Abstract
Two rice varieties PR-115 and Super-7 were imposed to water stress and different physiological traits were monitored to evaluate the performance of these varieties under drought. Under water stress condition although the relative water content, osmotic potential, chlorophyll content, photosynthesis rate, carbon discrimination and biomass decreased in both the varieties however, the reduction was more pronounced in Super-7 variety. Oryzanol a trans-ester of ferulic acid functions as antioxidant and it increased along with total phenolic and anthocyanin content in both the varieties under drought stress. However, gallic acid, 4 hydroxy benzoic acid, syringic acid and chlorogenic acid showed differential pattern in both of the varieties under water limiting conditions. Under drought, grain yield was penalized by 17 and 54% in PR-115 and Super-7 varieties, respectively in comparison to watered plants. Super-7 variety showed pronounced electrolyte leakage and MDA enhancement under water stress condition. High non photochemical quenching and reduction in Y(NO) and Y(II) indicated balanced energy management in tolerant PR-115 variety. The studies showed that PR-115 is a drought tolerant variety while Super-7 is drought sensitive in nature.
Keywords: Carbon discrimination, Drought, Oryza sativa, Oryzanol, Phenolic acids, Water use efficiency
Introduction
Rice (Oryza sativa L.) is one of the main grain crop of India and grown in almost all states. Rice contributes about 42% in country’s grain production. It not only forms the mainstay of people’s diet but the straw and bran is also an important cattle feed (Latha 2006). Rice is mainly grown under rainfed conditions in India. Thus the development of drought tolerant varieties and knowledge generation about tolerance has always been of higher priority. The best challenge for physiological researchers is to find out ways to reduce the water used in rice production. Water stress affects the growth and development of the plant and ultimately leads to a yield penalty or crop failure. Drought responses include alterations in their molecular, physiological, and biochemical mechanisms and also in their morphology (Chaves et al. 2003; Yousfi et al. 2016).
Drought stress is one of the major abiotic stress factors affecting the productivity of rice (Venuprasad et al. 2008). During drought stress osmotic pressure of the cells play a key role in maintaining the water potential of the plant (Osakabe et al. 2014). Plants adapt to water stress by increasing the osmotic pressure resulting due to increase in solutes. This osmotic adjustment ensures continuous water uptake during drought enabling the plant to maintain its turgidity, growth continuation and several physiological processes. During water stress, there is also increase in reactive oxygen species (ROS) which increases the amount of malondialdehyde (MDA). MDA is regarded as an indicator of oxidative damage caused by several stress (Møller et al. 2007). Thus crop productivity under water stress is dependent on relative water content (RWC) and osmotic potential. For better growth and development higher values of RWC and osmotic adjustment is required. The photosynthetic activity also decreases during drought stress (Blum 2009; Chaves et al. 2009) this is due to both stomatal and non-stomatal limitations, (Flexas and Medrano 2002). Water stress induces closure of stomata which reduces transpiration rate and uptake of CO2 (de Souza et al. 2013). An essential component under drought stress which determines plant productivity is water use efficiency (WUE). This factor is involved in improving performance of crop productivity under water stress conditions (Araus et al. 2002). Pigments also play a role in helping plants to withstand adversaries of drought. Chlorophyll an important component of photosynthetic apparatus absorbs light and transfers the light energy to the reaction centre of the photosystem. It has been reported that there is decrease in chlorophyll content and the maximum quantum yield of PSII (Fv/Fm) (Shukla et al. 2012) in drought stressed rice plants.
Carbon isotopic discrimination (Δ13C) has been used as selection marker for yield under drought (Adiredjo et al. 2014), which is the measure of 13C/12C ratio (δ13C) relative to the CO2 of atmosphere (Hall et al. 1994). It has been reported that drought increases δ13C and decreases Δ13C values in rice (Centritto et al. 2009) and the values vary with different genotypes. In C3 plants it is an indicator of the water use efficiency and this parameter is used by the plant breeders as a tool for selecting plants with high WUE under drought stress (Cattivelli et al. 2008).
Phytosterols are an important part of the lipid bilayer of cell membranes. These regulate fluidity of membranes and thus influence its properties structure and functions (Roche et al. 2008). It is reported that their levels increase in plants which are exposed to water stress which confirms the role of these phytosterols and their esters in tolerance of plants against water stress. Gamma-oryzanol, one of the phytochemical is composed of trans-ferulic acid esters (Lerma-Garcia et al. 2009) and triterpene alcohols (Cheruvanky 2003) that are found in high concentration in rice. γ-oryzanol scavenges ROS which are produced due to alteration in photosynthetic electron transport during water stress (Kumar et al. 2014).
In our present study we have studied two rice varieties PR-115 and Super-7 under control and drought condition. Our study includes physiological traits as RWC, osmotic potential, relative conductivity, pigment concentrations, photosynthetic response, including the transpiration rate and stomatal conductance, carbon discrimination along with biochemical assays associated with stress tolerance. Our study also analyzes the role of y-oryzanol and phenolic acids as antioxidants during drought stress.
Materials and methods
Plant material and growth conditions
Two rice (Oryza sativa L.) varieties namely PR-115 and Super-7 where procured from Directorate of Seed Research Institute, Mau (Uttar Pradesh, India). These two varieties were sown in trays for sapling formation in glass house. Saplings of 6-8 inches were transplanted into another pot after a period of one month. Plants were subjected to water stress after one month from the day of transplantation (2 months after germination). During water stress the controlled plants were watered daily with 750 ml of water while in drought treated plants watering was restricted by giving 200 ml of water every day. The plants were subjected to 10 days of water stress and later on watered normally and harvested.
Relative water content
Three to five mature leaves at 0, 1st, 4th, 7th, and 10th day of drought were harvested early morning and the fresh weight was recorded. These leaf samples were kept overnight in water to attain turgidity. Turgid weight (TW) was measured the next day and then these were kept in oven at 70 °C for 72 h. The dry weight was recorded andrelative water content was calculated as
Measurement of the osmotic potential
Osmotic potential at different level of subjected drought was determined in the rice leaves using a Wescor 5520 osmometer (Wescor, Logan, Utah, USA) as described by Ball and Oosterhuis (2005).
Measurement of membrane damage
Leaves discs of watered and 10 day drought treated plants were incubated in 5 ml of distilled water, and stored for 4 h at room temperature. Electrical conductivity (EC) was measured with a conductivity meter (Orion, Thermo Electron Corp.) and then the leaf tissues were boiled for 15 min to ensure that the residual electrolytes have been removed from leaf tissues, cooled to room temperature, and the EC was measured for the second time (Raymond et al. 1986). The % relative conductivity was calculated as:
where, Ci and Ct is the electrical conductivity before and after boiling of the sample.
Gas exchange measurements
The net photosynthesis rate (A), transpiration (E), stomatal conductance (gs) and WUE were measured in fully expanded rice leaves at the regular interval with portable photosynthesis system (Li-Cor, Lincoln, Nebraska, USA). The photosynthetic photon flux density was maintained at 800 μmol m−2 s−1, CO2 level in the leaf cuvette was 400 ppm, temperature of the leaf was at 30 °C and vapour pressure deficit level was less than 2 KPa.
Chlorophyll fluorescence studies
Dual PAM-100 (walz Effeltrich, Germany) was used for measuring chlorophyll fluorescence of PSII and P700 oxidation at the 10th day of drought.
Electron transport rates (ETR) were measured by using saturated pulse method under control and drought conditions. Different energy proportions of PSI and II were also recorded by Dual PAM-100. The cyclic electron flux was calculated as difference between ETR(I) and ETR(II) (Singh et al. 2014).
MDA estimation
Lipid peroxidation levels in plants was determined on the last day of drought as malondialdehyde (MDA) (Heath and Packer 1968). About 0.2 g leaves of rice were extracted in 1 ml of 0.25% TBA made in 10% TCA. This solution was heated for 30 min at 95 °C and quickly cooled in ice. This extract was centrifuged for 10 min at 10,000 rpm. The supernatant was taken for absorbance at 532 nm. This was subtracted from absorbance value at 600 nm which is correction for nonspecific turbidity. The level of lipid peroxidation is expressed as micromole of MDA formed using an extinction coefficient of 155 mM cm−1.
Pigments estimation
Leaf tissue of 20–25 mg was collected each from control and drought treated plants and were kept in 1 ml of 80% acetone in dark for overnight or till complete extraction of pigment, OD were recorded at 470, 647 and 663 nm for chl a, chl b and carotenoids determination according to Wellburn (1994).
For anthocyanin estimation, leaf samples of 30–35 mg were homoginized in 1% acidified methanol. The absorbance of supernatant was observed at 530 and 650 nm. Using the corrected OD the total anthocyanin content was calculated by (Murray and Hackett 1991).
Total phenolic content measurement
Total phenolic content (TPC) was estimated by Folin-Ciocalteu method. About 30 mg of leaf tissue were incubated in 1 ml of 95% methanol for 48 h in dark. The sample was then centrifuged at 10,000 rpm for 5 min at room temperature and supernatant was collected. Then 100 µl of each sample supernatant was taken and to this 200 µl 10% Folin-Ciocalteu reagent was added and vortexed. Then 800 µl 700 mM Na2CO3 was added and incubated for 2 h at room temperature in dark. The absorbance was read at 765 nm. Total phenolic content was expressed as µg g−1 of FW.
Isotope ratio mass spectrometry (IRMS) analysis
Leaf, stem, root, husk, filled and unfilled grain tissue were collected from control and 15 day drought treated rice plants at the end of the experiment after harvesting the crop. Samples were dried at 70 °C for a week. Dried samples were crushed in fine powder and carbon isotopic determination analyses were performed on the Isotope Ratio Mass Spectrometry (IRMS) according to Singh et al. (2016).
Oryzanol estimation
Mature grain samples which were partially dry were collected from well watered and drought treated plants after harvesting at the end of the experiment, samples were oven dried at 50 °C for 72 h and crushed to a fine powder. Sample powder of 15 gm was extracted with hexane by hot extraction method. Further 10 mg of oil was dissolved in hexane and OD was determined at 314 nm. Oryzanol content was calculated as mentioned by Misra and Nandi (2014).
where, 358.9 is the specific extinction coefficient of oryzanol.
Quantification of specific phenolic acids by HPLC
In 10 ml 50% methanol 100 mg leaf sample was crushed. Solution was centrifuged at 15,000 rpm kept for 15 min and then the supernatant was filtered through nylon filters (Milipore) of 0.45 mm. 20 µl of prepared sample was analyzed through an HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with two Shimadzu LC-10 ATVP reciprocating pumps, SPD-M20A PDA detector, an integrator, and Shimadzu 10VP software for data recording and processing. The analysis of Reverse-phase chromatography was carried by using a C-18 reverse-phase HPLC column [(250 × 4.6-mm id, particle size 5 micron) out in isocratic conditions, Phenomenex, US] at 25 °C. Elution was carried out at a flow rate of 0.6 ml min−1 with water:acetic acid (99.0:1.0 v/v) as solvent A and acetonitrile as solvent B. A gradient elution with 20–35% of solvent B in 0–14 min was used for 14–40 min with 35–50% of solvent B. In the sample loop samples were injected three times, and for quantification the mean of the peak areas of individual compounds was taken. Gallic, 4 hydroxybenzoic, coumaric, syringic, chlorogenic, rutin and ferulic acids were used as internal and external standards. Phenolic compounds present in the leaf samples were identified by comparing retention time (Rt) of standards. Comparing the peak areas of reference compounds with known concentrations were calculated which was run under the same elution conditions. Plain mobile phase was used as control for identification of blank peaks.
Statistical analysis
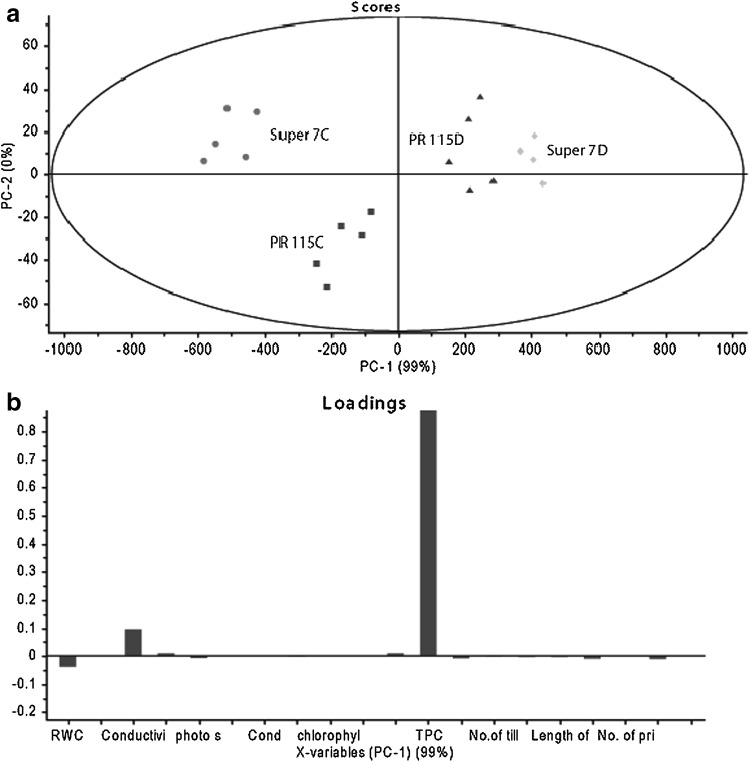
ANOVA was performed to study the level of significance between watered and drought-treated samples for all physiological data using SAS 9.1 (SAS institute). The PCA score plot has been ploted on the Unscrambler X Software package (Version 10.0.1, CAMO, USA). The Loading plots have been taken to differentiate the parameters between watered and drought. The five reading of all the parameter of control and treated sample were taken for the score plot of PCA.
Results
Changes in morphological characters
Drought stress reduces the plant growth in both rice varieties. Plant height, number of total tillers did not change in both rice varieties under water stress. However, number of productive tillers, primary and secondary spikelet and weight of 100 seeds were decreased in Super-7 varieties under drought stress. Overall grain yield reduced by 6 and 32% in PR-115 and Super-7 varieties respectively under water stress condition (Table 1).
Table 1.
The growth parameters in the two contrasting rice varieties
| Variety | Treatment | Plant height (cm) | No. of tillers/plant | Length of flag leaf (cm) | Length of spikelet (cm) | No. of spikelet | 100 seed weight (gm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | Productive | Primary | Secondary | ||||||
| PR-115 | Control | 73.44 ± 4.64 | 5 ± 1 | 5 ± 1 | 26.00 ± 2.65 | 28.11 ± 1.27 | 10 ± 1 | 21 ± 2 | 2.34 |
| Drought | 69.67 ± 5.85 | 5 ± 1 | 5 ± 1 | 24.89 ± 1.69 | 26.00 ± 2.24 | 10 ± 1 | 27 ± 2 | 2.18 | |
| Super-7 | Control | 72.22 ± 5.29 | 6 ± 1 | 5 ± 1 | 25.56 ± 2.19 | 29.78 ± 1.56 | 8 ± 1 | 34 ± 3 | 2.53 |
| Drought | 68.00 ± 11.11 | 5 ± 1 | 3 ± 1 | 26.00 ± 1.41 | 19.00 ± 1.58 | 6 ± 1 | 20 ± 2 | 1.70 | |
Physiological attributes of two rice varieties under water limiting conditions
Two rice varieties were imposed to drought stress and relative water content of the leaf was recorded. In PR-115 and Super-7 varieties RWC was decreased by 25 and 33% respectively during the 10 days of drought (Fig. 1a). Under drought stress with the decrease of RWC, osmotic potential of the leaf also decrease in Super-7 varieties, however, the relative decrease in osmotic potential in PR-115 variety was less as compared to that of Super-7 (Fig. 1b). Cell membrane damage monitored by electrolyte leakage and MDA (malondialdehyde) content of the leaf increased in both of the studied rice varieties under 10 days water stress condition (Fig. 1c, d). Rate of photosynthesis (A) decreased with drought stress in the studied rice varieties. Net photosynthesis (A) measured was 1.8 times higher in PR-115 variety in comparison to Super-7 variety at 10th day of drought (Fig. 2a). With the decrement in photosynthesis rate, transpiration and stomatal conductance also decreased under the drought condition. Stomatal conductance was 1.6 times higher in PR-115 variety while transpiration rates were almost similar (1.1 times higher in PR-115) in both of the varieties on the 10th day of drought (Fig. 2b, c). Water use efficiency (WUE) increased in PR-115 variety, however, in Super-7 variety it decreased rapidly at 4th, 7th and 10th day of drought (Fig. 2d). Photosynthesis rate mainly depends on the stomatal regulation and pigment concentration of the leaf tissue. Under drought stress (10th day) chlorophyll content decreased in PR-115 variety while did not show any change in Super-7 variety (Fig. 3a). Anthocyanin content showed an increase by 2.3 fold in PR-115 variety while carotenoids decreased in both of the rice varieties under drought stress (Fig. 3b, c). Total phenolic content (TPC) increased by 1.5 and 1.9 fold in both PR-115 and Super-7 varieties respectively, on 10th day of water stress condition (Fig. 3d).
Fig. 1.
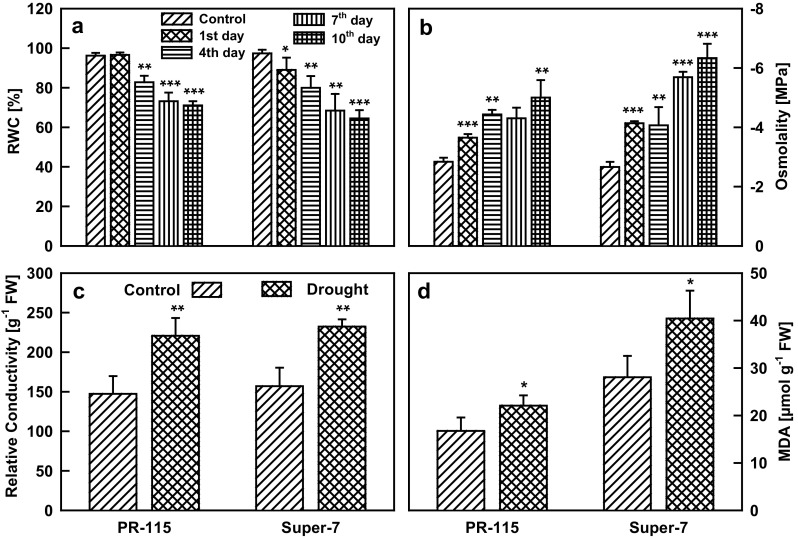
The relative water content (a), osmotic potential (b), electrolyte leakage as relative conductivity (c) and MDA content (d) in leaves of the two rice varieties under control and 1, 4, 7, 10 days drought treated conditions. Data showed as average ± standard deviation of five samples. Level of significance considered as P value *** <0.0001; ** <0.001; * <0.01
Fig. 2.
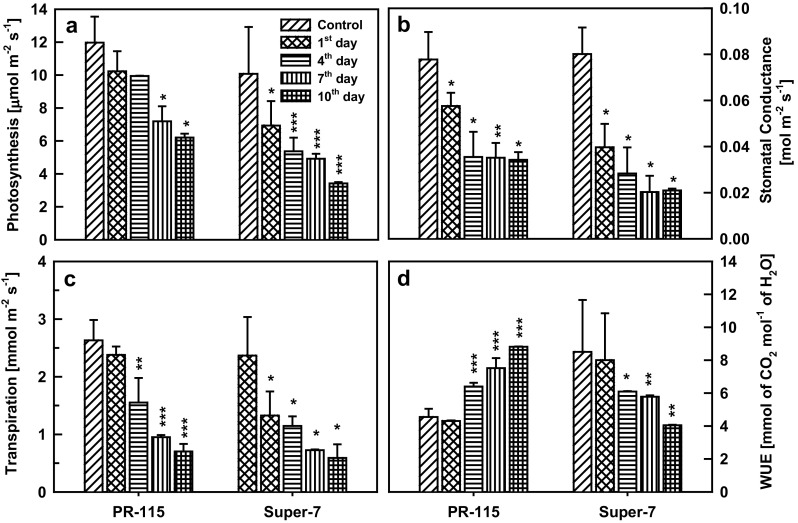
Net photosynthesis rate (a), stomatal conductance (b), transpiration rate (c), and water use efficiency (d) in leaves of PR-1115 and Super-7 varieties at 0, 1st, 4th, 7th and 10th day of drought. Data showed as average ± standard deviation of five samples. Level of significance considered as P value *** <0.0001; ** <0.001; * <0.01
Fig. 3.
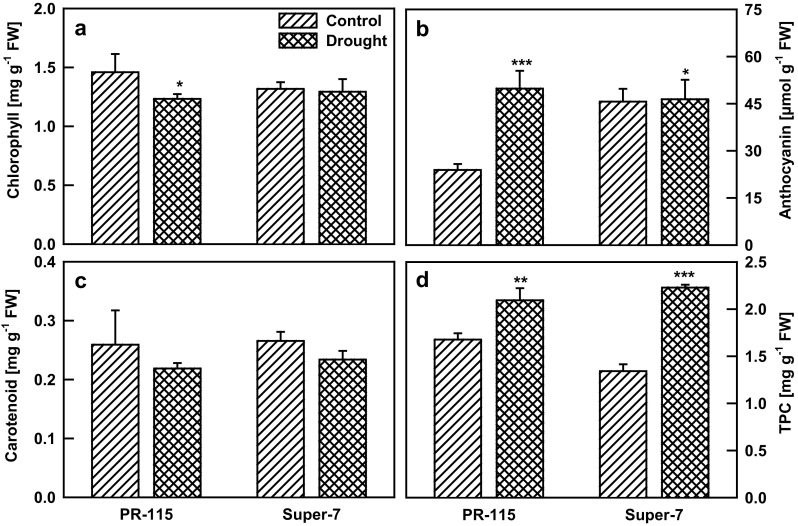
Chlorophyll (a), carotenoid (b), anthocyanin (c), and total phenolic content (d) of two rice varieties under control and 10 days drought stress. Data showed as average ± standard deviation of five samples. Level of significance considered as P value *** <0.0001; ** <0.001; * <0.01
Changes in energy fractions of PSI and PSII under water limiting conditions
Photosynthesis rate is affected by water stress as it affects both the PS (I) and PS (II) rates. Photochemical quantum yield of energy conversion of PSI, Y (I) slightly increased in PR-115 variety while decreased in Super-7 variety with increase in photochemical quantum yield of PSI due to donor side limitation Y(ND) (Fig. 4a, c). However, photochemical quantum yield of PSII decreased in both the varieties under drought stress condition (Fig. 4b). Non regulated energy dissipation, Y(NO) decreased in PR-115 variety while increased in Super-7 variety under drought stress (Fig. 4d). Photochemical quantum yield of PSI due to acceptor side limitation Y(NA) decreased in both of the varieties under drought stress (Fig. 4e). Regulated energy dissipation Y(NPQ) showed differential pattern in both of the studied rice varieties under drought stress condition (Fig. 4f).
Fig. 4.
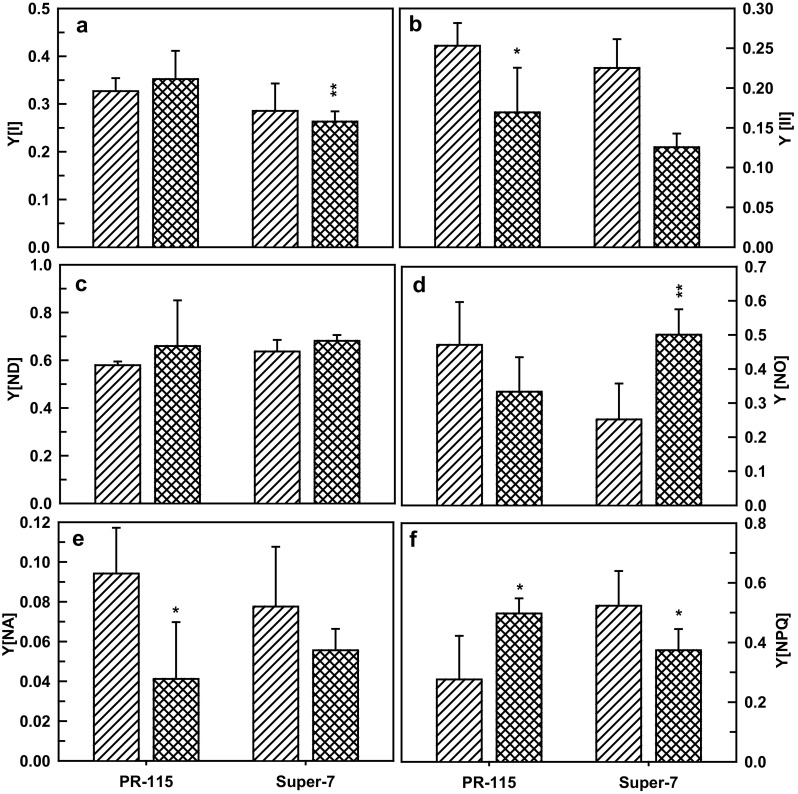
Photochemical quantum yield of PSI (a), photochemical quantum yield of PSII (b), photochemical quantum yield of PSI due to donor side limitations (c), photochemical quantum yield of PSII due to non-regulated energy dissipation (d), photochemical quantum yield of PSI due to acceptor side limitations (e), photochemical quantum yield of PSII due to regulated energy dissipation (f) in leaves of PR-1115 and Super-7 rice varieties under control and 10th day of drought stress. Data showed as average ± standard deviation of five samples. Level of significance considered as P value ** <0.001; * <0.01
Effect of water limitations on carbon discrimination and carbon translocation
Carbon discrimination (Δ13C) is measured as long term water use efficiency and depends on the capacity of carbon assimilation of the leaf tissue under stress condition. In our study Δ13C decreased in leaf, root and unfilled husk of the PR-115 variety while increased in stem, peduncle, filled husk and grain of the drought treated tissue under drought condition. However, in Super-7 variety Δ13C decreased in all tissues except root of the plant under water limiting condition (Fig. 5a, b). % carbon content increased in all tissues in PR-115 variety, while in Super-7 variety it increased only in root and peduncle tissue under drought condition (Fig. 5c, d). % Nitrogen content decreased in all tissues except unfilled husk in PR-115 variety under drought condition. However, in Super-7 variety % nitrogen increased in all tissues except peduncle under water stress condition (Fig. 5e, f). Drought stress showed decrease in leaf, stem, root and grain biomass accumulation in both of the varieties under water stress condition. Leaf biomass decreased by 12 and 30% in comparison to watered plants in PR-115 and Super-7 variety respectively (Fig. 6A). Reduction in stem biomass was similar in both of the varieties at 18% under drought stress (Fig. 6b). In PR-115 variety root biomass was comparatively higher in comparison to Super-7 varieties in control as well as drought condition (Fig. 6c). Grain yield penalty in PR-115 and Super-7 Variety was 17.5 and 54.8% respectively, under drought stress (Fig. 6d). The yield penalty persisted even on re-watering the plants after the water stress experiment at the time of harvesting.
Fig. 5.
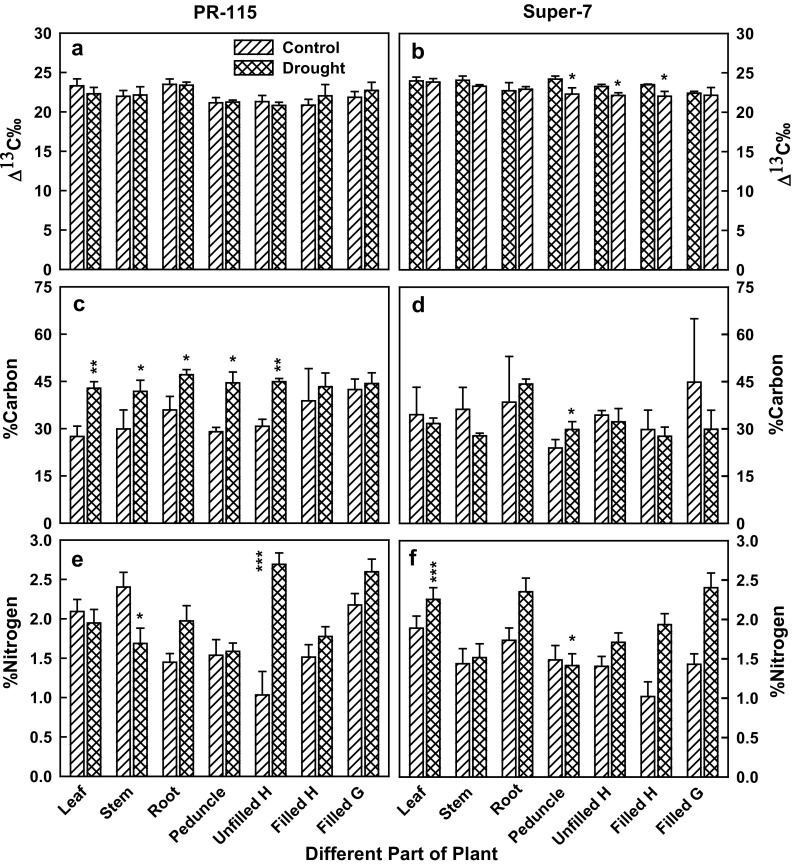
Carbon discrimination in PR-115 (a) and Super-7 (b), % carbon content of PR-115 (c) and Super-7 (d), % N content of PR-115 (e) and Super-7 (f) under control and 10th day drought condition in different parts of the plant. Data showed as average ± standard deviation of five samples. Level of significance considered as P value *** <0.0001; ** <0.001; * <0.01
Fig. 6.
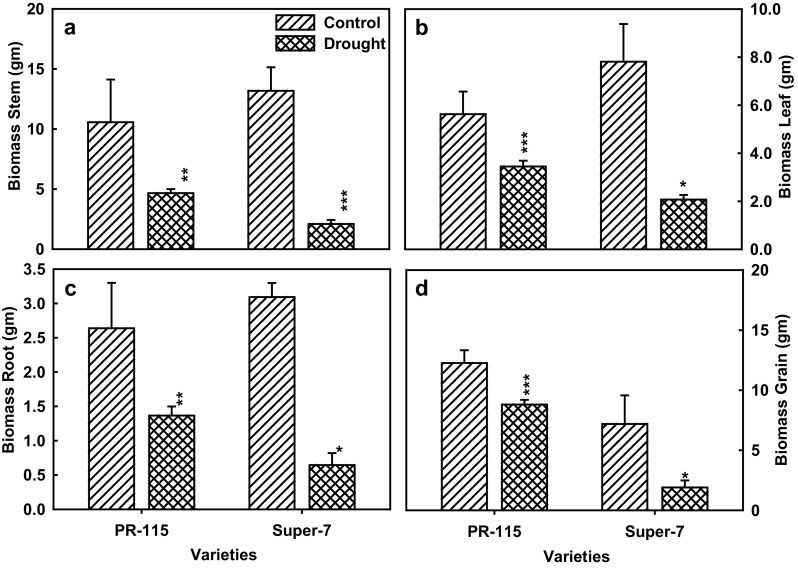
The % increase in biomass of leaf (a), stem (b), root (c) and grain (d) tissue of PR-115 and Super-7 rice varieties under control and 10th day drought. Data showed as average ± standard deviation of five samples
Effect of drought on oryzanol and its precursor ferulic acid content
Oryzanol a trans-ester of ferulic acid functions as antioxidant under water stress condition. Oryzanol content increased in both the studied rice varieties under drought stress (Fig. 7a). Chemical profiling of the leaf tissue showed an increase in ferulic acid and decrease in rutin and para-coumaric acid in both the varieties under drought stress condition. However, gallic acid, 4 hydroxy benzoic acid, syringic acid and chlorogenic acid showed differential pattern in both of the varieties under water limiting conditions (Fig. 7b, c).
Fig. 7.
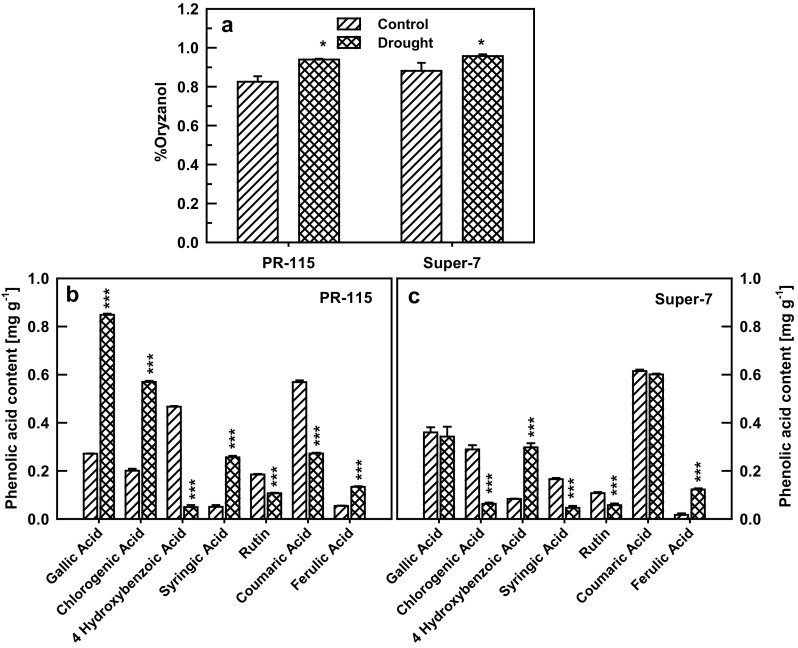
Bran oryzanol content (a) and chemical profiling of leaf tissue of PR-115 (b) and Super-7 (c) varieties under control and 10th day drought. Data showed as average ± standard deviation of five samples. Level of significance considered as P value *** <0.0001; * <0.01
Data analysis
Principal component analysis of both rice varieties showed that on the basis of physiological parameters under control condition PR-115 and Super-7 varieties grouped in different clusters. However, under drought stress both varieties grouped in single group due to higher TPC content under drought (Fig. 8a, b).
Fig. 8.
Principal component analysis of two rice varieties under control and drought conditions (a), Loadings plot of variance showed treated population on the +ve y axis, while control on –ve y axis (b)
Discussion
Drought is an important abiotic stress which affects all plant growth processes. It alters plant metabolism so that it overcomes the prolonged periods of drought for its survival. Drought stress causes limitation in various physiological activities these stress induced responses depend upon duration of exposure, stage of crop and their intensity rate on the crop. It is reported that the decrease in photosynthesis rate is due to stomatal closure in drought exposed plants. During drought stress the relative water content of plant decreases to an extent of 60–80% with increase in osmotic potential of the plant cells (Singh et al. 2015). This increases the level of osmolytes and ensures the plant to maintain its water content during drought enabling the plant to sustain its growth and yield (Blum 2005). Drought stress leads to increase in the amount of malondialdehyde (MDA) through lipid peroxidation which is due to increase in level of ROS. This increment in levels of MDA can be correlated with increase in relative conductivity (electrolyte leakage) of cell membranes under water stress (Fig. 1). Water use efficiency is an important component in determining yield of plant under drought stress. The decrease in photosynthesis, transpiration and stomatal conductance are directly correlated to it. Chlorophyll and carotenoids also help plant to withstand consequences of drought. It has been reported that the decrease in photosynthesis leads to degradation of these pigments. These absorb light and thus perform several photochemical processes of photosynthesis (Polívka and Frank 2010). These pigments also maintain CO2 assimilation rates and thus the proper functioning of the two photosystems of photosynthetic cells is ensured (Baker 2008). Carotenoids protect plants from oxidative damage caused by ROS production during drought and thus show several roles in drought tolerance (Loggini et al. 1999). Anthocyanin is another pigment which helps plant in withstanding adversaries of drought and during leaf development (Ranja et al. 2014) its increase in drought is an adaptive response of plant. Anthocyanin protects plant from photoinhibitionagainst high light stress (Hogewoning et al. 2012). Carotenoid pigments strengthen the photosystems during the water stress condition because it absorbs the excess light energy and transfers it to the photosystems. However, anthocyanin pigments function as attenuator, it absorbs the excess light but does not transfer light energy to the photosynthesis systems (Ranja et al. 2014).
Fluorescence and gas exchange measurements depicted as electron transport through PSI and PSII decreased in response to drought due to CO2 limitation caused by stomatal closer. Simultaneously Y(NPQ) increases in tolerant variety under drought stress, alleviating the excitation pressure placed on PSII; however, in sensitive variety Super-7, Y(NPQ) decreased (Fig. 4f) due to slowing of down-regulation of energy dissipation (Epron and Dreyer 1992). Regulation of energy dissipation by xanthophyll cycle decreased but non-regulated energy dissipation (YNO) increased (Fig. 4c) in sensitive variety during drought, this may be due to inhibition of proton gradient across the transthylakoidal membrane (Huang et al. 2012). Several studies have reported down-regulation of ETR(I) and ETR(II) and activation of Y(NPQ) and Y(ND) during drought (Zhang et al. 2011; Singh et al. 2014). NPQ is dependent on the formation of a proton gradient across the thylakoid membrane and protecting PSII against excess excitation pressure (Lehtimaki et al. 2010). However, an increase in electrolyte leakage along with MDA content showed structural damage in membranes and has often been used as a measure of drought tolerance (Pramachandra et al. 1990). Damaged membrane causes reduced mobility of the electron transport chain carriers and a reduced proton gradient across the thylakoid membrane (Kaiser 1987). Thus, lack of proton gradient decreased Y(NPQ) in sensitive variety under water stress (Fig. 4f). Significant decrease in photochemical quantum yield of PSII is related to a reduction in the reoxidation rate of the primary electron acceptor QA. Due to drought stress PSII reaction centers are unable to utilize the light energy and pass on electrons to the further e− acceptors.
The carbon isotope discrimination (Δ13C) technique for screening plants for their tolerance under drought conditions is well established (Wright et al. 1994; Scartazza et al. 1998; Centritto et al. 2009). It is reported to be negatively correlated to water use efficiency (WUE) as the diffusion of CO2 from stomata to the intercellular space affects the Δ13C of the whole plant (Farquhar et al. 1989). In the present study, the roots, leaves, stem, grains, and peduncle showed variations in carbon isotope discrimination (Fig. 5a, b). One of the several causes of these differences is the variation in developmental stages and condition of these organs which consequently assimilates carbon (Scartazza et al. 1998).The different lipid composition and fractionation processes during translocation of assimilates may also be the reason of such variations in Δ13C (Farquhar et al. 1989). Higher biomass and low carbon discrimination value of root and stem tissue were observed in drought plants of PR-115 and Super-7 varieties respectively (Figs. 5a, b, 6b, c). Accumulation of carbohydrates in stem and root tissue may be another alternative strategy for adapting to drought stress, the latter being beneficial where water is available at depth (Lopes and Reynolds 2010). Under drought stress condition larger carbohydrate accumulation in root tissue of tolerant variety suggest better osmotic adjustment in roots which may be of importance for the maintenance of vital processes and for recovery after drought (Nicolas et al. 1985). However, Rodrigues et al. (1995) also reported increase in stem biomass under drought stress in Lupinus albus and explained as maintenance of seed production in water-stressed plants by their ability to accumulate assimilates in the stem, which would be diverted to the ears during the seed filling stage.
Plants exposed to water stress have increased levels of phytosterols like oryzanol, which play a role in drought tolerance by strengthening the cell membrane (Kumar et al. 2014). This stabilizing property is due to its three-dimensional interactions with lipid layer of membranes and its polarity. The protective effect of oryzanol also includes scavenging of ROS produced due to oxidative stress. These functions provide tolerance to plants against water stress (Kumar et al. 2014). Both PR-115 and Super-7 showed significant increase in % oryzanol content under water stress (Fig. 7a). While the HPLC profiling of the oryzanol showed increase in ferulic acid under drought which serves as the precursor for the oryzanol biosynthesis (Fig. 7b, c). Enhanced antioxidant activity and oryzanol content is also reported to increase under elevated temperature and long term organic nutrient management condition (Britz et al. 2007; Zhou et al. 2002). Phenolics are believed to be involved in releasing oxidative stress which is caused due to accumulation of ROS during water stress (Sgherri et al. 2004). In both of the studied varieties the TPC increased in drought stress (Fig. 3d), this TPC serves as antioxidant in plant defense mechanism and protect the plant from molecular damage (Yu 1994; Halliwell 1994).
Conclusions
Two rice varieties having difference in tolerance potential against drought were studied on the basis of physiological attributes and oryzanol yield. Under water stress condition carbon assimilation decreased in both of the varieties but their translocation varied in different tissues. Tolerant PR-115 variety showed higher root biomass, low carbon discrimination and increased % oryzanol content along with higher phenolic acids. These function as antioxidants and protect the tissue from ROS damages.
Acknowledgements
Authors are thankful to The Director, CSIR-National Botanical Research Institute, Lucknow, for the facilities and for the financial support from the 12th Five Year Plan, Supra-Institutional project BSC-0204.
Authors’ contributions
All the experiments were performed by FK and PU. RS assisted in conceptualization guiding and supervising the experimental work, data collection and manuscript preparation. PKS assisted in conceptualization, guiding and supervising the experimental design and data collection. PAS was responsible for the overall conceptualization and supervision of the experiments, interpretation of the results and finalization of the manuscript.
Abbreviations
- ∆13C
Carbon isotope discrimination
- δ13C
13C/12C ratio relative to the CO2 of atmosphere
- A
Net photosynthesis rate
- DW
Dry weight
- E
Transpiration
- EC
Electrical conductivity
- ETR
Electron transport rate
- Fv/Fm
Maximum quantum yield of PSII
- FW
Fresh weight
- gs
Stomatal conductance
- IRMS
Isotopic ratio mass spectroscopy
- MDA
Malondialdehyde
- PS
Photosystem
- ROS
Reactive oxygen species
- RWC
Relative water content
- TPC
Total phenolic content
- TW
Turgid weight
- WUE
Water use efficiency
- Y(I)
Photochemical quantum yield of energy conversion of PSI
- Y(II)
Photochemical quantum yield of PSII
- Y(NA)
Photochemical quantum yield of PSI due to acceptor side limitation
- Y(ND)
Photochemical quantum yield of PSI due to donor side limitation
- Y(NPQ)
Regulated energy dissipation
- Y(NO)
Non-regulated energy dissipation
Compliance with ethical standards
Conflict of interest
The authors wish to state that we have no conflict of interest.
Contributor Information
Furqan Khan, Email: khanfurqan52@gmail.com.
Priyanka Upreti, Email: priyankaupreti1@gmail.com.
Ruchi Singh, Email: ruchi85.biotech@gmail.com.
Pradeep Kumar Shukla, Email: pradeepshuklak@yahoo.co.in.
Pramod Arvind Shirke, Phone: + 91 522 2297928, Email: pashirke@nbri.res.in.
References
- Adiredjo AL, Navaud O, Muños S, Langlade NB, Lamaze T, Grieu P. Genetic control of water use efficiency and leaf carbon isotope discrimination in sunflower (Helianthus annuus L.) subjected to two drought scenarios. PLoS One. 2014;9(7):e101218. doi: 10.1371/journal.pone.0101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. Plant breeding and drought in C3 cereals: what should we breed for? Ann Bot. 2002;89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Ball RA, Oosterhuis DM. Measurement of root and leaf osmotic potential using the vapor-pressure osmometer. Env Exp Bot. 2005;53:77–84. doi: 10.1016/j.envexpbot.2004.03.003. [DOI] [Google Scholar]
- Blum A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Crop Pasture Sci. 2005;56:1159–1168. doi: 10.1071/AR05069. [DOI] [Google Scholar]
- Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop Res. 2009;112:119–123. doi: 10.1016/j.fcr.2009.03.009. [DOI] [Google Scholar]
- Britz SJ, Prasad PVV, Moreau RA, Allen LH, Kremer DF, Boote KJ. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice.J Agr. Food Chem. 2007;55:7559–7565. doi: 10.1021/jf0637729. [DOI] [PubMed] [Google Scholar]
- Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Stanca AM. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res. 2008;105:1–14. doi: 10.1016/j.fcr.2007.07.004. [DOI] [Google Scholar]
- Centritto M, Lauteri M, Monteverdi MC, Serraj R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J Exp Bot. 2009;60:2325–2339. doi: 10.1093/jxb/erp123. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruvanky R. Phytochemical products: rice bran. In: Johnson I, Williamson G, editors. Phytochemical functional foods. Sawston: Woodhead Publishing; 2003. pp. 347–376. [Google Scholar]
- de Souza TC, de Castro EM, Magalhães PC, Lino LDO, Alves ET, de Albuquerque PEP. Morphophysiology, morphoanatomy, and grain yield under field conditions for two maize hybrids with contrasting response to drought stress. Acta Physiol Plant. 2013;35:3201–3211. doi: 10.1007/s11738-013-1355-1. [DOI] [Google Scholar]
- Epron D, Dreyer E. Effects of severe dehydration on leaf photosynthesis in Quercus petruea (Matt.) Liebl: photosystem II efficiency, photochemical and nonphotochemical fluorescence quenching and electrolyte leakage. Tree Physiol. 1992;10:273–284. doi: 10.1093/treephys/10.3.273. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol. 1989;40:503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Richards RA, Condon AG, Wright GC, Farquhar GD. Carbon isotope discrimination and plant breeding. In: Janick J, editor. Plant breeding reviews. New York: Wiley; 1994. pp. 81–113. [Google Scholar]
- Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–190. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hogewoning SW, Wientjes E, Douwstra P, Trouwborst G, Van Ieperen W, Croce R, Harbinson J. Photosynthetic quantum yield dynamics: from photosystems to leaves. Plant Cell. 2012;24:1921–1935. doi: 10.1105/tpc.112.097972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yang SJ, Zhang SB, Zhang JL, Cao KF. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta. 2012;235:819–828. doi: 10.1007/s00425-011-1544-3. [DOI] [PubMed] [Google Scholar]
- Kaiser WM. Effects of water deficit on photosynthetic capacity. Physiol Plant. 1987;71:142–149. doi: 10.1111/j.1399-3054.1987.tb04631.x. [DOI] [Google Scholar]
- Kumar MS, Dahuja A, Rai RD, Walia S, Tyagi A. Role of γ-oryzanol in drought-tolerant and susceptible cultivars of rice (Oryza sativa L.) Indian J Biochem Bio. 2014;51:75–80. [PubMed] [Google Scholar]
- Latha M. Germplasm characterization and evaluation of horticultural crops. New Delhi: Annu Rep NBPGR; 2006. [Google Scholar]
- Lehtimaki N, Lintala M, Allahverdiyeva Y, Aro EM, Mulo P. Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. J Plant Physiol. 2010;167:1018–1022. doi: 10.1016/j.jplph.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lerma-Garcia MJ, Herrero-Martinez JM, Simó-Alfonso EF, Mendonça CR, Ramis-Ramos G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009;115:389–404. doi: 10.1016/j.foodchem.2009.01.063. [DOI] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MS, Reynolds MP. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct Plant Biol. 2010;37:147–156. doi: 10.1071/FP09121. [DOI] [Google Scholar]
- Misra G, Nandi S. Enzymatic deacidification of rice bran oil containing high free fatty acids with recycling. Chem Sci Rev Lett. 2014;2:376–381. [Google Scholar]
- Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Murray JR, Hackett WP. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol. 1991;97:343–351. doi: 10.1104/pp.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas ME, Lambers H, Simpson RJ, Dalling MJ. Effect of drought on metabolism and partitioning of carbon in two wheat varieties differing in drought-tolerance. Annal Bot. 1985;55:727–742. doi: 10.1093/oxfordjournals.aob.a086951. [DOI] [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran LSP. Response of plants to water stress. Front plant sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polívka T, Frank HA. Molecular factors controlling photosynthetic light harvesting by carotenoids. Accounts chem Res. 2010;43:1125–1134. doi: 10.1021/ar100030m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramachandra GS, Saneoka G, Ogata S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J Agric Sci. 1990;115:63–66. doi: 10.1017/S0021859600073925. [DOI] [Google Scholar]
- Ranja S, Singh R, Singh M, Pathre UV, Shirke PA. Characterizing photoinhibition and photosynthesis in juvenile-red versus mature-green leaves of Jatropha curcas L. Plant Physiol Biochem. 2014;79:48–59. doi: 10.1016/j.plaphy.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Raymond CA, Harwood CE, Owen JV. A conductivity method for screening populations of eucalypts for frost damage and frost tolerance. Aust J Bot. 1986;34:377–393. doi: 10.1071/BT9860377. [DOI] [Google Scholar]
- Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti J, Mongrand S, Perrier-Cornet JM, Simon-Plas F. Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB. 2008;J22:3980–3991. doi: 10.1096/fj.08-111070. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Pacheco CMA, Chaves MM. Soil-plant water relations, root distribution and biomass partitioning in Lupinus albus L. under drought conditions. J Exp Bot. 1995;46:947–956. doi: 10.1093/jxb/46.8.947. [DOI] [Google Scholar]
- Scartazza A, Lauteri M, Guido MC, Brugnoli E. Carbon isotope discrimination in leaf and stem sugars, water-use efficiency and mesophyll conductance during different developmental stages in rice subjected to drought. Funct Plant Biol. 1998;25:489–498. [Google Scholar]
- Sgherri C, Stevanovic B, Navari-Izzo F. Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol Plantarum. 2004;122:478–485. doi: 10.1111/j.1399-3054.2004.00428.x. [DOI] [Google Scholar]
- Shukla N, Awasthi RP, Rawat L, Kumar J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem. 2012;54:78–88. doi: 10.1016/j.plaphy.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Singh R, Naskar J, Pathre UV, Shirke PA. Reflectance and cyclic electron flow as an indicator of drought stress in cotton (Gossypium hirsutum) Photochem Photobiol. 2014;90:544–551. doi: 10.1111/php.12213. [DOI] [PubMed] [Google Scholar]
- Singh R, Pandey N, Naskar J, Shirke PA. Physiological performance and differential expression profiling of genes associated with drought tolerance in contrasting varieties of two Gossypium species. Protoplasma. 2015;252:423–438. doi: 10.1007/s00709-014-0686-0. [DOI] [PubMed] [Google Scholar]
- Singh R, Pandey N, Kumar A, Shirke PA. Physiological performance and differential expression profiling of genes associated with drought tolerance in root tissue of four contrasting varieties of two Gossypium species. Protoplasma. 2016;253:163–174. doi: 10.1007/s00709-015-0800-y. [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Cruz MS, Amante M, Magbanua R, Kumar A, Atlin GN. Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crop Res. 2008;107:232–244. doi: 10.1016/j.fcr.2008.02.004. [DOI] [Google Scholar]
- Wellburn A. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wright GC, Rao RC, Farquhar GD. Water-use efficiency and carbon isotope discrimination in peanut under water deficit conditions. Crop Sci. 1994;34:92–97. doi: 10.2135/cropsci1994.0011183X003400010016x. [DOI] [Google Scholar]
- Yousfi S, Márquez AJ, Betti M, Araus JL, Serret MD. Gene expression and physiological responses to salinity and water stress of contrasting durum wheat genotypes. J Integr Plant Biol. 2016;58:48–66. doi: 10.1111/jipb.12359. [DOI] [PubMed] [Google Scholar]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Hu YY, Luo HH, Chow WS, Zhang WF. Two distinct strategies of cotton and soybean differing in leaf movement to perform photosynthesis under drought in the field. Funct Plant Biol. 2011;38:567–575. doi: 10.1071/FP11065. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Robards K, Helliwell S, Blanchard C. Ageing of stored rice: changes in chemical and physical attributes. J Cereal Sci. 2002;35:65–78. doi: 10.1006/jcrs.2001.0418. [DOI] [Google Scholar]