Abstract
Ajuga bracteosa is a medicinally important plant globally used in the folk medicine against many serious ailments. In the present study, effects of two significant elicitors, methyl jasmonate (Me-J) and phenyl acetic acid (PAA) were studied on growth parameters, secondary metabolites production, and antioxidant potential in adventitious root suspension cultures of A. bracteosa. The results showed a substantial increase in biomass accumulation, exhibiting longer log phases of cultures growth in response to elicitor treatments, in comparison to control. Maximum dry biomass formation (8.88 DW g/L) was recorded on 32nd day in log phase of culture when 0.6 mg/L Me-J was applied; however, PAA at 1.2 mg/L produced maximum biomass (8.24 DW g/L) on day 40 of culture. Furthermore, we observed the elicitors-induced enhancement in phenolic content (total phenolic content), flavonoid content (total flavonoid content) and antioxidant activity (free radical scavenging activity) in root suspension cultures of A. bracteosa. Application of 0.6 mg/L and 1.2 mg/L of Me-J, root cultures accumulated higher TPC levels (3.6 mg GAE/g DW) and (3.7 mg GAE/g DW) in the log phase and stationary phase, respectively, while 2.5 mg/L Me-J produced lower levels (1.4 mg GAE/g DW) in stationary phase of growth stages. Moreover, TFC and FRSA values were found in correspondence to TPC values in the respective growth phases at the similar elicitor treatment. Thus, a feasible protocol for establishment of adventitious roots in A. bracteosa was developed and enhancement in biomass and metabolite content in adventitious root was promoted through elicitation.
Keywords: Elicitation, Adventitious roots, Methyl jasmonate, Phenyl Acetic Acid, Phenolics, Ajuga
Introduction
Ajuga bracteosa Wall ex Benth. is a highly valued endangered medicinal herb of the family Labiatae (Lamiaceae), distributed in subtropical and temperate regions from Kashmir to Nepal in western Himalaya at an altitude of 1300–2000 m in Bhutan, Pakistan, Afghanistan, China and Malaysia (Arfan et al. 1996; Gautam et al. 2011). It is found in northern hilly areas of Pakistan and locally called as ‘‘Kori Booti’’ due to its acrid taste (Arfan et al. 1996). This herb has a vast variety of medicinal uses and is being used since ancient times for treatment of multiple diseases such as malaria, gout, rheumatism, palsy diabetes, hypertension, fever, stomach pain and amenorrhoea and as tonic and in the treatment of agues (Chopra et al. 1956). It is said to prevent cancer, gastrointestinal disorders, worm infestations, urinary disorders, fungal infections, inflammation and tuberculosis (Jan et al. 2014). Extracts of A. bracteosa contain a substantial number of valuable natural products such as neo-clerodane diterpenoids, phytoecdysteroids (20-Hydroxyecdysone), iridoid glycosides, flavonoids, isoflavonoids and withanolides (Pala et al. 2011). Among these, 20-Hydroxyecdysone is the primary bioactive constituent in A. bracteosa extract and is reported as anti-microbial, anti-tumor, anti-inflammatory and insect anti-feedant agent (Dinan 2001). Withanolides have proven to be effective in treatment against bronchial asthma, inflammation, cancer, and autoimmune diseases (Misico et al. 2011). In the past decade, the market demand for A. bracteosa has increased dramatically not only in the subcontinent region but also in other Asian countries. A. bracteosa is at the verge of eminent danger of extinction in Pakistan due to habitat destruction, over exploitation, illegal collection and has been enlisted in red book of endangered and rare plant species (Kayani et al. 2014). Considering its paramount medicinal significance, novel means and ways should be implemented on the conservation and sustainable utilization of A. bracteosa. In comparison to conventional cultivation procedures, plant cell cultures have emerged as a promising platform for the biosynthesis of valuable metabolites in limited time and space (Yang and Stöckigt 2010). Adventitious roots act as biosynthetic factories for production and accumulation of much valuable health promoting phytochemicals including phenolics, flavonoids, alkaloids etc. In vitro root culture is an efficient way for metabolite production with faster growth rate and active secondary metabolism (Khan et al. 2015b). Plants accumulate secondary metabolites under different stresses like temperature, elicitors or signal molecules, irradiation of different intensities of UV or visible light, injury, nutrient deficiencies, pathogen attack, herbicide treatment etc. (Dixon and Paiva 1995). Elicitation is proven as an effective effective strategy for the improvement and production of bioactive secondary metabolites (Paek et al. 2001). Methyl jasmonate (Me-J) and phenyl acetic acid (PAA) have been employed in vitro for the biosynthesis of many constitutive and inducible health promoting secondary metabolites, notably anti-cancerous compounds through plant cell culture technology (Mulabagal and Tsay 2004). Therefore, the aim of the current study was the establishment of an efficient and reproducible protocol for formation of adventitious roots in A. bracteosa. Furthermore, evaluation of the essential components of plant antioxidant system was elucidated to understand the phenomenon of elicitation for production of secondary metabolites through root suspension cultures.
Materials and methods
Plant material and explant preparation
The wild grown plants of A. bracteosa were collected from Shinkiari situated near by Abbottabad city in Khyber Pakhtunkhwa, Pakistan during November 2015. Explants (nodal regions of ~2.5 cm size) were obtained from wild grown plants of A. bracteosa. During sterilization, the explants were thoroughly rinsed with ethanol (70%) for 5 min followed by rinsing for 3 min in 0.1% (w/v) mercuric chloride solution (HgCl2). Finally, the explants were washed three times with sterile distilled water and were dried on sterile blotting paper. These surface dried explants were placed on MS medium (Murashige and Skoog 1962) solidified with agar (0.8%) (pH 5.8) in sterilized magenta jars. Conditions of growth room were: 16 h of photoperiod of ~40 μM m−2 s−1, 25 ± 1 °C temperature and 70% relative humidity.
Adventitious root induction
Initially, to determine the most pertinent explant type for adventitious roots formation, leaf explants (~3.5 mm2), stem explants (~3.0 mm2) and root segments (~2.5 mm2) were isolated from 28 days old in vitro grown plantlets grown on solid MS medium containing 1.2 mg/L α-naphthalene acetic acid (NAA). Leaf explants were subsequently used in later experiments due to its higher responding frequency than other explants.
To evaluate the impacts of plant growth regulators (PGRs) on induction of adventitious roots, leaf explants were cultured on MS medium supplemented with 4% sucrose (w/v) and solidified at 0.8% (w/v) agar in 150 ml conical flask. Before pouring the media in sterilized petri plates, three concentrations of 2,4-Dichlorophenoxyacetic acid (2,4-D) (0.6, 1.2 or 2.5 mg/L) andindole-3-acetic acid (IAA) or NAA was added to the media. Media was adjusted at 5.8 pH (Eutech pH 510, Singapore) followed by autoclaving at 121 °C for 20 min at 1 atm pressure (System VX 100, Germany). MS medium devoid of any growth regulator (MS0) was used as control treatment. Data on the growth parameters during adventitious rooting were recorded as (1) the frequency of the induction of adventitious roots (%) (2) number of roots per explant (3) biomass accumulation (g/L).
Elicitation and growth kinetics of adventitious root culture
For the development of inoculum culture, 28 days old adventitious roots were transferred into liquid MS medium containing 2.1 mg/L NAA and placed on a gyratory shaker (110 rpm) at room temperature. Inoculum from two weeks old cultures was subsequently used in further experiments, which were carried out by inoculating 2 g fresh root suspension in each flask (Erlenmeyer; 250 mL) containing 50 mL MS medium, 30 g/L sucrose and 1.2 mg/L NAA. The effect of elicitors on various parameters of adventitious roots were studied by adding varying levels (0.6, 1.2, 2.5 mg/L) of methyl jasmonate (Me-J) and phenyl acetic acid (PAA) into culture media. For control treatment, MS medium supplemented with 1.2 mg/L NAA (devoid of elicitors) was used.
Data concerning the growth kinetics and accumulation of secondary metabolites was recorded after every 4 days for a period of 44 days. In all the experiments triplicate culture flasks were used.
Biochemical characterization
Samples for biochemical characterization were collected from the different distinct growth stages of the growth curve during adventitious root culture as control, lag phase, log phase and stationary phase. At each growth stage, adventitious roots were harvested at the onset of maximum biomass accumulation in response to each elicitor treatment.
Samples were collected in triplicates for each growth stage and fresh biomass (FBM) was recorded. Each root suspension culture was filtered through a 0.45 µm stainless steel sieve followed by washing with sterile distilled water. Excess water was removed by gently pressing the samples on filter paper and finally oven dried at 60 °C for 24 h. Dry biomass (DBM) was taken by weighing these dried roots and dried root suspension cultures were subjected to metabolite extraction as per the method of Khan et al. (2013). Briefly, 100 mg of each finely ground dried powder of the samples was soaked in 10 ml methanol (80%; v/v) and sonicated 3 times for ten min with an interval of 30 min. The mixtures were subjected to centrifugation at 8 × 103 rpm for 10 min. The supernatants were decanted into sterile storage tubes and were immediately used or stored at 4 °C for further analysis.
For the estimation of total flavonoid content (TFC), aluminum chloride colorimetric method (ACCM) was used with some modifications in Chang et al. (2002). Briefly, methanolic crude extract prepared in 0.5 ml DMSO (1000 ppm extract) was gently mixed with 0.1 ml of 10% aluminum chloride (AlCl3), 1.5 ml of methanol, 0.1 ml of 1.0 M potassium acetate (CH3CO2K) and 2.8 ml of sterile distilled water and kept at room temperature for 30 min. Finally, the change in absorbance was recorded at 415 nm using UV/VIS-DAD spectrophotometer (Agilent 8453). Quercetin was used as positive control to draw the calibration curve.
Folin-Ciocalteu reagent method (FCRM) was used for the assessment of total phenolic content (TPC) as per the method of Velioglu et al. (1998). Briefly, 0.2 mL of methanolic extract (1000 ppm) of the plant prepared in DMSO was treated with 1.5 mL of Folin-Ciocalteu reagent (10-fold diluted) and kept at room temperature for 5 min. Further, 1.5 mL of 6% (w/v) sodium carbonate (Na2CO3) was added to the mixture, swirled gently and finally kept at room temperature for 90 min. After this incubation, the change in absorbance was recorded at 725 nm by using UV/VIS-DAD spectrophotometer (Agilent 8453). Gallic acid was used as positive control to draw the calibration curve.
1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay (FRSA) was used for determination of antioxidant potential as described by Abbasi et al. (2010). Briefly, 20–30 mg of fresh tissue was weighed followed by maceration in 0.5 mL ethanol (80%) for 10 min, and finally centrifuged at 1 × 104 rpm for 4 min to obtain the crude extract. The reaction was initiated by addition of 0.5 mL diluted test sample to 0.5 mL of 2000 µ mol/L of a DPPH solution for estimation of FRSA. The test tube was kept for 20 min at room temperature, and the absorbance of the reaction mixture was measured at 520 nm using UV–visible spectrophotometer (Agilent 8453, CA USA). The antioxidant potential of each biological sample was calculated as % DPPH free radical scavenging activity.
Statistical analysis
The data given in the manuscript are the mean values of triplicate experiments. Statistical analysis was carried out by GraphPad Prism 5.01 and Statistics 8.1. Significance in variations between treatments was determined by one-way ANOVA (analysis of variance) at P < 0.05.
Results and discussion
Induction and establishment of adventitious roots
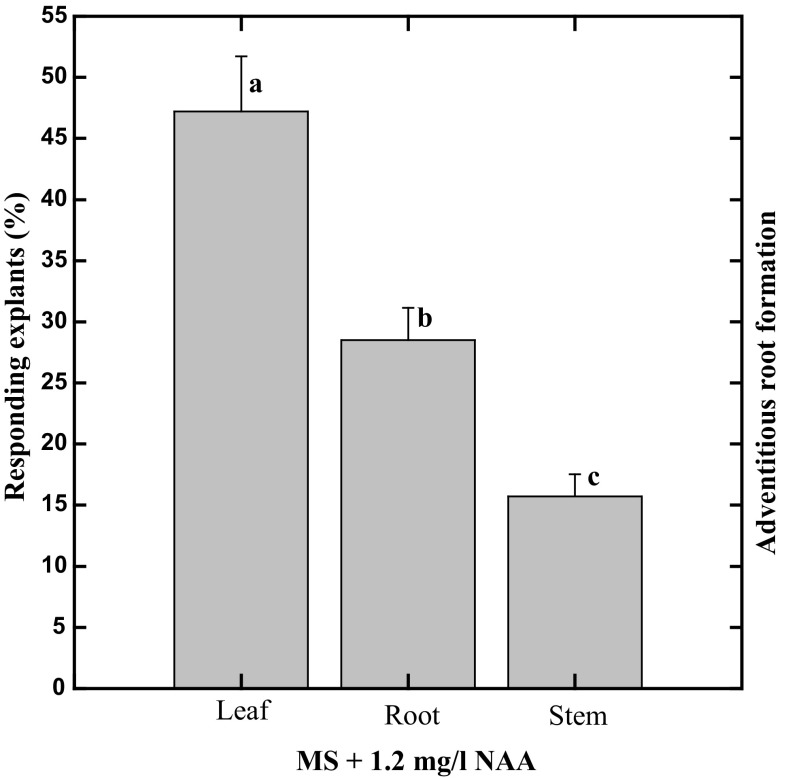
For the determination of the best explant for adventitious roots formation, different explants including leaf, root and stem sections were tested in vitro. It was observed that leaf explants were more effective for adventitious root induction (47%) than other explants i.e. root (27%) and stem (16%) when cultured on MS medium combined with 1.2 mg/L NAA (Fig. 1).
Fig. 1.
Effects of explant type on solid MS media containing 1.2 mg/L NAA on adventitious root formation in A. bracteosa. Columns annotated with common letter/s are similar significantly at P < 0.05
The selection of suitable explant type is a pre-requisite for achieving optimal results in any in vitro morphogenetic processes (Khan et al. 2015b). The differential morphogenetic shifts exhibited by different explants at the same growth conditions may be linked with the genetic variability of the explant types (Abbasi et al. 2016).
The different levels of auxins (2,4 D, NAA or IAA) resulted in induction of the adventitious roots at the cut ends of leaf explants in varying frequency (Table 1). Auxins are commonly reported as potent growth regulators for production of adventitious roots in plants (Bathoju and Giri 2012).
Table 1.
Effects of different levels of auxins on induction of adventitious roots from leaf explant of A. bracteosa in solid media
| S.# | MS + PGRS (mg/L) | Root induction frequency (%) | Roots per explant (mean) | Fresh bio mass (g/L) | Dry bio mass (g/L) |
|---|---|---|---|---|---|
| 1 | MS (0) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2 | 2,4_D (0.6) | 28.1 ± 1.4 c | 8.2 ± 0.4 b | 13.2 ± 0.7 cd | 0.8 ± 0.08 cd |
| 3 | 2,4_D (1.2) | 18.1 ± 1.2d | 6.6 ± 0.08c | 10.3 ± 0.8d | 0.6 ± 0.08d |
| 4 | 2,4_D (2.5) | 12.5 ± 0.6d | 4.2.1 ± 0.4cd | 5.2 ± 0.8de | 0.3 ± 0.08d |
| 5 | NAA (0.6) | 28.2 ± 2.3c | 7.4 ± 0.8c | 13.3 ± 0.7cd | 0.9 ± 0.08cd |
| 6 | NAA (1.2) | 47.2 ± 2.1 b | 18.6 ± 1.1a b | 15.2 ± 1.2 bc | 1.5 ± 0.8 c |
| 7 | NAA (2.5) | 37.2 ± 1.2bc | 14.2 ± 0.8b | 12.6 ± 0.8cd | 0.8 ± 0.02cd |
| 8 | IAA (0.6) | 22.1 ± 1.6cd | 9.6 ± 0.8bc | 10.3 ± 0.8cd | 0.7 ± 0.08b |
| 9 | IAA (1.2) | 30.5 ± 2.6 c | 13.1 ± 1.4 bc | 25.2 ± 1.8 b | 3.3 ± 0.8 ab |
| 10 | IAA (2.5) | 21.7 ± 2.1c | 7.1 ± 0.9c | 12.4 ± 1.1cd | 0.8 ± 0.08cd |
Data represent mean values from three replicates with ±standard error. Values with common letter/s are similar significantly at P < 0.05. Bold letters show highest root induction frequency in comparison
Among the auxins tested, NAA was the most effective for obtaining higher growth parameters of adventitious roots in the present study. Highest root induction frequency (47%), number of roots per explant (18.6), fresh weight (15.2 g/L) and dry weight (1.5 g/L) were observed at 1.2 mg/L NAA after two weeks of the culture period (Table 1). The formation and proliferation of adventitious roots result from primary growth, which has comparatively few vascular and mechanical tissues while in secondary growth relatively thickening of primary roots occur (Zhang et al. 2011, 2012). One reason for the higher activity of NAA compared to other auxins might be that NAA is the more persistent type of auxins, possessing a higher half-life compared to IAA and 2, 4-D in the plant tissues (De Klerk et al. 1997). In comparison to IAA, which is irreversibly conjugated and oxidize quickly in the plant cells, NAA conjugates well, do not oxidize and may be released as free auxin at any time (Peeters et al. 1991). Furthermore, the fact that NAA uptake is faster than other auxins for instance 2,4-D and IAA in our experiments, supported the positive effects of NAA during adventitious roots formation (De Klerk et al. 1997). Significantly lower values of induction frequency and biomass accumulation were observed on 2,4-D (Table 1). Numerous plant in vitro studies have indicated the pivotal role of NAA in adventitious root induction in many medicinally significant plant species (Khan et al. 2015b; Yan et al. 2014). NAA has also been reported to be more inductive than IBA for formation of adventitious roots in milk thistle (Khan et al. 2015b). When IAA was used, 30.5% root induction was observed on 1.2 mg/L (Table 1). The higher biomass accumulation in response to 1.2 mg/L IAA may be ascribed to the rapid photo-oxidation of IAA and thus promoting faster root initiation and cell division. In addition to light induced oxidation, the MS salts too have degrading effect on the lesser stable IAA in the media, depleting it up to 50% in 24 h. On the other hand, 2,4-D and NAA is unaffected by MS salts and light and does not easily oxidized in the medium (Dunlap et al. 1986). Moreover, higher levels of any auxin (2.5 mg/L) significantly reduced the growth parameters during adventitious rooting (Table 1).
Effects of elicitors on rooting parameters
Rooting frequency and biomass formation were further enhanced by exploitation of the leaf explants on MS media supplemented with Me-J or PAA. Highest rooting frequency (63.4%) and maximum biomass accumulation (41.2 FBM) were observed in response to lower levels of Me-J (Table 2). However, PAA was more effective in growth parameters at 1.2 mg/L. The increase in biomass accumulation as a result of elicitation with Me-J can be linked to the triggering response induced by its presence on the endogenous IAA production which is highly involved in rooting of plants (Yan et al. 2014).
Table 2.
Effects of different levels of elicitors on induction of adventitious roots from leaf explant of A. bracteosa in solid media
| S.# | MS + PGRS (mg/L) | Root induction frequency (%) | Roots per explant (mean) | Fresh bio mass (g/L) | Dry bio mass (g/L) |
|---|---|---|---|---|---|
| 1 | PAA (0.6) | 26.1 ± 1.3c | 9.2 ± 0.6c | 18.2 ± 1.1c | 1.8 ± 0.08c |
| 2 | PAA (1.2) | 52.2 ± 2.9 ab | 16.2 ± 1.8 ab | 26.2 ± 2.4 b | 3.8 ± 0.9 b |
| 3 | PAA (2.5) | 23.3 ± 1.4cd | 8.3 ± 1.2c | 12.2 ± 1.2cd | 1.7 ± 0.5c |
| 4 | Me-J (0.6) | 63.4 ± 3.1 a | 24.3 ± 1.9 a | 41.2 ± 2.2 a | 4.9 ± 0.9 a |
| 5 | Me-J (1.2) | 36.4 ± 1.1bc | 14.5 ± 0.8bc | 28.1 ± 2.1b | 3.8 ± 0.8b |
| 6 | Me-J (2.5) | 21.6 ± 1.7cd | 8.7 ± 1.4c | 18.7 ± 1.7bc | 2.7 ± 0.6bc |
Data represent mean values from three replicates with ± standard error. Values with common letter/s are similar significantly at P < 0.05. Bold letters show highest root induction frequency in comparison
Elicitation of adventitious roots in liquid medium
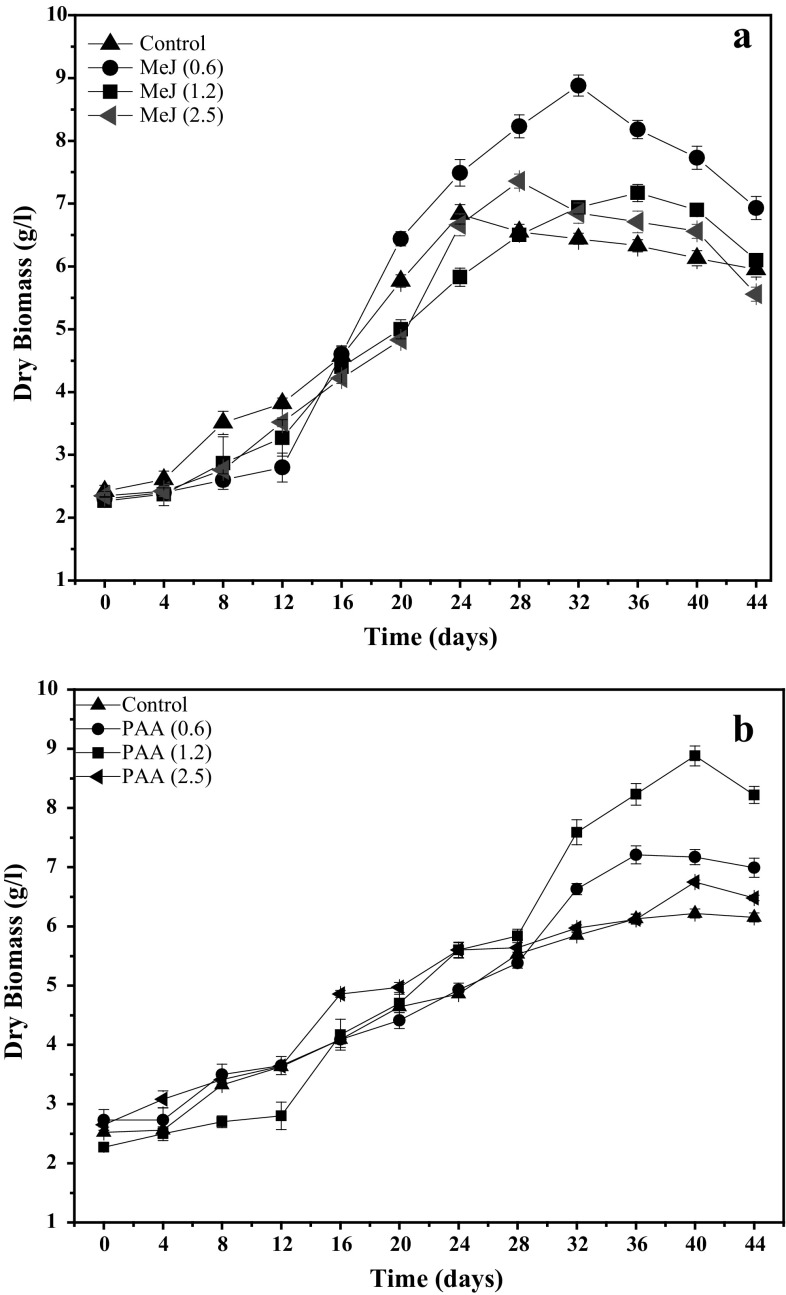
Effects of elicitation on growth kinetics of adventitious roots in suspension cultures showed significant variations in the growth patterns in response to different concentrations of Me-J and PAA (Fig. 2a and b). When treated with 0.6 mg/L Me-J, biomass formation displayed a parabolic growth curve, which represented a lag phase of 8 days, log phase of 24 days, and a stationary phase of 12 days for dry weight (DW) formation during the 44-day study period (Fig. 2a).
Fig. 2.
Growth kinetics of root suspension cultures of A. bracteosa on MS medium combined with 0.6, 1.2 and 2.5 mg/L of methyl jasmonate (a) and Phenyl acetic acid (b). Data represent mean values from three replicates with ±standard error
Maximum biomass formation (8.88 DW g/L) was observed on the 32nd day in log phase of culture. This was almost 4 times greater than the inoculum fresh weight. Furthermore, adventitious roots in suspension culture were found white in log phase, while pale brownish in stationary phase. In response to 1.2 mg/L Me-J, a longer log phase followed by shorter stationary phase was observed with maximum biomass accumulation of (7.17 DW g/L), harvested on 36th day of the growth curve (Fig. 2a). When treated with phenyl acetic acid, 4.2-fold, 3.2-fold and 1.8-fold increment in dry weight was recorded at 1.2, 0.6 and 2.5 mg/L, respectively (Fig. 2b). The involvement of elicitors in root growth in the present study may be attributed to the production of reactive oxygen intermediates (ROIs) in response to elicitors treatment at low level. ROIs such as (–OH) are usually produced by NADPH Oxidase (NOX) in the plant cells, result in wall loosening, cell extension and thus elongation and growth of roots (Gapper and Dolan 2006; Liszkay et al. 2004). However, higher amount of elicitor specifically Me-J strongly inhibits root growth and thus decrease biomass accumulation (Thanh et al. 2005). In the current study, Me-J as low as 0.6 mg/L showed the best response in terms of root induction, rooting frequency and fresh and dry biomass accumulation.
Within all the levels tested, PAA at 1.2 mg/L produced maximum biomass (8.24 g/L) on the 40th day of culture. However, increased levels (2.5 mg/L) of both the elicitors declined the peaks of log phases by 4 and 3 days, respectively, when compared to control. PAA is suggested to be less potent in ROS production than Me-J, a feature that explains the comparatively lower response of PAA. Nonetheless, an inverse relation of the culture volume to fresh biomass of roots was observed with passage of time which may be due to the consumption of nutrients and water from culture medium (Khan et al. 2015b).
Biochemical characterization of the distinct growth phases during adventitious root suspension culture
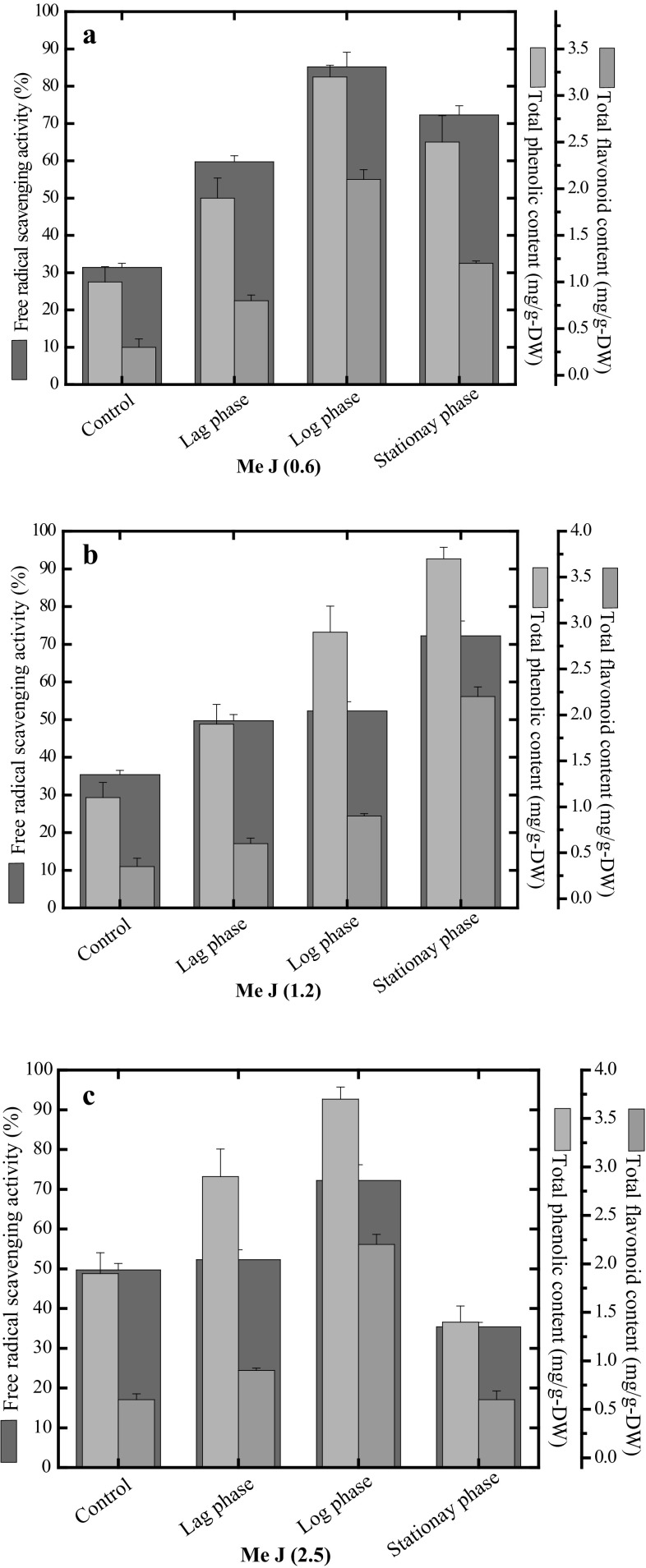
DPPH-FRSA, TPC and TFC were used as biochemical markers for estimation of secondary metabolite content in the distinct growth phases during adventitious root suspension culture in response to different elicitor treatments. Different levels (0.6, 1.2, 2.5 mg/L) of Me-J and PAA were tested in vitro to check their effects on antioxidant potential and accumulation of phenolics and flavonoids in the root cultures harvested from four distinct growth phases i.e. control, lag, log and stationary phase.
In response to 0.6 and 1.2 mg/L of Me-J, root cultures produced higher amounts of TPC i.e. 3.6 and 3.7 mg GAE/g DW in the log phase and stationary phase, respectively, however 2.5 mg/L Me-J declined TPC levels with the lower value of 1.4 mg GAE/g DW detected in stationary phase of growth stages (Fig. 3a, b and c). The fact that Me-J influences the secondary metabolism of plants is already established and reported (Ali et al. 2007). Generally, phenolics and flavonoids are anti-oxidative in nature and are produced in the phenyl-propanoid pathway (Heller and Forkmann 1988; Lister et al. 1996). Biosynthesis of phenolics and flavonoids in plants initiates through the deamination of l-phenylalanine to trans-cinnamic acid and ammonia by a strategic enzyme phenylalanine ammonia-lyase (PAL). (Khan et al. 2015a). Me-J interacts with the surface receptors of plant cell triggering a cascade of plant defense reactions as a result of the de novo transcription of many important secondary metabolism genes such as PAL gene (Gundlach et al. 1992). The role of Me-J as signaling molecule is well documented in literature for their morphological and physiological attributes in plant cell. (Jalalpour et al. 2014). We observed that lower concentrations of Me-J have a stimulating effect on production of phenolic compounds when compared to control (1.9 mg GAE/g DW) (Fig. 3a). The antioxidant activity was demonstrated as percent (%) DPPH free radical scavenging activity (FRSA) in the present study. TFC and FRSA values were found in correspondence to TPC values in the respective growth phases at the similar elicitor treatment. Data in Fig. 3a, b reveals higher FRSA (85.2%) with maximum TFC level (2.1 mg QAE/g DW) in the log phase followed by stationary phase (FRSA; 72.2% and TFC; 2.2 mg QAE/g DW) elicited with 0.6 and 1.2 mg/L of Me-J. Plants cope with the vital stress conditions including biotic and abiotic (elicitor exposure) through a variety of defense responses, which are mediated by production of different secondary metabolites for instance phenolics, flavonoids, and alkaloids etc.
Fig. 3.
Evaluation of secondary metabolite content during growth kinetics of root suspension cultures of A. bracteosa in response to a 0.6 mg/L, b 1.2 mg/L and c 2.5 mg/L of Me-J. Data represent mean values from three replicates with ±standard error
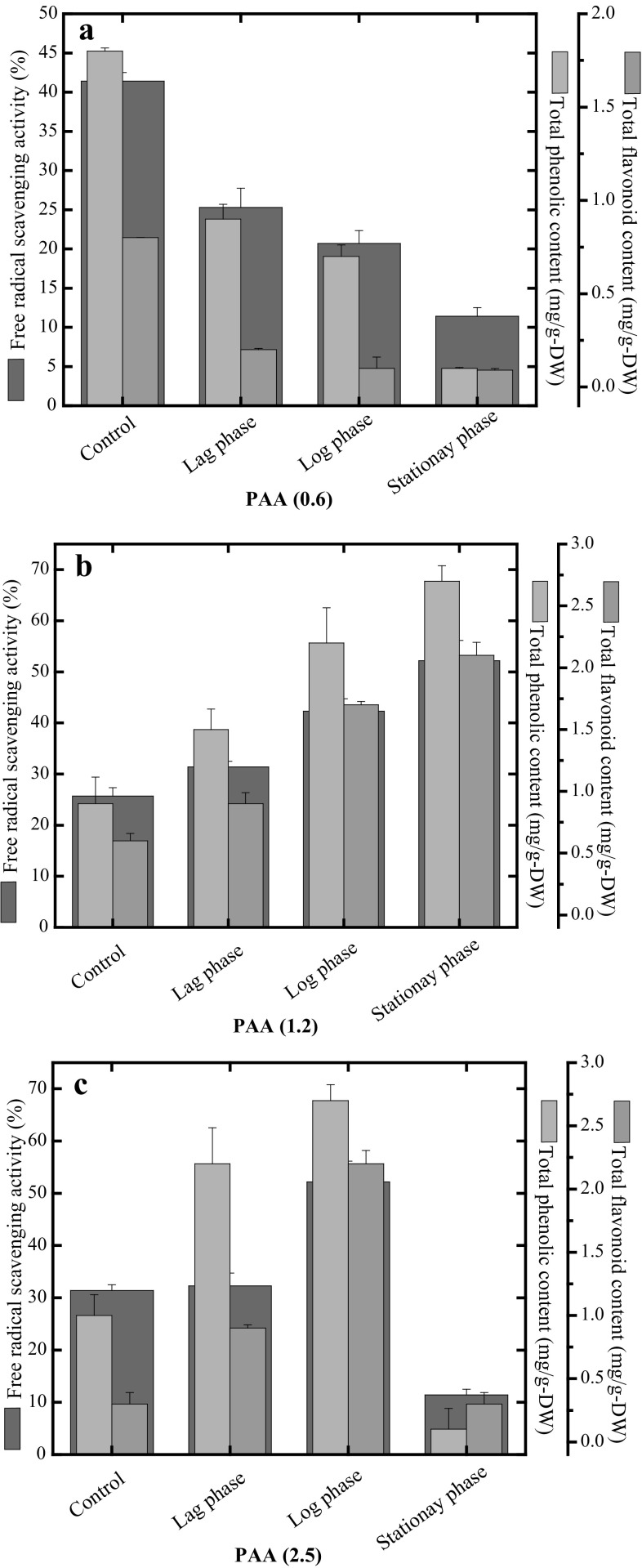
Interestingly, PAA at 0.6 mg/L resulted in higher levels of TPC, TFC and FRSA in the control roots as compared to other growth stages and lower values were detected in the stationary phase (Fig. 4a). The production of phenolic acids and flavonoids in response to PAA induced elicitation highlights the involvement of its role in phenylpropanoid pathway and subsequent production of plant secondary metabolites. When treated with 1.2 mg/L PAA, maximum production of the secondary metabolite content was observed in the stationary phase. At this treatment, higher values of FRSA (52.2%), TFC (2.7 mg QAE/g DW) and TFC (2.1 mg QAE/g DW) were recorded in the root suspension culture (Fig. 4b). Similarly, increased concentration of PAA at 2.5 mg/L resulted in decreased levels of these biochemical markers in the stationary phase of the growth curve (Fig. 4c).
Fig. 4.
Evaluation of secondary metabolite content during growth kinetics of root suspension cultures of A. bracteosa in response to a 0.6 mg/L, b 1.2 mg/L and c 2.5 mg/L of phenyl acetic acid. Data represent mean values from three replicates with ±standard error
Conclusions
An efficient and feasible protocol for the formation of adventitious roots in A. bracteosa was established successfully. Significant enhancement in biomass and metabolite content in adventitious root was promoted through elicitation by Me-J and PAA. This protocol will lead into further research for the commercial production of metabolites from root cultures of A. bracteosa. Further, the molecular mechanism underlying adventitious root formation and proliferation need to be elucidated for determining the putative genes responsible for enhanced production of biomass having higher metabolite content.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abbasi BH, Khan MA, Mahmood T, Ahmad M, Chaudhary MF, Khan MA. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult (PCTOC) 2010;101:371–376. doi: 10.1007/s11240-010-9692-x. [DOI] [Google Scholar]
- Abbasi BH, Ali H, Yücesan B, Saeed S, Rehman K, Khan MA. Evaluation of biochemical markers during somatic embryogenesis in Silybum marianum L. 3 Biotech. 2016;6:1–8. doi: 10.1007/s13205-016-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Hahn E-J, Paek K-Y. Methyl Jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules. 2007;12:607. doi: 10.3390/12030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfan M, Khan GA, Ahmad N. Steroids and terpenoids of the genus Ajuga. J Chem Soc Pak. 1996;18:170–174. [Google Scholar]
- Bathoju G, Giri A. Production of medicinally important secondary metabolites (stigmasterol and hecogenin) from root cultures of Chlorophytum borivilianum (Safed musli) Recent Res Sci Technol. 2012;4:45–48. [Google Scholar]
- Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10(3):178–182
- Chopra RN, Chopra IC, Nayar SL. Glossary of Indian medicinal plants. New Delhi: Council of Scientific and Industrial Research; 1956. [Google Scholar]
- De Klerk G-J, Brugge JT, Marinova S. Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in Malus ‘Jork 9’. Plant Cell Tissue Organ Cult. 1997;49:39–44. doi: 10.1023/A:1005850222973. [DOI] [Google Scholar]
- Dinan L. Phytoecdysteroids: biological aspects. Phytochemistry. 2001;57:325–339. doi: 10.1016/S0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JR, Kresovich S, McGee RE. The effect of salt concentration on auxin stability in culture media. Plant Physiol. 1986;81:934–936. doi: 10.1104/pp.81.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiol. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of Ajuga bracteosa Wall. ex Benth. mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol. 2011;133:928–930. doi: 10.1016/j.jep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Gundlach H, Muller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Forkmann G (1988) Biosynthesis. In: Harborne JB, Mabry TJ, Mabry H (eds) The flavonoids. Springer, pp 399–425
- Jalalpour Z, Shabani L, Afghani L, Sharifi-Tehrani M, Amini S-A. Stimulatory effect of Methyl Jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turk J Biol. 2014;38:76–82. doi: 10.3906/biy-1306-91. [DOI] [Google Scholar]
- Jan M, Singh S, Kaloo ZA, Maqbool F. Callus induction and multiple shoot regeneration in Ajuga bracteosa Wall. ex Benth.—an important medicinal plant growing in Kashmir Himalaya. J Sci Innov Res. 2014;3:319–324. [Google Scholar]
- Kayani WK, Rani R, Ihsan-ul H, Mirza B. Seasonal and geographical impact on the morphology and 20-hydroxyecdysone content in different tissue types of wild Ajuga bracteosa Wall. ex Benth. Steroids. 2014;87:12–20. doi: 10.1016/j.steroids.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Khan MA, Abbasi BH, Ahmed N, Ali H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind Crops Prod. 2013;46:105–110. doi: 10.1016/j.indcrop.2012.12.035. [DOI] [Google Scholar]
- Khan MA, Abbasi BH, Ali H, Ali M, Adil M, Hussain I. Temporal variations in metabolite profiles at different growth phases during somatic embryogenesis of Silybum marianum L. Plant Cell Tissue Organ Cult (PCTOC) 2015;120:127–139. doi: 10.1007/s11240-014-0587-0. [DOI] [Google Scholar]
- Khan MA, Abbasi BH, Shah NA, Yücesan B, Ali H. Analysis of metabolic variations throughout growth and development of adventitious roots in Silybum marianum L. (Milk thistle), a medicinal plant. Plant Cell Tissue Organ Cult (PCTOC) 2015;123:501–510. doi: 10.1007/s11240-015-0854-8. [DOI] [Google Scholar]
- Lee YS, Yang T-J, Park SU, Baek JH, Wu SQ, Lim K-B. Induction and proliferation of adventitious roots from ‘Aloe vera’ leaf tissues for ‘in vitro’ production of aloe-emodin. Plant Omics. 2011;4:190. [Google Scholar]
- Lister CE, Lancaster JE, Walker JR. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J Sci Food Agric. 1996;71:313–320. doi: 10.1002/(SICI)1097-0010(199607)71:3<313::AID-JSFA586>3.0.CO;2-N. [DOI] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2−, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136:3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misico RI, Nicotra VE, Oberti JC, Barboza G, Gil RR, Burton G (2011) Withanolides and related steroids. In: Progress in the chemistry of organic natural products, vol 94. Springer, pp 127–229 [DOI] [PubMed]
- Mulabagal V, Tsay H-S. Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng. 2004;2:29–48. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Paek K-Y, Hahn E-J, Son S-H. Application of bioreactors for large-scale micropropagation systems of plants. In Vitro Cell Dev Biol Plant. 2001;37:149–157. doi: 10.1007/s11627-001-0027-9. [DOI] [Google Scholar]
- Pala A, et al. Ajuga bracteosa wall: a review on its ethnopharmacological and phytochemical studies. Der Pharm Sin. 2011;2:1–10. [Google Scholar]
- Peeters AJ, Gerards W, Barendse GW, Wullems GJ. In vitro flower bud formation in tobacco: interaction of hormones. Plant Physiol. 1991;97:402–408. doi: 10.1104/pp.97.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh N, Murthy H, Yu K, Hahn E, Paek K. Methyl Jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol. 2005;67:197–201. doi: 10.1007/s00253-004-1759-3. [DOI] [PubMed] [Google Scholar]
- Velioglu Y, Mazza G, Gao L, Oomah B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Yan Y-H, et al. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS ONE. 2014;9:e90700. doi: 10.1371/journal.pone.0090700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Stöckigt J. Trends for diverse production strategies of plant medicinal alkaloids. Nat Prod Rep. 2010;27:1469–1479. doi: 10.1039/c005378c. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao W-Y, Wang J, Li X-L. Effects of explant types and media salt strength on growth and secondary metabolite accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant. 2011;33:2447–2452. doi: 10.1007/s11738-011-0785-x. [DOI] [Google Scholar]
- Zhang J, Gao W-Y, Wang J, Li X. Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant. 2012;34:1345–1351. doi: 10.1007/s11738-012-0931-0. [DOI] [Google Scholar]