Abstract
Antioxidant enzymes are known to play a significant role in scavenging reactive oxygen species and maintaining cellular homeostasis. Activity of four antioxidant enzymes viz., superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) was examined in the flag leaves of nine Aegilops tauschii and three Triticum dicoccoides accessions along with two bread wheat cultivars under irrigated and rain-fed conditions. These accessions were shortlisted from a larger set on the basis of field performance for a set of morpho-physiological traits. At anthesis, significant differences were observed in enzyme activities in two environments. A 45% elevation in average GR activity was observed under rain-fed conditions. Genotypic variation was evident within each environment as well as in terms of response to stress environment. Aegilops tauschii accession 3769 (86% increase in SOD, 41% in CAT, 72% in APX, 48% in GR activity) and acc. 14096 (37% increase in SOD, 32% CAT, 25% APX, 42% GR) showed up-regulation in the activity of all the four studied antioxidant enzymes. Aegilops tauschii accessions—9809, 14189 and 14113 also seemed to have strong induction mechanism as elevated activity of at least three enzymes was observed in them under rain-fed conditions. T. dicoccoides, on the other hand, maintained active antioxidative machinery under irrigated condition with relatively lower induction under stress. A significant positive correlation (r = 0.760) was identified between change in the activity of CAT and GR under stress. Changes in plant height, spike length and grain weight were recorded under stress and non-stress conditions on the basis of which a cumulative tolerance index was deduced and accessions were ranked for drought tolerance. Overall, Ae. tauschii accession 3769, 14096, 14113 (DD-genome) and T. dicoccoides accession 7054 (AABB-genome) may be used as donors to combine beneficial stress adaptive traits of all the three sub-genomes into a synthetic hexaploid for improving wheat for water stress conditions.
Keywords: Wild wheats, Antioxidant enzymes, Reactive oxygen species, Water deficit stress
Introduction
Bread wheat is an allohexaploid (AABBDD) that arose from interspecific hybridization of tetraploid Triticum turgidum (AABB, syn. T. dicoccoides) and diploid Aegilops tauschii Coss. (DD) (Mcfadden and Sears 1946). The interspecific cross and subsequent spontaneous chromosome doubling that formed the foundation of hexaploid common wheat was a rare event (Warburton et al. 2006). The genetic diversity within D-genome of wheat progenitor, Aegilops tauschii (wild goat grass) is much higher than that of bread wheat (Caldwell et al. 2004). Similarly, the AB genome donor, Triticum turgidum subsp T. dicoccoides (wild emmer wheat), native of eastern Mediterranean region, had accumulated over its long evolutionary history, genetic diversity and adaptations to multiple abiotic and biotic stresses (Peleg et al. 2005).
Natural genetic variation in Ae. tauschii and T. dicoccoides population holds potential for improving drought tolerance of modern varieties of common wheat since unpredictable moisture deficit during crop growth is one of the major constraints to productivity and stability of crop performance. Water deficiency and the concomitant heat stress at anthesis and grain filling disturb the steady state levels of reactive oxygen species (ROS) in the cell. Excessive accumulation of re active oxygen species represented predominately by superoxide anion (O2 −), hydrogen peroxide (H2O2), hydroxyl radical (·OH) and singlet oxygen (1O2) leads to the generation of oxidative stress. Oxidative burst is known to damage membranes through lipid peroxidation besides causing protein denaturation and DNA damage (Mittler 2002). It might also impair the machinery that works to maintain cellular homeostasis and ameliorate the damage caused due to stress post recovery (Hernandez and Almansa 2002). To overcome the cytotoxic effects of reactive oxygen species, a highly efficient antioxidative enzymatic defense system- superoxide dismutase (EC 1.15.1.1), catalase (EC 1.11.1.16), peroxidase (1.11.1.7), ascorbate peroxidase (EC 1.11.1.11), glutathione reductase (1.6.4.2), glutathione-S-transferase (GST) is present in plant cells. These enzymes are located in different compartments of cell except catalase which is located exclusively in peroxisomes (Willekens et al. 1997). Superoxide dismutase (SOD) is the front line of defense that rapidly dismutates superoxide but produces yet another ROS i.e. H2O2 in this process. H2O2 scavengers are catalase (CAT) and ascorbate peroxidase (APX) but these enzymes seem to have distinct roles because they are located in different compartments of the cell. APX works as a fine regulator of ROS under steady state level but catalase might cause bulk removal of H2O2 under stress conditions (Gill and Tuteja 2010). Glutathione reductase (GR) catalyses the rate limiting step of ascorbate glutathione pathway. When the activity of this enzyme gets upregulated, the ratio of NADP+/NADPH increases. Consequently, high NADP+ pool accept excess electrons and limit their flow to oxygen in photosynthetic electron transport chain (Asada and Takahashi 1987).
Modulation in the activity of these enzymes may be important in plant’s resistance to environmental stresses (Allen 1995). Genotypes respond differentially to oxidative injury as a result of variation in their antioxidant systems (Pastori and Trippi 1992; Kraus et al. 1995). Wheat genotypes have been reported to have different levels of tolerance to water deficit induced oxidative stress (Sairam et al. 1998; Loggini et al. 1999; Lascano et al. 2001; Sgherri et al. 2000; HongBo et al. 2005; Khanna-Chopra and Selote 2007; Simova-Stoilova et al. 2009; Hameed et al. 2011; Varga et al. 2012; Singh et al. 2012). Comparison between tetraploid and hexaploid wheat cultivars with respect to antioxidant response under water stress has also been reported (Sairam et al. 2001).
During slow process of evolution from wild type to domestic, plants have undergone a series of changes in morphology, physiology and biochemical mechanisms (Evans 1981). Because man-made selections in breeding and heavy use of agricultural inputs in crop cultivation were mainly directed towards improving grain yield and end-use quality, as a result many useful alleles for stress tolerance were left behind (Shimshi et al. 1982). Accordingly, drought tolerance potential of wild wheat relatives and landraces is now being examined using several drought adaptive indicator traits like root system architecture, cell membrane stability, osmotic adjustment, relative water content, water use efficiency (WUE) and stomatal conductance, content of proline, ABA, chlorophyll and carbohydrate remobilization (Peleg et al. 2005; Reynolds et al. 2007; Kurahashi et al. 2009). With regard to variability in antioxidant system and use of this trait as a surrogate for selections in breeding, a study conducted by Badiani et al. 1990 considered relation between drought stress and enzymatic antioxidant system in different species of wheat. Zhang and Kirkham (1994) first reported that hexaploids (T. aestivum, T. compactum) had higher peroxidase and malondialdehyde levels and were found to have less efficient antioxidant system (Asa-Glu cycle) than tetraploid (T. carthilicum, T. dicoccum, T. dicoccoides and T. durum) and diploid wheat (T. monococcum). However, DD-genome species, i.e. Ae. tauschii was not a part of this studies. Also, the studies were conducted at seedling stage and not under field conditions. In a recent study, Osipova et al. (2011) investigated the relationship between dehydro ascorbate reductase (DHAR) and catalase (CAT) in chromosome substitution lines of cv. Chinese spring carrying separate chromosomes from donor synthetic hexaploid wheat (6x) under water deficit stress. However, genotypic difference in the activity of different ROS scavenging enzymes in wild wheat in contrasting environments during crop development has not been a subject of active investigation though several studies have been carried out in cultivated wheat and other cereal and legume species.
Breeding wheat for enhancing resilience to environmental stresses, maintaining growth and sustaining yield under unfavourable conditions particularly drought, requires strengthening the genetic makeup of present day wheat cultivars The untapped genetic diversity inherent in these wild progenitors may be introgressed into common wheat by the ‘bridge’ of synthetic hexaploids derived from cross of tetraploid wheat x Ae. tauschii (Trethowan and Mujeeb-Kazi 2008). Keeping these points in mind, a chosen set of accessions of immediate wheat progenitors, i.e. Ae. tauschii and T. dicoccoides were taken to evaluate the degree of resilience of these wild accessions in terms of the activity of key reactive oxygen species scavenging enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) and relate them to plant performance under water deficit stress.
Materials and methods
Plant material
The present study was conducted on nine accessions of Aegilops tauschii (diploid, DD-genome) and three accessions of Triticum dicoccoides (tetraploid, AABB-genome) (Table 1). These accessions were chosen from a larger set of fifty-seven Ae. tauschii and twenty-six T. dicoccoides accessions that were initially evaluated under irrigated and rain-fed conditions for key drought adaptive traits (membrane injury, relative water content and content of proline) and phenotypic traits (plant height, spike length and grain weight) for two crop seasons (2010–2011 and 2011–2012).
Table 1.
Aegilops tauschii and Triticum dicoccoides accessions used for the study
| Accessions | Species | Origin | Source/supplier | Supplier’s accession no. |
|---|---|---|---|---|
| 3769 | Aegilops tauschii (diploid) | Iran | Dr. JP Gustafson, University of Missouri, USA | – |
| 9809 | Iran | Dr. BS Gill, Kansas State University, USA | TA2468 | |
| 14096 | Azerbaijan | Dr. Andreas Boerner, IPK, Gatersleben, Germany | AE145 | |
| 14113 | Azerbaijan | AE195 | ||
| 14129 | Azerbaijan | AE211 | ||
| 14130 | Turkmenistan | AE212 | ||
| 14189 | Afghanistan | AE278 | ||
| 14219 | Turkmenistan | AE499 | ||
| 14231 | Soviet Union | AE692 | ||
| 7054 | Triticum dicoccoides (tetraploid) | Israel | National Bureau of Plant Genetic Resources (NBPGR), New Delhi | EC 171812 |
| 7079 | Israel | EC 171837 | ||
| 14004 | Israel | Centre for Plant Breeding Research, Wageningen, Netherlands | G194-3 M-6 M |
Information generated from these morpho-physiological traits was used to constitute the present set of genotypes. Within the Ae. tauschii group, accession 3769, 9809, 14130 and 14189 had performed well under rain-fed conditions reflecting their inherent tendency to sustain growth despite stressful conditions. Accessions 14113, 14129 and 14219 displayed stable performance under both irrigated and rain-fed environments. However, accessions 14096 and 14231 depicted relatively poorer response for field studied physiological and biochemical traits (Suneja 2014). With respect to Triticum dicoccoides, three accessions- 7054, 7079 and 14004 were shortlisted for enzymatic analysis. Accession 7054 ranked high for phenotypic traits. Accession 7079 suffered minimum membrane damage and accumulated higher levels of proline. Accession 14004 had shown greater membrane stability but a relatively poorer phenotypic response (Suneja 2014). These T. dicoccoides accessions had also been evaluated for the activity of key carbohydrate metabolizing enzymes under irrigated and rain-fed conditions (Suneja et al. 2015).
In addition to diploid and tetraploid progenitor accessions, two landmark wheat cultivars- PBW-343 and C-306 (hexaploid, AABBDD-genome) were also included in the set. C-306 is a tall, traditional, pre-green revolution variety (released in 1965) which continues to be grown as a rain-fed cultivar. PBW-343, an Attila ‘sib’ and a derivative of Veery group of wheats developed through spring wheat x winter wheat crosses at CIMMYT, Mexico, was released in 1995. It is one of the most widely grown wheat cultivars under irrigated conditions of North West Plain Zone of India.
Raising of Aegilops tauschii and Triticum dicoccoides plants in the field
Germinated seeds of Ae. tauschii accessions were vernalized in cold chambers maintained at 4 °C with 8/16 h light/dark regime for six weeks. Vernalized seedlings of Ae. tauschii and germinated seedlings of T. dicoccoides were transplanted in the experimental fields of Department of Plant Breeding and Genetics, P.A.U. Ludhiana (30°54′N and 75°48′E) in two sets (later demarcated as irrigated and rain-fed set) in the last week of October. Three replications per set were sown in randomized complete block design. The natural day length was supplemented with artificial light to ensure 14–15 h of light per day. Pre-sowing irrigation was given and thereafter, one set was irrigated at periodic intervals, whereas, plants in the adjacent set received water only available through rainfall and all irrigations were withheld from sowing to maturity. Soil moisture content was determined at different stages of crop development. The rain-fed plot was found to have 70% less moisture content than irrigated plot at anthesis. Although crop season received a total rain-fall of 108.8 mm (October 2011 to May 2012), the month of March (period of anthesis and active grain-filling) received no rainfall, which allowed sufficient build up of water deficit stress in crop.
Membrane injury
Percent membrane damage was measured at vegetative stage, sixty days after sowing (DAS). The assay was performed according to Blum and Ebercon (1981) with some modifications. Cut end of the leaves were submerged in deionised water and stoppered in control set, whereas those in stressed set were kept in 40% PEG for 24 h. After 24 h, in case of stressed samples, 40% PEG was drained; samples washed (2–3) times with deionised water and then kept at 10 °C for 24 h with cut end of the leaf submerged in deionised water. After incubation, the conductivity (µ siemens) was recorded respectively for control and stressed samples using a digital conductivity meter. After the measurements were taken, vials were autoclaved for 15 min at 121 °C/0.10 MPa and their conductance was measured again. Membrane damage was expressed in percent units
T1 = Mean conductivity of stressed sample before autoclaving, T2 = Mean conductivity of stressed sample after autoclaving, C1 = Mean conductivity of control sample before autoclaving, C2 = Mean conductivity of control sample after autoclaving.
Relative water content
Relative water content from the leaves of irrigated and rain-fed plants was recorded at vegetative stage, sixty days after sowing (DAS) using the method of Weatherly (1950).
Extraction and estimation of proline
Filtrate obtained after homogenizing leaf tissue (100 mg) in 3% aqueous sulfosalicyclic acid was used for proline estimation by the procedure of Bates et al. (1973).
Extraction and estimation of antioxidant enzymes
Antioxidant enzymes were extracted from flag leaf at anthesis stage corresponding to Zadok stage GS65 (Zadoks et al. 1974). Since flowering time of the accessions varied, 90–100 days after sowing, when anthers of 50% of the spikes have extruded out, the flag leaf sample was taken for enzyme extraction. All the four enzymes- SOD, CAT, APX and GR were extracted in triplicate (replication-wise) with relevant extraction buffers at 4 °C to minimize denaturation and were assayed at 37 °C. The components of assay system except enzyme extract were pre-incubated at 37 °C for 15 min before starting the assay.
Superoxide dismutase (SOD)
Leaf sample was extracted with chilled 0.1 M Tris HCl buffer (pH 7.5) containing EDTA, PVP, β-mercaptoethanol using pre-chilled pestle and mortar and assayed after centrifugation as described by Marklund and Marklund (1974). A unit of enzyme activity was defined as the amount of enzyme causing 50% inhibition of auto-oxidation of pyrogallol observed in blank in one minute in one gram of fresh tissue.
Catalase (CAT)
Catalase was extracted from leaf tissue with ice cold 0.05 M sodium phosphate buffer (pH 7.5) containing 1% polyvinyl pyrrolidone. The activity of CAT was determined following the method of Chance and Maehly (1955). One unit of catalase activity was expressed as μmoles of H2O2 decomposed min−1 g−1FW.
Ascorbate peroxidase (APX)
Enzyme was extracted from leaf tissue with 0.05 M sodium phosphate buffer (pH 7.0) containing EDTA, polyvinyl pyrrolidone (PVP) and ascorbic acid and assayed from the supernatant obtained after centrifugation at 10,000g for 15 min (Asada and Takahashi 1987). One unit of ascorbate peroxidase activity was expressed as µmoles of monodehydroascorbate formed min−1 g−1FW.
Glutathione reductase (GR)
Extraction procedure for glutathione reductase was similar to that of superoxide dismutase. Activity of the enzyme was determined by adding 0.2 M sodium phosphate buffer (pH 7.5), MgCl2, EDTA, NADPH and oxidized glutathione to the enzyme extract following the decrease of absorbance at 340 nm as described by Esterbauer and Grill (1978). One unit of glutathione reductase activity was expressed as µmoles of NADP+ formed min−1 g−1FW.
Observation of plant phenotypic traits indicative of water stress tolerance
Data was recorded for the plant height and spike length in field grown crop (under both irrigated and rain-fed conditions), a week before crop attained maturity. Later, the seeds were dehulled from spikelets of Ae. tauschii and T. dicoccoides and threshed out from ears of cultivated wheats to obtain 100-grain weight. Consequently overall plant performance was evaluated in terms of change observed in these phenotypic characters under stress, expressed as a percentage by taking values in non-stress environment as 100. These were referred to as Tolerance index 1(percent resistance to change in plant height), Tolerance index 2 (percent resistance to change in spike length) and Tolerance index 3 (percent resistance to change in grain weight). Thereafter, values of tolerance index1 (TI1), tolerance index 2 (TI2) and tolerance index 3(TI3) were averaged to obtain a cumulative tolerance index (CTI). Cumulative tolerance index that served as a measure of drought tolerance was used for ranking the genotypes in each ploidy level.
Statistical analysis
Results were expressed as Mean ± S.D. (n = 3). Two-way analysis of variance (ANOVA) was performed to determine the role of environments (E), genotypes (G) and genotype x environment (G x E) interaction on the activity of different antioxidant enzymes in contrasting environments (P ≤ 0.05, DSASTAT software1.101). Fisher LSD test of significance (P ≤ 0.05, DSASTAT software1.101) was used to indicate significant differences between genotypes and treatments. Correlation coefficients were worked out (using MS Excel 2007) for associating tolerance behaviour with change in the activity of enzyme under stress. Mean values of enzyme activity were calculated and percent increase/decrease in activity under stress was determined (taking values in non-stress environment as 100). Using NTSYS pc 2.02, multivariate principal component analysis (PCA) was used to cluster the genotypes and assess association between all evaluated traits under water stress conditions (Rohlf 1998).
Results
The set of Ae. tauschii, T. dicoccoides and cultivated wheat accessions revealed significant effect of environment (irrigated and rain-fed), genotypes as well as genotype x environment interaction on the degree of membrane injury, relative water content, proline content (vegetative stage), activities of all the four antioxidant enzymes- superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) (at anthesis) and phenotypic characters- plant height, spike length and grain weight (at maturity) (Table 2).
Table 2.
Analysis of variance (ANOVA) for the studied traits in a set of Aegilops tauschii, Triticum dicoccoides accessions and cultivated wheats
| Environment (E) | Genotype (G) | G X E | Error | |
|---|---|---|---|---|
| Degrees of freedom (df) | 1 | 13 | 13 | 26 |
| Membrane injury | – | 443.03* | – | 45.746 |
| Relative water content | 431.14* | 13.11* | 4.70* | 5.7 |
| Proline content | 6.578* | 0.996* | 0.309* | 0.26 |
| Superoxide dismutase | 108.42* | 78.06* | 48.18* | 16.54 |
| Catalase | 2588672.00* | 573026.40* | 670552.60* | 184971.3 |
| Ascorbate peroxidase | 80.18* | 64.90* | 16.36* | 3.72 |
| Glutathione reductase | 4281464.00* | 395458.50* | 225713.20* | 105350 |
| Plant height | 3404* | 4586.71* | 328.22* | 13.27 |
| Spike length | 52.03* | 60.76* | 12.54* | 0.496 |
| Grain weight | 9360.29* | 168491.76* | 8002.06* | 1241.89 |
* Indicates values significant at P < 0.05
Genotypic variation in membrane injury, relative water content and proline content
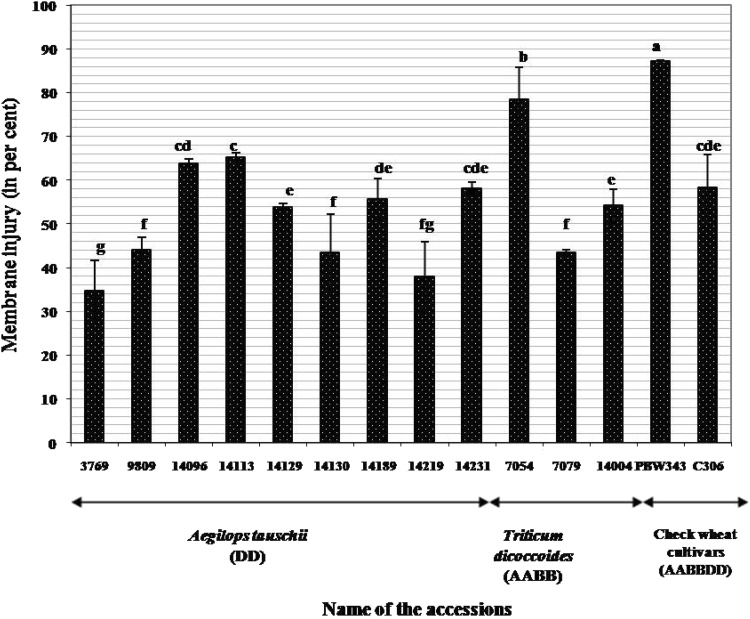
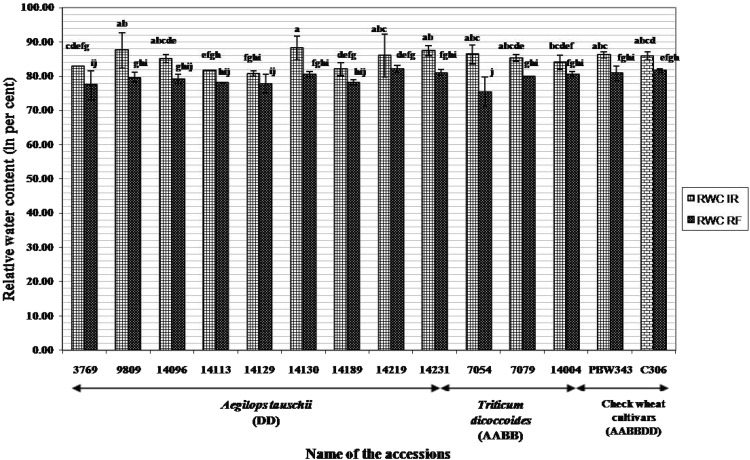
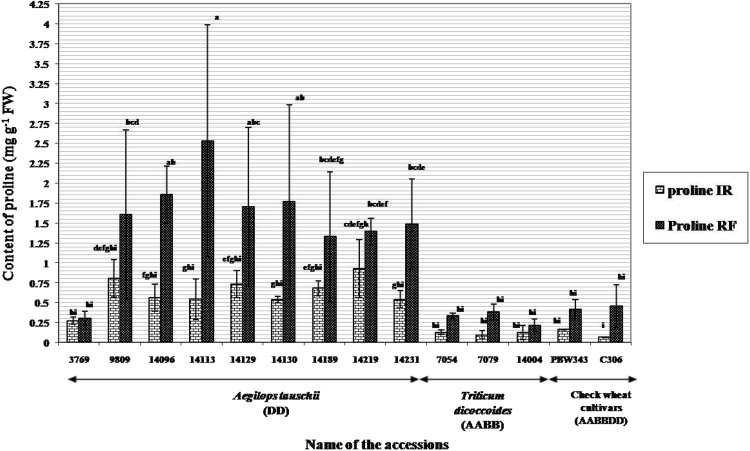
Ae. tauschii acc 3769 (34.7%) and 14219 (37.9%) revealed minimum membrane damage followed closely by Ae. tauschii acc. 9809 (44%), acc. 14130 (43.4%) and T. dicoccoides acc. 7079 (43.5%). Membrane injury index for progenitor accessions was much lower than that observed in check wheat cultivars PBW-343 and C-306 (Fig. 1). An average decrease in relative water content (from 85% under irrigated to 79.5% under rain-fed conditions) was observed across the two environments. Minimum decrease in RWC under water stress conditions was seen in Ae. tauschii acc. 14129 and T. dicoccoides acc. 141004 (Fig. 2). Significant genotypic differences were obtained in selected accessions of three species with respect to the content of proline. Ae. tauschii as a group exhibited higher proline levels under both irrigated and rain-fed conditions. While Ae. tauschii acc. 14219 accumulated maximum amount of proline under irrigated conditions, acc 14113 had its highest content under rain-fed conditions (Fig. 3). On the other hand, T. dicoccoides acc. 7079 and check wheat cultivar C-306 revealed fourfold and sevenfold increase in proline content respectively under rain-fed conditions (Fig. 3).
Fig. 1.
Membrane injury (percent) in Aegilops tauschii, Triticum dicoccoides and check wheat cultivars under rain-fed conditions
Fig. 2.
Relative water content (in percent) in Aegilops tauschii, Triticum dicoccoides and check wheat cultivars under irrigated and rain-fed conditions
Fig. 3.
Proline content (mg g−1 FW) in Aegilops tauschii, Triticum dicoccoides and check wheat cultivars under irrigated and rain-fed conditions
Main effects of environmental factors on the activity of antioxidant enzymes
Environmental influence on the up-regulation in the enzyme activity was prominent as evidenced by a net increase in the activity of all four antioxidant enzymes- SOD, CAT, APX and GR under rain-fed conditions when compared with their respective mean activities under irrigated conditions (Table 3). Average activity of the superoxide dismutase increased from 15.94 to 18.72 units under stress. Catalase activity showed an increase from 3144.36 to 3574.37 units while that of ascorbate peroxidase rose from 9.28 to 11.68 units. Glutathione reductase revealed almost 45% elevation in enzyme activity (1231.66–1784.67 units) under rain-fed conditions. This clearly indicates the development of water deficit stress to the levels necessary to trigger enzyme up-regulation. With regard to genotypic differences as well as genotype x environment interaction, several key insights were obtained for each of the following studied enzymes.
Table 3.
Activity of superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase in flag leaves of Aegilops tauschii, Triticum dicoccoides and cultivated wheats under irrigated and rain-fed conditions
| Species | Accessions | Superoxide dismutase | Catalase | Ascorbate peroxidase | Glutathione reductase | ||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Rain-fed | Irrigated | Rain-fed | Irrigated | Rain-fed | Irrigated | Rain-fed | ||
| MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | ||
| Aegilops tauschii (diploid) | 3769 | 16.89 ± 5.64 | 31.48 ± 2.06 | 3007.45 ± 240.66 | 4264.26 ± 581.97 | 7.66 ± 0.35 | 13.19 ± 2.49 | 1495.18 ± 68.21 | 2218.65 ± 30.32 |
| (0.39 ± 0.07) | (0.65 ± 0.02) | (70.87 ± 5.01) | (87.85 ± 9.52) | (0.182 ± 0.04) | (0.272 ± 0.04) | (35.57 ± 6.94) | (45.80 ± 1.92) | ||
| bcdef | a (186.38%) | efgh | a (141.79%) | ij | cdef (172.19%) | defghi | ab (148.39%) | ||
| 14189 | 17.70 ± 4.50 | 12.57 ± 1.93 | 2397.53 ± 192.30 | 3698.87 ± 516.75 | 4.39 ± 1.05 | 11.35 ± 3.86 | 1085.21 ± 102.31 | 2067.26 ± 316.42 | |
| (0.42 ± 0.17) | (0.27 ± 0.02) | (55.63 ± 13.92) | (79.37 ± 4.87) | (0.103 ± 0.04) | (0.242 ± 0.06) | (24.80 ± 1.94) | (44.33 ± 3.32) | ||
| bcdef | defgh (71.02%) | hi | abcde (154.28%) | k | efgh (258.54%) | hijk | bc (190.49%) | ||
| 9809 | 10.38 ± 3.14 | 19.38 ± 6.82 | 2905.66 ± 283.39 | 2929.12 ± 429.03 | 5.81 ± 1.13 | 12.89 ± 2.05 | 699.36 ± 34.10 | 1188.37 ± 252.00 | |
| (0.15 ± 0.02) | (0.54 ± 0.10) | (42.62 ± 2.55) | (83.47 ± 1.81) | (0.087 ± 0.03) | (0.367 ± 0.01) | (10.38 ± 2.13) | (33.68 ± 1.51) | ||
| gh | bc (186.71%) | fghi | fghi (100.81%) | jk | cdef (221.86%) | k | fghijk (169.92%) | ||
| 14130 | 16.57 ± 0.67 | 19.20 ± 4.81 | 2903.67 ± 225.48 | 3286.16 ± 92.78 | 6.19 ± 0.69 | 5.95 ± 2.43 | 1542.07 ± 202.73 | 1346.47 ± 28.42 | |
| (0.25 ± 0.01) | (0.30 ± 0.09) | (44.33 ± 2.95) | (51.49 ± 0.45) | (0.095 ± 0.01) | (0.22 ± 0.05) | (23.57 ± 3.36) | (21.12 ± 1.23) | ||
| bcdefg | bc (115.87%) | fghi | cdefg (113.17%) | jk | jk (96.12%) | defghi | efghi (87.32%) | ||
| 14096 | 21.49 ± 0.86 | 29.55 ± 4.53 | 2505.68 ± 140.57 | 3307.63 ± 503.25 | 6.63 ± 0.52 | 8.31 ± 1.86 | 1574.23 ± 229.26 | 2244.11 ± 403.57 | |
| (0.55 ± 0.01) | (0.73 ± 0.03) | (64.25 ± 6.87) | (82.22 ± 2.93) | (0.170 ± 0.04) | (0.214 ± 0.09) | (40.46 ± 7.92) | (57.54 ± 20.77) | ||
| b | a (137.51%) | hi | cdefg (132.01%) | jk | hij (125.34%) | cdefgh | ab (142.55%) | ||
| 14113 | 16.34 ± 3.71 | 29.37 ± 0.13 | 2286.60 ± 652.82 | 3757.32 ± 50.61 | 8.64 ± 0.19 | 8.55 ± 0.81 | 1228.94 ± 636.31 | 2676.85 ± 306.94 | |
| (0.36 ± 0.07) | (0.54 ± 0.01) | (50.98 ± 13.33) | (69.46 ± 0.75) | (0.193 ± 0.02) | (0.158 ± 0.01) | 35.61 ± 10.53 | (49.49 ± 5.80) | ||
| bcdefg | a (179.74%) | i | abc (164.32%) | hij | hij (98.96%) | fghij | a (217.82%) | ||
| 14219 | 13.71 ± 1.14 | 12.27 ± 1.24 | 2241.67 ± 291.27 | 4073.41 ± 32.05 | 13.97 ± 0.56 | 12.61 ± 3.48 | 819.94 ± 477.47 | 1944.00 ± 20.84 | |
| (0.21 ± 0.03) | (0.28 ± 0.12) | (35.03 ± 6.93) | (90.61 ± 30.37) | (0.218 ± 0.02) | (0.293 ± 0.17) | (12.50 ± 6.55) | (43.26 ± 14.62) | ||
| cdefgh | efgh (89.5%) | i | ab (181.71%) | cde | defg (90.26%) | jk | bcd (237.09%) | ||
| 14129 | 14.43 ± 1.01 | 19.24 ± 5.03 | 3301.27 ± 405.41 | 3592.32 ± 581.97 | 5.99 ± 0.56 | 6.48 ± 0.71 | 1350.48 ± 151.58 | 2072.62 ± 20.84 | |
| (0.30 ± 0.03) | (0.32 ± 0.05) | (67.88 ± 9.34) | (59.78 ± 3.56) | (0.123 ± 0.01) | (0.108 ± 0.01) | (27.72 ± 2.70) | (34.76 ± 3.23) | ||
| cdefgh | bc (133.33%) | cdefg | abcdef (108.82%) | jk | jk (108.18%) | efghi | bc (153.47%) | ||
| 14231 | 18.74 ± 7.10 | 16.51 ± 4.94 | 2856.36 ± 292.39 | 3572.04 ± 58.48 | 6.12 ± 1.08 | 10.72 ± 1.75 | 1116.03 ± 418.73 | 1619.78 ± 835.57 | |
| (0.28 ± 0.06) | (0.28 ± 0.08) | (43.31 ± 2.23) | (60.39 ± 1.71) | (0.095 ± 0.03) | (0.54 ± 0.08) | (17.55 ± 9.02) | (27.30 ± 13.80) | ||
| bcd | bcdefg (88.10%) | ghi | abcdef (125.06%) | jk | fgh (175.16%) | ghijk | cdefg (145.14%) | ||
| Triticum dicoccoides (tetraploid) | 7054 | 14.55 ± 3.78 | 18.42 ± 1.68 | 4201.84 ± 213.67 | 4082.56 ± 516.18 | 15.47 ± 1.88 | 17.77 ± 3.05 | 818.60 ± 66.32 | 1070.47 ± 96.63 |
| (0.34 ± 0.09) | (0.41 ± 0.03) | (98.90 ± 6.63) | (90.23 ± 9.16) | (0.364 ± 0.05) | (0.392 ± 0.06) | (19.27 ± 1.87) | (23.72 ± 2.73) | ||
| cdefgh | bcde (126.60%) | a | ab (97.16%) | bcd | ab (114.87%) | jk | hijk (130.77%) | ||
| 7079 | 9.50 ± 0.71 | 13.76 ± 4.32 | 3596.29 ± 581.97 | 3253.96 ± 829.94 | 18.72 ± 1.75 | 15.09 ± 4.46 | 1828.78 ± 483.15 | 1532.69 ± 49.26 | |
| (0.24 ± 0.03) | (0.46 ± 0.30) | (90.85 ± 11.48) | (97.39 ± 12.36) | (0.473 ± 0.03) | (0.448 ± 0.04) | (46.11 ± 10.58) | (47.89 ± 16.56) | ||
| h | cdefgh (144.84%) | abcdef | cdefg (90.48%) | a | bcd (80.61%) | bcde | defghi (83.81%) | ||
| 14004 | 11.68 ± 5.44 | 9.84 ± 2.27 | 3752.55 ± 237.29 | 3023.75 ± 241.22 | 15.70 ± 1.39 | 18.03 ± 1.40 | 1321.01 ± 64.42 | 1463.03 ± 49.26 | |
| (0.27 ± 0.09) | (0.30 ± 0.06) | (89.49 ± 6.75) | (94.38 ± 12.01) | (0.374 ± 0.02) | (0.561 ± 0.02) | (31.54 ± 2.83) | (45.54 ± 0.64) | ||
| fgh | h (84.25%) | abc | defgh (80.58%) | abc | ab (114.84%) | efghij | defghi (110.75%) | ||
| Cultivated checks | C-306 | 22.16 ± 3.59 | 11.87 ± 0.38 | 4123.91 ± 919.91 | 3478.20 ± 229.41 | 8.39 ± 0.39 | 9.69 ± 1.34 | 1334.41 ± 128.84 | 1893.09 ± 449.05 |
| (hexaploids) | (0.48 ± 0.01) | (0.28 ± 0.08) | (88.36 ± 5.59) | (83.17 ± 25.66) | (0.182 ± 0.02) | (0.234 ± 0.09) | (28.89 ± 1.87) | (43.60 ± 0.35) | |
| b | fgh (53.56%) | ab | bcdefg (84.34%) | hij | ghi (115.49%) | efghij | bcd (141.87%) | ||
| PBW-343 | 19.03 ± 4.16 | 18.70 ± 7.07 | 3940.61 ± 372.80 | 3721.54 ± 489.54 | 6.28 ± 0.71 | 17.81 ± 2.22 | 1028.94 ± 606.31 | 1647.91 ± 170.52 | |
| (0.58 ± 0.21) | (0.59 ± 0.29) | (117.88 ± 6.91) | (116.19 ± 30.14) | (0.188 ± 0.01) | (0.547 ± 0.00) | (29.61 ± 13.53) | (50.67 ± 1.36) | ||
| bc | bcd (98.27%) | abc | abcd (94.44%) | jk | ab (283.6%) | ijk | cdef (160.16%) | ||
Accessions have been arranged in descending order of ranking (based on cumulative tolerance index) in each respective ploidy level
Values are mean ± S.D. of three replicates. Value without parenthesis is the activity of the respective enzyme, whereas value within the parenthesis is the specific activity of that enzyme. Percentages in bold represents change in the activity of the respective enzymes under rain-fed conditions (keeping base as 100 for irrigated conditions). Values with same alphabet(s) are not significantly different at P ≤ 0.05 (Fisher’s LSD test)
Superoxide dismutase activity is represented as units min-1 g-1 FW and specific activity as units min-1 mg-1 protein.
A unit of catalase activity is represented as µmole of H2O2 decomposed min-1 g-1 FW and specific activity as µmole of H2O2 decomposed min-1 mg-1 protein
A unit of ascorbate peroxidase activity is represented as µmole of monodehydroascorbate formed min-1 g-1FW and specific activity as µmole of monodehydroascorbate formed min-1 mg-1 protein.
A unit of glutathione reductase activity is represented as µmole of NADP+ formed min-1 g-1 FW and specific activity as µmole of NADP+ formed min-1 mg-1 protein
Superoxide dismutase
Activity of the superoxide dismutase ranged from 9.50 units (Ae. tauschii accession 7079) to 22.16 units (C-306) under irrigated conditions and 9.84 units (T. dicoccoides 14004) to 31.48 units (Ae. tauschii accession 3769) under rain-fed conditions (Table 3) clearly indicating both environmental and genotypic influence. Some of the Ae. tauschii accessions exhibited almost two-fold increase in the activity of SOD. The activity of SOD rose from 16.89 to 31.48 units in Ae. tauschii accession 3769, 10.38 to 19.38 units in Ae. tauschii accession 9809 and 16.34 to 29.37 units in Ae. tauschii accession 14113 under rain-fed conditions. Triticum dicoccoides accession 7079, despite having lowest SOD activity within the irrigated set, showed a 45% increase in activity (from 9.50 to 13.76 units) under rain-fed conditions. In Triticum dicoccoides accession 7054 a 27% increase in enzyme activity could be seen under rain-fed conditions (Table 3). C-306, though had the highest activity of the SOD in the irrigated set, it, however, showed a marked dip in the enzyme activity, i.e. from 22.16 to 11.87 units under rain-fed conditions.
Catalase
Activity of catalase (CAT) varied from 2241.67 units (Ae. tauschii accession 14219) to 4201.84 units (T. dicoccoides accession 7054) under irrigated conditions and from 2929.12 units (Ae. tauschii accession 9809) to 4264.26 units (Ae. tauschii accession 3769) under rain-fed conditions (Table 3). Genotypic variation between the two wild species could be seen for the activity of enzyme catalase. Within the irrigated set, all T. dicoccoides accessions outperformed Ae. tauschii accessions by displaying higher enzyme activity. Highest activity of catalase under irrigated conditions was observed in T. dicoccoides accession- 7054 (4201.84 units). However, within the T. dicoccoides set, no significant differences in enzyme activity could be identified in the irrigated and rain-fed counterparts of the respective accessions. An inclination towards stress induced enzyme up-regulation was clearly revealed in Ae. tauschii accessions- 3769 (3007.45 to 4264.26 units), 14113 (2286.60 to 3757.32 units), 14189 (2397.53 to 3698.87 units) and 14219 (2241.67 to 4073.41 units) (Table 3). Among the cultivated wheats, C-306 had higher enzyme activity under irrigated conditions. No significant up-regulation could be seen in activity of these wheats under stress.
Ascorbate peroxidase
Activity of the ascorbate peroxidase ranged from 4.39 (Ae. tauschii accession 14189) to 18.72 units (T. dicoccoides 7079) under irrigated conditions and from 5.95 (Ae. tauschii accession 14130) to 18.03 units (T. dicoccoides 14004) under rain-fed conditions (Table 3). With regard to inter-species differences, significant genotypic variation could be identified between Ae. tauschii and T. dicoccoides within the irrigated set. As for catalase, T. dicoccoides had higher enzyme activity than Ae. tauschii as a group. T. dicoccoides accessions 7079 (18.72 units) and 14004 (18.03 units) had the highest activity of APX within irrigated and rain-fed set respectively. Significant induction in APX activity was observed in Ae. tauschii accessions- 3769 (72%), 9809 (121%) and 14189 (158%) under rain-fed conditions (Table 3). Ae. tauschii accessions3769 and 14189 had higher catalase activity as well. Furthermore, 3769 and 9809 depicted an almost 50% augmentation in SOD activity. Check wheat cultivars- PBW-343 and C-306 had lower enzyme activity than T. dicoccoides within the irrigated set, but were found to up-regulate enzymatic expression under stress with PBW-343 showing threefold induction of APX, i.e. from 6.28 to 17.81 units (Table 3).
Glutathione reductase
Activity of the enzyme glutathione reductase under both irrigated and rain-fed conditions depicted a wide spectrum of genetic variability in studied accessions of Ae. tauschii and T. dicoccoides. Under irrigated conditions, activity of glutathione reductase in Ae. tauschii group varied from 699.36 units (Ae. tauschii accession 9809) to 1574.23 units (Ae. tauschii accession 14096) showing almost double the difference in enzyme activity between the two extremes (Table 3). Under rain-fed conditions almost all the Ae. tauschii accessions presented an up-regulation in enzyme activity. Maximum stimulation in the enzyme activity under stress was presented by Ae. tauschii accession 14219 (137% increase, from 819 to 1944 units under rain-fed conditions) followed closely by accession 14113 (118% increase from 1228.94 to 2676.85 units). Almost two-fold increase in CAT activity was also observed in Ae. tauschii accession 14219. In T. dicoccoides accessions, the activity of enzyme glutathione reductase spanned from 818.60 units to 1828.78 units under irrigated conditions and varied from 1070.47 to 1463.03 units under rain-fed conditions revealing significant genotypic variation both within and between the two species. T. dicoccoides accession 7054 showed about 31% increase in GR activity. It had also shown an increase in the activity of superoxide dismutase under rain-fed conditions. For wheat cultivars, significant but relatively moderate up-regulation was seen. In PBW-343, activity of GR increased from 1028.94 to 1647.91units, while in C-306 activity rose from 1334.41 to 1893.09 units under rain-fed conditions.
Tolerance indices
Wild, winter habit of Ae. tauschii and seed shattering in both Ae. tauschii and T. dicoccoides do not lend themselves to meaningful yield trials, particularly in our environment. Therefore, as a measure of field performance under rain-fed conditions, observations were recorded for plant height, spike length and grain weight for accessions of Ae. tauschii, T. dicoccoides and bread wheat cultivars under irrigated and rain-fed conditions (Table 4). A general reduction in plant height was observed for the studied accessions. Within Ae. tauschii group, accession 14096 was tallest under irrigated and acc. 3769 under rain-fed conditions. Nearly 35% curtailment in plant height could be recorded in Ae. tauschii accession 14231 and T. dicoccoides accession 14004. Spike length too got reduced in most of the cases with T. dicoccoides accession 7079 and bread wheat cultivar- PBW-343 showing an almost 30% reduction in length of the spike. However, an increase in grain weight was observed for some of these accessions, for instance, ≥10% increase in Ae. tauschii accessions 14096, 14130, 14189 and T. dicoccoides accession 7054 (Table 4). This may probably be due to stress induced early flowering and consequently longer grain-filling duration. Larger grain weight under stress may also accrue from re-allocation of resources per floret on account of smaller spikes under stress. Changes in these phenotypic characters under water stress conditions indicated tolerance levels of each genotype. On the basis of tolerance index calculated for each of these traits, a cumulative tolerance index was deduced. Since growth habit, phenology of both the progenitor species is distinct from each other as well as from modern wheat cultivars, accessions within each ploidy level were ranked separately for drought tolerance. Within A. tauschii group, acc. 3769 (CTI = 101%) and 14189 (CTI = 99%) were found most tolerant to field induced water stress. Acc. 14231 performed relatively poor (CTI = 83%) with regard to these yield component traits (Table 4). Amongst T. dicoccoides, acc. 7054 achieved highest cumulative tolerance index (105%) as against acc. 14004 that ranked lowest in the group. Incidentally, these values were the highest and the lowest in progenitor set.
Table 4.
Phenotypic characters- plant height (in cm), spike length (in cm) and grain weight (in mg) of Aegilops tauschii, Triticum dicoccoides and cultivated wheats under irrigated and rain-fed conditions
| Species | Accessions | Plant height (Tolerance Index1) | Spike length (Tolerance Index2) | Grain weight (Tolerance Index3) | Cumulative tolerance index (%) | |||
|---|---|---|---|---|---|---|---|---|
| Irrigated | Rain-fed | Irrigated | Rain-fed | Irrigated | Rain-fed | |||
| MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | MEAN ± S.D. | |||
| Aegilops tauschii (diploid) | 3769 | 84.33 ± 2.31 | 82.00 ± 1.00 | 13.00 ± 0.00 | 13.00 ± 0.00 | 371.5 ± 7.78 | 388.50 ± 0.71 | 100.60 |
| g | ghi (97.23%) | ijkl | ijkl (100.00%) | fghi | fg (104.58%) | |||
| 14189 | 73.00 ± 3.46 | 62.33 ± 0.58 | 12.33 ± 0.58 | 12.67 ± 0.58 | 348.5 ± 7.78 | 383.00 ± 86.27 | 99.33 | |
| klm | o (85.39%) | klm | jkl (102.7%) | fghij | fgh (109.9%) | |||
| 9809 | 74.67 ± 0.58 | 76.33 ± 1.15 | 14.67 ± 0.58 | 14.33 ± 0.58 | 323.5 ± 33.23 | 314.50 ± 10.61 | 99.06 | |
| jklm | ijk (102.23%) | g | gh (97.73%) | hijkl | ijkl (97.22%) | |||
| 14130 | 77.33 ± 1.53 | 64.33 ± 4.16 | 11.00 ± 0.00 | 11.33 ± 0.58 | 244.0 ± 70.71 | 269.00 ± 1.41 | 98.82 | |
| hijk | no (83.19%) | n | mn (103.03%) | m | lm (110.25%) | |||
| 14096 | 84.67 ± 2.52 | 69.67 ± 2.08 | 14.00 ± 0.00 | 13.67 ± 0.58 | 250.0 ± 12.73 | 282.50 ± 3.54 | 97.63 | |
| g | lmn (82.28%) | ghi | ghij (97.62%) | m | klm (113.00%) | |||
| 14113 | 83.00 ± 1.73 | 73.00 ± 1.00 | 13.00 ± 0.00 | 13.67 ± 0.58 | 341.5 ± 94.05 | 285.00 ± 24.04 | 92.18 | |
| gh | klm (87.95%) | ijkl | ghij (105.13%) | ghijk | klm (83.46%) | |||
| 14219 | 80.14 ± 1.23 | 69.23 ± 2.77 | 13.39 ± 1.61 | 12.52 ± 0.98 | 290.5 ± 23.33 | 248.00 ± 24.04 | 88.88 | |
| ghij | mn (86.92%) | hijk | ijkl (94.35%) | jklm | m (85.37%) | |||
| 14129 | 78.00 ± 1.73 | 63.00 ± 1.73 | 13.00 ± 0.00 | 13.33 ± 0.58 | 407.0 ± 25.46 | 336.50 ± 12.02 | 88.67 | |
| hijk | o (80.77%) | ijkl | hijk (102.56%) | f | ghijk (82.68%) | |||
| 14231 | 75.33 ± 0.58 | 50.00 ± 2.65 | 12.67 ± 0.58 | 12.00 ± 0.00 | 364.0 ± 72.12 | 324.50 ± 20.51 | 83.42 | |
| jkl | p (66.37%) | jkl | lmn (94.74%) | fghi | hijkl (89.15%) | |||
| Triticum dicoccoides (tetraploid) | 7054 | 134.00 ± 13.89 | 135.00 ± 7.21 | 21.33 ± 2.08 | 21.00 ± 0.00 | 734.50 ± 24.75 | 850.00 ± 16.97 | 104.97 |
| c | c (100.75%) | b | bc (98.44%) | bc | a (115.72%) | |||
| 7079 | 132.00 ± 1.00 | 126.00 ± 2.00 | 20.00 ± 0.00 | 14.00 ± 0.00 | 715.0 ± 26.87 | 772.00 ± 33.94 | 91.14 | |
| c | d (95.45%) | cd | ghi (70.00%) | bc | b (107.97%) | |||
| 14004 | 155.33 ± 3.05 | 97.33 ± 2.52 | 25.00 ± 1.00 | 16.00 ± 0.00 | 651.50 ± 28.99 | 649.00 ± 29.70 | 75.43 | |
| a | f (62.66%) | a | f (64.00%) | de | de (99.62%) | |||
| Cultivated checks (hexaploids) | C-306 | 142.00 ± 1.00 | 133.33 ± 2.08 | 19.67 ± 0.58 | 18.17 ± 1.04 | 610.00 ± 19.09 | 882.50 ± 40.31 | 110.31 |
| b | c (93.9%) | d | e (92.37%) | e | a (144.67%) | |||
| PBW-343 | 103.33 ± 2.08 | 97.33 ± 2.52 | 17.00 ± 0.00 | 12.33 ± 0.58 | 675.00 ± 7.07 | 690.00 ± 24.04 | 104.26 | |
| e | f (94.19%) | f | klm (72.55%) | cd | cd (146.03%) | |||
Values are means of three replicates. Values with same alphabet(s) are not significantly different at P ≤ 0.05 (Fisher’s LSD test)
Accessions have been arranged in descending order of ranking (on the basis of cumulative tolerance index) in each ploidy level
Association between enzyme activity and tolerance indices
Correlation coefficients were worked out between different measures of plant performance (tolerance index 1, tolerance index 2 and tolerance index 3) and change in the activity of enzymes (SOD, CAT, APX and GR) under stress. A significant positive correlation (r = 0.569) was found between change in the activity of catalase and change in spike length (tolerance index 2). Alongside, a significant negative correlation (r = -0.513) was observed for change in catalase activity with change in grain weight under stress (tolerance index 3). A highly significant positive correlation (r = 0.760) was detected between catalase and glutathione reductase for respective changes in their enzyme activity. Small sample size might have thwarted the detection of some associations. Three correlation coefficients just below the chosen significance threshold (p value 0.05) were noticed between change in the SOD and change in plant height (r = 0.441, p = 0.09); change in SOD and change in grain weight (r = -0.391, p = 0.15); and change in GR and change in spike length (r = 0.413, p = 0.13).
Grouping of genotypes on the basis of multivariate principal component analysis
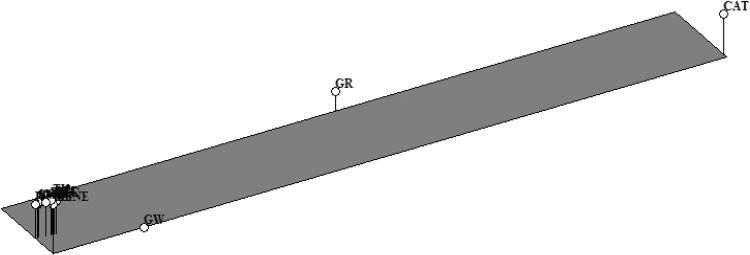
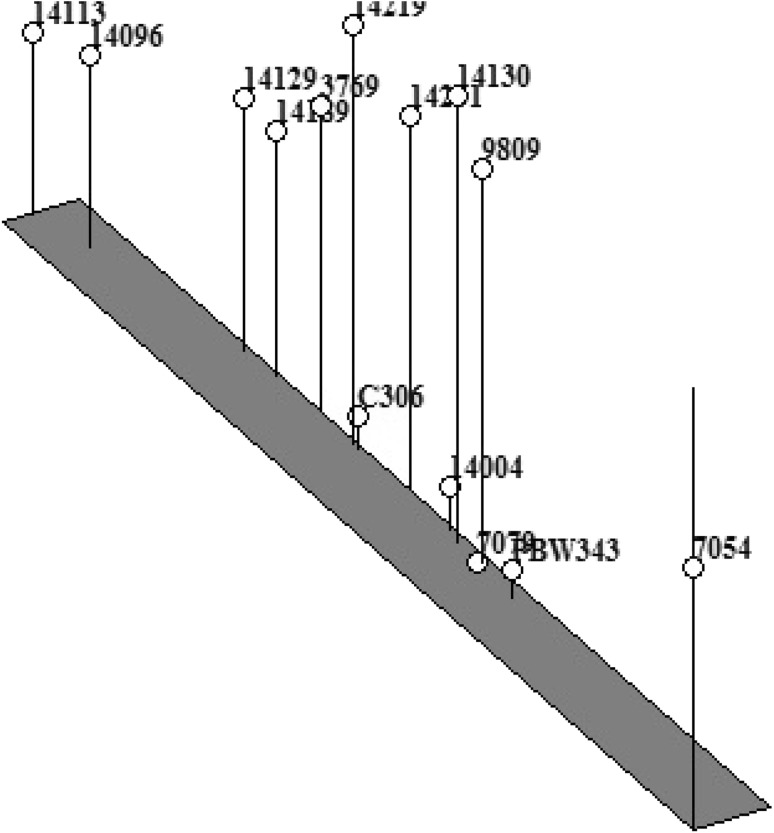
Multivariate principal component analysis was used to study the extent of genotypic variation contributed by different traits—membrane injury (MI), relative water content (RWC), proline content, superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), plant height (PH), spike length (SL), grain weight (GW), tolerance index 1(TI 1), tolerance index 2 (TI 2), tolerance index 3 (TI 3), cumulative tolerance index (CTI) as well as to identify association between these traits evaluated at different stages of crop development under rain-fed conditions. Of the 14 component drought adaptive parameters, activity of the enzyme catalase, glutathione reductase and grain weight under stress corresponded to 23, 16 and 12% of variation respectively. Three of these traits occupied distinct position on the 3D-plot (Fig. 5). Rest eleven drought adaptive characters grouped together into a single cluster and found separate location on the plot. With regard to genotype clustering, the selected set of accessions (of each ploidy level) grouped into three major clusters. Most of the accessions of both the Ae. tauschii and T. dicoccoides clustered in the centre (along with hexaploid wheat cultivars PBW-343 and C-306). Two A. tauschii accessions 14096 and 14113 occupied a separate position on the 3D plot (Fig. 4). Activity of all the four antioxidant enzymes had increased in acc. 14096 (37% SOD, 32% CAT, 25% APX and 42% GR) under water stress whereas 80% increase in SOD, 64% increase in CAT and 118% increase in GR was observed in acc. 14113 under stress. Both these accessions occupied middle position in ranking based on CTI. T. dicoccoides accession 7054 seemed unique as was positioned exclusively on the other end of the 3D plot. Between the two groups of progenitor species, this accession had revealed the highest drought tolerance levels (CTI = 105%, Table 4).
Fig. 5.
Multivariate principal component analysis (PCA) of 14 component traits- membrane injury (MI), proline, relative water content (RWC), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), plant height (PH), spike length (SL), grain weight (GW), tolerance index1 (TI1), tolerance index 2 (TI2), tolerance index 3 (TI3) and cumulative tolerance index (CTI)) under water stress
Fig. 4.
Multivariate principal component analysis (PCA) of Aegilops tauschii (acc. 3769, 9809, 14096, 14113, 14129, 14130, 14189, 14219and 14231), Triticum dicoccoides (7054, 7079 and 14004) and check wheat cultivars (PBW-343 and C-306) under water stress
Discussion
When a plant encounters water deficit stress, it not only experiences disruption of cell turgor or constraints in general growth and development, but it also suffers severely due to oxidative stress that usually presents itself as a secondary stress, an outcome of external environmental fluctuations. Accordingly, plant cells respond defensively to this oxidative burst by actively removing excess of reactive oxygen species and maintaining anti-oxidant defense compounds at levels that reflect ambient cellular metabolism. In the present investigation, a representative set of Ae. tauschii and T. dicoccoides accessions were explored for their antioxidant potential in terms of the activity of four enzymes- SOD, CAT, APX and GR in flag leaf at anthesis under both irrigated and rain-fed conditions during the crop season (2011–2012). At anthesis and subsequent grain-filling, carbon assimilation in flag leaf makes the most significant contribution to formation of healthy and bold grains. In our case, enhanced activity of all four antioxidant enzymes in flag leaf under rain-fed conditions hints at the role of these enzymes in buffering the deleterious effects of redox perturbations and contributing to genotype’s resilience to stress environment. Genotypic differences in enzyme activity were however evident. Maximum enzyme up-regulation under stress for three of the four studied enzymes- SOD (87% in Ae. tauschii accession 9809), CAT (82% in Ae. tauschii accession 14219) and GR (137% in Ae. tauschii accession 14219) was observed in Ae. tauschii as a group. A differential pattern of enzyme expression could be identified in the diploid and tetraploid wheat progenitors with respect to up-regulation or down-regulation of enzyme activity under stress (Fig. 5).
Activity of four enzymes rose by varying levels in different genotypes. Nevertheless, exceptional genotypes such as Aegilops tauschii accession 3769 and 14096 showed up-regulation in the activity of all the four studied antioxidant enzymes under rain-fed conditions. Aegilops tauschii accession 3769 had displayed highest SOD and CAT activity under rain-fed conditions, with lower membrane injury at tillering stage. It was also identified as a heat tolerant genotype in the study of Gupta et al. (2010). Higher activity of antioxidant enzymes like SOD, APX, DHAR, MDHAR, GR and CAT has been reported in tolerant genotypes of wheat (Sairam et al. 1997, 1998, Almeselmani et al. 2006). Compared to Aegilops tauschii accession 3769, acc. 14096 had revealed higher membrane injury and lower relative water content at tillering stage (Suneja 2014). Accordingly, an 18% reduction in plant height (indicative of vegetative biomass accumulation) was also evident. But, efficient up-regulation in the activity of all four enzymes at anthesis stage seemed to cushion cellular machinery from ROS activity, resulting in a 13% increase in grain weight under stress. Dynamic modulation in enzyme activity in response to stress might have contributed to regulation of steady state levels of ROS in chloroplast, enabling active photosynthesis in flag leaf, thus contributing to adequate grain-filling despite conditions of water deficit stress. It may be said that these wild accessions may harbor more effective alleles for the genes involved in the synthesis and degradation of these enzymes that may be accordingly up- or down-regulated in response to stress.
Aegilops tauschii accessions 9809 (86% SOD, 122% APX and 70% GR), 14113 (79% SOD, 39% CAT and 54% GR) and 14189 (159% CAT, 54% APX and 90% GR) too seemed to possess a strong induction mechanism leading to enhanced activity of three of the four studied enzymes. Among all the progenitor accessions, 14113 had displayed maximum induction of proline accumulation (fivefold) and maximum increase in spike length under stress. It also occupied a distinct position in 3D plot. Acc 9809 had displayed higher proline content at vegetative stage. In previous studies by other authors, Ae. tauschii accession 9809 was also found highly resistant to one or more of the four studied diseases- leaf rust, stem rust, powdery mildew, and tan spot (Cox et al. 1992). Since the up-regulation of these enzymes is in common between these accessions, these enzymes seem to work in fine concert with each other to prevent accumulation of toxic levels of H2O2 in cell. However, unlike Aegilops tauschii accessions 9809 and 14113, Aegilops tauschii accession 14189 revealed a 10% increase in grain weight under stress indicating that the tolerance to the oxidative burst may be by the virtue of inherent genetic potential of this accession. Hence, in terms of variability at intra-species level, genotypic differences in Ae. tauschii accessions for tolerance to water stress may mainly be attributed to their unique allelic composition, discrete water stress threshold levels and differential ability to trigger antioxidant defense consequently balancing ROS formation and its consequent consumption.
With regard to T. dicoccoides as a group, this tetraploid progenitor presented higher enzyme activity under irrigated conditions. T. dicoccoides accession 7079 revealed highest constitutive levels of APX and GR. It had suffered lower membrane damage, maintained higher proline content in leaves at tillering stage, exhibited efficient mobilization of stem reserves and higher acid and alkaline invertase activity in developing grain (Suneja et al. 2015). In addition to higher constitutive levels, metabolic plasticity in terms of a modest increase in the activity of SOD, APX and GR was evident in T. dicoccoides accession 7054 which eventually translated into a 16% increase in grain weight under water stress conditions. This accession topped in CTI index (for progenitor species) and occupied a unique position in 3D plot. Triticum dicoccoides accession 14004 did not reveal any significant up-regulation for the studied enzymes. Finally, though a small number of T. dicoccoides accessions were used, a pattern of higher constitutive activity of enzymes and relatively milder induction was indicated in comparison to Aegilops tauschii as a group. Since these accessions did not show dramatic difference in enzyme activity between the two environments, probably, their genetic makeup maintains fairly active antioxidant defense machinery even under normal conditions. The origin of T. dicoccoides accessions used in the study in drier climes of Israel may be pertinent to this observed pattern. Genomic buffering provided by the tetraploid status may be another likely cause for the steadier enzyme activity levels.
As far as the cultivated wheat varieties are concerned, highest SOD activity under irrigated conditions was seen in C-306, while PBW-343 revealed maximum degree of induction for APX. Expression of higher APX activity in drought susceptible genotype PBW-343 (relative to C-306) could be a consequence of excessive superoxide and hydrogen peroxide generation, which also acts as a signal for antioxidant enzyme induction as also evidenced by higher induction of GR in PBW-343. Anti-oxidative enzyme activity has previously been studied in C-306 (Sairam et al. 1998, Kaur et al. 2012) and PBW-343 where C-306 was found to increase anti-oxidant activity and reveal drought tolerant behaviour, whereas PBW-343 was found susceptible to high temperature stress (Almeselmani et al. 2006, Kaur et al. 2013), water (Kaur et al. 2013) and salt stress (Bhagi et al. 2013).
Though significant genotypic differences came forth both at intra-species and inter-species level, the activity of four enzymes did not show a similar pattern of response to stress in all the genotypes except for CAT and GR (r = 0.760). Both catalase and glutathione reductase had occupied distinct positions in 3D plot. Catalase is known to protect cells from H2O2 by catalyzing its decomposition into O2 and H2O. An increase in temperature, higher light intensity, stomatal closure due to higher transpiration (Foyer and Noctor 2000) increases photorespiration that elevates the levels of H2O2 in the peroxisomes. Activity of catalase is therefore crucial in removing photorespiratory H2O2 and an increase in its activity may be related to ability of the accessions to cope with photorespiratory H2O2 (Cavalcanti et al. 2004). Glutathione reductase, on the other hand, is an important member of Asa-GSH pathway, known to play its role in recovery of reduced glutathione which is used by glutathione peroxidase to reduce H2O2. The highly significant positive correlation between change in the activity of CAT and GR may be suggestive of a similar regulatory stimulus, involvement of a common set of molecular sensors and redox-sensitive transcription factors in different branches of ROS regulatory networks to protect the plant from oxidative damage caused due to water deficit stress.
In addition to association between respective enzyme activities, a significant positive correlation of CAT activity with spike length under water stress was noteworthy. Its negative correlation with grain weight may in part accrue from inherently negative association of spike length and grain weight. Early flowering under drought generally results in smaller spikes but longer grain filling periods with fewer but bolder grains as a consequence.
Hence, the ability of a plant to tolerate water deficit may be underpinned by a myriad of morphological, physiological and biochemical adaptations, some of which are likely to be plastic. Early flowering, developing deep root system, accumulation of osmolytes, stomatal conductance and retention of chlorophyll (photosynthetic pigments) are some of the well recognized examples of adaptive plasticity to drought stress (Nicotra and Davidson 2010; Des Marais et al. 2013; Aspinwall et al. 2014). Environmental variability modulates antioxidant enzyme expression that may serve as a means to maintain steady state levels of ROS and sustain cellular homeostasis (Jaleel et al. 2009). Physiological plasticity exhibited in the form of enzyme induction may thus contribute to enhanced performance under water deficit stress.
Therefore, to conclude, it can be said that Aegilops tauschii and T. dicoccoides are widely adaptable direct ancestors of bread wheat that harbor wide genetic diversity required to improve drought resistance in cultivated wheat. The results from the present study revealed higher activity and greater variability in the expression of antioxidative enzymes in wild species relative to cultivated wheats. While T. dicoccoides seem to have a well-assembled and functionally active antioxidant machinery that works constitutively to maintain cellular redox balance, in Ae. tauschii, up-regulation as a response to stress seems to be the dominant mechanism contributing to their tolerant behaviour and maintenance of oxidative balance in the cell. These species level differences might result from different adaptation mechanisms developed in them in due course of time to cope with unique aspects of local management and incurred environmental constraints. Hence, this genetic variability in anti-oxidant enzyme system could be gainfully exploited by crossing Ae. tauschii accessions 3769, 9809, 14096, 14113 with T. dicoccoides accessions 7054 and 7079 to develop synthetic wheats. These neo-hexaploid wheats will combine together the beneficial stress adaptive traits of all three sub-genomes (AB and D) into a single genetic background. The work is already in progress at our centre. This elite genetic material may then be used for genome mapping, chromosome localization and cloning of these novel stress responsive alleles for their eventual introgression and deployment in commercial check wheat cultivars.
Acknowledgements
Author thanks the financial support received under Innovation in Science Pursuit for Inspired Research (INSPIRE) Programme, Department of Science and Technology, Government of India [Grant no. DST/INSPIRE Fellowship/2010 [162]]. School of Agricultural Biotechnology, Punjab Agricultural University, Ludhiana is acknowledged for sharing the seed of Aegilops tauschii and Triticum dicoccoides accessions.
References
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171:382–388. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in chloroplasts. In: Klye DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 227–287. [Google Scholar]
- Aspinwall MJ, Loik ME, de Dios VR, Tjoelker MG, Payton PR, Tissue DT. Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ. 2014;38:1752–1764. doi: 10.1111/pce.12424. [DOI] [PubMed] [Google Scholar]
- Badiani M, De Biasi MG, Colognola M, Artemi F. Catalase, peroxidase and superoxide dismutase activities in seedlings submitted to increasing water deficit. Agrochimica. 1990;34:90–102. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline content for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bhagi P, Zhawar VK, Gupta AK. Antioxidant response and Lea genes expression under salt stress and combined salt plus water stress in two wheat cultivars contrasting in drought tolerance. Ind J Exp Bot. 2013;51:746–757. [PubMed] [Google Scholar]
- Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981;21:43–47. doi: 10.2135/cropsci1981.0011183X002100010013x. [DOI] [Google Scholar]
- Caldwell KS, Dvorak J, Lagudah ES, Akhunov E, Luo M, Wolters P, Powell W. Sequence polymorphism in polyploid wheat and their D genome diploid ancestor. Genetics. 2004;167:941–947. doi: 10.1534/genetics.103.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viegas RA, Silveira JAG. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 2004;163(563):571. doi: 10.1111/j.1469-8137.2004.01139.x. [DOI] [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. Meth Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- Cox TS, Raupp WJ, Wilson DL, Gill BS, Leath S, Bockus WW, Browder LE. Resistance to foliar diseases in a collection of Triticum tauschii germplasm. Plant Dis. 1992;76:1061–1064. doi: 10.1094/PD-76-1061. [DOI] [Google Scholar]
- Des Marais DL, Hernandez KM, Juenger TE. Genotype-by-environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annu Rev Eco Evol Syst. 2013;44:5–29. doi: 10.1146/annurev-ecolsys-110512-135806. [DOI] [Google Scholar]
- Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiol. 1978;61:119–121. doi: 10.1104/pp.61.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. Yield improvement in wheat: empirical or analytical? In: Evans LT, Peacock WJ, editors. Wheat science—today and tomorrow. Cambridge: Cambridge University Press; 1981. pp. 203–222. [Google Scholar]
- Foyer CH, Noctor G. Tansley Review 112 Oxygen processing in photosynthesis: regulation and signaling. New Phytol. 2000;146:359–388. doi: 10.1046/j.1469-8137.2000.00667.x. [DOI] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kaur S, Seghal S, Sharma A, Chhuneja P, Bains NS. Genotypic variation for cellular thermotolerance in Aegilops tauschii Coss., the D-genome progenitor of wheat. Euphytica. 2010;175:373–381. doi: 10.1007/s10681-010-0185-0. [DOI] [Google Scholar]
- Hameed A, Bibi N, Akhter J, Iqbal N. Differential changes in antioxidant, proteases and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol Biochem. 2011;49:178–185. doi: 10.1016/j.plaphy.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Almansa MS. Short term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant. 2002;115:251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- HongBo S, ZongSuo L, MingAn S. Changes of antioxidative enzymes and MDA content under soil water deficit among ten wheat (T. aestivum L.) genotypes at maturation stage. Colloids Surf B Biointerfaces. 2005;45:7–13. doi: 10.1016/j.colsurfb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R. Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant. 2009;31:427–436. doi: 10.1007/s11738-009-0275-6. [DOI] [Google Scholar]
- Kaur M, Gupta AK, Zhawar VK. Antioxidant response and Lea genes expression under exogenous ABA and water deficit stress in wheat cultivars contrasting in drought tolerance. J Plant Biochem Biotechnol. 2012 [PubMed] [Google Scholar]
- Kaur M, Bhagi P, Gupta AK, Zhawar VK. Antioxidant potential and expression of Lea genes under heat stress in two wheat cultivars differing in heat tolerance. Plant Stress. 2013;7:19–29. [Google Scholar]
- Khanna-Chopra R, Selote DS. Acclimation to drought stress generates oxidative stress tolerance in drought resistant than susceptible wheat cultivar under field conditions. Environ Exp Bot. 2007;60:276–283. doi: 10.1016/j.envexpbot.2006.11.004. [DOI] [Google Scholar]
- Kraus TE, McKersie BD, Fletcher RA. Paclobutrazol induced tolerance of wheat leaves to paraquat may involve increased antioxidant enzyme activity. J Plant Physiol. 1995;145:570–576. doi: 10.1016/S0176-1617(11)81790-6. [DOI] [Google Scholar]
- Kurahashi Y, Terashima A, Takumi S. Variation in dehydration tolerance, ABA sensitivity and related gene expression patterns in D-genome progenitor and synthetic hexaploid wheat lines. Intl J Mol Sci. 2009;10:2733–2751. doi: 10.3390/ijms10062733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano HR, Antonicelli GE, Luna CM, Melchiorre MN, Gomes LD, Racca RW, Trippi VS, Casano LM. Antioxidative system response of different wheat cultivars under drought: field and in vitro studies. Aus J Plant Physiol. 2001;28:1–9. [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidant defense system, pigment composition and photosynthetic efficiency two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1099. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McFadden ES, Sears ER. The origin of Triticum spelta and its free threshing hexaploid relatives. J Hered. 1946;37:81–107. doi: 10.1093/oxfordjournals.jhered.a105590. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Davidson A. Adaptive phenotypic plasticity and plant water use. Funcy Plant Biol. 2010;37:117–127. doi: 10.1071/FP09139. [DOI] [Google Scholar]
- Osipova SV, Permyakov AV, Peryakova MD, Pshenichnikova TA, Borner A. Leaf dehydroascorbate reductase and catalase activity is associated with soil drought tolerance in bread wheat. Acta Physiol Plant. 2011;33:2169–2177. doi: 10.1007/s11738-011-0756-2. [DOI] [Google Scholar]
- Pastori GM, Trippi VS. Oxidative stress induces high rate of glutathione reductase synthesis in a drought resistant maize strain. Plant Cell Physiol. 1992;33:957–961. [Google Scholar]
- Peleg Z, Fahima T, Abbo S, Krugman T, Nevo E, Yakir D, Saranga Y. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ. 2005;28:176–191. doi: 10.1111/j.1365-3040.2005.01259.x. [DOI] [Google Scholar]
- Reynolds M, Dreccer F, Trethowan R. Drought adaptive traits derived from wheat wild relatives and land races. J Exp Bot. 2007;58:177–186. doi: 10.1093/jxb/erl250. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ (1998) NTSYS-pc version 2.0. Exeter Software. Setauket, NY
- Sairam RK, Shukla DS, Saxena DC. Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol Plant. 1997;40:357–364. doi: 10.1023/A:1001009812864. [DOI] [Google Scholar]
- Sairam RK, Deshmukh PS, Saxena DC. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol Plant. 1998;41:387–394. doi: 10.1023/A:1001898310321. [DOI] [Google Scholar]
- Sairam RK, Chandrasekhar V, Srivastava GC. Comparison of hexaploid and tetraploid wheat cultivars in their responses to water stress. Biol Plant. 2001;44:89–94. doi: 10.1023/A:1017926522514. [DOI] [Google Scholar]
- Sgherri CLM, Maffei M, Navari-Izzo F. Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol. 2000;157:273–279. doi: 10.1016/S0176-1617(00)80048-6. [DOI] [Google Scholar]
- Shimshi D, Mayoral ML, Atsmon D. Responses to water stress in wheat and related wild species. Crop Sci. 1982;22:123–128. doi: 10.2135/cropsci1982.0011183X002200010028x. [DOI] [Google Scholar]
- Simova-Stoilova L, Demirenska K, Petrova T, Tsenov N, Feller U. Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (T. aestivum L.) varieties subjected to long term field drought. Plant Growth Regul. 2009;58:107–117. doi: 10.1007/s10725-008-9356-6. [DOI] [Google Scholar]
- Singh S, Gupta AK, Kaur N. Differential responses of antioxidative defence system to long term field drought in wheat (Triticum aestivum L.) genotypes difference in drought tolerance. J Agron Crop Sci. 2012;198:185–195. doi: 10.1111/j.1439-037X.2011.00497.x. [DOI] [Google Scholar]
- Suneja Y (2014) Physio-biochemical responses and allelic diversity for water deficit tolerance related traits in Aegilops tauschii and Triticum dicoccoides. Ph.D. Thesis, Punjab Agricultural University, Ludhiana, India
- Suneja Y, Sharma A, Gupta AK, Bains NS. Differential response of wild and cultivated wheats to water deficits during grain development: changes in soluble carbohydrates and invertases. Physiol Mol Biol Plants. 2015;21:169–177. doi: 10.1007/s12298-015-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethowan RM, Mujeeb-Kazi A. Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Sci. 2008;48:1255–1265. doi: 10.2135/cropsci2007.08.0477. [DOI] [Google Scholar]
- Varga B, Janda T, Laszlo E, Veisz O. Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol Plant. 2012;34:849–858. doi: 10.1007/s11738-011-0882-x. [DOI] [Google Scholar]
- Warburton ML, Crossa J, Franco J, Kazi M, Trethowan R, Rajaram S, Pfeiffer W, Zhang P, Dreisigacker S, van Ginkel M. Bringing wild relatives back into the family: recovering genetic diversity in CIMMYT improved wheat germplasm. Euphytica. 2006;149:289–301. doi: 10.1007/s10681-005-9077-0. [DOI] [Google Scholar]
- Weatherly PE. Studies in the water relation of cotton plants. I. The field measurement of water deficit in leaves. New Phytol. 1950;49:81–87. doi: 10.1111/j.1469-8137.1950.tb05146.x. [DOI] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Montagu MV, Inze D, Camp WV. Catalase is a sink for H2O2 and is indispensible for stress defence in C3 plants. EMBO. 1997;16:4808–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- Zhang J, Kirkham MB. Drought stress induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol. 1994;35:785–791. doi: 10.1093/oxfordjournals.pcp.a078658. [DOI] [Google Scholar]