Abstract
Starch occupies the maximal component of cereal grains and is pivotal for maize yield and quality. However, the regulatory mechanism of starch synthesis is still poorly understand. In this study, a GRAS transcription factor, ZmGRAS20, was isolated from maize inbred line B73 based on transcriptome sequencing. Quantitative real-time PCR indicated that ZmGRAS20 is specifically expressed in maize endosperm. Transient expression of ZmGRAS20-green fluorescent protein fusion protein in tobacco cells showed a nucleus and membrane localization of the protein. Transactivation assay of ZmGRAS20 demonstrated that it has no transactivation activity in yeast cells. Overexpression of ZmGRAS20 led to a chalky region of ventral endosperm with decreased starch content and defective agronomic characters in transgenic seeds. Moreover, ZmGRAS20-overexpression plants had fewer fractions of long-branched starch chains. Further scanning electron microscopy observation of ZmGRAS20 transgenic seeds exhibited altered starch granules morphology compared with wide type plants. Taken together, these results suggested that ZmGRAS20 may function as a starch synthesis regulatory factor in rice endosperm.
Keywords: Maize, ZmGRAS20, Endosperm, Starch synthesis, Quality
Introduction
Maize (Zea mays L.) has become one of the most important cereal crop around the world. In general, maize grain is composed of 71% starch which accounted for a large proportion, 9% protein, and 4% oil on a dry weight basis (Orman and Rajr 2002). It has been proved that starch is pivotal for maize yield and quality since the starch type and physicochemical properties can directly influence them (Chen et al. 2016; Morley-Smith et al. 2008; Sonnewald and Kossmann 2013). Hence, starch biosynthesis in corn storage organs has become a research hotspot in recent years.
Starch is a kind of carbohydrate which is insoluble, non-structural and comprises two polymers of glucose, amylose and amylopectin (Pfister and Zeeman 2016; Zeeman et al. 2010). Amylose is a linear polysaccharide with α-1,4-linked d-glucose units, <1% α-1,6 linkages and a degree of polymerization (DP) of 500-5000. Amylopectin is a highly branched molecule, with α-1,4-linked d-glucose backbones, 4–6% α-1,6 linkages and a DP of 50,000–500,000 (Juliano 1998; Perez and Bertoft 2010; Regina et al. 2004; Smith 1999). The process of starch biosynthesis is very complicated (Emes et al. 2003) involving numerous enzymes such as starch synthase (SS, EC2.4.1.21), ADP-glucose pyrophosphorylase (AGPase, EC 2.7.7.27), starch branching enzyme (SBE, EC 2.4.1.18), starch debranching enzyme (DBE, 3.2.1.41), granule-bound starch synthase (GBSS, EC 2.4.1.21) and starch phosphorylase (PHO, EC 2.4.1.1) (Fujita 2014; Jeon et al. 2010; Nelson and Pan 1995; Pfister and Zeeman 2016; Satoh et al. 2008; Tian et al. 2009). Studies of these key genes in starch synthesis suggested that they will influence crop yield and quality by changing starch content or structure. For instance, overexpression of AtSSIV in Arabidopsis increased total leaf starch content (Gamez-Arjona et al. 2011). Overexpression of BEII in potato enhanced the abundance of short amylopectin chains and reduced the phosphate content (Brummell et al. 2015). SSII and SSIII of rice isoforms was proved to synthesize the intermediate chains of amylopectin (Craig et al. 1998; Zhang et al. 2004, 2005, 2008). Besides, lots of reviews on starch synthesis were also reported (Beck and Ziegler 1989; Keeling and Myers 2010; Tetlow 2011; Tetlow and Emes 2014), but the regulatory mechanism of starch synthesis is still poorly understand.
In addition to the aforementioned key genes, some transcription factors (TFs) were also demonstrated to play a crucial role in starch biosynthesis. SUSIBA2, a WRKY TF, is a regulatory transcription factor in starch synthesis (Sun et al. 2003). Rice starch regulator1 (RSR1), an APETALA2 (AP2)/EREBP-type TF, negatively regulates starch biosynthesis (Fu and Xue 2010). OsbZIP58 can regulate starch biosynthesis in rice endosperm as well (Wang et al. 2013). With the development of transcriptome sequencing and genome sequencing technology, enormous data are available for researchers, and the analyses based on these massive transcriptome data have been performed for gene discovery and functional characterization (Jiang et al. 2012; Sekhon et al. 2011, 2013). Thus, we screened 36 candidate TF genes high-expressed in maize endosperm out of 2298 TF genes identified in maize by previous transcriptome sequencing (Jiang et al. 2012). Moreover, among these 36 TFs, a few of their function had been analysed in recent researches. ZmaNAC36 was proven to be involved in co-expression of starch synthetic genes and strongly expressed in maize endosperm (Zhang et al. 2014). ZmbZIP91 was validated to act as a core regulatory factor in starch synthesis by binding to ACTCAT elements in the promoters of starch synthesis genes (Chen et al. 2016). Additionally, ZmGRAS20 was validated to have high expression in endosperm according to the heatmap of qRT-PCR data (Li et al. 2014). In our current study, ZmGRAS20 was demonstrated to belong to GRAS family in maize by bioinformatics analysis. It is reported that a GRAS transcription factor (TF) coding gene was correlated with type I rice starch synthesis genes in rice endosperm (Fu and Xue 2010). The family name GRAS was derived from three initially identified members, GAI, RGA and SCR (Bolle 2004). Sequence analysis of the GRAS family indicates that it has a mobile N terminus and a highly conserved C terminus (Pysh et al. 1999). GRAS protein can regulate a number of important growth and development process in plants, such as meristem formation, hormone and light signal transduction and root growth. Arabidopsis thaliana SHORT ROOT (SHR) protein is necessary for cell division and endodermis specification (Helariutta et al. 2000; Wang et al. 2011). PAT1 protein is involved in phytochrome A signal transduction (Bolle et al. 2000). Although GRAS proteins are implicated to function as transcription factors, the above analysis suggests that they instead might either modify or bind small molecules (Zhang et al. 2012).
In this study, we analysed the transcriptome data from previous research (Jiang et al. 2012; Sekhon et al. 2011) and screened a TF (transcript name GRMZM2G023872_T01) which was highly expressed in maize endosperm so that we conjectured that it is likely to be involved in starch synthesis. We isolated this gene from maize inbred line B73, and designated it as ZmGRAS20 because of previous report (Li et al. 2014). The expression pattern of ZmGRAS20 was further confirmed by qRT-PCR analysis. The molecular characteristics and functional properties of ZmGRAS20 were analysed and its regulation effect was identified by its overexpression in rice. Chalkiness character was found in seeds endosperm of ZmGRAS20 transgenic rice with decreased starch content, defective agronomic characters and altered starch structure. These results suggested a new functional gene of starch synthesis in rice endosperm.
Materials and methods
Plant materials
Maize inbred line B73 was used to clone and check the expression levels of ZmGRAS20 in this study. Plants were grown in a greenhouse at 28 °C with a 14 h light/10 h dark photoperiod. Twelve representative tissues, including root, stem, leaf, tassel, filament, cluster, 10 DAP_Embryo, 15 DAP_Embryo, 20 DAP_Embryo, 10 DAP_Endosperm, 15 DAP_Endosperm and 20 DAP_Endosperm, were collected from a life cycle of maize and frozen into liquid nitrogen directly and stored at −80 °C for future study as described earlier by Cai et al. (2014).
Rice materials (Oryza sativa L. japonica cv. Zhonghua 11) used in the study was grown in a greenhouse under standard growth conditions. The same numbers of plants were grown in the same pot to minimize experimental variations.
RNA isolation and qRT-PCR
Total RNA was isolated from tissue samples of maize with Trizol Reagent (invitrogen) using its Manuals and Protocols and treated with DNase I (invitrogen) to remove genomic DNA contamination. 1 μL of the isolated RNA was used to detect its concentration and purity on a spectrophotometer (One Drop OD-1000). RNA was subsequently reverse-transcribed to first-strand cDNA with Reverse Transcription System (Promega, USA).
For qRT-PCR (quantitative real-time PCR) analysis, the primers of ZmGRAS20 used in qRT-PCR are as follows: 5′-TCCTTATCTGGCTCTGGGTTCA-3′ (forward), 5′-GGCGATTATGCCACGCTAGT-3′ (reverse). The maize actin1 gene (J01238) was used as an internal control with the following primers: 5′-GGGATTGCCGATCGTATGAG-3′ (forward), 5′-GAGCCACCGATCCAGACACT-3′ (reverse). The qRT-PCR was carried out using SYBR Green Master (Roche) on an ABI 7300 Real Time PCR System (Applied Biosystems), and the reactions were performed as described previously (Zhao et al. 2011, 2014). The thermal cycle conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. At the end of the 40 cycles, a melting curve was generated to analyze the specificity of the reactions. Each cDNA sample was tested in three replicates. The achieved data were calculated by method as described previously (Livak and Schmittgen 2001).
Subcellular localization
The full-length open reading frame (ORF) of ZmGRAS20 without the termination codon was amplified by PCR using the following primers: 5′-GCTCTAGAATGGATGCTCACACCACTCA-3′ (forward, XbaI site underlined), 5′-CGGGATCCCTCCATGGGTCCATTACGGT-3′ (reverse, BamHI site underlined). PCR products were digested with XbaI/BamHI and inserted into pCAMBIA1305 vector under the control of cauliflower mosaic virus (CaMV) 35S promoter. The 35S::ZmGRAS20-GFP fusion and control group 35S::GFP were transiently introduced into leaves from 4 to 6-week-old tobacco (Nicotiana bethamiana) using a syringe (2 mL) without needle respectively. The infiltrated tobacco plants were cultured in dark for 12 h and then moved to normal illumination greenhouse for two days. GFP signals were detected by a confocal laser scanning biological microscope (Olympus FV1000).
Transcriptional activation assay
Gene-specific primers: 5′-GGAATTCCATATGATGGATGCTCACACCACTCA-3′ (forward, NdeI site underlined) and 5′-CGGGATCCCTACTCCATGGGTCCATTAC-3′ (reverse, BamHI site underlined) were used to amplified the 1527 bp coding sequence (CDS) of ZmGRAS20. The products were then inserted into pGBKT7 vector (Clontech) which contains the GAL4 DNA-binding domain. The fused construct pGBKT7-ZmGRAS20 was then transformed into yeast strain AH109 containing HIS3, ADE2 and MEL1 reporter genes, with the transformants of pGBKT7 as the negative control, the co-transformants of pGBKT7-53 and pGADT7-T as the positive control by using Clontech’s yeast hybrid User Manual. The transformed yeast cells were examined on SD/-Trp and SD/-Trp/-His/-Ade/X-α-GAL medium for 2–3 days at 30 °C.
Generation of rice transgenic plants
The ORF of ZmGRAS20 was cloned using our forenamed reverse-transcribed cDNA with designed primers 5′-CGGGATCCATGGATGCTCACACCACTCAC-3′ (forward, BamHI site underlined) and 5′-GCTCTAGACTACTCCATGGGTCCATTACG-3′ (reverse, XbaI site underlined). PCR products were digested with BamHI and XbaI restriction enzymes, and then inserted into an overexpression vector pCAMBIA1301a reconstructed by our laboratory (insert a CaMV 35S promoter and a NOS). This recombination construct pCAMBIA1301a-ZmGRAS20 harboured a GUS reporter gene and overexpressed a full-length c-DNA of ZmGRAS20 driven by CaMV 35S promoter and was subsequently transformed into rice japonica cv. Zhonghua 11 using Agrobacterium-mediated transformation (Lin and Zhang 2005). Zhonghua 11 as a wide type (WT) and rice contains pCAMBIA1301a as a control check (CK). ZmGRAS20 transgenic plants were confirmed by histochemical staining of GUS activity, PCR analysis using its specific primers and qRT-PCR.
Determination of agronomic characters and measurement of grain quality
A hundred seeds were analysed for grain length, width and thickness by using a vernier caliper. The 1000-grain weight was determined by counting ten replicates of 100-grain samples independently on an electronic balance. Every measurement was repeated three times.
Separating embryos and pericarps from the grains and grinding them into powder. Using the manufacturer’s instruction of Total Starch Assay Kit (K-TSTA; Megazyme) to measure the starch content. Using iodine colorimetric method (K-AMYL; Megazym) to measure the apparent amylose content (AAC) of the sample (Juliano 1971). As for the soluble sugar content, we measured it using anthrone methods (Wang et al. 2013).
Measurement of starch blue value (BV) and maximum absorption wavelength (λmax)
Isolation of amylose and amylopectin and measurement of blue value (BV) and maximum absorption wavelength (λmax) of starch were consulted by modified alkaline steeping method (Fu and Xue 2010; Gilbert and Spragg 1964; Lu et al. 1997). 5 mg of separated amylose powder was dissolved in 8 mL 90% dimethyl sulfoxide (DMSO) and diluted to 100 mL with water. The absorbance spectrum of the starch-iodine complexes was recorded from 500 to 800 nm, and the wavelength at λmax was determined. The BV was A600. 15 mg of separated amylopectin powder was diluted to 100 mL with water. The absorbance spectrum of the starch-iodine complexes was recorded from 500 to 700 nm, and the wavelength at λmax was determined. The BV was A680.
Observation of starch granules by scanning electron microscopy (SEM)
The morphology of starch granules were observed on a Hitachi S-3000N scanning electron microscopy (SEM) (Hitachi, Tokyo, Japan) according to the methods of Fu and Xue (2010). SEM images of ventral, central and dorsal area of mature endosperm were detected through cross-sections of mature rice seeds.
Results
Identification and sequence analysis of ZmGRAS20
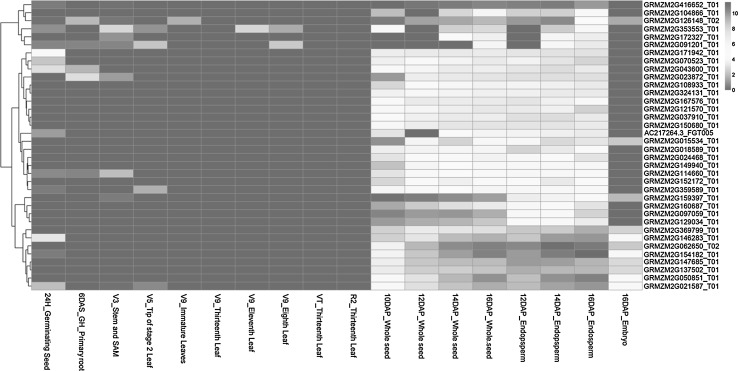
According to previous transcriptome analysis of 2298 TFs in maize (Jiang et al. 2012; Sekhon et al. 2011, 2013), 36 members were shown to have endosperm-specific expression among the 18 representative tissues (Fig. 1). ZmGRAS20 (transcript name GRMZM2G023872_T01), in the midst of these 36 genes, was also demonstrated to have high expression in endosperm based on qRT-PCR data (Li et al. 2014). These findings suggested that ZmGRAS20 may be involved in the regulation of starch synthesis in endosperm. To determine this hypothesis, ZmGRAS20 was cloned from maize inbred line B73 using the gene specific primers, and a subsequently functional analysis was performed in this study. Bioinformatics analysis demonstrated that ZmGRAS20 contains an open reading frame (ORF) of 1527 bp that encodes a protein of 508 amino acids and belongs to a GRAS protein. Its theoretical molecular weight (Mw) and isoelectric point (pI) is 56.08 kD and 5.91 (http://web.expasy.org/compute_pi/), respectively.
Fig. 1.
Heatmap of 36 candidate genes at different developmental stages. The colour scale at the right of the figure represents the RPKM normalized log2 transformed values based on the transcriptome data (colour figure online)
Expression patterns of ZmGRAS20
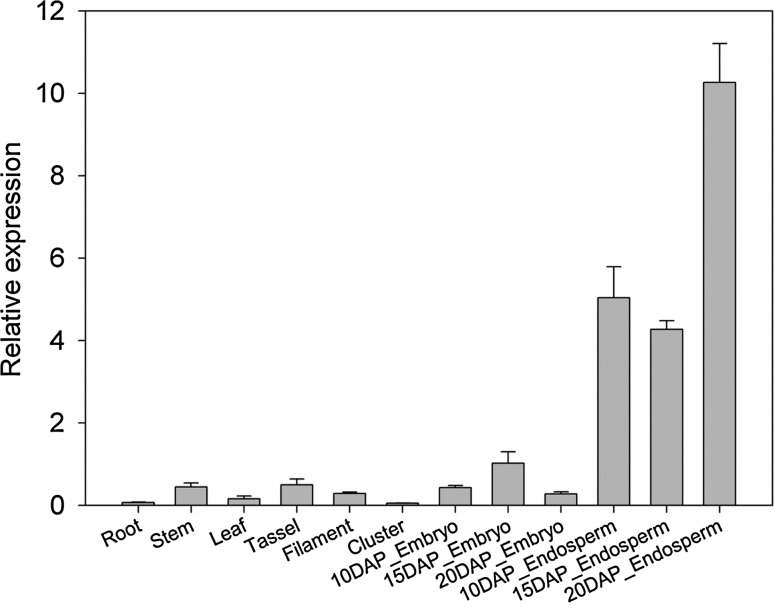
ZmGRAS20 was shown to have an endosperm-specific expression pattern in maize by transcriptome sequencing and qRT-PCR data (Li et al. 2014; Sekhon et al. 2011, 2013). To further validate whether ZmGRAS20 gene is specifically expressed in maize endosperm, total RNAs from different tissues were isolated and a qRT-PCR was carried out. The expression of endosperms in different developmental stages was detected as well to better understand the function of this gene. The result showed that ZmGRAS20 was highly expressed in 10 DAP_Endosperm, 15 DAP_Endosperm and 20 DAP_Endosperm, especially in 20 DAP_Endosperm (Fig. 2). qRT-PCR analysis of ZmGRAS20 revealed the feature of endosperm-specific expression.
Fig. 2.
Tissue-specific expression patterns of ZmGRAS20 in maize root, stem, leaf, tassel, filament, cluster, 10 DAP_Embryo, 15 DAP_Embryo, 20 DAP_Embryo, 10 DAP_Endosperm, 15 DAP_Endosperm and 20 DAP_Endosperm analysed by qRT-PCR. The maize actin1 gene (J01238) was used as an internal control. Error bars are standard deviations of three technical repeats and two biological repeats
Subcellular localization and transcription activity of ZmGRAS20
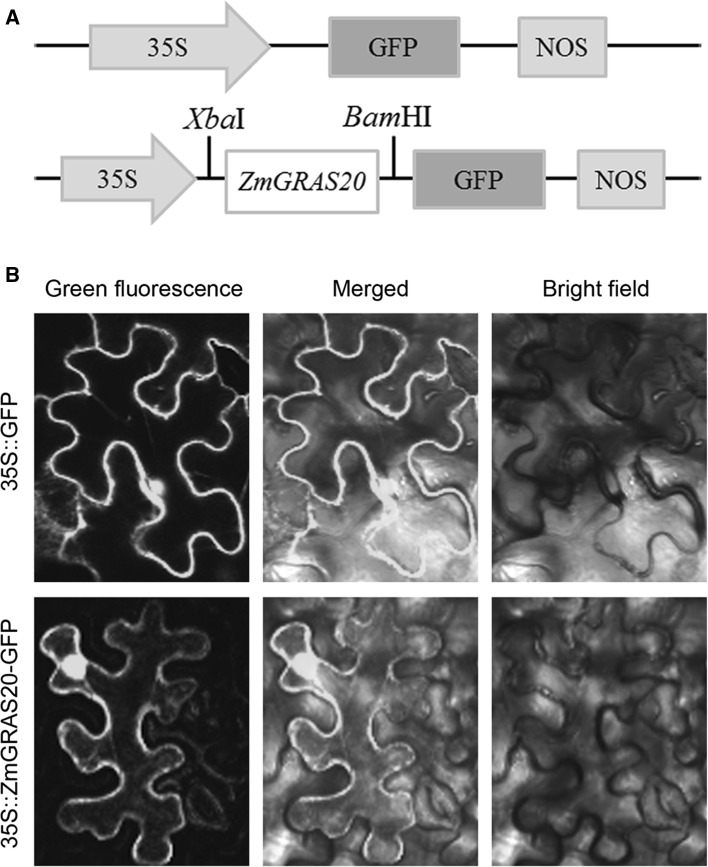
To examine its subcellular localization, the 35S::ZmGRAS20-GFP fusion was transiently expressed in tobacco leaves with 35S::GFP act as a control (Fig. 3a). The result indicated that the green fluorescence of 35S::ZmGRAS20-GFP fusion protein can be observed in the membrane and nucleus, and the fluorescence of 35S::GFP control was also visualized both in nucleus and membrane (Fig. 3b), showing that ZmGRAS20 is a nucleus and membrane-localized protein.
Fig. 3.
Subcellular localization of ZmGRAS20. a Schematic diagram of ZmGRAS20 subcellular localization construct. 35S, cauliflower mosaic virus 35S; GFP, green fluorescence protein; NOS, nopaline synthase gene terminator. b The 35S::ZmGRAS20-GFP fusion protein and 35S::GFP were transiently expressed in tobacco epidermal cells and visualized by a confocal laser scanning biological microscope, respectively
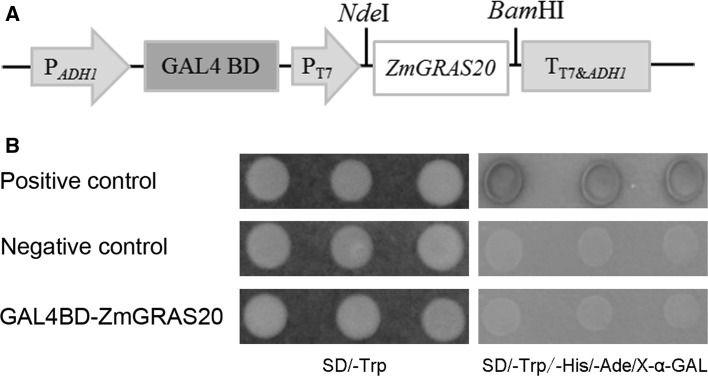
To detect if it has transcription activity, a fused construct: pGBKT7-ZmGRAS20, containing the GAL4 DNA-binding domain and the ORF of ZmGRAS20, was generated and transformed into yeast strain AH109, with the transformants of pGBKT7 as the negative control and the co-transformants of pGBKT7-53 and pGADT7-T as the positive control according to the Clontech’s yeast hybrid User Manual (Fig. 4a). As the Fig. 4b shows, all of the three kinds of yeast cells can grow well on the SD/-Trp medium, and the transformants of pGBKT7-ZmGRAS20 and pGBKT7 cannot grow on the SD/-Trp/-His/-Ade/X-α-GAL medium and showed no α-galactosidase activity while only the poitive control can survive on the same medium. It is suggested that ZmGRAS20 has no transcription activity in yeast cell.
Fig. 4.
Transcriptional activation assay of ZmGRAS20. a Schematic diagram of ZmGRAS20 transcriptional activation construct. PADH1, ADH1 promoter; GAL4 BD, GAL4 DNA-binding domain; PT7, T7 promoter; TT7&ADH1, T7 and ADH1 transcription termination signals. b The construct pGBKT7-ZmGRAS20 was transformed into yeast strain AH109, containing HIS3, ADE2 and MEL1 reporter genes. Positive control, the co-transformants of pGBKT7-53 and pGADT7-T; negative control, the transformants of pGBKT7. The results were examined on SD/-Trp and SD/-Trp/-His/-Ade/X-α-GAL medium for 2–3 days at 30 °C
Analysis of agronomic characters of ZmGRAS20 transgenic rice
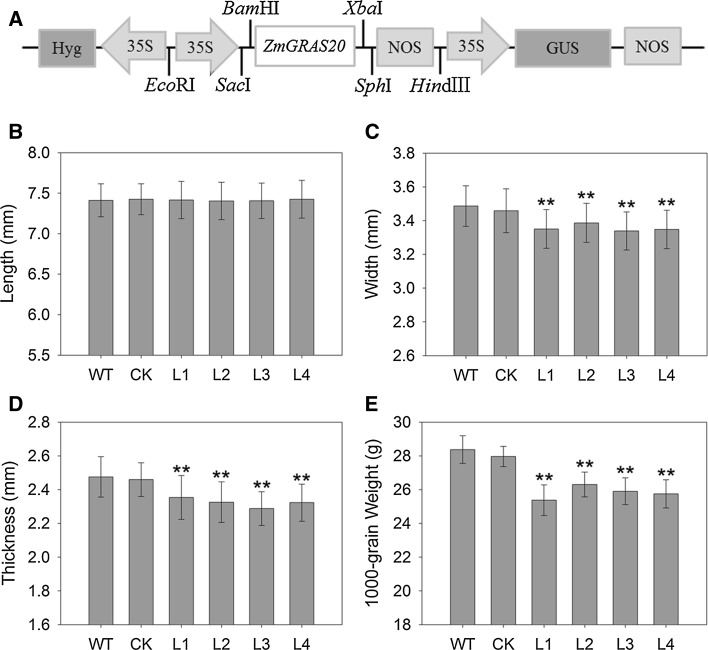
In order to understand the role of ZmGRAS20 in seed starch accumulation, ten independent overexpression rice plants were obtained using Agrobacterium-mediated transformation, and the transformed lines of rice were confirmed by hygromycin selection and molecular detection (Fig. 5a). The expression of ZmGRAS20 in transgenic rice was confirmed by qRT-PCR (data not shown). Four lines of them were selected for future study. To investigate whether ZmGRAS20 affects the trait of transgenic rice plants, statistical analysis of some agronomic traits, including 1000-grain weight, grain length, grain width and grain thickness, was performed using the T2 generation of transgenic plants, WT plants and CK plants. There was no significant change in grain length between transgenic plants, WT and CK plants (Fig. 5b). However, grain width, grain thickness and 1000-grain weight of transgenic plants were all significantly less than that of WT and CK plants (Fig. 5c–e).
Fig. 5.
Overexpression construct and agronomic characters of ZmGRAS20. a Schematic diagram of ZmGRAS20 overexpression construct. b–e Grain agronomic characters including grain length (b), grain width (c), grain thickness (d), and 1000-grain weight (e). WT, rice japonica cv. Zhonghua 11 as a wide type; CK, rice contains pCAMBIA1301a as a control check; L1-L4, four independent transgenic lines of ZmGRAS20. Data are presented as mean ± SD of three replicates. **Significant differences between WT and transgenic plants at P < 0.01, respectively
Analysis of starch content of ZmGRAS20 transgenic rice seed
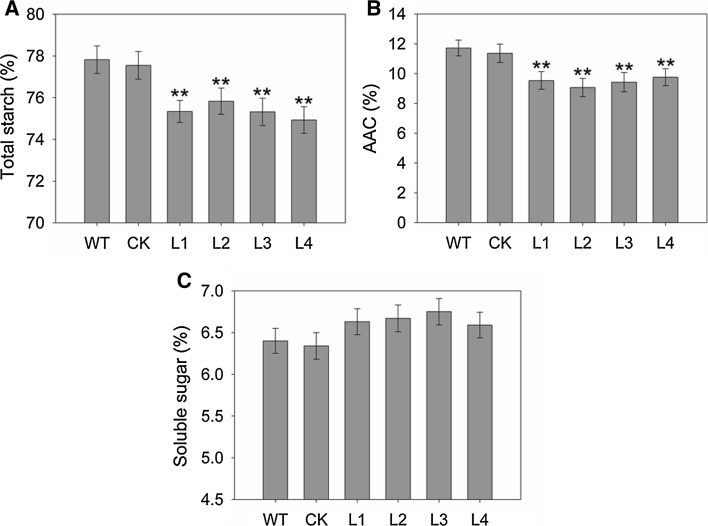
In order to study whether ZmGRAS20 has an effect on starch synthesis, the total starch content, the apparent amylose content (AAC) and the soluble sugar content of WT, CK and transgenic plants were measured according to the mentioned methods. The results showed that the average total starch content of overexpression lines L1, L2, L3, and L4 were 75.34, 75.83, 75.32 and 75.34%, respectively, and WT and CK plants were 77.82 and 77.55%, respectively (Fig. 6a). The AAC of four transgenic lines was 9.53, 9.07, 9.42 and 9.78%, respectively and WT and CK plants were 11.72 and 11.36%, respectively (Fig. 6b). Compared with WT and CK plants, overexpression lines had an extremely significant reductions of seed starch content and AAC, and the maximum average difference of total starch content and AAC were 2.89 and 2.65% compared with WT plants, respectively. In contrast, the soluble sugar content in the WT and CK plants were slightly lower than that in transgenic plants but not reach a significant level (Fig. 6c).
Fig. 6.
Altered starch content in transgenic rice of ZmGRAS20. a Total starch content in rice endosperm. b Apparent amylose content (AAC) in rice endosperm. c Soluble sugar content in rice endosperm. WT, rice japonica cv. Zhonghua 11 as a wide type; CK, rice contains pCAMBIA1301a as a control check; L1–L4, four independent transgenic lines of ZmGRAS20. Data are presented as mean ± SD of three replicates. **Significant differences between WT and transgenic plants at P < 0.01, respectively
Overexpression of ZmGRAS20 influences starch structure in transgenic rice
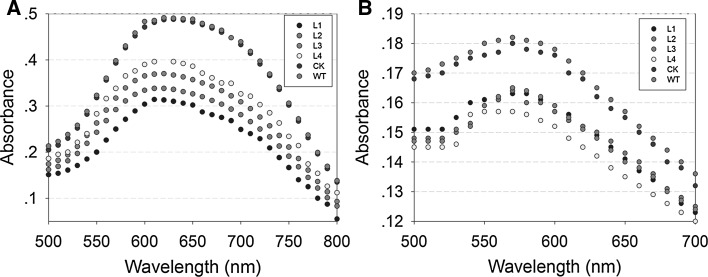
Blue value (BV) and maximum absorption wavelength (λmax) of starch is reported to represent the ability to combine starch and iodine. Larger values of BV and λmax imply the larger fractions of long chains. Thus, the different BV and λmax can give an index to the distinction of starch structure (Hizukuri 1986, 1988; Takeda et al. 1986). To verify if the structure of amylose and amylopectin were altered by overexpression of ZmGRAS20, we determined BV and λmax of starch in WT, CK and four ZmGRAS20 overexpression plants. The data gave the result that λmax of amylose and amylopectin was 620 nm and 570 nm respectively and BV of which was 0.348 and 0.129, respectively. As shown in Fig. 7a, b, BV and λmax of amylose and amylopectin in four transgenic lines were significantly less than WT and CK plants, suggesting that ZmGRAS20 overexpression causes fewer fractions of long-branched starch chains.
Fig. 7.
Blue value (BV) and maximum absorption wavelength (λmax) of ZmGRAS20. a Absorbance spectra of isolated amylose-iodine complexes of ZmGRAS20-overexpression plants. BV at 600 nm and λmax represent the ability to combine with iodine. b Absorbance spectra of isolated amylopectin-iodine complexes of ZmGRAS20-overexpression plants. BV at 680 nm and λmax represent the ability to combine with iodine. WT, rice japonica cv. Zhonghua 11 as a wide type; CK, rice contains pCAMBIA1301a as a control check; L1–L4, four independent transgenic lines of ZmGRAS20. Data are presented as mean ± SD of three replicates. **Significant differences between WT and transgenic plants at P < 0.01, respectively
Overexpression of ZmGRAS20 influences starch granules morphology
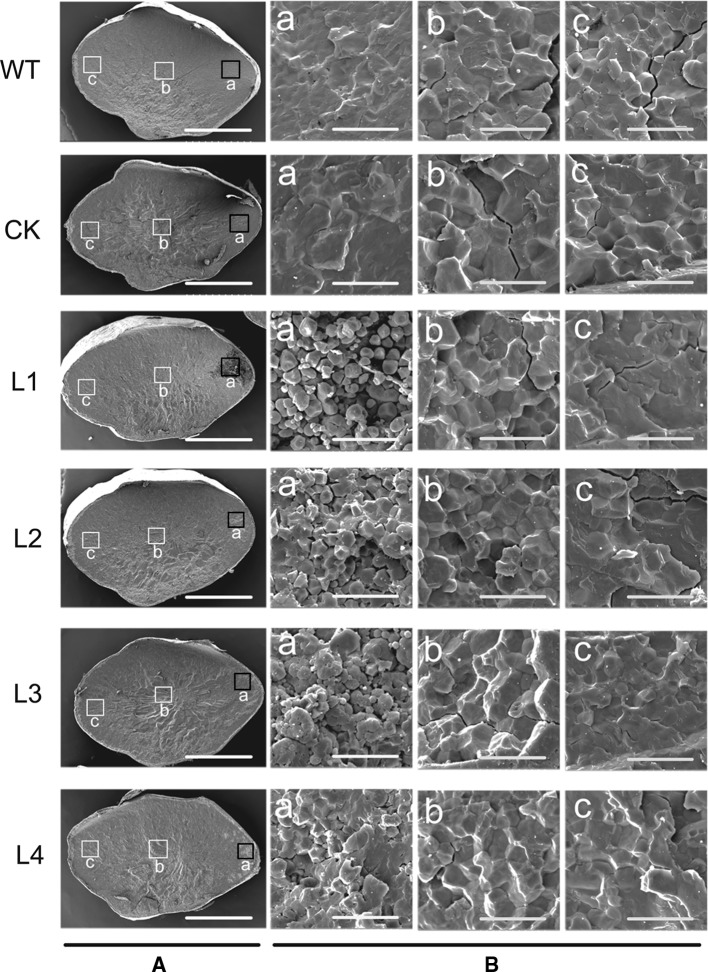
Since the starch content was significantly changed in transgenic rice, we further examined the starch granules morphology by Scanning Electron Microscopy (SEM). SEM images of transverse sections of rice grains overexpressing ZmGRAS20 gene showed a chalky region of ventral endosperm (Fig. 8A). It had no changes in the central and dorsal endosperms compared with WT and CK seeds,which was composed of densely packed, polyhedral starch granules, but obvious differences in the ventral endosperm (Fig. 8B). SEM of the ventral area of endosperm showed that the starch granules of WT and CK seeds were evenly distributed, compactly arranged, presented polyhedron structure and had no free starch granules, while which of four transgenic lines were loosely packed and had lots of spherical single starch granules, is consistent with the chalky region of endosperm. These results suggested that the flawed seed phenotypes may be caused by the ZmGRAS20-overexpression.
Fig. 8.
Scanning electron microscopy (SEM) images of the transverse sections of rice seeds. Cross-sections of mature seeds are shown in A. SEM of the ventral area of mature endosperm is shown in a of B and indicated by a red square in A. SEM of the central area of mature endosperm is shown in b of B. SEM of the dorsal area of mature endosperm is shown in c of B. WT, rice japonica cv. Zhonghua 11 as a wide type; CK, rice contains pCAMBIA1301a as a control check; L1–L4, four independent transgenic lines of ZmGRAS20. Bars 1 mm (A); 20 μm (B) (colour figure online)
Discussion
Starch is the main component of maize seeds and closely related to maize yield and quality, which accounted for about 70% of the dry weight. Many researches have been made to study the synthesis and accumulation mechanism of starch. Increasing evidence indicated that starch biosynthesis is a complex system composed of multiple genes during endosperm development, such as soluble starch synthase (SS), ADP-glucose pyrophosphorylase (AGPase), granule-bound starch synthase (GBSS), starch branching enzyme (SBE) and starch debranching enzyme (DBE), and most of them are highly conserved in crop seeds (Tian et al. 2009). Besides, many researchers have reported that these genes involved in starch synthesis are regulated by transcription factors through interacting with the upstream of gene promoter (Chen et al. 2016; Wang et al. 2013).
To identify the possible maize TFs involved in regulating starch biosynthesis, we performed a screening of endosperm-specific expression TFs according to the result of transcriptome sequencing in this study. A total of 36 candidate genes highly expressed in endosperm were identified. Among them, ZmGRAS20 were chosen for further functional analysis (Jiang et al. 2012; Sekhon et al. 2011, 2013). Moreover, the specific expression in endosperm of ZmGRAS20 was also confirmed by qRT-PCR data (Li et al. 2014; Sekhon et al. 2011, 2013) and this study (Fig. 2). So we hypothesized that ZmGRAS20 may be related to starch synthesis in maize endosperm. Protein anaysis demonstrated that ZmGRAS20 carries a GRAS conserved domain. The 35S::ZmGRAS20-GFP showed a nucleus and membrane-localized GFP signal (Fig. 3b), indicating that ZmGRAS20 might work both in cell nucleus and membrane. Transcriptional activation assay of ZmGRAS20 manifested no transcription activity in yeast (Fig. 4b). Hao et al. reported that a soybean NAC TF gene GmNAC20 has a repression domain of the C-terminal activation domain so that the full length of GmNAC20 does not have transcriptional activation activity, but when the repression domain was removed, it will have transcription activity (Hao et al. 2010). For this reason, we speculated ZmGRAS20 may have a similar repression domain or act as a transcriptional repressor to inhibit the transcription of target gene. Specific reason remains to be seen by further experiment.
Transgenic rice plants were generated to assess the regulatory role of ZmGRAS20 in starch synthesis. As expected, transgenic rice seeds displayed altered grain morphology and declining starch content, decreasing agronomic characters in seeds, and slightly increasing soluble sugar content, implying a possible role for ZmGRAS20 in starch synthesis (Figs. 5, 6). As we know, soluble sugar will convert into starch during starch synthesis and this process needs multiple enzymes involvement (Nelson and Pan 1995) so we speculated that there is a negative correlation between soluble sugar content and starch content. Certainly, more experiments are required to verify our conjecture in the future. Since the starch content was significantly changed in transgenic rice, we wanted to further examine the starch structure change of ZmGRAS20-overexpression rice. As reported, BV and λmax are two important indexes of starch indicating its capacity to combine with iodine. The higher BV and λmax are, the more fractions of long chains have (Hizukuri 1986), which means different BV and λmax reflect the differences of starch structure (Hizukuri 1988; Takeda et al. 1986). Our result indicated that the BV and λmax of amylose and amylopectin were all lower in ZmGRAS20 transgenic lines than in WT and CK plants (Fig. 7), suggesting that ZmGRAS20-overexpression lines give rise to fewer fractions of long-branched starch chains, that is to say, overexpression of ZmGRAS20 influenced starch structure. Meanwhile, a chalky region of ventral endosperm was examined (Fig. 8A). According to some research, chalky rice usually had decreasing agronomic characters (Kim et al. 2000) and lower total starch content and AAC compared with normal grains (Ashida et al. 2009; Lisle et al. 2000). Besides, chalky kernels also contained less long branch-chain amylopectin than normal kernels (Patindol and Wang 2003). These reports lead us to conclude that during amylopectin synthesis, the blocked process of chain extension can cause the accumulation of amylopectin short-chains and the reduction of long-chains, thus inducing the abnormal accumulation of starch and finally form chalkiness. In our research, this chalky phenomenon of ZmGRAS20 may also arise from the altered starch structure. Further observation of transverse sections of transgenic rice seeds using SEM showed loosely packed, spherical starch granules on the ventral area in seed endosperm corresponding with the belly chalky region in endosperm (Fig. 8). Increasing evidence suggested that the altered morphology of starch granules can lead to the formation of chalkiness (Lisle et al. 2000; Qiao et al. 2011). The SEM images revealed that ZmGRAS20 transgenic seeds exhibited altered starch granules morphology, further proving the role of ZmGRAS20 in the formation of chalkiness. Considering that starch is actively accumulated at the periphery that is belly at the late stage (Qiao et al. 2011; Tsukaguchi and Iida 2008), which is consistent with our tissue-specific expression pattern result that ZmGRAS20 is highly expressed in maize different developmental stages endosperm especially in 20 DAP_Endosperm, implying that ZmGRAS20 may work at the late stage of starch synthesis and eventually form a ventral chalk.
It is indispensable for people to improve crop grain quality but the process of seed quality formation is very complex. Genes related to starch synthesis team up with each other to define the relationship of main properties that determine cereal eating and cooking quality (Tian et al. 2009). We studied an endosperm-specific TF ZmGRAS20 and found out that overexpression of ZmGRAS20 in rice significantly influences seeds starch content and structure and the quality of endosperm. On the whole, all these data from our research suggest that ZmGRAS20 may be a regulatory gene related to starch synthesis. Nevertheless, more complicated regulatory mechanism needed to be further studied.
Acknowledgements
This research was supported by grants from the National Program on Key Basic Research Project (973 Program, No. 2014CB138204) and Genetically Modified Organisms Breeding Major Projects (2013ZX003-002). We would like to thank the members of the Key Laboratory of Crop Biology of Anhui province for their technical assistance in this study.
Authors’ contribution
YZ, QM and BC designed the research, HC conducted the molecular experiments, MZ performed the rice transformation, YC analyzed agronomic characters, detected starch content and analyzed the data, RC participated in the design of this study, YZ and HC drafted the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Huilin Cai and Yulong Chen have contributed equally to this work.
References
- Ashida K, Iida S, Yasui T. Morphological, physical, and chemical properties of grain and flour from chalky rice mutants. Cereal Chem. 2009;86(2):225–231. doi: 10.1094/CCHEM-86-2-0225. [DOI] [Google Scholar]
- Beck E, Ziegler P. Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Biol Plant Mol Biol. 1989;128(40):95–117. doi: 10.1146/annurev.pp.40.060189.000523. [DOI] [Google Scholar]
- Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218(5):683–692. doi: 10.1007/s00425-004-1203-z. [DOI] [PubMed] [Google Scholar]
- Bolle C, Koncz C, Chua NH. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 2000;14(10):1269–1278. [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Watson LM, Zhou J, McKenzie MJ, Hallett IC, et al. Overexpression of STARCH BRANCHING ENZYME II increases short-chain branching of amylopectin and alters the physicochemical properties of starch from potato tuber. BMC Biotechnol. 2015;15:28. doi: 10.1186/s12896-015-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai RH, Zhao Y, Wang YF, Lin YX, Peng XJ, et al. Overexpression of a maize WRKY58 gene enhances drought and salt tolerance in transgenic rice. Plant Cell Tissue Org. 2014;119(3):565–577. doi: 10.1007/s11240-014-0556-7. [DOI] [Google Scholar]
- Chen J, Yi Q, Cao Y, Wei B, Zheng LJ, et al. ZmbZIP91 regulates expression of starch synthesis-related genes by binding to ACTCAT elements in their promoters. J Exp Bot. 2016;67(5):1327–1338. doi: 10.1093/jxb/erv527. [DOI] [PubMed] [Google Scholar]
- Craig J, Lloyd JR, Tomlinson K, Barber L, Edwards A, et al. Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell. 1998;10(3):413–426. doi: 10.1105/tpc.10.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes MJ, Bowsher CG, Hedley C, Burrell MM, Scrase-Field ES, et al. Starch synthesis and carbon partitioning in developing endosperm. J Exp Bot. 2003;54(382):569–575. doi: 10.1093/jxb/erg089. [DOI] [PubMed] [Google Scholar]
- Fu FF, Xue HW. Coexpression analysis identifies rice starch regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010;154(2):927–938. doi: 10.1104/pp.110.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N. Starch biosynthesis in rice endosperm. Agri Biosci Monogr. 2014;4:1–18. doi: 10.5047/agbm.2014.00401.0001. [DOI] [Google Scholar]
- Gamez-Arjona FM, Li J, Raynaud S, Baroja-Fernandez E, Munoz FJ, et al. Enhancing the expression of starch synthase class IV results in increased levels of both transitory and long-term storage starch. Plant Biotechnol J. 2011;9(9):1049–1060. doi: 10.1111/j.1467-7652.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- Gilbert GA, Spragg SP. Iodimetric determination of amylose. In: Whister RL, editor. Methods in carbohydrate chemistry. New York: Academic Press; 1964. pp. 168–169. [Google Scholar]
- Hao YJ, Song QX, Chen HW, Zou HF, Wei W, et al. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta. 2010;232(5):1033–1043. doi: 10.1007/s00425-010-1238-2. [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101(5):555–567. doi: 10.1016/S0092-8674(00)80865-X. [DOI] [PubMed] [Google Scholar]
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res. 1986;147(2):342–347. doi: 10.1016/S0008-6215(00)90643-8. [DOI] [Google Scholar]
- Hizukuri S. Recent advances in molecular structures of starch. J Appl Glycosci. 1988;35(3):185–198. [Google Scholar]
- Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y. Starch biosynthesis in cereal endosperm. Plant Physiol Biochem. 2010;48(6):383–392. doi: 10.1016/j.plaphy.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zeng BA, Zhao HN, Zhang M, Xie SJ, et al. Genome-wide transcription factor gene prediction and their expressional tissue-specificities in maize. J Integr Plant Biol. 2012;54(9):616–630. doi: 10.1111/j.1744-7909.2012.01149.x. [DOI] [PubMed] [Google Scholar]
- Juliano BO. A simplified assay for milled-rice amylose. Cereal Sci Today. 1971;16:334–360. [Google Scholar]
- Juliano BO. Varietal impact on rice quality. Cereal Foods World. 1998;43(4):207–222. [Google Scholar]
- Keeling PL, Myers AM. Biochemistry and genetics of starch synthesis. Annu Rev Food Sci Technol. 2010;1:271–303. doi: 10.1146/annurev.food.102308.124214. [DOI] [PubMed] [Google Scholar]
- Kim SS, Lee SF, Kim OW, Kim DC. Physicochemical characteristics of chalky kernels and their effects on sensory quality of cooked rice. Cereal Chem. 2000;77(3):376–379. doi: 10.1094/CCHEM.2000.77.3.376. [DOI] [Google Scholar]
- Li GS, Wang DF, Yang RL, Logan K, Chen H, et al. Temporal patterns of gene expression in developing maize endosperm identified through transcriptome sequencing. Proc Natl Acad Sci USA. 2014;111(21):7582–7587. doi: 10.1073/pnas.1406383111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Zhang QF. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005;23(8):540–547. doi: 10.1007/s00299-004-0843-6. [DOI] [PubMed] [Google Scholar]
- Lisle AJ, Martin M, Fitzgerald MA. Chalky and translucent rice grains differ in starch composition and structure and cooking properties. Cereal Chem. 2000;77(5):627–632. doi: 10.1094/CCHEM.2000.77.5.627. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu S, Chen LN, Lii CY. Correlations between the fine structure, physicochemical properties, and retrogradation of amylopectins from Taiwan rice varieties. Cereal Chem. 1997;74(1):34–39. doi: 10.1094/CCHEM.1997.74.1.34. [DOI] [Google Scholar]
- Morley-Smith ER, Pike MJ, Findlay K, Koeckenberger W, Hill LM, et al. The transport of sugars to developing embryos is not via the bulk endosperm in oilseed rape seeds. Plant Physiol. 2008;147(4):2121–2130. doi: 10.1104/pp.108.124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Pan D. Starch synthesis in maize endosperms. Ann Rev Plant Physiol Plant Mol Biol. 1995;46(1):475–496. doi: 10.1146/annurev.pp.46.060195.002355. [DOI] [Google Scholar]
- Orman BA, Rajr S. Comparison of near-infrared spectroscopy calibration methods for the prediction of protein, oil, and starch in maize grain. J Agric Food Chem. 2002;39(5):592–598. [Google Scholar]
- Patindol J, Wang YJ. Fine structures and physicochemical properties of starches from chalky and translucent rice kernels. J Agric Food Chem. 2003;51(9):2777–2784. doi: 10.1021/jf026101t. [DOI] [PubMed] [Google Scholar]
- Perez S, Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch Starke. 2010;62(8):389–420. doi: 10.1002/star.201000013. [DOI] [Google Scholar]
- Pfister B, Zeeman SC. Formation of starch in plant cells. Cell Mol Life Sci: CMLS; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18(1):111–119. doi: 10.1046/j.1365-313X.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- Qiao JF, Liu ZH, Deng SY, Ning HF, Yang XY, et al. Occurrence of perfect and imperfect grains of six japonica rice cultivars as affected by nitrogen fertilization. Plant Soil. 2011;349(1–2):191–202. doi: 10.1007/s11104-011-0861-4. [DOI] [Google Scholar]
- Regina A, Rahman S, Li Z, Morell MK (2004) STARCH synthesis. In: Wrigley C, Corke H, Walker C (eds) Encyclopedia of grain science. Elsevier, Oxford, pp 224–232
- Satoh H, Shibahara K, Tokunaga T, Nishi A, Tasaki M, et al. Mutation of the plastidial alpha-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell. 2008;20(7):1833–1849. doi: 10.1105/tpc.107.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon RS, Lin HN, Childs KL, Hansey CN, Buell CR, et al. Genome-wide atlas of transcription during maize development. Plant J. 2011;66(4):553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- Sekhon RS, Briskine R, Hirsch CN, Myers CL, Springer NM, et al. Maize gene atlas developed by RNA sequencing and comparative evaluation of transcriptomes based on RNA sequencing and microarrays. PLoS ONE. 2013;8(4):e61005. doi: 10.1371/journal.pone.0061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM. Making starch. Curr Opin Plant Biol. 1999;2(3):223–229. doi: 10.1016/S1369-5266(99)80039-9. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Kossmann J. Starches-from current models to genetic engineering. Plant Biotechnol J. 2013;11(2):223–232. doi: 10.1111/pbi.12029. [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, et al. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15(9):2076–2092. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Hizukuri S, Juliano BO. Purification and structure of amylose from rice starch. Carbohydr Res. 1986;148(2):299–308. doi: 10.1016/S0008-6215(00)90397-5. [DOI] [Google Scholar]
- Tetlow IJ. Starch biosynthesis in developing seeds. Seed Sci Res. 2011;21(1):5–32. doi: 10.1017/S0960258510000292. [DOI] [Google Scholar]
- Tetlow IJ, Emes MJ. A review of starch-branching enzymes and their role in amylopectin biosynthesis. IUBMB Life. 2014;66(8):546–558. doi: 10.1002/iub.1297. [DOI] [PubMed] [Google Scholar]
- Tian ZX, Qian Q, Liu QQ, Yan MX, Liu XF, et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc Natl Acad Sci USA. 2009;106(51):21760–21765. doi: 10.1073/pnas.0912396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaguchi T, Iida Y. Effects of assimilate supply and high temperature during grain-filling period on the occurrence of various types of chalky kernels in rice plants (Oryza sativa L.) Plant Prod Sci. 2008;11(2):203–210. doi: 10.1626/pps.11.203. [DOI] [Google Scholar]
- Wang JH, Andersson-Gunneras S, Gaboreanu I, Hertzberg M, Tucker MR, et al. Reduced expression of the SHORT-ROOT gene increases the rates of growth and development in hybrid poplar and Arabidopsis. PLoS ONE. 2011;6(12):e28878. doi: 10.1371/journal.pone.0028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Xu H, Zhu Y, Liu QQ, Cai XL. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J Exp Bot. 2013;64(11):3453–3466. doi: 10.1093/jxb/ert187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol. 2010;61:209–234. doi: 10.1146/annurev-arplant-042809-112301. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Colleoni C, Ratushna V, Sirghle-Colleoni M, James MG, et al. Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol Biol. 2004;54(6):865–879. doi: 10.1007/s11103-004-0312-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Myers AM, James MG. Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol. 2005;138(2):663–674. doi: 10.1104/pp.105.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Szydlowski N, Delvalle D, D’Hulst C, James MG, et al. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol. 2008;8:96. doi: 10.1186/1471-2229-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Iyer LM, Aravind L. Bacterial GRAS domain proteins throw new light on gibberellic acid response mechanisms. Bioinformatics. 2012;28(19):2407–2411. doi: 10.1093/bioinformatics/bts464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Chen J, Yi Q, Hu YF, Liu HM, et al. Novel role of ZmaNAC36 in co-expression of starch synthetic genes in maize endosperm. Plant Mol Biol. 2014;84(3):359–369. doi: 10.1007/s11103-013-0153-x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhou YQ, Jiang HY, Li XY, Gan DF, et al. Systematic analysis of sequences and expression patterns of drought-responsive members of the HD-Zip gene family in maize. PLoS ONE. 2011;6(12):e28488. doi: 10.1371/journal.pone.0028488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ma Q, Jin XL, Peng XJ, Liu JY, et al. A novel maize homeodomain-leucine zipper (HD-Zip) I gene, Zmhdz10, positively regulates drought and salt tolerance in both rice and Arabidopsis. Plant Cell Physiol. 2014;55(6):1142–1156. doi: 10.1093/pcp/pcu054. [DOI] [PubMed] [Google Scholar]