Abstract
Chloroplast genome sequences are very useful for species identification and phylogenetics. Chuanminshen (Chuanminshen violaceum Sheh et Shan) is an important traditional Chinese medicinal plant, for which the phylogenetic position is still controversial. In this study, the complete chloroplast genome of Chuanminshen violaceum Sheh et Shan was determined. The total size of Chuanminshen chloroplast genome was 154,529 bp with 37.8% GC content. It has the typical quadripartite structure, a large single copy (17,800 bp) and a small single copy (84,171 bp) and a pair of inverted repeats (26,279 bp). The whole genome harbors 132 genes, which includes 85 protein coding genes, 37 tRNA genes, eight rRNA genes, and two pseudogenes. Thirty-nine SSR loci, 32 tandem repeats and 49 dispersed repeats were found. Phylogenetic analyses results with the help of MEGA showed a new insight for the Chuanminshen phylogenetic relationship with the reported chloroplast genomes in Apiales plants.
Keywords: Chuanminshen violaceum Sheh et Shan, Chloroplast genome, Genome features, Phylogenetic analysis
Introduction
Chloroplast plays an important role in the plant photosynthesis and carbon fixation (Neuhaus and Emes 2000). The size of the major angiosperm chloroplast genome is 110–165 kb and contains 90–110 unigenes (Sugiura 1992). They consist of four parts, a large single copy region (LSC), a small single copy region (SSC), and two inverted repeats (IRs; Jansen et al. 2005). Chloroplast genomes are highly conserved in sequence and structure due to their non-recombinant, haploid and uniparentally (i.e., maternally) inherited nature (Birky 2001; Wicke et al. 2011). Therefore, chloroplast genome sequence was widely used in the phylogenetic analyses, organelle-scale barcodes research and evolutionary studies. Presently, chloroplast sequences of 30 species of Apiales plants were reported in NCBI (http://www.ncbi.nlm.nih.gov/genome/organelle/).
Chuanminshen is a Chinese endemic genera of the Apiaceae plant family which has only one species. Chuanminshen violaceum Sheh et Shan is a typical species of the Apiaceae family (Flora 1979), and is considered a ‘medicine food homology’ plant. The rhizome of the plant is popularly used as a traditional Chinese medicine in China. Chuanminshen has significant effects in reducing phlegm, anti-cough and nourishing yin. Modern pharmacognosy research showed that the roots of Chuanminshen are rich in bioactive components such as Chuanminshen violaceum polysaccharides (CVPs; Lei and Zhang 2012). These components function as antioxidants, antimutations, antifatigue (Chen and Peng 2011), and antivirals (Song et al. 2013), and have expectorant and antitussive properties as well, along with reports of physique enhancement (Feng et al. 2015; Zhang et al. 2007). The latest study also found that polysaccharides obtained from Chuanminshen could improve the immune responses of foot-and-mouth disease vaccine in mice (Feng et al. 2015).
Previously, research of Chuanminshen mostly focused on the field of pharmacognosy, but very rarely on the phylogenetics. To date, the phylogenetic analysis of Chuanminshen (She and Shan 1980; Song et al. 2014; Tao et al. 2008) is still controversial. In the current study, we sequenced the Chuanminshen chloroplast genome, and made a survey of its general features. Furthermore, we investigated the phylogenetic relationships by Chuanminshen and all other Apiales plants whose chloroplast genome have been reported.
Materials and methods
Plant materials
The sequenced plant was originated from our own breeding parents, the inbred line CMS-1. The plant was confirmed by Shangqin Hu (Director of Chinese herbal medicine planting research center of Sichuan province, China) through plant phenotype indentification.
Methods
Total genomic DNA was extracted from fresh, clean Chuanminshen leaves by plant genomic DNA extraction kit ™. cpDNA amplification was carried out through nine universal effective primer pairs by long-range PCR (Yang et al. 2014). The PCR products were fragmented to construct 500 bp short-insert libraries according to the Illumina manufacturer’s manual. Each DNA library was labeled with a barcode and pooled together in one lane and the sequencing was executed using Illumina Hiseq 2000 in Kunming Institute of Botany, Chinese Academy of Sciences. Raw data were filtered using the Next Generation Sequencing (NGS) QC toolkit (Patel and Jain 2012), high-quality short reads were assembled into the complete chloroplast genome using SOAPdenovo software (Luo et al. 2015) andthe chloroplast genome was annotated using the Dual Organellar GenoMe Annotator (DOGMA) tool (Wyman et al. 2004) and CpGAVAS (Liu et al. 2012). The start/stop codons and intron/exon boundaries were manually corrected. The perl script MicroSAtellite (MISA; Parida et al. 2010) was used to analyze the distributing frequency of chloroplast genome simple sequence repeat (SSR; parameter setting: the minimum repeat number of each unit are as follows, 1–10, 2–6, 3–5, 4–5, 5–5, 6–5). Tandem repeat finder (Benson 1999; the default parameters were used) and REPuter (Kurtz and Schleiermacher 1999; the setting parameters of REPuter to the minimal repeat size of 30 bp, hamming distance to 3) was used to analyze the repeat incidents in Chuanminshen cp DNA. In order to avoid the influences of the IR regions, we used only a single IR region, meanwhile, the redundant results of REPuter were manually removed. Chuanmingshen cp genome, 30 Apiales chloroplast genome and an outgroup chloroplast genome was downloaded from NCBI, 80 common proteins were used to carry out the maximum likelihood (ML) analysis based on the JTT matrix-based model and 1000 bootstraps by MEGA7 (Kumar et al. 2016), all positions containing gaps and missing data were eliminated. There were a total of 12,409 positions in the final dataset, the branch lengths measured in the number of substitutions per site.
Results and discussion
Cp genome general features of Chuanminshen
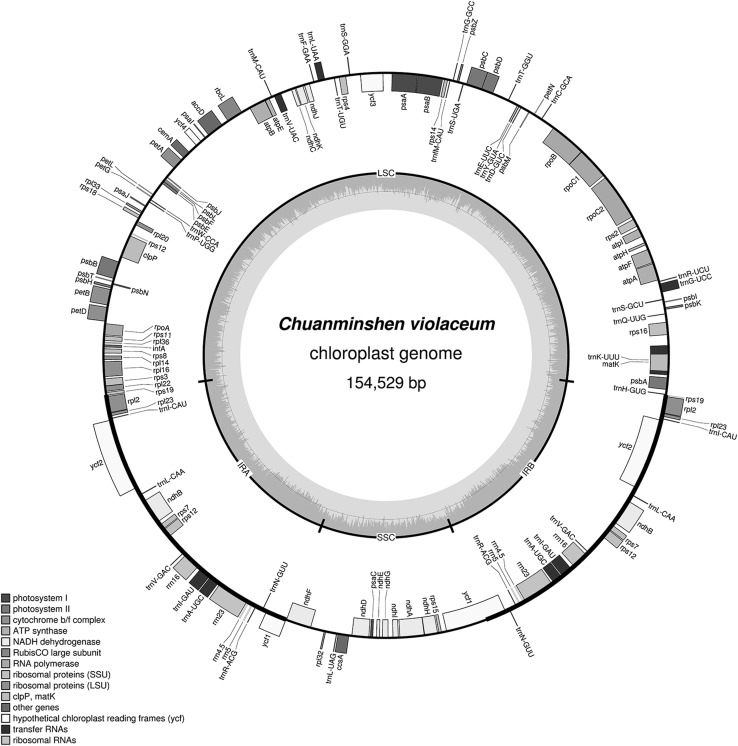
The full length of chloroplast genome sequence for Chuanminshen (with the accession number in GenBank is KU921430) was 154,529 bp, constructed with a quadripartite structure (Fig. 1). The four parts were LSC with 17,800 bp, SSC with 84,172 bp, IR regions (IRa and IRb) with 26,279 bp. The whole Chuanminshen cp genome contained 132 genes, including 85 protein-coding genes, 37 transfer RNAs, eight ribosomal RNAs and two pseudogenes (ycf1 and rps19). Whereas only 112 unigenes were harbored in Chuanminshen cp genome due to a multi-copy of six protein-coding genes, four rRNAs and 10 tRNAs. The total GC content in Chuanminshen cp genome was 37.8%. Of these, the LSC region, SSC region and IR region was 35.9, 31.5 and 42.9%, respectively. Most genes in Chuanminshen cp genome contained only one or no intron. In addition, three genes had two introns, which are rps12 (ribosomalproteinS12), clpP (clp protease proteolytic subunit), ycf3 (hypothetical chloroplast RF34).
Fig. 1.
Gene map of the Chuanminshen violaceum Sheh et Shan chloroplast genome: Outside of the outer circle was drawn with the counter clockwise genes, and inside was drawn with transcribed clockwise genes, various colors indicated the genes in different functional groups. LSC The large single copy region, SSC the small single copy region, IRa and IRb the inverted repeats. The two innermost gray area: The darker gray represented GC content and the lighter represented AT content
Compared with other cp genome sequences, Chuanminshen cp genome was smaller than Panax ginseng 156,318 bp (Kim and Lee 2004), Nicotiana tabacum 155,943 bp (Shinozaki et al. 1986), and much longer than Salvia miltiorrhiza Bge151328 bp (Qian et al. 2013), a model plant of Chinese herbal medicine (Zhang et al. 2015). Statistical analysis showed that Chuanminshen is the sixth largest cp genome in size and has the highest GC content among the 15 chloroplast genome sequences (including Chuanminshen) of Apiaceae plant family. Similar to the Sesamum indicum L (Yi and Kim 2012), Epimedium (Zhang et al. 2016), Daucus carota (Ruhlman et al. 2006), it is rich in GC content in IR regions owing to four rRNA genes. Through the annotation, all the genes were classified into four categories, including the gene for self replication such as rRNA genes and tRNA genes, genes for photosynthesis such as psaA and ndhA, other genes such as clpP, and genes of unknown functions such as ycf1.
Cp SSR and repeat sequence analysis
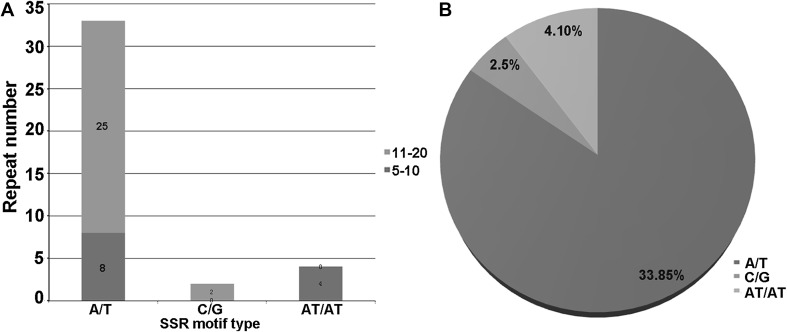
SSR markers are widely used in phylogenetic analysis, population genetics and ecological studies (Cavalier-Smith 2002). Using MISA, 39 SSR loci were identified in the Chuanmingshen cp genome. Three compound formations of SSR were presented, 10.3% di-nucleotide (all was AT/AT) and 89.7% mono-nucleotide (84.6% A/T, 5.1% G/C; Fig. 2). Eleven repeats of A/T motif is the most frequent and included up to 12. The second abundant is 10 repeats of A/T motif, which was found in eight loci. Once in eight repeats and thrice in six repeats were found in AT/AT motif. Obtaining SSR from the cp genome was common in the study, because it exhibited highly polymorphic results due to the diversity levels in repeat unit copy numbers among the same species (Grassi et al. 2002; Powell et al. 1995). In accordance with most cp SSR research results, Chuanminshen cp SSR was rich in homopolymers as well. Surprisingly, we did not find any tri-nucleotide repeats or larger repeat units in the Chuanmingshen cp genome, we suspected that this result was relevant due to the usage of the default MISA tool. Subsequently, we analyzed the Daucus carota, Salvia miltiorrhiza and Epimedium cp genome based on our own set of parameters. In contrast to the previous SSR analysis reported in the literature, the statistical results confirmed our speculation that only two types of the repeat units were observed.
Fig. 2.
The distribution information of SSR motif repeat in Chuanminshen cp genome. a The number of distributions of SSR motif repeat. x-axis SSR repeat types; y-axis Number of SSR types; red bars stand for 11–20 motif repeats, blue bars stand for 5–10 motif repeats. b The percentage distribution of SSR motif type. Blue sector represented A/T, green sector represented AT/AT, red sector represented G/C
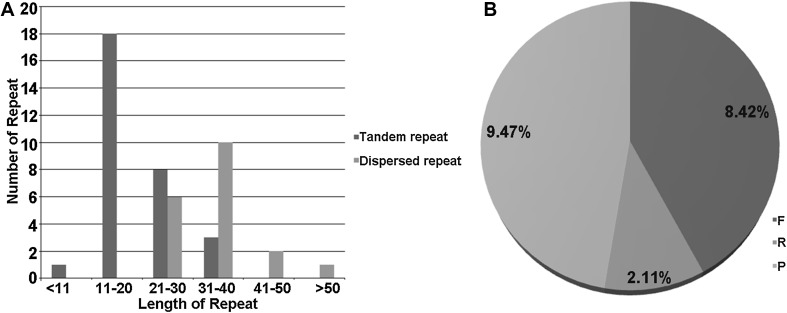
In most angiosperm plants, repeat regions regularly occurred in non-coding regions and frequent variation occurred due to illegitimate recombination and slipped-strand mispairing (Asano et al. 2004; Timme et al. 2007). The tandem repeats and dispersed repeat sequence analysis were realized by Tandem repeat finder and REPuter respectively in this study. Thirty-two tandem repeats were found, ranging from 9 to 40 bp. Twenty-one bp repeats were most common within five times, which was thrice in LSC and twice in IRs. The major distribution of repeat size was 11–21 bp (Fig. 3a). The number of tandem repeats in Chuanminshen cp genome was equivalent to that of crofton weed (Nie et al. 2012) and slightly more than bamboo (Zhang et al. 2011) whereas the longest tandem repeat size was shorter than the crofton weed with 85 bp and bamboo with 65 bp. To investigate the dispersed repeats in Chuanminshen cp genome, we adjusted the setting parameters of the REPuter described above. In total, 19 repeats were found and were divided into three categories, eight forward repeats, nine palindromic repeats and two reverse repeats. The maximum size of repeat was 64 bp. Repeat unit length in 30–40 bp was 16 including two reverse repeats, 41–50 bp was two, and the rest was one with 64 bp (Fig. 3).
Fig. 3.
Distribution frequency of disperse and tandem repeats in Chuanminshen cp genome. a The distribution frequency of repeats, x-axis Length of repeats; y-axis Number of repeats; Red bars stand for dispersed repeats, blue bars stand for tandem repeats. b The percentage distribution of dispersed repeat type. F Forward, P palindromic, R reverse
The results present here in regarding to the SSR and the repeat sequences promoted the identification of Chuanminshen species which laid the foundation for further phylogenetic studies and diversity analyses.
Phylogenetic analysis
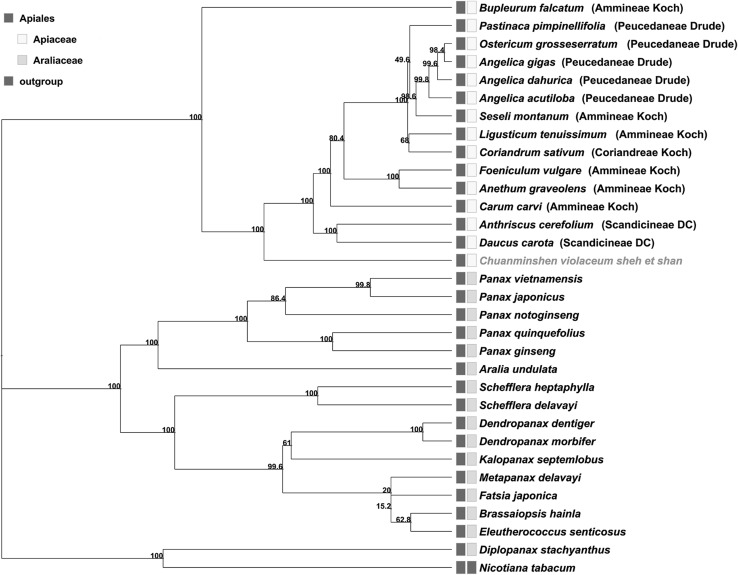
Chloroplast genome was successfully applied to phylogenetics in several angiosperms (Samson et al. 2007). Previous studies had two viewpoints in the phylogenetic analysis of Chuanminshen. In the first report, the phylogenetic position of Chuanminshen was placed in the Peucedaneae Drude based on the microstructural features of the fruit (She and Shan 1980). Beyond the traditional classification, by using the molecular markers ITS, RAPD and ISSR, Chuanminshen was clustered closely to Changium smyrnioides (Tao et al. 2008) and belonged to the genera Smyrnieae Koch which was supported based on the variation of psbA-trnH sequence (Song et al. 2014). In order to provide more evidence to solve the dispute throughout the current study, we performed phylogenetic analysis based on 80 common protein coding sequences, 30 plants of Araliaceae and Apiaceae with Nicotiana tabacum as an outgroup (Fig. 4). The phylogenetic tree constructed with Maximum Likelihood (ML) algorithms was carried out using MEGA7, the results suggested that the closest relationship to Chuanminshen was Bupleurum falcatum which belonged to Ammineae Koch, Anthriscus cerefolium and Daucus carota belonging to the Scandicineae DC. The three closer species are Carum carvi, Anethum graveolens, and Foeniculum vulgare which also were classified in the Ammineae Koch. Our analysis results suggested that Chuanminshen’s phylogenetic position was distant from the Peucedaneae Drude, whereas only the chloroplast genome represents maternal genetic code, so further confirmation of the phylogenetic relationship is still needed to combine the nuclear genome information.
Fig. 4.
ML phylogenetic tree of 31 plants of Apiales based on 80 protein coding sequence of cp genomes. Numbers above each node are bootstrap support values from 1000 replicates. The gray box indicates the taxa that belong to the Apliales, the yellow box indicates the taxa that belongs to Apiaceae, the green box indicates the taxa that belongs to Araliaceae, the purple box indicates the taxa that belongs to the outgroup
Acknowledgements
This work was supported by the Science and Technology Support Program of Sichuan Province, China (No. 2014FZ0053). The authors would like to thank Shangqing Hu director (Chinese herbal medicine planting research center of Sichuan province, China) for the Chuanminshen species identification.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no actual or potential conflicts of interest.
Footnotes
Can Yuan, Wenjuan Zhong and Fangsheng Mou have contributed equally to this work.
References
- Asano T, Tsudzuki T, Takahashi S, Shimada H, Kadowaki K. Complete nucleotide sequence of the sugarcane (Saccharum officinarum) chloroplast genome: a comparative analysis of four monocot chloroplast genomes. DNA Res. 2004;11:93–99. doi: 10.1093/dnares/11.2.93. [DOI] [PubMed] [Google Scholar]
- Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu Rev Genet. 2001;35:125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Chloroplast evolution: secondary symbiogenesis and multiple losses. Curr Biol. 2002;12:R62–R64. doi: 10.1016/S0960-9822(01)00675-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Peng C. Study on anti-fatigue and anti-oxidant effects of Chuanminshen violaceum. Res Pract Chin Med. 2011;25:28–30. [Google Scholar]
- Feng H, Fan J, Qiu H, Wang Z, Yan Z, Yuan L, et al. Chuanminshen violaceum polysaccharides improve the immune responses of foot-and-mouth disease vaccine in mice. Int J Biol Macromol. 2015;78:405–416. doi: 10.1016/j.ijbiomac.2015.04.044. [DOI] [PubMed] [Google Scholar]
- Flora C. China flora editorial board. Beijing: Academic Press by Beijing science, CHN, ISBN; 1979. [Google Scholar]
- Grassi F, Labra M, Scienza A, Imazio S. Chloroplast SSR markers to assess DNA diversity in wild and cultivated grapevines. Vitis. 2002;41:157–158. [Google Scholar]
- Jansen RK, Raubeson LA, Boore JL, dePamphilis CW, Chumley TW, Haberle RC, et al. Methods for obtaining and analyzing whole chloroplast genome sequences. Methods Enzymol. 2005;395:348–384. doi: 10.1016/S0076-6879(05)95020-9. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Lee HL. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004;11:247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S, Schleiermacher C. REPuter: fast computation of maximal repeats in complete genomes. Bioinformatics. 1999;15:426–427. doi: 10.1093/bioinformatics/15.5.426. [DOI] [PubMed] [Google Scholar]
- Lei X, Zhang M. Materia Medica. Pharm Clin Chin. 2012;3:34–38. [Google Scholar]
- Liu C, Shi L, Zhu Y, Chen H, Zhang J, Lin X, et al. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genom. 2012;13:715. doi: 10.1186/1471-2164-13-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. Erratum: SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2015;4:30. doi: 10.1186/s13742-015-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ. Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- Nie X, Lv S, Zhang Y, Du X, Wang L, Biradar SS, et al. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora) PLoS ONE. 2012;7:e36869. doi: 10.1371/journal.pone.0036869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida SK, Yadava DK, Mohapatra T. Microsatellites in Brassica unigenes: relative abundance, marker design, and use in comparative physical mapping and genome analysis. Genome. 2010;53:55–67. doi: 10.1139/G09-084. [DOI] [PubMed] [Google Scholar]
- Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W, Morgante M, McDevitt R, Vendramin GG, Rafalski JA. Polymorphic simple sequence repeat regions in chloroplast genomes: applications to the population genetics of pines. Proc Natl Acad Sci U S A. 1995;92:7759–7763. doi: 10.1073/pnas.92.17.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Song J, Gao H, Zhu Y, Xu J, Pang X, et al. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PLoS ONE. 2013;8:e57607. doi: 10.1371/journal.pone.0057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Lee SB, Jansen RK, Hostetler JB, Tallon LJ, Town CD, et al. Complete plastid genome sequence of Daucus carota: implications for biotechnology and phylogeny of angiosperms. BMC Genom. 2006;7:222. doi: 10.1186/1471-2164-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson N, Bausher MG, Lee SB, Jansen RK, Daniell H. The complete nucleotide sequence of the coffee (Coffea arabica L.) chloroplast genome: organization and implications for biotechnology and phylogenetic relationships amongst angiosperms. Plant Biotechnol J. 2007;5:339–353. doi: 10.1111/j.1467-7652.2007.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She M, Shan R. Cyclorhiza and Chuanminshen: two newly proposed genera in Umbelliferae (Apiaceae) Acta Phytotax Sin. 1980;18:45–49. [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, et al. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Yin Z, Li L, Cheng A, Jia R, Xu J, et al. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antivir Res. 2013;98:344–351. doi: 10.1016/j.antiviral.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Song C, Wu B, Zhou W, Liu Q. Analyses on relationship and taxonomic position of Chuanminshen Sheh et Shan (Apiaceae) based on variation of psbA-trnH sequence. Plant Resour Environ. 2014;23:19–26. [Google Scholar]
- Sugiura M. The chloroplast genome. Plant Mol Biol. 1992;19:149–168. doi: 10.1007/BF00015612. [DOI] [PubMed] [Google Scholar]
- Tao X, Gui X, Fu C, Qiu Y. Analysis of genetic differentiation and phylogenetic relationship between Changium smyrnioides and Chuanminshen violaceum using molecular markers and ITS sequences. J Zhejiang Univ (Agric Life Sci) 2008;34:473–481. [Google Scholar]
- Timme RE, Kuehl JV, Boore JL, Jansen RK. A comparative analysis of the Lactuca and Helianthus (Asteraceae) plastid genomes: identification of divergent regions and categorization of shared repeats. Am J Bot. 2007;94:302–312. doi: 10.3732/ajb.94.3.302. [DOI] [PubMed] [Google Scholar]
- Wicke S, Schneeweiss GM, dePamphilis CW, Muller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76:273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 2014;14:1024–1031. doi: 10.1111/1755-0998.12165. [DOI] [PubMed] [Google Scholar]
- Yi DK, Kim KJ. Complete chloroplast genome sequences of important oilseed crop Sesamum indicum L. PLoS ONE. 2012;7:e35872. doi: 10.1371/journal.pone.0035872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yu T, Su X, Zhang H. Physiochemical properties and the immunological activity of the Chuanmingshen polysaccharide. West China J Pharm Sci. 2007;22:396–398. [Google Scholar]
- Zhang YJ, Ma PF, Li DZ. High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae) PLoS ONE. 2011;6:e20596. doi: 10.1371/journal.pone.0020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Luo H, Xu Z, Zhu Y, Ji A, Song J, et al. Genome-wide characterisation and analysis of bHLH transcription factors related to tanshinone biosynthesis in Salvia miltiorrhiza. Sci Rep. 2015;5:11244. doi: 10.1038/srep11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Du L, Liu A, Chen J, Wu L, Hu W, et al. The complete chloroplast genome sequences of five Epimedium species: lights into phylogenetic and taxonomic analyses. Front Plant Sci. 2016;7:306. doi: 10.3389/fpls.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]