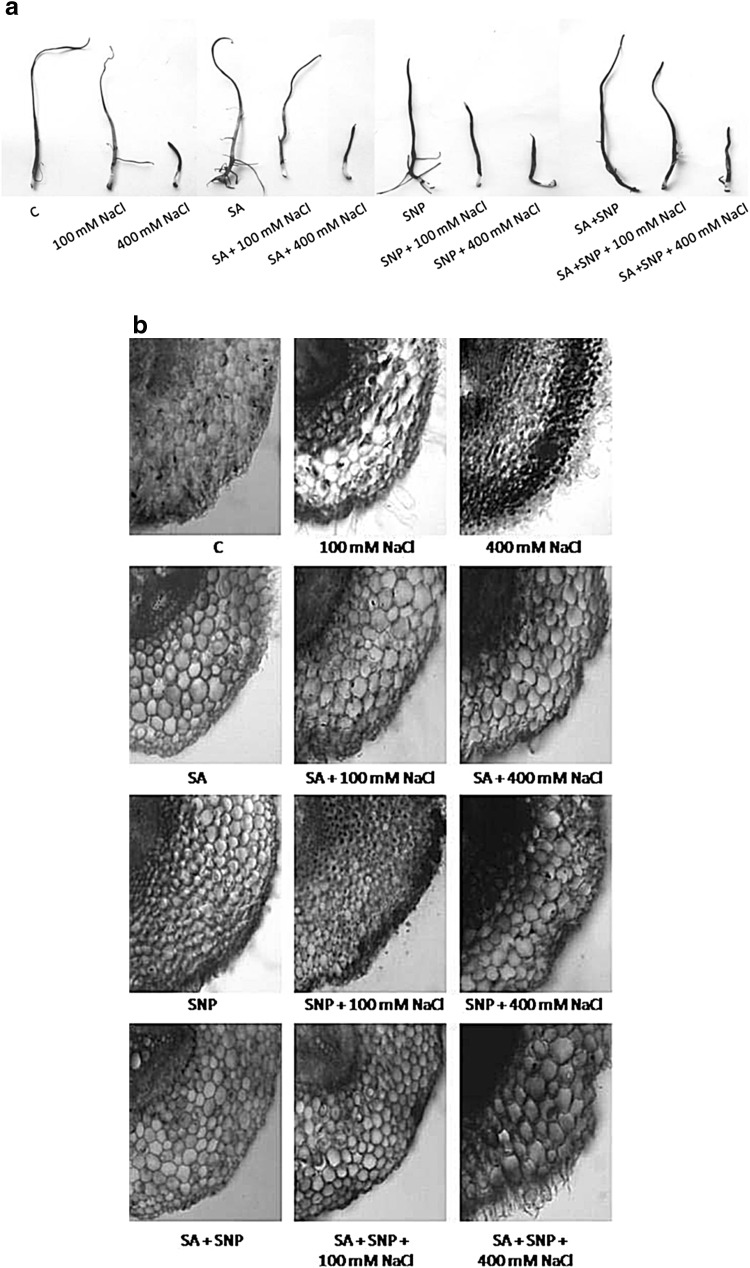
Fig. 1.
Localization (a), anatomical study (b) of superoxide radical by nitroblue tetrazolium in P. sativum L. radicles exposed to various treatments for 7 days
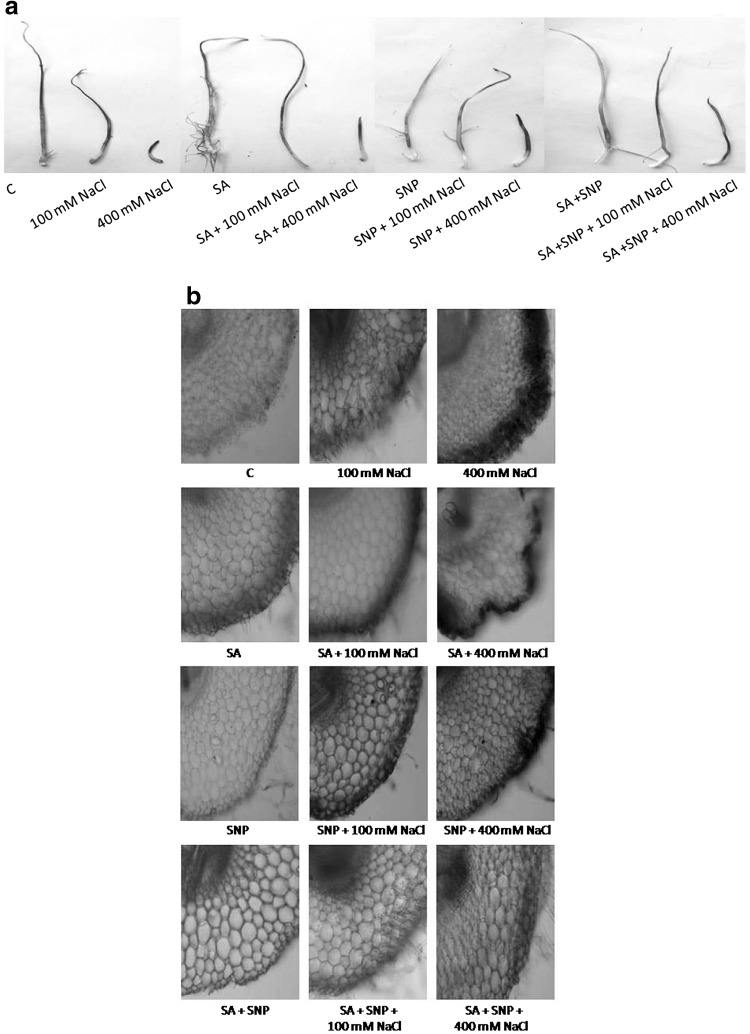
Fig. 2.
Localization (a), anatomy (b) of hydrogen peroxide synthesis sites using 3,3-diaminobenzidine in P. sativum L. radicles exposed to various treatments for 7 days
Markers of oxidative injury
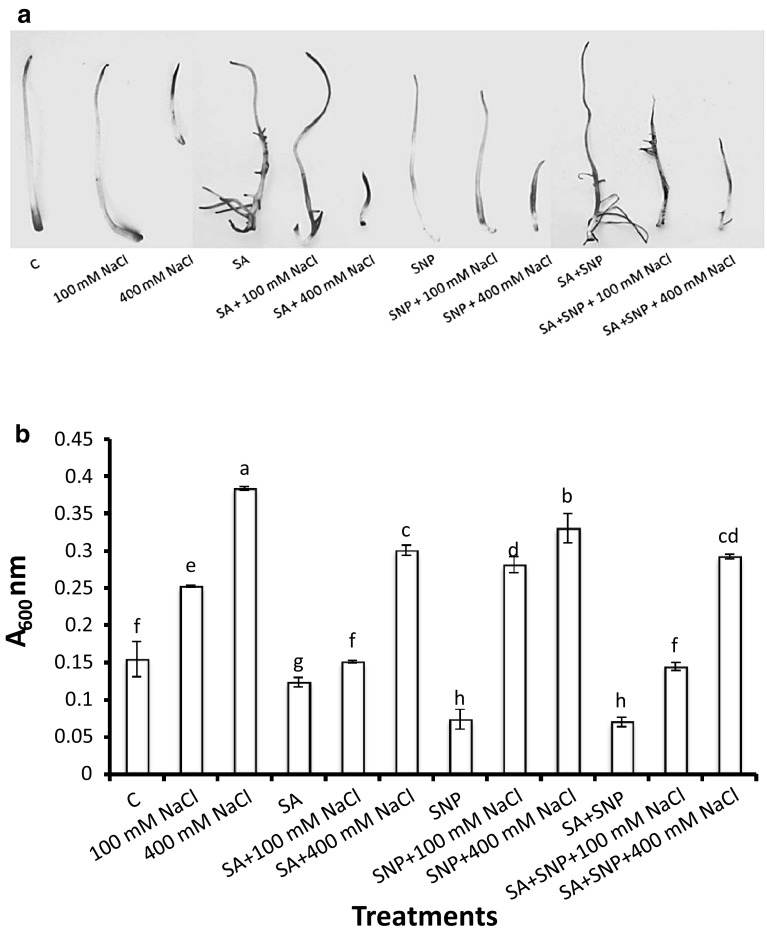
Evans blue staining was performed to assess cell death in the salt-treated and non-treated (control) radicles of P. sativum L. The salt-treated radicles had more intense blue staining than the control radicles, indicating loss of membrane integrity (Fig. 3a). Uptake of Evans blue increases by 153%, as compared to control, when radicles were exposed to 400 mM NaCl for 7 days (Fig. 3b). The radicles subjected to SA and/or SNP along with NaCl had faintly stained radicles thereby approving the protective effects of both the potential compounds (Fig. 3). Additionally, Evans blue staining of radicles showed that exogenous addition of SA revealed comparatively less staining in salt treated P. sativum L. than those treated with SNP, suggesting SA to be more efficient in the amelioration of salt-induced injury symptoms (Fig. 3).
Fig. 3.
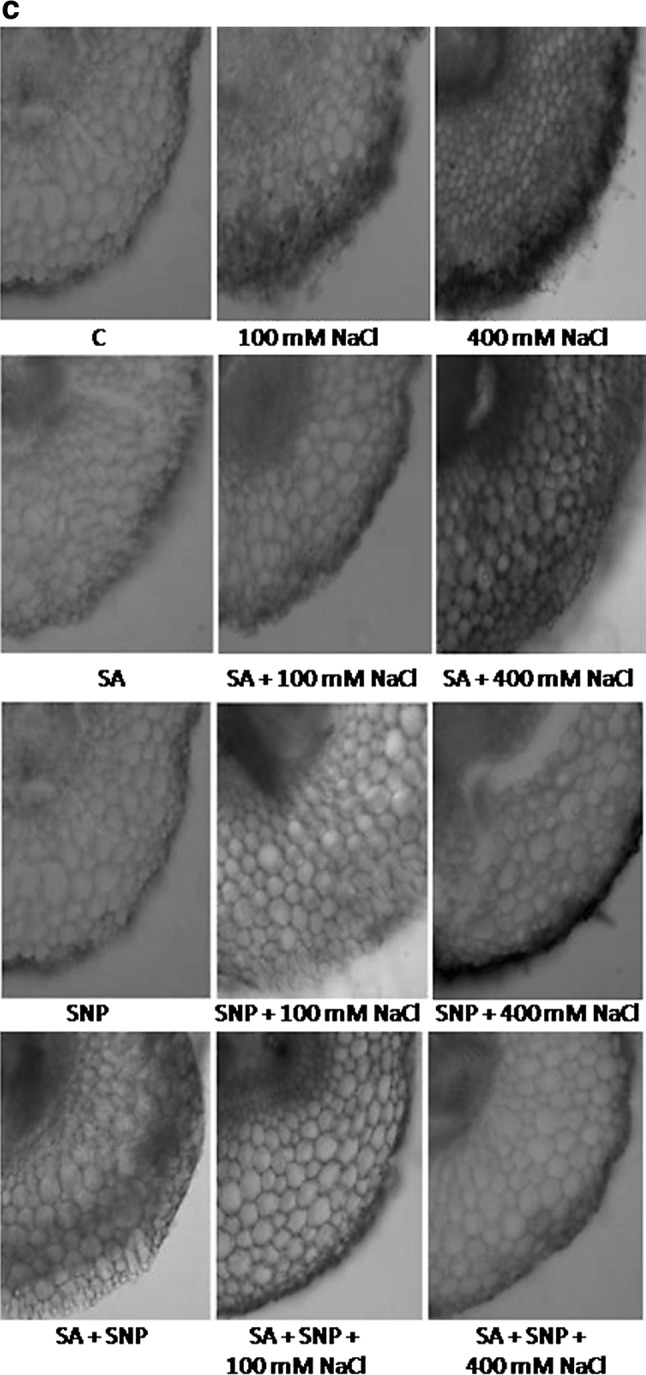
Cell death assessment in P. sativum L. radicles treated with different concentrations of salt, salicylic acid and/or sodium nitroprusside, by Evans blue staining (a), uptake (b), anatomical observations (c). Data presented are mean ± SE of five separate observations. Different letters indicate significant difference between treatments at P < 0.05
Changes in the contents of MDA, 4-HNE and PCO in respect to NaCl treatment, either alone or in combination with each of SA, SNP or SA + SNP, were monitored (Table 2). Documented results indicated that MDA, 4-HNE and PCO were significantly (P < 0.05) increased in salt subjected P. sativum L. radicles as compared to control. Soaking of the radicles in SA, SNP or SA + SNP, suppressed stress responses thereby lesser accumulations of MDA, 4-HNE and PCO as compared to the corresponding salinity level. Their least levels were recorded in SA + SNP exposed radicles. At the moment, SA + SNP have more pronounced control than SNP or SA on MDA, 4-HNE and PCO accumulations (Table 2).
Table 2.
Levels of malondialdehyde, 4-hydroxy-2-nonenal and protein carbonyls in P. sativum L. subjected to salicylic acid and/or sodium nitroprusside coupled salt-solutions
| Treatments | Malondialdehyde (nmol g−1 FW) | 4-Hydroxy-2-nonenal (mmol g−1 FW) | Protein carbonylation (mmol g−1 FW) |
|---|---|---|---|
| C | 25.98f ± 0.06 | 2.03g ± 0.04 | 12.56i ± 0.54 |
| 100 mM NaCl | 32.58d ± 0.02 | 3.65c ± 0.09 | 31.14b ± 0.50 |
| 400 mM NaCl | 44.16a ± 0.35 | 5.18a ± 0.28 | 36.27a ± 0.21 |
| SA | 24.01gh ± 1.97 | 1.93g ± 0.02 | 11.25j ± 0.18 |
| SA + 100 mM NaCl | 30.40e ± 1.45 | 2.86e ± 0.06 | 22.42f ± 0.74 |
| SA + 400 mM NaCl | 38.58b ± 0.19 | 3.85b ± 0.11 | 27.79c ± 0.28 |
| SNP | 19.81i ± 0.87 | 1.65h ± 0.10 | 10.76jk ± 0.30 |
| SNP + 100 mM NaCl | 25.50fg ± 0.98 | 2.56f ± 0.06 | 20.08g ± 0.37 |
| SNP + 400 mM NaCl | 35.86c ± 1.37 | 3.52cd ± 0.05 | 26.50d ± 0.28 |
| SA + SNP | 16.76j ± 0.71 | 1.55h ± 0.03 | 10.31k ± 0.47 |
| SA + SNP + 100 mM NaCl | 22.46h ± 0.86 | 2.43f ± 0.01 | 18.83h ± 0.26 |
| SA + SNP + 400 mM NaCl | 32.14d ± 0.29 | 3.39d ± 0.01 | 25.06e ± 0.23 |
Data are means of five replicates ± SE. Different letters indicate significant difference between treatments at P < 0.05
Protein content
Content of protein was reduced considerably with increased NaCl concentration (Table 3). The percentages of decline were 46 and 87% for 100 and 400 mM NaCl, respectively as compared to the control. Nevertheless, addition of SA, SNP or SA + SNP as seed soaking, with or without NaCl, caused negligible change in protein levels than those of the radicles grown under NaCl alone. A maximum of it (95%) was measured in the SA + SNP + NaCl exposed samples, compared to all other treatments (Table 3).
Table 3.
Variations in content of protein, proline, sugar and glycinebetaine in P. sativum L. subjected to salicylic acid and/or sodium nitroprusside coupled salt-solutions
| Treatments | Protein content (µg g−1 FW) | Proline content (mg g−1 FW) | Sugar content (mg g−1 FW) | Glycine betaine (µmol g−1 FW) |
|---|---|---|---|---|
| C | 66.86b ± 1.42 | 0.28h ± 0.02 | 49.69k ± 1.63 | 13.63h ± 0.22 |
| 100 mM NaCl | 36.45e ± 3.22 | 0.55g ± 00 | 76.16h ± 5.48 | 16.93g ± 0.59 |
| 400 mM NaCl | 8.94h ± 0.72 | 2.44d ± 0.03 | 177.53d ± 3.93 | 19.54f ± 0.25 |
| SA | 69.63a ± 1.06 | 0.31h ± 00 | 57.35j ± 1.81 | 16.33g ± 0.15 |
| SA + 100 mM NaCl | 61.10d ± 1.90 | 0.91f ± 0.05 | 101.73g ± 3.28 | 19.56f ± 0.44 |
| SA + 400 mM NaCl | 19.21g ± 1.91 | 3.10c ± 0.19 | 206.74c ± 1.56 | 20.62e ± 0.31 |
| SNP | 70.02a ± 1.38 | 0.34h ± 00 | 64.44i ± 2.05 | 18.85f ± 0.55 |
| SNP + 100 mM NaCl | 62.84cd ± 1.04 | 1.17e ± 0.06 | 110.87f ± 0.71 | 21.93d ± 0.98 |
| SNP + 400 mM NaCl | 21.88f ± 0.83 | 3.33b ± 0.04 | 213.84b ± 3.92 | 24.51b ± 1.12 |
| SA + SNP | 71.03a ± 1.27 | 0.35h ± 00 | 65.43i ± 1.55 | 20.60e ± 0.32 |
| SA + SNP + 100 mM NaCl | 63.80c ± 0.71 | 1.28e ± 0.02 | 121.85e ± 3.23 | 23.25c ± 0.69 |
| SA + SNP + 400 mM NaCl | 23.41f ± 0.55 | 3.50a ± 0.06 | 227.93a ± 2.18 | 26.61a ± 0.34 |
Data are mean ± SE (n = 5). Values in the same column followed by different letters are significantly different (P < 0.05)
Antioxidant enzymes
Enzymes like SOD, CAT, POX and APX were performed differently in P. sativum L. subjected to NaCl alone and in combination with SA, SNP or SA + SNP (Fig. 4). Data indicated that activities of CAT, POX and APX declined considerably (94, 57 and 86%, respectively) while SOD was raised (174%) under NaCl-stress compared to non-treated control. Priming of radicles by soaking in SA, SNP or SA + SNP stimulated stress tolerance by increasing CAT, POX, APX and SOD activities as compared with corresponding NaCl level. Highest activities of all the enzymes were measured in the SA + SNP grown radicles. However, SA + SNP revealed more stimulatory impact than the SNP or SA alone on enzymic activities (Fig. 4a, c, e, g).
Fig. 4.
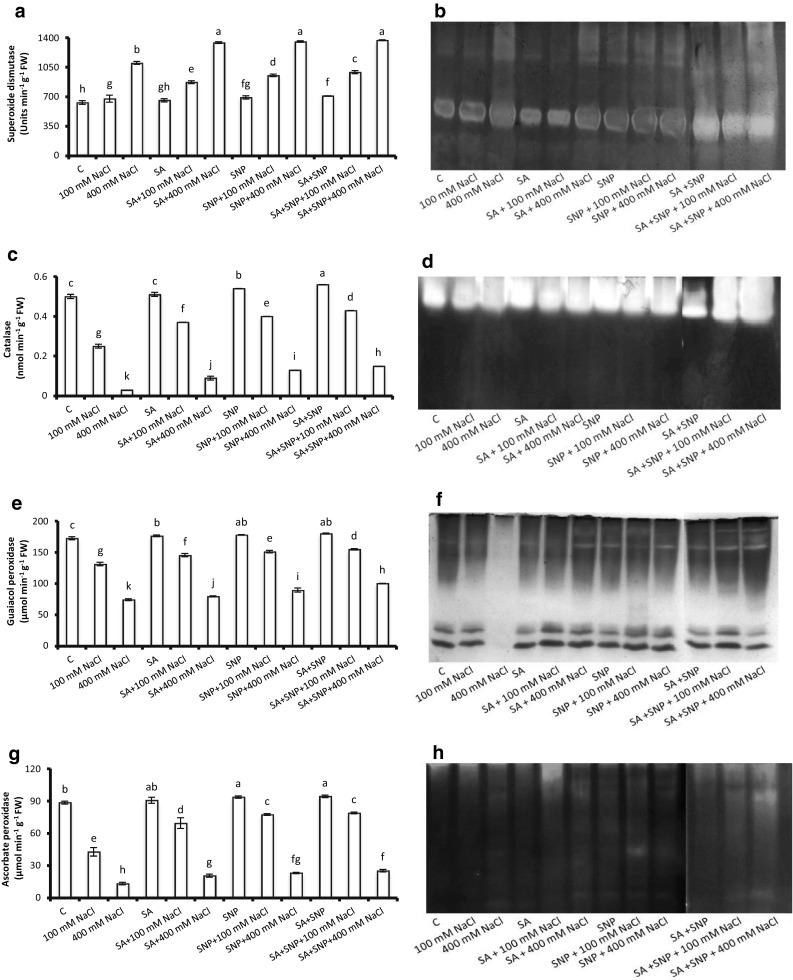
Activities and isozymic patterns of superoxide dismutase (a, b), catalase (c, d), guaiacol peroxidase (e, f) and ascorbate peroxidase (g, h) in P. sativum L. radicles exposed to various experimental solutions for 7 days. Bars presented in the graphs are mean ± SE of five separate observations. Different letters indicate significant difference between treatments at P < 0.05
To further strengthen the spectrophotometric data of SOD, CAT, POX and APX, their isozymes were analysed using non-denaturing-PAGE. Analysis of Native-PAGE gel revealed four isoforms each of SOD, POX and APX whereas only one of CAT, in response to NaCl and, SA and/or SNP. Exposure of P. sativum L. radicles to salt solution decreased the intensities of SOD isozymes (Fig. 4b). While, intense bands were appeared in the samples exposed to SA + SNP + NaCl (400 mM), and this pattern was well supported by its biochemical data also (Fig. 4a). No any significant change in the CAT isozyme was observed in response to any of the treatments (Fig. 4d). Further, relative to the POX and APX of non-treated controls, band number and intensity both were found to be reduced with increased supply of NaCl, but was restored considerably when SA and/or SNP was applied in the medium exogenously (Fig. 4f, h). The salinity-induced changes in POX and APX isozymes were in parallel to their spectrophotometric data (Fig. 4e, g).
Osmolytes
The levels of sugar, proline and glycinebetaine in P. sativum L. radicles significantly (P < 0.05) enhanced in response to saline stress, and compared to their respective controls (Table 3). The quantities of sugar, proline and glycinebetaine were found to be higher (357, 871 and 143%, respectively) in response to high NaCl concentration than that of its low level (153, 196 and 124%, respectively). Maximum accruals of sugar (227.93 mg g−1 FW), proline (3.5 mg g−1 FW) and glycinebetaine (26.61 µmol g−1 FW) were discernible in the SA + SNP + NaCl grown samples than the SNP or SA + NaCl grown (Table 3).
Discussion
The present investigation was undertaken to improve our understanding of the physiological and biochemical mechanisms implicated in salinity tolerance, and stimulation of such mechanisms by exogenous applications of SA and/or SNP. Addition of SA and/or SNP exhibited ameliorative effects under both saline and non-saline conditions. Application of SA and/or SNP on NaCl subjected P. sativum L. radicles considerably (P < 0.05) decreased the amounts of ROS, lipid–protein oxidised products like MDA, 4-HNE and PCO, altered the activities of antioxidant enzymes (SOD, CAT, POX and APX) and increased accumulation of osmolytes (sugar, proline and glycinebetaine). Applied treatments revealed reduced negative influence of NaCl-stress on growth traits. However, amongst the tested treatments, combined addition of SA and SNP was found to be the best than their alone application in regard to amelioration of NaCl-induced injury symptoms.
Accumulated data unveiled that NaCl strongly inhibited growth and DW accumulation in P. sativum L. radicles. At low salt concentration (100 mM) growth traits were reduced slightly. While, marked (P < 0.05) inhibitory impacts were discernible with increased (400 mM) NaCl (Table 1). However, a negative association (r = −0.98, P < 0.05) between NaCl-concentration and growth trait could be observed. Existing reports testifies that roots may be the foremost sites of salt injury (Sarkar et al. 2013). Moreover, few studies have also indicated that shoot growth is more susceptible to salt-stress than the root growth, while others have suggested that roots of even xero-halophytes have a high susceptibility to NaCl (Maimaiti et al. 2014). The reductions in radicle length and biomass may possibly be due to the ionic stress, which reduces the water potential of the growing medium and hinders water absorption by roots (Maimaiti et al. 2014). Salinity-induced growth suppression could also be due to the scarcity of energy because mechanisms implicated in salt transport and repair of salt-induced damages on membranes or cellular proteins are energy consuming (Hayat et al. 2012). Our results are in harmony with that of Hayat et al. (2012) and Khan et al. (2014) who have observed significant reduction in growth indices of salt stressed Brassica juncea L. and Vigna radiata L. However, addition of SA and/or SNP remarkably improved the growth of P. sativum L. (Table 1). Addition of SA into stressed plants is shown to increase the physiological responses like DW, root and shoot length, and leaf area compared to those grown without SA (Ismail 2013). It has also been shown to enhance the growth of Zea mays L. (Ismail 2013) and B. juncea L. (Hayat et al. 2012) seedlings under NaCl-stress. Likewise, SNP was shown to improve the seed germination and root/shoot growth in various groups of salt affected plants (Lamattina et al. 2003; Sheokand et al. 2010).
Abiotic stress promoted ROS directly affects the cell membrane integrity via inducing oxidative damage. In the current study, a negative correlation (r = −0.86, P < 0.05) between ROS accumulation and cell membrane integrity of P. sativum L. was revealed under salinity stress. Investigation carried out in the radicles of P. sativum L. exhibited that with rising concentration of NaCl, there was an increment in the absorption of Evans blue dye, exhibiting increased rate of cell death (148%). On the other hand, cell’s integrity was maintained up to an extent (89%) in the radicles of P. sativum L. when SA and SNP were jointly applied with salt solution, as these molecules are shown to possess membrane stabilising properties (Fig. 3).
In addition to detrimental effects on cell metabolism and osmotic adjustment, high salt concentration also encouraged the production of ROS (Keshavkant et al. 2012). The probable reason for increased ROS generation under salinization is a respiratory disorder in affected tissues (Efimova et al. 2014). Moreover, excessive accumulations of Na+ and Cl− ions, stomatal closure, reduced availability of carbon dioxide, and increased energy of electron excitation are also contributing ROS in salt-stressed seedlings (Keshavkant et al. 2012). In this investigation, NaCl-induced substantial (P < 0.05) upsurge in the ROS levels (O2˙−: 407%, H2O2: 249%), which was measured to be quite lesser in SA (343 and 201%), SNP (221 and 184%) or SA + SNP (164 and 170%) treated seeds (Table 1). Matching observations were also noted for salt-exposed Cicer arietinum L. (Keshavkant et al. 2012) and Oryza sativa L. (Sarkar et al. 2013). However, SA or SNP supplementation in salt-stressed C. arietinum L., and Z. mays L. seedlings considerably reduced oxidative injury (Sheokand et al. 2010; Agami 2013). SA or SNP regulated elevation in the activities of antioxidant enzymes and accumulations of osmolytes are shown to be the prime reasons for this reduced oxidative injury (Kazemi et al. 2010; Agami 2013).
ROS are well known to induce perturbation of nucleic acids, oxidation of proteins and lipids and alterations of key enzymes (Chandrakar et al. 2016b). ROS-exerted membrane deterioration is the foremost reason of intoxication of plant cells (Yadu et al. 2016). Contents of both MDA and 4-HNE, two popularly used biomarkers of oxidative stress and structural integrity of cellular membranes, in P. sativum L. subjected to NaCl-stress were 169 and 255% higher than their respective controls (Table 2), suggesting the involvement of oxidative stress. A close association (r = 0.99, P < 0.05) between extent of lipid peroxidation and NaCl-concentration was observed in this study. Salt-dependent increases in MDA and 4-HNE are possibly the resultant of NaCl-imposed alterations in defence enzymes. Additionally, stress-induced enhanced lipoxygenase was shown to be responsible for higher lipolytic activity and oxidation of lipid moieties in membranes (Kazemi et al. 2010). Consistent rise in lipid oxidised products were also measured in salt-affected seedlings of Brassica napus L. and C. sativus L. (Efimova et al. 2014; Naliwajski and Sklodowska 2014). Exogenous addition of SA and/or SNP substantially reduced MDA and 4-HNE accumulations in P. sativum L. grown in NaCl (Table 2). Like ours, significant fall in lipid peroxidation reaction was also noticed following exogenous application of SA or SNP in salt-stressed seedlings of Kosteletzkya virginica, V. radiata L. and B. napus L. (Guo et al. 2009; Khan et al. 2014; Efimova et al. 2014). Lamattina et al. (2003) reported that NO readily reacts with lipid alcoxyl and peroxyl radicals thereby terminating the propagation of ROS-mediated lipid oxidation reaction, hence reduced MDA accumulation. However, SA actively participates in the stabilisation of cell membranes, and by enhancing the antioxidant enzymes, facilitates alleviation of NaCl-induced oxidative damage (Agami 2013; Khan et al. 2014).
Under NaCl-imposed oxidative condition, cellular proteins may also undergo the process of degradation and/or oxidation (Sarkar et al. 2013). Attack of ROS on cellular proteins may results modifications on it via distinct pathways, out of which few are direct and others are indirect amendments (Parkhey et al. 2014a). In the conducted study, salinity-induced marked (87%, P < 0.05) reduction in the protein content while, PCO accumulation increased substantially (Tables 2, 3). The magnitude of both protein reduction and PCO accumulation rose considerably along with NaCl concentration. Consistent results were also documented for O. sativa L. (Sarkar et al. 2013) and C. sativus L. (Naliwajski and Sklodowska 2014) in response to salt-exposure. The reduced protein under salt-stress is possibly due to hindrance in the activity of nitrate reductase or increased activity of proteases or both (Keshavkant et al. 2012). Moreover, during abiotic stresses, exposure of proteins to ROS exerts detrimental conformational changes that lead to the decrease or loss of protein function and increased PCO accumulation (Parkhey et al. 2014a). However, application of SA and/or SNP alleviated the adverse effects of salt-stress by up-regulating the process of protein synthesis and antioxidant machinery in P. sativum L. radicles. Both SNP and SA were found to accelerate the pace of protein synthesis and defence enzymes of salt-exposed C. arietinum L. and Hordeum vulgare L. plants resulting higher levels of protein (Sheokand et al. 2010; Pirasteh-Anosheh et al. 2014) and lower PCO content. SA is also shown to confer resistance to various abiotic stresses by its ability to express genes that specifically codes for stress responsive proteins like PR-proteins (Merkouropolous et al. 1999). Moreover, SA could improve acclimation in Solanum lycopersicum L. to salt-stress by stimulating abscisic acid accumulation (Song et al. 2011). Previously, SNP was found to up-regulate the expression of NtGRAS1 gene in Nicotiana tabacum, that play a critical role in the transcriptional regulation of genes involved in the oxidative tress tolerance (Czikkel and Maxwell 2007). Therefore, besides induction of antioxidant responses and stress responsive gene expression, the interactions between SA, NO (SNP) and ABA may also contribute in mitigation of protein degradation/oxidation reaction during salt-stress (Song et al. 2011).
Maintaining intactness of the cellular membranes under NaCl-stress is suggested as an integral part and pre-requisite for the salinity tolerance (Keshavkant et al. 2012). Both SA and SNP may retain membrane integrity by improving the levels of the antioxidant systems that protects the plants from the oxidative injury (Sheokand et al. 2010; Ismail 2013). In this study, both spectrophotometric data and isozyme charting showed that activities of CAT, POX and APX were declined, while SOD was increased in P. sativum L. under salt-stress. However, activities of above antioxidants were increased significantly (P < 0.05) after SA and/or SNP addition (Fig. 4). The reduction in activities indicated that these antioxidants were unable to completely detoxify the H2O2 that resulted from the salt-stress (Keshavkant et al. 2012). Salt-exposure accumulates the ROS, particularly H2O2, in affected cells (Agami 2013). The metabolism of H2O2 is dependent on coordinated functioning of various antioxidants viz. CAT, POX and APX. The acceleration in the activities of antioxidant enzymes by SA or SNP is a gene regulated phenomenon. Following molecular analysis of stressed Arabidopsis thaliana, Cao et al. (2005) suggested that elevation in antioxidant enzyme was the consequence of enhanced expression of a stress responsive gene DET2. Previous reports showed that the addition of SA or SNP modified antioxidant enzymes activities under salinity-stress (Sheokand et al. 2010; Ismail 2013). Guo et al. (2009) found that application of SNP increased SOD, CAT and POX activities in salt-stressed K. virginica. Similarly, SA was shown to enhance CAT, POX and glutathione in NaCl-exposed Z. mays L. seedlings (Agami 2013; Ismail 2013). Researches have also evidenced that NO itself serves as an antioxidant and can result low levels of ROS accumulation (Lamattina et al. 2003).
The accruals of sugar, proline and glycinebetaine under salt-stress prevent the cell by balancing the osmoticum of cytosol with that of vacuole and external environment (Hong et al. 2000; Naliwajski and Sklodowska 2014). Besides, above molecules interact with cellular enzymes and stabilise their structures and functions. Higher osmolyte concentration was also correlated with higher relative water content and ability of plants to survive under saline-conditions. These osmolytes has also been proven to serve as molecular chaperons involved in safeguard of protein integrity and activation of key enzymes (Pirasteh-Anosheh et al. 2014). As osmoprotectors, sugars stabilize membranes and proteins, possibly by substituting the water in the formation of hydrogen bonds with polar residues of polypeptides and phosphate groups of phospholipids (Maimaiti et al. 2014). Similarly, glycinebetaine also play role in salt-stressed plants by osmotic adjustment, photosynthetic apparatus protection and reduction of ROS (Maimaiti et al. 2014). Moreover, proline is shown to protect the protein turnover machinery against stress injury and also by up-regulating the stress responsive genes, improves the stress tolerance mechanisms of plants (Efimova et al. 2014). In this investigation, although osmolyte (sugar, proline and glycinebetaine) content increased in response to salinity, the treatments with SA and/or SNP proved to be quite effective in further raising the osmolytes level in P. sativum L. (Table 3). It is probable that after removal of stress condition, a rapid breakdown of proline and sugar may make available a desired level of reducing agents that can efficiently up-regulate oxidative phosphorylation leading synthesis of adenosite triphosphate (ATP) in mitochondria. The ATPs so produced can play a prominent role in rapid recovery of seedlings from salt-stress (Pirasteh-Anosheh et al. 2014). SA- or SNP-induced enhanced synthesis of sugar, proline or glycinebetaine were also observed in salt-affected K. virginica (Guo et al. 2009), Z. mays L. (Agami 2013), H. vulgare L. (Pirasteh-Anosheh et al. 2014) and V. radiata L. (Khan et al. 2014). The enhanced level of proline in SA-/SNP-subjected germinating seeds might be due to a decline in dissolved proteins (Guo et al. 2009; Agami 2013). In salt-stressed plants, SA has been supposed to inhibit polysaccharide hydrolyzing enzymes and/or hasten the incorporation of soluble sugars into polysaccharides (Pirasteh-Anosheh et al. 2014). Similarly, SA improves glycinebetaine accrual in NaCl-subjected plants through increased availability of methionine and suppression of ethylene synthesis (Khan et al. 2014). Moreover, increased osmolyte accumulation cannot be considered as a sole criterion for salinity tolerance, as it also accumulates in response to several other stresses like high temperature, chilling, drought, starvation, etc. (Hong et al. 2000).
Conclusions
The role of SA and SNP in resuming abiotically constrained seedling growth has been independently proven in many studies (Guo et al. 2009; Hayat et al. 2012). It was apparent that these two compounds cross talk to induce defensive functions countering abiotic stress responses. Application of SA and/or SNP significantly improved growth traits and elevated the levels of antioxidants and osmoprotectants in salt-stressed P. sativum L. Thus, conducted study revealed that cautious addition of these two compounds synergizes the stress-ameliorating properties of SA and SNP at the physiological as well as biochemical level.
Acknowledgements
The authors would like to thanks the Department of Science and Technology, New Delhi, for awarding INSPIRE fellowship (No. DST/INSPIRE Fellowship/2013/791, dated 23 November 2013) to Vibhuti Chandrakar. Authors are also grateful to Department of Science and Technology, New Delhi, for financial support under DST-FIST scheme (Sanction No. 2384/IFD/2014-15, dated 31 July 2014).
References
- Agami RA. Alleviating the adverse effects of NaCl stress in maize seedlings by pretreating seeds with salicylic acid and 24-epibrassinolide. South Afr J Bot. 2013;88:171–177. doi: 10.1016/j.sajb.2013.07.019. [DOI] [Google Scholar]
- Bates LS, Walrow RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, Segundo BS. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2013;165:688–704. doi: 10.1104/pp.113.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzen H, Zhao B. Loss of function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant. 2005;123:57–64. doi: 10.1111/j.1399-3054.2004.00432.x. [DOI] [Google Scholar]
- Chance M, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–817. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- Chandra J, Keshavkant S. Physiological and biochemical changes during seed development and maturation in Madhuca latifolia Roxb. Bangladesh J Bot. 2016;45:335–343. [Google Scholar]
- Chandra J, Tandon M, Keshavkant S. Increased rate of drying reduces metabolic inequity and critical water content in radicles of Cicer arietinum L. Physiol Mol Biol Plants. 2015;21:215–223. doi: 10.1007/s12298-015-0294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakar V, Dubey A, Keshavkant S (2016a) Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J Soil Sci Plant Nut 16:662–676
- Chandrakar V, Naithani SC, Keshavkant S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: a review. Biologia. 2016;71:367–377. doi: 10.1515/biolog-2016-0052. [DOI] [Google Scholar]
- Chaudhuri A, Singh KL, Kar RK. Interaction of hormones with reactive oxygen species in regulating seed germination of Vigna radiata. J Plant Biochem Physiol. 2013;1:1–5. [Google Scholar]
- Czikkel BE, Maxwell DP. NtGRAS1, a novel stress-induced member of the GRAS family in tobacco, localizes to the nucleus. J Plant Physiol. 2007;164:1220–1230. doi: 10.1016/j.jplph.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Efimova MV, Savchuk AL, Hasan JAK, Litvinovskaya RP, Khripach VA, Kholodova VP, Kuznetsov VV. Physiological mechanisms of enhancing salt tolerance of oilseed rape plants with brassinosteroids. Russ J Plant Physiol. 2014;61:733–743. doi: 10.1134/S1021443714060053. [DOI] [Google Scholar]
- FAO . Global network on integrated soil management for sustainable use of salt-affected soils. Rome: FAO Land and Plant Nutrition Management Service; 2005. [Google Scholar]
- Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. doi: 10.1007/BF02374789. [DOI] [Google Scholar]
- Guo Y, Tian Z, Yan D, Zhang J, Qin P. Effects of nitric oxide on salt stress tolerance in Kosteletzkya virginica. Life Sci J. 2009;6:67–75. [Google Scholar]
- Hayat S, Maheshwari P, Wani AS, Irfan M, Alyemeni MN, Ahmad A. Comparative effect of 28 homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol Biochem. 2012;53:61–68. doi: 10.1016/j.plaphy.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Hong ZL, Lakkineni K, Zhang ZM, Verma DPS. Removal of feedback inhibition of delta 1-pyrrolidone-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail MA. Alleviation of salinity stress in white corn (Zea mays L.) plant by exogenous application of salicylic acid. Am J Life Sci. 2013;1:248–255. doi: 10.11648/j.ajls.20130106.12. [DOI] [Google Scholar]
- Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hortic. 2010;126:402–407. doi: 10.1016/j.scienta.2010.07.037. [DOI] [Google Scholar]
- Keshavkant S, Padhan J, Parkhey S, Naithani SC. Physiological and antioxidant responses of germinating Cicer arietinum seeds to salt stress. Russ J Plant Physiol. 2012;59:206–211. doi: 10.1134/S1021443712010116. [DOI] [Google Scholar]
- Khan MIR, Asgher M, Khan NA. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb CJ. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Maimaiti A, Yunus Q, Iwanaga F, Mori N, Tanaka K, Yamanaka N. Effects of salinity on growth, photosynthesis, inorganic and organic osmolyte accumulation in Elaeagnus oxycarpa seedlings. Acta Physiol Plant. 2014;36:881–892. doi: 10.1007/s11738-013-1466-8. [DOI] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Merkouropolous G, Barnett DC, Shirasat AH. The Arabidopsis expensin gene is developmentally regulated, is induced by wounding, methyl jasmonate, abscissic acid, salicylic acid and codes for protein with unusual motifs. Planta. 1999;208:212–219. doi: 10.1007/s004250050552. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Naliwajski MR, Skłodowska M. The oxidative stress and antioxidant systems in cucumber cells during acclimation to salinity. Biol Plant. 2014;58:47–54. doi: 10.1007/s10535-013-0378-1. [DOI] [Google Scholar]
- Parkhey S, Naithani SC, Keshavkant S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol Biochem. 2012;57:261–267. doi: 10.1016/j.plaphy.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Parkhey S, Naithani SC, Keshavkant S. Protein metabolism during natural ageing in desiccating recalcitrant seeds of Shorea robusta. Acta Physiol Plant. 2014;36:1649–1659. doi: 10.1007/s11738-014-1540-x. [DOI] [Google Scholar]
- Parkhey S, Tandan M, Keshavkant S. Salicylic acid and acquisition of desiccation tolerance in Pisum sativum seeds. Biotechnology. 2014;13:217–225. doi: 10.3923/biotech.2014.217.225. [DOI] [Google Scholar]
- Pirasteh-Anosheh H, Ranjbar G, Emam Y, Ashraf M. Salicylic acid-induced recovery ability in salt-stressed Hordeum vulgare plants. Turkish J Bot. 2014;38:112–121. doi: 10.3906/bot-1302-20. [DOI] [Google Scholar]
- Ray S, Roy K, Sengupta C. Evaluation of protective effects of water extract of Spirulina platensis on cisplatin-induced lipid peroxidation. Ind J Pharma Sci. 2007;69:378–383. doi: 10.4103/0250-474X.34546. [DOI] [PubMed] [Google Scholar]
- Roach T, Kranner I, Bechett R, Whitaker C, Minibayeva F. Extracellular production of ROS during seed germination and early seedling growth in Pisum sativum. J Plant Physiol. 2010;167:805–811. doi: 10.1016/j.jplph.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Sarkar RK, Mahata KR, Singh DP. Differential responses of antioxidant system and photosynthetic characteristics in four rice cultivars differing in sensitivity to NaCl stress. Acta Physiol Plant. 2013;35:2915–2926. doi: 10.1007/s11738-013-1322-x. [DOI] [Google Scholar]
- Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheokand S, Bhankar V, Sawhney V. Ameliorative effect of exogenous nitric oxide on oxidative metabolism in NaCl treated chickpea plants. Braz J Plant Physiol. 2010;22:81–90. doi: 10.1590/S1677-04202010000200002. [DOI] [Google Scholar]
- Shi QH, Ding F, Wang XF, Wei M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem. 2007;45:542–550. doi: 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Song SY, Chen Y, Chen J, Dai XY, Zhang WH. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta. 2011;234:331–345. doi: 10.1007/s00425-011-1403-2. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Analysis of sugars found in glycoproteins. Methods Enzymol. 1966;8:3–26. doi: 10.1016/0076-6879(66)08005-4. [DOI] [Google Scholar]
- Srivastava OP, Huystee RB. An inter-relationship among peroxidise, IAA oxidase and polyphenol oxidase from peanut cells. Canadian J Bot. 1977;55:2630–2635. doi: 10.1139/b77-301. [DOI] [Google Scholar]
- Suzuki K, Yano A, Shinshi H. Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 1999;119:1465–1472. doi: 10.1104/pp.119.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Woodbury W, Spencer AK, Stahmann MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- Yadu B, Chandrakar V, Keshavkant S. Responses of plants towards fluoride: an overview of oxidative stress and defense mechanisms. Fluoride. 2016;49:293–302. [Google Scholar]