Abstract
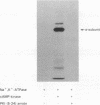
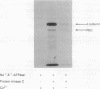
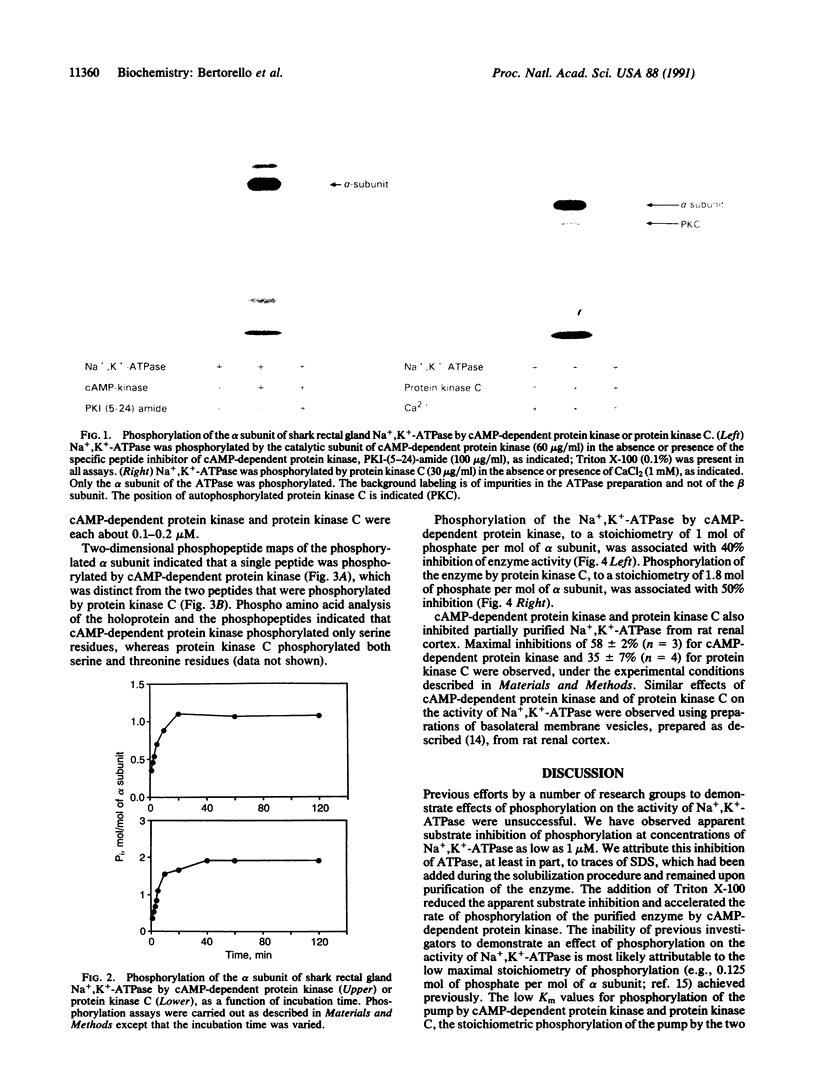
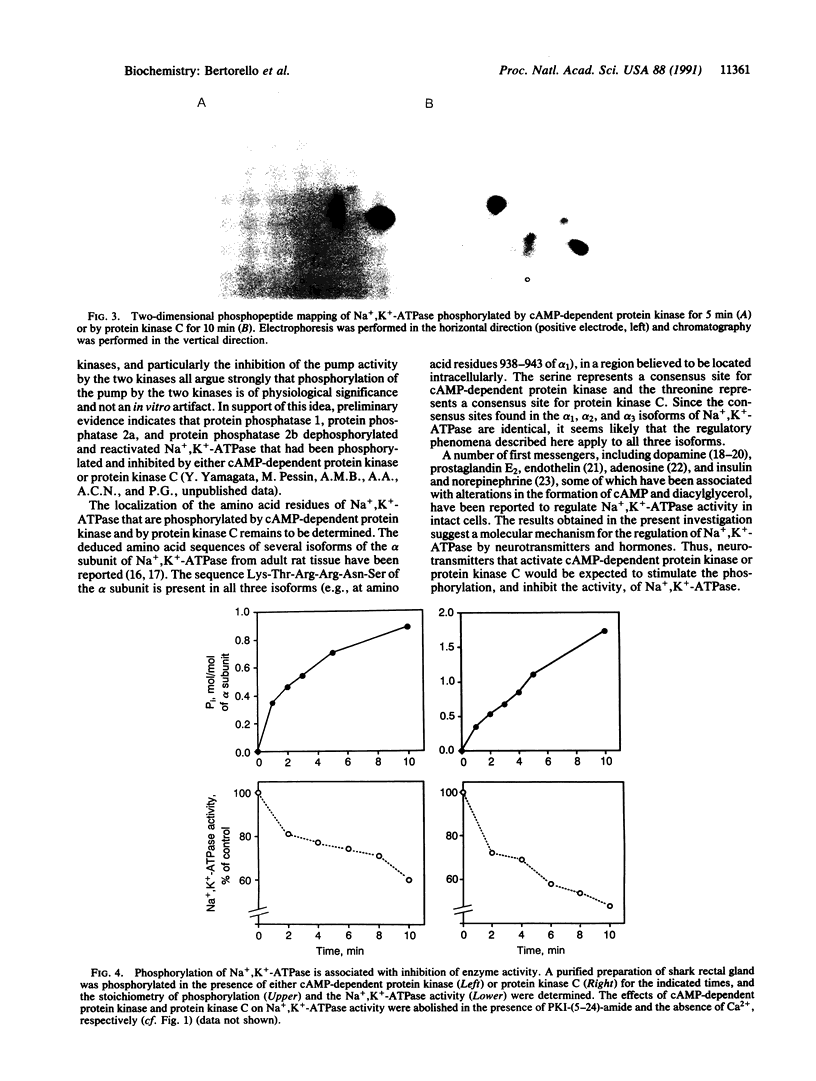
We have examined two distinct protein kinases, cAMP-dependent protein kinase and protein kinase C, for their ability to phosphorylate and regulate the activity of three different types of Na+,K(+)-ATPase preparation. cAMP-dependent protein kinase phosphorylated purified shark rectal gland Na+,K(+)-ATPase to a stoichiometry of approximately 1 mol of phosphate per mol of alpha subunit. Protein kinase C phosphorylated purified shark rectal gland Na+,K(+)-ATPase to a stoichiometry of approximately 2 mol of phosphate per mol of alpha subunit. The phosphorylation by each of the kinases was associated with an inhibition of Na+,K(+)-ATPase activity of about 40-50%. These two protein kinases also inhibited the activity of a partially purified preparation of Na+,K(+)-ATPase from rat renal cortex and the activity of Na+,K(+)-ATPase present in preparations of basolateral membrane vesicles from rat renal cortex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Aperia A., Fryckstedt J., Svensson L., Hemmings H. C., Jr, Nairn A. C., Greengard P. Phosphorylated Mr 32,000 dopamine- and cAMP-regulated phosphoprotein inhibits Na+,K(+)-ATPase activity in renal tubule cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2798–2801. doi: 10.1073/pnas.88.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Na+-K+-ATPase is an effector protein for protein kinase C in renal proximal tubule cells. Am J Physiol. 1989 Feb;256(2 Pt 2):F370–F373. doi: 10.1152/ajprenal.1989.256.2.F370. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Hökfelt T., Goldstein M., Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988 Jun;254(6 Pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- Brodie C., Sampson S. R. Regulation of the sodium-potassium pump in cultured rat skeletal myotubes by intracellular sodium ions. J Cell Physiol. 1989 Jul;140(1):131–137. doi: 10.1002/jcp.1041400116. [DOI] [PubMed] [Google Scholar]
- Celsi G., Nishi A., Akusjärvi G., Aperia A. Abundance of Na(+)-K(+)-ATPase mRNA is regulated by glucocorticoid hormones in infant rat kidneys. Am J Physiol. 1991 Feb;260(2 Pt 2):F192–F197. doi: 10.1152/ajprenal.1991.260.2.F192. [DOI] [PubMed] [Google Scholar]
- Clausen T., Everts M. E. Regulation of the Na,K-pump in skeletal muscle. Kidney Int. 1989 Jan;35(1):1–13. doi: 10.1038/ki.1989.1. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem. 1984 Dec 10;259(23):14491–14497. [PubMed] [Google Scholar]
- Herrera V. L., Emanuel J. R., Ruiz-Opazo N., Levenson R., Nadal-Ginard B. Three differentially expressed Na,K-ATPase alpha subunit isoforms: structural and functional implications. J Cell Biol. 1987 Oct;105(4):1855–1865. doi: 10.1083/jcb.105.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowndes J. M., Hokin-Neaverson M., Bertics P. J. Kinetics of phosphorylation of Na+/K(+)-ATPase by protein kinase C. Biochim Biophys Acta. 1990 Apr 9;1052(1):143–151. doi: 10.1016/0167-4889(90)90069-p. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem. 1987 May 25;262(15):7273–7281. [PubMed] [Google Scholar]
- Scalera V., Huang Y. K., Hildmann B., Murer H. A simple isolation method for basal-lateral plasma membranes from rat kidney cortex. Membr Biochem. 1981;4(1):49–61. doi: 10.3109/09687688109065422. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Preparation of membrane-bound and of solubilized (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. The effect of preparative procedures on purity, specific and molar activity. Biochim Biophys Acta. 1979 Apr 12;567(2):436–444. doi: 10.1016/0005-2744(79)90129-3. [DOI] [PubMed] [Google Scholar]
- Skou J. C. The Na,K-pump. Methods Enzymol. 1988;156:1–25. doi: 10.1016/0076-6879(88)56004-4. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Scharschmidt B. F. (Na,K)-ATPase-mediated cation pumping in cultured rat hepatocytes. Rapid modulation by alanine and taurocholate transport and characterization of its relationship to intracellular sodium concentration. J Biol Chem. 1983 Nov 10;258(21):12912–12919. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- Zeidel M. L., Brady H. R., Kone B. C., Gullans S. R., Brenner B. M. Endothelin, a peptide inhibitor of Na(+)-K(+)-ATPase in intact renaltubular epithelial cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]