Abstract
Moringa oleifera Lam., the miracle tree, is widely used as a traditional medicine. The analyses of phytochemicals and antioxidant potential of hydroethanolic extract of various plant parts of M. oleifera revealed that leaves possessed the highest content of total phenolics (9.58 mg/g), β-carotene (14.10 mg/g) and lycopene (2.60 mg/g). Flowers and bark showed the highest content of total flavonoids (3.5 mg/g) and anthocyanin (52.80 mg/g), respectively. Leaves also showed maximum antioxidant potential using nitric oxide scavenging assay (IC50 - 120 µg/ml) and deoxyribose degradation assay (IC50—178 µg/ml). Highest DPPH radical scavenging activity was observed in flowers (IC50—405 µg/ml). The GC–MS study revealed the presence of 29, 36 and 24 compounds in bark, leaf and flower, respectively. The major constituent identified were epiglobulol (41.68% in bark), phytol (23.54% in leaf) and β-sitosterol (15.35% in flower).The phytochemicals identified possess several therapeutic activity, including antioxidant potential, which was confirmed through earlier reports. Moreover, the presence of 1,1,3-triethoxubutane in all the plant parts analyzed, projects it as an important source of waste water treatment as hydrophobic modifiers.
Keywords: Moringa oleifera, Phytochemicals, Antioxidant potential, GC–MS
Introduction
Reactive oxygen species (ROS) or free radicals are molecular species having an unpaired electron in an atomic orbital with independent existence. ROS are produced during oxidative processes and their rate of production increases under pathological conditions (Weidinger and Kozlov 2015). These free radicals initiate the chain reaction and damage the cell. Antioxidants check the concentration of ROS in the cell through scavenging free radical, inhibiting lipid peroxidation, and chelating catalytic metal ions (Valko et al. 2016). Phyto-antioxidants have lesser side effects as compared to the synthetic antioxidants (Nasri et al. 2015) and thus search for novel natural antioxidants becomes inevitable.
Moringa oleifera Lam. (Moringaceae), commonly known as miracle tree, is a good source of proteins, vitamins and minerals (Saini et al. 2016). Every part of the plant is palatable and is considered as the ‘natural nutrition of the tropics’. The plant possesses important metabolites like quercetin, kaempferol, zeatin, campesterol, sitosterol etc., which confer various medicinal uses, including antihypertensive, anticancer, hepatoprotective, anti-inflammatory and cholesterol lowering activities (reviewed by Koul and Chase 2015; Kumar et al. 2016; Saini et al. 2016). The hydroethanolic extract of M. oleifera has been reported to modulate the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages (Fard et al. 2015). In Rajasthan (a semi-arid region of India), parts of this plant form a common ingredient of many dishes cooked in a household kitchen (Bhargave et al. 2015). Studies on humans and animals have revealed that the M. oleifera is quite safe for consumption (Stohs and Hartman 2015).
It is a well established fact that geographic variation affects the production of phyto-compounds with regard to the amount and type, together with its bioactivities (Figueiredo et al. 2008; Vongsak et al. 2015). Thus, exploring a plant for its bioactive components from different regions of the world, in search of new active principles, always holds significance. There are scanty reports on the identification of metabolites of M. oleifera from Rajasthan, which focus only on pods and in vivo antioxidant activity (Paliwal et al. 2011; Mathur and Kamal 2012; Singh et al. 2014). The present work was undertaken to investigate and compare the phytochemicals and antioxidant potential of plant parts (Flower, bark and leaves) of M. oleifera from Rajasthan. This study of the experimental plant has been reported for the first time from this region to the authors’ best knowledge.
Materials and methods
Extraction
Samples of bark and leaves of M. oleifera were collected in the month of February, 2014 and flowers in March, 2014 from the campus of Banasthali University, Rajasthan, India. A voucher specimen was deposited at the herbarium of the Department of Bioscience & Biotechnology, Banasthali University (Specimen no. BURI-890/2015). Various plant parts were dried in oven at 35–40 °C for 4 days and then finely powdered. 5 g of powdered samples were taken in 95% ethanol and kept in an orbital shaker (Metrex, MRS-100C—37 °C; 120 rpm) for 24 h. Thereafter, the contents were centrifuged at 3000 g for 15 min. The supernatant of each sample was collected and stored at 4 °C for further analysis.
Total phenolic content (TPC)
TPC was determined according to the method of Singleton and Rossi (1965). 0.125 ml of ethanolic extract was added to 0.125 ml of Folin–Ciocalteu reagent. Then sodium bicarbonate was added and the mixture was diluted to 3 ml. Test tubes were then incubated for 90 min and absorbance was recorded at 760 nm. The result was expressed as mg of Gallic acid equivalents GAE/g dry weight of sample.
Total flavonoid content (TFC)
The assay was carried out by the method of Vats (2016) and expressed as Quercetin equivalents in mg QE/g dry weight of sample. Extracts were mixed with 95% ethanol, 10% aluminum chloride, 1 M potassium acetate and distilled water. After incubation at room temperature, the absorbance of the reaction mixture was measured at 415 nm.
β-carotene and Lycopene content
1 ml of extract was vigorously shaken with 10 ml of acetone hexane mixture (4:6) for 1 min and filtered through Whatman No. 4 filter paper. The absorbance of the filtrate was measured at 453, 505, and 663 nm and β-carotene and lycopene contents were calculated according to following formula (Nagata and Yamashita 1992):
Ascorbic acid content
1 g of dried and powdered sample was macerated in 10 ml of extracting solution (3% metaphosphoric acid in 1.39 N acetic acid) for 5 min. The solution was centrifuged and the supernatant was titrated against indophenol solution (0.25 mg/ml) till a distinct rose pink color persisted for >5 min (end point) and compared with blank. Ascorbic acid content was calculated as per given formula (AOAC 1990):
X = average milliliter for test solution titration; B = average milliliters for test blank titration; F = milligrams of ascorbic acid equivalent to 1.0 ml indophenol solution; E = grams of sample assayed; V = volume of the initial test solution; and Y = volume test solution titrated.
Anthocyanin content
1 ml of sample was diluted to 5 ml using potassium chloride buffer (0.025 M; pH 1.0) and sodium acetate buffer (0.4 M; pH 4.5), each in different test tubes (Horwitz and Latimer 2006). Absorbance was recorded for both the dilutions at 520 and 700 nm. Anthocyanin pigment concentration (cyanidin-3-glucoside equivalents, mg/ml) was determined as follows:
where, A = (A520 − A700) pH 1.0 − (A520 − A700) pH 4.5, MW = molecular weight of cyanidin-3-glucoside i.e. 449.2 g/mol, DF = dilution factor, 1 = path length in cm, ε = 26,900 molar extinction coefficient in Lmol−1cm−1, 103 = conversion factor from g to mg.
DPPH radical scavenging assay
The assay was carried out according to the method of Vats and Kamal (2014). 1 ml plant extract was mixed with 1 ml of 0.3 mM DPPH and allowed to stand for 30 min at room temperature in dark. The absorbance was taken at 517 nm and IC50 (μg/ml) was calculated.
Nitric oxide scavenging assay (NOSA)
2 ml of sodium nitroprusside (10 mM) in 0.5 ml phosphate buffer saline (1 M; pH 7.4) was mixed with 0.5 ml extract and the mixture was incubated at 25 °C for 150 min. From the incubated mixture, 0.5 ml was taken out and added to 1 ml of sulphanilic acid reagent. 1 ml of naphthylethylenediaminedihydrochloride (0.1%) was added and incubated at room temperature for 30 min. Absorbance was measured at 540 nm. Nitric oxide radical scavenging activity was calculated and expressed as IC50 (μg/ml; Badami et al. 2003).
Deoxyribose degradation assay (DDA)
The reaction mixture contained in a final volume of 1.0 ml, 100 µl of 2-deoxy-2-ribose (28 mM), 500 µl solution of various concentrations of test sample in potassium phosphate buffer (50 mM; pH = 7.4), 200 µl of EDTA (1.04 mM) and FeCl3 (200 μM) solution (1:1 v/v), 100 µl of H2O2 (1 mM) and 100 µl of ascorbic acid and the mixture was incubated at 37 °C for 1 h. 1 ml of TBA (1%) and TCA (2.8%) each was added to the test tubes and was incubated at 100 °C for 20 min. After cooling, absorbance was measured at 532 nm. Results were expressed as IC50 (μg/ml; Halliwell et al. 1987).
FRAP Assay
300 mM acetate buffer (pH 3.6), 10 mM 2, 4, 6-tripyridyl-striazine (TPTZ) in 40 mM HCl and 20 mM FeCl3.6H2O in distilled water was prepared. 25 ml of acetate buffer, 2.5 ml TPTZ solution and 2.5 ml FeCl3.6H2O solution was mixed to make the working solution.50 µl of extract was mixed with 1.5 ml of FRAP reagent. Absorbance was recorded after 5 min at 593 nm (Benzie and Strain 1996).
GC–MS analysis
The analysis was done on a Shimadzu system GC–MS QP2010 on an omega wax column. The following program was used: helium was used as the carrier gas at an injection temperature 250 °C (with a split ratio of 10.0), ion-source temperature 230 °C. The oven temperature was programmed from 80 °C (isothermal for 3 min), with an increase of 10 °C/min, to 250 °C (isothermal for 5 min), then 15 °C/min to 280 °C, ending with a 18 min isothermal at 280 °C. MS was programmed to have a scan interval of 0.5 s.The peaks were identified from NIST (National Institute of Standards and Technologies) or WILEY8 libraries of the mass spectrometer.
Statistical analysis
Experimental results were expressed as mean ± standard error (n = 3). Statistical analysis was done using Duncan’s MRT at P < 0.05.
Results and discussion
TPC and TFC
TPC was found to be 0.59 and 1.36 folds more in the leaves of the experimental plant as compared to flower and bark, respectively. On the other hand highest content of TFC was observed in flower (3.5 ± 0.1 mg/g; P < 0.05) as lowest in bark (1.47 ± 0.08 mg/g; P < 0.05; Table 1). Total polyphenols were found to be more in the aqueous methanolic extract of M. oleifera leaves grown in Chad, Sahrawi refugee camps (Southwestern Algeria), and Haiti as compared to the present study (Leone et al. 2015). Fakurazi et al. (2012) reported lesser TPC in flowers (0.24 mg GAE/g) and leaves (0.19 mg GAE/g) in 80% ethanolic extract of M. oleifera collected from Malaysia. Moreover, they reported a low TPC content in aqueous extract than ethanolic extract. The alcoholic leaf and flower extracts of miracle tree showed the presence of 4.44 and 4.41 mg/ml of TFC, and 2.28 and 1.08 mg/ml of TPC, respectively (Sankhalkar and Vernekar 2016). Singh et al. (2009) reported 9.9 and 12.63 folds higher TPC and TFC, respectively in the aqueous extract of leaves of M. oleifera collected from Lucknow, India. This shows that the selection of solvent for extraction is important for getting more amount of phytometabolites of interest. The difference can also be attributed to the different geographical locations from where the samples have been collected because environmental factors influence the production of bioactive compounds (Akula and Ravishankar 2011).
Table 1.
Amount of various phytometabolites (mg/g) in plant parts of M. oleifera
| Plant parts | TPC | TFC | β-carotene | Lycopene | Ascorbic acid | Anthocyanin |
|---|---|---|---|---|---|---|
| Leaf | 9.58c ± 0.29 | 2.3b ± 0.09 | 14.10c ± 0.05 | 2.60c ± 0.04 | 2.80b ± 0.18 | 9.40a ± 0.98 |
| Flower | 6.03b ± 0.06 | 3.5c ± 0.1 | 1.40b ± 0.10 | 0.40b ± 0.10 | 7.10c ± 0.46 | 40.90b ± 1.88 |
| Bark | 4.06a ± 0.04 | 1.47a ± 0.08 | 0.60a ± 0.04 | 0.30a ± 0.05 | 2.60a ± 0.25 | 52.80c ± 1.46 |
Values are mean ± S.E. (n = 3). Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
Carotenoid and lycopene content
Leaves showed 5.5 and 7.67 folds more lycopene content as compared to flower and bark, respectively. Similarly, the highest content of β-carotene was observed in leaves (14.1 ± 0.05 mg/g) and lowest in bark (0.60 ± 0.04 mg/g; Table 1). Leone et al. (2015) observed the presence of 0.28 mg/g of β-carotene in the leaves of the experimental plant. Lower content of β-carotene in M. oleifera leaves has also been reported by other workers (Charan and Gupta 2013; Raghavendra et al. 2015). The lycopene content in different plant parts of the experimental plant has been reported for the first time. Carotenoids widely distributed in plants, giving red, orange or yellow color to fruits/leaves and are a precursor of vitamin A. Lycopene is a carotenoid which protects biomolecules like DNA, proteins and lipids against adverse effects of free radicals (Reshmitha et al. 2017). It has the highest potential to scavenge singlet oxygen. Thus, it plays a significant role in disease management (Lin et al. 2016).
Ascorbic acid content
Ascorbic acid reduces the indicator dye 2, 6-dichloroindophenol, to colorless solution. Excess unreduced dye gives rose pink color in an acid solution and indicates the end point (AOAC 1990). The highest amount of ascorbic acid was found in flower (7.10 ± 0.46 mg/g) and the lowest amount in bark (2.60 ± 0.25 mg/g; Table 1). The content was found to be more than the Moringa samples collected from Nicaragua and Pakistan (Siddhuraju and Becker 2003; Iqbal and Bhanger 2006), which reveals its high nutritional property. Raghavendra et al. (2015) reported a lower content of Ascorbic acid (0.75 mg/g) in the leaves of M. oleifera. As ascorbic acid is an important and widely studied dietary antioxidant present in the extracellular fluids. It neutralizes ROS in aqueous phase before the initiation of lipid peroxidation and also regenerates α-tocopherol, which in turn inhibits peroxyl radical and singlet oxygen (Percival 1998).
Anthocyanin content
Anthocyanin is responsible for the blue, purple and red colour of flower, fruits and leaves (Lu et al. 2015). Monomeric anthocyanin reversibly change color with a change in pH. The difference in the absorbance of the pigments at 520 nm is proportional to the pigment concentration (Lee et al. 2005). The least amount of all the metabolites analyzed, except anthocyanin, was observed in bark. The anthocyanin content was evaluated to be 4.62 and 0.29 folds more than leaves and flower, respectively (Table 1). Anthocyanins have been reported to be an antidiabetic and insulinotropic agent, inhibitor of lens opacity caused due to diabetic retinopathy and lipid lowering agent interfering with obesity (Ghosh and Konishi 2007).
Antioxidant assays
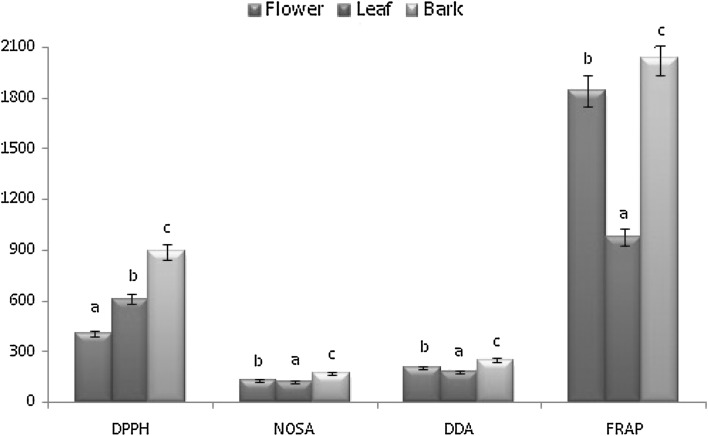
DPPH, NOSA and DDA were expressed as IC50. Lower the IC50 value, higher is the antioxidant activity. Highest DPPH scavenging activity was observed in flower (405 µg/ml) followed by leaf (610 µg/ml) and bark (890 µg/ml; Fig. 1). Leaves collected from different provinces in Thialand showed better DPPH scavenging activity, which ranged from 39.73–150.64 µg/ml (Vongsak et al. 2015). Santos et al. (2012) reported relatively lower scavenging activity of the ethanolic extract of the flower of Moringa collected from Brazil. This difference may be attributed to a different geographical location of sample collection, which often leads to a differential metabolite profile, especially antioxidants (Iqbal and Bhanger 2006). The antiradical efficiency of leaves of the experimental plant procured from Rajasthan has been reported (Kamal et al. 2012). NO scavenging assay is based on the scavenging of nitric oxide radicals generated from sodium nitroprusside by Griess reagent (sulphanilamide and NED that compete for nitrite in the Griess reaction). Leaf (120 µg/ml) and flower (130 µg/ml) showed better activity against nitric oxide radical (Fig. 1). Sreelatha and Padma (2009) reported a much lower value of IC50 for NO scavenging assay for aqueous extract of leaves of Moringa from Tamil Nadu, India, using soxhlet process. It can be concluded that the method of extraction and solvent used plays a vital role in asserting the antioxidant potential of the plant extract. Hydroxyl radical scavenging activity was found to be highest in the leaves (178 µg/ml) followed by flower and bark (Fig. 1). Florence et al. (2014) reported very high IC50 value for OH scavenging assay in the leaf sample from Brazil using methanol as solvent. Thus, it can be concluded that hydroethanolic extract is better solvent for M. oleifera for hydroxyl radical scavenging activity. FRAP assay depends upon the reduction of ferric tripyridyltriazine (Fe3+-TPTZ) complex to ferrous tripyridyltriazine (Fe2+-TPTZ) complex, which has an intense blue color which can be monitored at 593 nm (Vats 2016). Unlike the above results highest ferric reduction (FRAP) was shown by bark (2036.6 ± 3.3 μM) followed by flower (1845 ± 16.25 μM) and leaf (978.3 ± 20.8 μM; Fig. 1). There is no previous report on FRAP activity of bark. There are few reports on the FRAP activity of leaves and flower of Moringa (Fakurazi et al. 2012; Florence et al. 2014). Effective antioxidant potential of M. oleifera shown in various assays can be attributed to the presence of significant amounts of antioxidants (TPC, TFC, acorbic acid, anthocyanins, lycopene and carotenoids) as reported earlier in the present study.
Fig. 1.
Antioxidant potential of M. oleifera (DPPH, NOSA & DDA-µg/ml; FRAP-µM)
GC–MS analysis
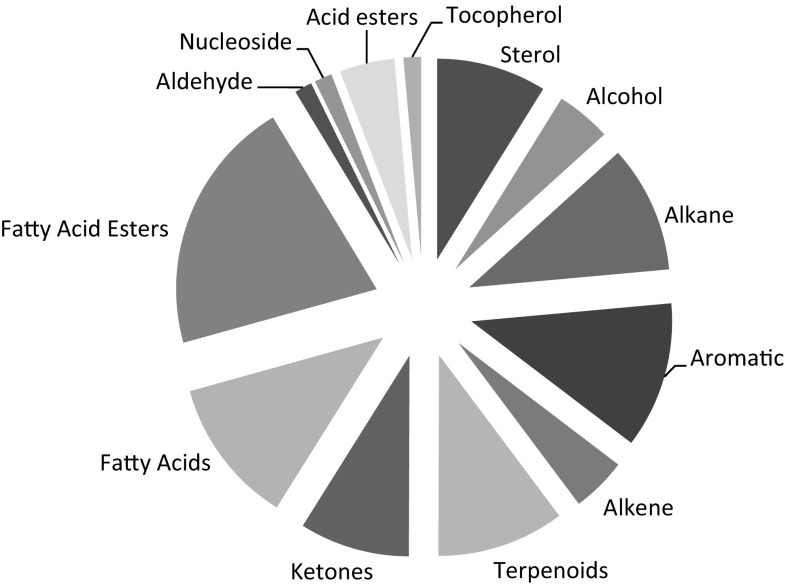
The GC–MS study revealed the presence of 29, 36 and 24 compounds in bark, leaf and flower of M. oleifera, respectively (Table 2). Overall, four compounds were found to be present in all the plant parts analyzed (1,1,3-triethoxybutane; acetophenone; epiglobulol and n-hexadecanoic acid); bark and leaf possessed 6 common compounds (2,4-hexadiene, 1,1-diethoxy; cis-3-hexenal diethyl acetal; cytidine; 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester; hexadecanoic acid, methyl ester; and cis-9,12-linoleic acid); 3 compounds were present in both bark and flower (3,8-dimethyl-2,7-dioxaspiro[4.4]nonane-1,6-dione;2-methyloctacosane and stigmasterol), and leaf and flower (detradecanoic acid; hexadecanoic acid, ethyl ester; and β-sitosterol). Out of 69 compounds identified (compounds present in more than one plant parts were considered only once) from different plant parts only 18 have been reported earlier (discussed in the next paragraph), the rest has been reported for the first time. Major phytochemical groups identified have been shown in Fig. 2.
Table 2.
Constituents of hydroethanolic extract of plant parts of M. oleifera
| No. | R. time | Area % | Components (Bark) |
|---|---|---|---|
| 1. | 4.525 | 0.82 | cis-4-Cyclopentene-1,3-diol# |
| 2. | 4.715 | 0.84 | 3,8-Dimethyl-2,7-dioxaspiro[4.4]nonane-1,6-dione# |
| 3. | 4.934 | 1.71 | 1,1,3-Triethoxybutane# |
| 4. | 5.872 | 6.19 | Acetophenone# |
| 5. | 6.170 | 0.64 | 2-Phenylpropan-2-ol# |
| 6. | 7.226 | 0.41 | 2,4-Hexadiene, 1,1-diethoxy# |
| 7. | 8.171 | 0.47 | cis-3-Hexenal diethyl acetal# |
| 8. | 9.115 | 6.44 | Alpha citral# |
| 9. | 10.783 | 0.68 | Tridecane |
| 10. | 10.890 | 0.38 | Heptadecane |
| 11. | 11.686 | 5.88 | Cytidine# |
| 12. | 12.075 | 0.24 | Benzeneacetonitrile, 4-hydroxy- |
| 13. | 13.638 | 41.68 | Epiglobulol# |
| 14. | 16.083 | 0.19 | Neophytadiene# |
| 15. | 16.168 | 0.35 | 2-Pentadecanone, 6,10,14-trimethyl-# |
| 16. | 16.492 | 0.44 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester# |
| 17. | 16.888 | 0.42 | 2-Phenyltridecane# |
| 18. | 16.976 | 0.38 | Hexadecanoic acid, methyl ester# |
| 19. | 17.328 | 1.72 | n-Hexadecanoic acid |
| 20. | 17.642 | 1.20 | Heptadecanoic acid, ethyl ester |
| 21. | 18.625 | 1.01 | 9-Hexadecyn-1-ol# |
| 22. | 19.153 | 0.28 | Docosanoic acid |
| 23. | 19.242 | 0.34 | cis-9,12-Linoleic acid# |
| 24. | 19.283 | 0.99 | Ethyl 9-hexadecenoate# |
| 25. | 20.446 | 0.27 | Palmitate |
| 26. | 22.599 | 0.62 | 2-Methyloctacosane# |
| 27. | 23.463 | 1.04 | Bis(2-ethylhexyl) phthalate |
| 28. | 34.718 | 4.78 | Stigmasterol |
| 29. | 36.126 | 13.55 | γ-Sitosterol# |
| Leaf | |||
| 1. | 4.319 | 0.74 | Phenol# |
| 2. | 4.515 | 0.66 | 2-Hydroxy-gamma-butyrolactone# |
| 3. | 4.929 | 0.82 | 1,1,3-Triethoxybutane# |
| 4. | 5.867 | 4.49 | Acetophenone# |
| 5. | 6.166 | 0.34 | Phenylpropane-2-ol# |
| 6. | 7.136 | 0.16 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4 h-pyran-4-one |
| 7. | 7.224 | 0.19 | 2,4-Hexadiene, 1,1-diethoxy-# |
| 8. | 8.169 | 0.18 | cis -3-hexenal diethyl acetal# |
| 9. | 11.593 | 1.57 | Cytidine# |
| 10. | 12.837 | 0.50 | Phosphoric acid, diethyl octyl ester# |
| 11. | 13.643 | 4.03 | Epiglobulol# |
| 12. | 14.510 | 0.75 | 2,6-Bis(1,1-Dimethylethyl)-4-Methylphenol# |
| 13. | 15.250 | 0.54 | Tetradecanoic acid |
| 14. | 16.093 | 0.74 | 2,6,10-Trimethyl,14-ethylene-14-pentadecne# |
| 15. | 16.490 | 0.14 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester# |
| 16. | 16.974 | 1.21 | Hexadecanoic acid, methyl ester# |
| 17. | 17.061 | 0.24 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione# |
| 18. | 17.337 | 5.04 | n-Hexadecanoic acid |
| 19. | 17.638 | 0.95 | Hexadecanoic acid, ethyl ester |
| 20. | 18.633 | 0.56 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester# |
| 21. | 18.702 | 4.37 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-# |
| 22. | 18.825 | 23.54 | Phytol |
| 23. | 19.054 | 1.18 | cis,cis,cis-7,10,13-Hexadecatrienal# |
| 24. | 19.233 | 0.51 | cis,cis-Linoleic acid# |
| 25. | 19.309 | 4.33 | Linolenic acid, ethyl ester# |
| 26. | 20.292 | 0.23 | Isopropyl(dimethyl)silylpalmitate# |
| 27. | 20.443 | 1.36 | Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester# |
| 28. | 21.067 | 0.64 | 4,8,12,16-Tetramethylheptadecan-4-olide# |
| 29. | 21.197 | 0.70 | Methyl (Z)-5,11,14,17-eicosatetraenoate# |
| 30. | 22.245 | 0.58 | 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester# |
| 31. | 22.367 | 0.39 | Hexadecadienoic acid, methyl ester# |
| 32. | 22.594 | 2.28 | Eicosane |
| 33. | 31.920 | 20.57 | Vitamin E |
| 34. | 36.101 | 1.74 | β-Sitosterol |
| 35. | 36.554 | 2.82 | Stigmasta-5,24(28)-dien-3-ol, (3.beta.)-# |
| 36. | 38.344 | 1.57 | Lupeol# |
| Flower | |||
| 1. | 4.380 | 7.08b | 1,2,3-Propanetriol# |
| 2. | 4.711 | 0.37 | 3,8-Dimethyl-2,7-dioxaspiro[4.4]nonane-1,6-dione# |
| 3. | 4.933 | 1.03 | 1,1,3-Triethoxybutane# |
| 4. | 5.869 | 14.39 | Acetophenone# |
| 5. | 6.166 | 0.87 | Phenylpropan-2-ol# |
| 6. | 8.971 | 0.54 | dl-Mevalonic acid lactone# |
| 7. | 13.644 | 2.62 | Epiglobulol# |
| 8. | 14.510 | 1.04 | Propanoic acid, 2-methyl-3-[4-t-butyl]phenyl-# |
| 9. | 15.248 | 0.91 | Tetradecanoic acid |
| 10. | 17.338 | 8.14 | n-Hexadecanoic acid |
| 11. | 17.639 | 5.25 | Hexadecanoic acid, ethyl ester |
| 12. | 19.058 | 3.07 | (Z,Z)-6,9-cis-3,4-epoxy-nonadecadiene# |
| 13. | 19.281 | 14.77 | Ethyl Oleate# |
| 14. | 19.491 | 1.49 | Octadecanoic acid, ethyl ester# |
| 15. | 20.404 | 1.85 | Tetratetracontane |
| 16. | 21.385 | 0.79 | Docosanoic acid, ethyl ester# |
| 17. | 22.593 | 4.74 | 2-Methyloctacosane# |
| 18. | 24.042 | 0.85 | Docosanoic acid, ethyl ester# |
| 19. | 25.745 | 4.36 | Hexatriacontane |
| 20. | 33.959 | 1.85 | Campesterol# |
| 21. | 34.718 | 3.64 | Stigmasterol |
| 22. | 36.084 | 15.35 | β-sitosterol# |
| 23. | 37.044 | 1.43 | Methyl commateC# |
| 24. | 39.939 | 1.77 | Stigmast-4-en-3-one# |
#Reported first time in M. oleifera
Fig. 2.
Major phytochemical groups identified from GC–MS analysis of hydroethanolic extract of M. oleifera (compounds present in more than one plant parts have been considered only once)
Chuang et al. (2007) reported the presence of tridecane, n-hexadecanoic acid and palmitate in the volatile component of leaves of M. oleifera from Taiwan. Mukunzi et al. (2011) identified tetradecanoic acid and heptadecane together with tridecane from the samples collected from Rwanda and China. Few other compounds, which have been already identified are-stigmasterol; β-sitosterol (Anwar et al. 2007); 2,3-dihydro-3,5-dihydroxy-6-methyl-4 h-pyran-4-one (Dev et al. 2011); phytol; eicosane; hexatroacontane (Marrufo et al. 2013); benzeneacetonitrile, 4-hydroxy-; heptadecanoic acid, ethyl ester; docosanoic acid; bis(2-ethylhexyl) phthalate; tetratetracontane (Mathur et al. 2014); hexadecanoic acid, ethyl ester (Nepolean et al. 2009) and vitamin E (Atawodi et al. 2010). In another study GC–MS analysis showed the presence of cis-vaccenic acid; 9,12,15-octadecatrienoic acid ethyl ester; 6-octadecenoic acid; and 2-octyl-cyclopropaneoctanal in the methanolic extract of M. oleifera leaves (Jayanthi et al. 2015). The present study shows a difference in the amount of the already reported compounds in the plant parts analyzed together with the occurrence of non-reported compounds. The climatic zone where the plant is grown potentially affects the presence of metabolites and its bioactivities (Figueiredo et al. 2008).
In order to ascertain the ‘miracle’ behind the miracle tree, the bioactivity/use of the identified compounds were explored from previously reported studies (Table 3). Components of bark showed potential as antioxidant, antimicrobial, antiseptic, cytotoxic, HIV Type 1 reverse transcriptase inhibitors, etc. Bioactive compounds from leaf possess antimicrobial, antioxidant, hypoglycemic, anti-inflammatory, antidiarrhoeal and others. Metabolites from flower were reported to have antioxidant, antidiabetic, anticataract, antiearwax, antineuralgic, antiseptic, cytotoxic and others (Table 3). Moreover, the presence of 1,1,3-Triethoxybutane in all the plant parts analyzed, projects it as an important source of waste water treatment, as hydrophobic modifiers, which finds relevance in the present day global water pollution scenario (Soane et al. 2012).
Table 3.
Activities of the identified compounds as reported from from previous studies.
Sources: ** Soane et al. (2012); *Dr. Duke’s Phytochemical and Ethno botanical Database (2014); 1Ramasamy and Gopalakrishnan (2013); 2 Goclik et al. (1999); 3Gohar et al. (2010); 4Kumar et al. (2010); 5Asghar et al. (2011)
| Components | Previously reported bioactivity |
|---|---|
| cis-4-cyclopentene-1,3-diol (B) | NR |
| 3,8-Dimethyl-2,7-dioxaspiro[4.4]nonane-1,6-dione (B, F) | NR |
| 1,1,3-Triethoxybutane (B, L, F) | Hydrophobic modifier in waste water treatment** |
| Acetophenone (B, L, F) | Antibacterial, fungicide* |
| 2-Phenylpropan-2-ol (B) | Flavor, fragrance* |
| 2,4-Hexadiene, 1,1-diethoxy (B, L) | NR |
| cis-3-Hexenal diethyl acetal (B, L) | NR |
| Alpha citral (B) | Antibacterial, pesticide* |
| Tridecane (B) | Antimicrobial* |
| Heptadecane (B) | Antimicrobial, cytotoxic to HeLa and MCF-7 cell lines1 |
| Cytidine (B, L) | NR |
| Benzeneacetonitrile, 4-hydroxy- (B) | HIV type 1 reverse transcriptase and tyrosine kinase inhibitor2 |
| Epiglobulol (B, L, F) | Antiseptic, cytotoxic* |
| Neophytadiene(B) | Antipyretic, analgesic, anti-inflammatory, antimicrobial, antioxidant* |
| 2-Pentadecanone, 6,10,14-trimethyl- (B) | Fragrance* |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (B, L) | Antibacterial3 |
| 2-Phenyltridecane (B) | NR |
| Hexadecanoic acid, methyl ester (B, L) | Anti inflammatory* |
| n-Hexadecanoic acid (B, L, F) | Antioxidant, hypocholesterolemic, pesticide, anti-androgenic factor4 |
| Heptadecanoic acid, ethyl ester (B) | Antioxidant* |
| 9-Hexadecyn-1-ol (B) | NR |
| Docosanoic acid (B) | Cosmetics* |
| cis-9,12-linoleic acid (B, L) | Antioxidant, hypocholesterolemic* |
| Ethyl 9-hexadecenoate (B) | Antioxidant, anti androgenic, flavor, hemolytic* |
| Palmitate (B) | Antioxidant, Hypocholesterolemic4 |
| 2-Methyloctacosane (B,F) | Pheromone* |
| Bis(2-ethylhexyl) phthalate (B) | Oral toxicity during pregnancy and suckling in long -Evans rat5 |
| Stigmasterol (B,F) | Antioxidant, antiviral, Antihepatotoxic, anti-inflammatory* |
| γ-sitosterol (B) | Anti-diabetic, antiangeogenic, anticancer, anti-inflammatory, antimicrobial* |
| Phenol (L) | Analgesic, antioxidant, Antibacterial* |
| 2-Hydroxy-gamma-butyrolactone (L) | NR |
| Phenylpropane-2-ol (L) | Flavor, fragrance* |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4 h-pyran-4-one (L) | Antimicrobial, anti-inflammatory, antioxidant* |
| Phosphoric acid, diethyl octyl ester (L) | NR |
| 2,6-Bis(1,1-Dimethylethyl)-4-Methylphenol (L) | Antioxidant* |
| Tetradecanoic acid (L, F) | Antioxidant, cancer preventive, lubricant, cosmetics* |
| 2,6,10-Trimethyl,14-ethylene-14-pentadecne (L) | Antipyretic, analgesic, anti-inflammatory, antimicrobial, antioxidant* |
| 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (L) | NR |
| Hexadecanoic acid, ethyl ester (L, F) | Antioxidant, anti androgenic, flavor, hemolytic* |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (L) | Anticarcinogenic, antiatherogenic, antioxidant, anti-inflammatory* |
| 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- (L) | Anti-inflammatory, antieczemic, anticoronary, insectifuge* |
| Phytol (L) | Antimicrobial, anticancer, anti-inflammatory, diuretic4 |
| cis,cis,cis-7,10,13-hexadecatrienal (L) | NR |
| cis,cis-linoleic acid (L) | Antioxidant, hypocholesterolemic* |
| Linolenic acid, ethyl ester (L) | Anti-inflammatory, hypocholesterolemic, hepatoprotective* |
| Isopropyl(dimethyl)silylpalmitate (L) | NR |
| Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester (L) | Flavor* |
| 4,8,12,16-Tetramethylheptadecan-4-olide (L) | Antimicrobial* |
| Methyl (Z)-5,11,14,17-eicosatetraenoate (L) | Antibacterial, to treat dysentery and diarrhea* |
| 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester (L) | NR |
| Hexadecadienoic acid, methyl ester (L) | Anti-inflammatory* |
| Eicosane (L) | Antimicrobial and larvicidal* |
| Vitamin E (L) | Antioxidant, immunostimulant, Hypocholesterolemic4 |
| β-Sitosterol (L,F) | Anti diabetic, antiangeogenic, anti-inflammatory, antidiarroheal* |
| Stigmasta-5,24(28)-dien-3-ol, (3.beta.)- (L) | Antioxidant* |
| Lupeol (L) | Antioxidant, antihypoglycemic, Anti-tumor* |
| 1,2,3-Propanetriol (F) | Anticataract, antiearwax, antineuralgic, antiketotic* |
| dl-Mevalonic acid lactone (F) | NR |
| Propanoic acid, 2-methyl-3-[4-t-butyl]phenyl- (F) | Antioxidant* |
| (Z,Z)-6,9-cis-3,4-epoxy-nonadecadiene (F) | Pheromone* |
| Ethyl Oleate (F) | Flavor* |
| Octadecanoic acid, ethyl ester (F) | 5-alpha reductase inhibitor, hypocholesterolemic, lubricant, flavor* |
| Tetratetracontane (F) | Antimalarial and antibacterial |
| Docosanoic acid, ethyl ester (F) | Cosmetics* |
| Hexatriacontane (F) | Antioxidant* |
| Campesterol (F) | Antioxidant, hypocholesterolemic, anti-inflammatory* |
| Methyl commate C (F) | Antidiabetic, antihyperlipidemic* |
| Stigmast-4-en-3-one (F) | Antiprostatitic* |
Within parentheses—B Bark; L Leaf; F Flower; NR No report
In conclusion, the study projects M. oleifera as a very rich source of bioactive compounds having multiple therapeutic activities including antioxidant potential, which was elucidated through the GC–MS and other biochemical studies. Moreover, the role of the plant in waste water treatment can also be explored. The study also emphasizes the need to study plants from various geographical regions in search of new therapeutic molecules. Overall, the plant is a good candidate for the search of future nutraceuticals.
Acknowledgements
The authors thank Prof. Aditya Shastri, Vice Chancellor and Prof. Vinay Sharma, Head Department of Bioscience and Biotechnology, Banasthali Vidyapith, India, for providing the necessary facilities. Ms. Tanya Gupta is grateful to the DBT, Govt. of India for providing M.Sc. fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Akula R, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;611:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of the Association of Official analytical chemists. 15. Arlington: A Association of Official analytical chemists; 1990. [PubMed] [Google Scholar]
- Asghar SF, Rehman H, Choudahry MI, Rahman A. Gas chromatography–mass spectrometry (GC–MS) analysis of petroleum ether extract oil and bio-assays of crude extract of Iris germanica. Int J Genet Mol Bio. 2011;37:95–100. [Google Scholar]
- Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, Bartsch H, Owen RW. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J Med Food. 2010;13:710–716. doi: 10.1089/jmf.2009.0057. [DOI] [PubMed] [Google Scholar]
- Badami S, Moorkoth S, Rai SR, Kannan E, Bhojraj S. Antioxidant activity of Caesalpinia sappan heartwood. Biol Pharm Bull. 2003;26(11):1534–1537. doi: 10.1248/bpb.26.1534. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhargave A, Pandey I, Nama KS, Pandey M. Moringa oleifera Lam.—Sanjana (Horseradish Tree)—A miracle food plant with multipurpose uses in Rajasthan-India—an overview. Int J Pure App Biosci. 2015;3(6):237–248. doi: 10.18782/2320-7051.2169. [DOI] [Google Scholar]
- Charan AA, Gupta P. Comparative analysis of antibacterial, antioxidant and photosynthetic activity of Azadirachta indica, Rosa indica and Moringa oleifera cultivars. Int J Curr Res. 2013;53:556–561. [Google Scholar]
- Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Biores Technol. 2007;98:232–236. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dev SR, Geetha P, Orsat V, Gariépy Y, Raghavan GS. Effects of microwave-assisted hot air drying and conventional hot air drying on the drying kinetics, color, rehydration, and volatiles of Moringa oleifera. Drying Technol. 2011;29:1452–1458. doi: 10.1080/07373937.2011.587926. [DOI] [Google Scholar]
- Duke J (2014) Dr Duke’s phytochemical and ethnobotanical databases. http://www.ars-grin.gov/duke/
- Fakurazi S, Sharifudin SA, Arulselvan P. M. oleifera hydroethanolic extracts effectively alleviate acetaminophen-induces hepatotoxicity in experimental rats through their antioxidant nature. Molecules. 2012;17:8334–8350. doi: 10.3390/molecules17078334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard MT, Arulselvan P, Karthivashan G, Adam SK, Fakurazi S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn Mag. 2015;11(Suppl 4):S556–S563. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- Florence IF, Adeboye AO, Stephen IO. Comparative evaluation of in vitro antioxidant properties of Cajanus cajanseed and M. oleifera leaf extracts. Int J Biochem Res Rev. 2014;42:163–172. doi: 10.9734/IJBCRR/2014/6460. [DOI] [Google Scholar]
- Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;162:200–208. [PubMed] [Google Scholar]
- Goclik E, Konig GM, Wright AD. Collection and secondary metabolite investigations of marine organisms from the two Azorean island Faial and São Jorge Arquipélago. Life Mar Sci. 1999;17A:43–49. [Google Scholar]
- Gohar YM, El-Naggar MMA, Soliman MK, Barakat KM. Characterization of marine Burkholderia cepacia antibacterial agents. J Nat Prod. 2010;3:86–94. [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. Thedeoxyribose methods: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:210–215. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Horwitz W, Latimer GW (2006) Liquids. In: Official methods of analysis of AOAC international (18thedn). AOAC International: Gaithersburg, Maryland
- Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Comp Anal. 2006;196:544–551. doi: 10.1016/j.jfca.2005.05.001. [DOI] [Google Scholar]
- Jayanthi M, Garg SK, Yadav P, Bhatia AK, Goel A. Some newer marker phytoconstituents in methanolic extract of Moringa oleifera leaves and evaluation of its immunomodulatory and splenocytes proliferation potential in rats. Indian J Pharmacol. 2015;47(5):518–523. doi: 10.4103/0253-7613.165199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal R, Yadav S, Mathur M, Katariya P. Antiradical efficiency of 20 selected medicinal plants. Nat Prod Res. 2012;26:1054–1062. doi: 10.1080/14786419.2011.553720. [DOI] [PubMed] [Google Scholar]
- Koul B, Chase N. Moringa oleifera Lam.: panacea to several maladies. J Chem Pharm Res. 2015;7(6):687–707. [Google Scholar]
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity total phenolics and GC–MS study of Vitex negundo. Afr J Biochem Res. 2010;4(7):191–195. [Google Scholar]
- Kumar A, Naaz F, Kushwaha A, Chaudhary P, Srivastav P. Present review on phytochemistry, neutraceutical, antimicrobial, antidiabetic, biotechnological and pharmacological characteristics of Moringa oleifera Linn. BMR Phytomed. 2016;2(1):1–17. [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269–1278. [PubMed] [Google Scholar]
- Leone A, Fiorillo G, Criscuoli F, Ravasenghi S, Santagostini L, Fico G, Spadafranca A, Battezzati A, Schiraldi A, Pozzi F, di Lello S. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi refugee camps, and Haiti. Int J Mol Sci. 2015;16(8):18923–18937. doi: 10.3390/ijms160818923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Zhao HS, Xiang LR, Xia J, Wang LL, Li XN, Li JL, Zhang Y. Lycopene protects against atrazine-induced hepatic ionic homeostasis disturbance by modulating ion-transporting ATPases. J Nutr Biochem. 2016;27:249–256. doi: 10.1016/j.jnutbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Meng X, Wan H, Zhang J, Tian J, Hao S, Jin K, Yao Y. Photoperiod and shading regulate coloration and anthocyanin accumulation in the leaves of malus crabapples. Plant Cell Tissue Organ Cult. 2015;121(3):619–632. doi: 10.1007/s11240-015-0733-3. [DOI] [Google Scholar]
- Marrufo T, Nazzaro F, Mancini E, Fratianni F, Coppola R, De Martino L, Agostinho AB, DeFeo V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules. 2013;18:10989–11000. doi: 10.3390/molecules180910989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur M, Kamal R. Studies on trigonelline from Moringa oleifera and its in vitro regulation by feeding precursor in cell cultures. Rev Bras Farmacogn. 2012;22:994–1001. doi: 10.1590/S0102-695X2012005000041. [DOI] [Google Scholar]
- Mathur M, Yadav S, Katariya PK, Kamal R. In vitro propagation and biosynthesis of steroidal sapogenins from various morphogenetic stages of Moringa oleifera Lam., and their antioxidant potential. Acta Physiol Plant. 2014;36:1749–1762. doi: 10.1007/s11738-014-1549-1. [DOI] [Google Scholar]
- Mukunzi D, Nsor-Atindana J, Xiaoming Z, Gahungu A, Karangwa E, Mukamurezi G, Al-Domi H, Princewill-Ogbonna IL, Ogbonna PC, Arief NJ. Comparison of volatile profile of Moringa oleifera leaves from Rwanda and China using HS-SPME. Pak J Nutr. 2011;10:602–608. doi: 10.3923/pjn.2011.602.608. [DOI] [Google Scholar]
- Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Jpn Soc Food Sci Technol. 1992;39:925–928. doi: 10.3136/nskkk1962.39.925. [DOI] [Google Scholar]
- Nasri H, Shirzad H, Baradaran A, Rafieian-kopaei M. Antioxidant plants and diabetes mellitus. J Res Med Sci. 2015;20(5):491–502. doi: 10.4103/1735-1995.163977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepolean P, Anitha J, Emilin RR. Isolation, analysis and identification of phytochemicals of antimicrobial activity of Moringa oleifera Lam. Curr Biotica. 2009;3:33–37. [Google Scholar]
- Paliwal R, Sharma V, Pracheta SH. Hepatoprotective and antioxidant potential of Moringa oleifera pods against DMBA-induced hepatocarcinogenesis in male mice. Int J Drug Dev Res. 2011;3:128–138. [Google Scholar]
- Percival M. Antioxidants. Clin Nutr Insights. 1998;1098:54–58. [Google Scholar]
- Raghavendra S, Rajashekara E, Nagaraj MS, Ramesh CK, Paramesha M, Rao SJ. Evaluation of phytoconstituents, nutrient composition and antioxidant properties in Moringa oleifera-BhagyaKDM 01 variety. Curr Trends Biotechnol Pharm. 2015;9(4):369–379. [Google Scholar]
- Ramasamy V, Gopalakrishnan VK. Identification of bioactive compounds from Spirulina by gas chromatography coupled with mass spectrophotometer (GC–MS) Am J PharmTech Res. 2013;3(6):497–506. [Google Scholar]
- Reshmitha TR, Thomas S, Geethanjali S, Arun KB, Nisha P. DNA and mitochondrial protective effect of lycopene rich tomato (Solanum lycopersicum L.) peel extract prepared by enzyme assisted extraction against H2O2 induced oxidative damage in L6 myoblasts. J Funct Foods. 2017;28:147–156. doi: 10.1016/j.jff.2016.10.031. [DOI] [Google Scholar]
- Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6(2):203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankhalkar S, Vernekar V. Quantitative and Qualitative analysis of Phenolic and Flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacog Res. 2016;8(1):16–21. doi: 10.4103/0974-8490.171095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AF, Argolo AC, Paiva PM, Coelho LC. Antioxidant activity of Moringa oleifera tissue extracts. Phytother Res. 2012;269:1366–1370. doi: 10.1002/ptr.4591. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey D, Upadhyay G, Singh HB. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol. 2009;476:1109–1116. doi: 10.1016/j.fct.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Singh D, Arya PV, Aggarwal VP, Gupta RS. Evaluation of antioxidant and hepatoprotective activities of Moringa oleifera Lam. leaves in carbon tetrachloride-intoxicated rats. Antioxidants. 2014;3(3):569–591. doi: 10.3390/antiox3030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. [Google Scholar]
- Soane DS, Mahoney RP, Slattery I (2012) Treatment of wastewater. U.S. Patent Application, 13/713,671
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 2009;644:303–311. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29(6):796–804. doi: 10.1002/ptr.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90(1):1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Vats S. Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Appl Biochem Biotechnol. 2016;178:504–512. doi: 10.1007/s12010-015-1890-x. [DOI] [PubMed] [Google Scholar]
- Vats S, Kamal R. Identification of flavonoids and antioxidant potential of Cassia tora L. Am J Drug Discov Dev. 2014;4(1):50–57. doi: 10.3923/ajdd.2014.50.57. [DOI] [Google Scholar]
- Vongsak B, Mangmool S, Gritsanapan W. Antioxidant activity and induction of mRNA expressions of antioxidant enzymes in HEK-293 cells of Moringa oleifera leaf extract. Planta Med. 2015;81(12/13):1084–1089. doi: 10.1055/s-0035-1546168. [DOI] [PubMed] [Google Scholar]
- Weidinger A, Kozlov AV. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]