Abstract
Objective This study was conducted to investigate whether the add-on treatment of allergic rhinitis (AR) based on the Self-assessment of Allergic Rhinitis and Asthma (SACRA) questionnaire for assessing AR control improves both AR and asthma control in asthmatic patients with AR.
Methods This multi-center prospective study was performed in Nagano prefecture, Japan. Two hundred five asthmatic patients and 23 respiratory physicians participated in the study. We administered add-on AR treatments based on the results of the SACRA questionnaire. After the first SACRA questionnaire, 67 asthmatic patients agreed to receive an add-on AR treatment. Three months after the AR treatment, a secondary SACRA questionnaire, asthma control test (ACT), and pulmonary function tests were performed.
Results After the add-on AR treatment, the visual analogue scales (VASs) for AR and asthma, as assessed by the SACRA questionnaire and ACT score, were significantly improved in the patients of the AR+ group. With regard to the pulmonary function tests, the percent predicted vital capacity, and percent predicted forced expiratory volume in one second were also significantly improved. Regardless of whether the patients had previously undergone leukotriene receptor antagonists (LTRA) treatment, the VASs for AR and asthma and the ACT score were significantly improved in the AR+ group. However, the vital capacity (VC), forced vital capacity (FVC) and forced expiratory volume (FEV1) were only significantly improved in the AR+ group that had previously undergone LTRA treatment.
Conclusion SACRA questionnaire-based add-on AR treatment would be convenient for the detection of AR by respiratory physicians and would offer improved asthma control. This questionnaire can also be used to assess the therapeutic effects.
Keywords: allergic rhinitis, asthma, asthma control test (ACT), pulmonary function tests, SACRA questionnaire, visual analogue scale
Introduction
The relationship between asthma and allergic rhinitis (AR) has been widely and clinically recognized (1-3). The “One airway, one disease” concept was first described by Grossman in 1997 (4). He described the concept mainly from the pathophysiological roles of leukotriene inflammation in the upper and lower airways (4). Although the rate of comorbidities varies among different regions and countries, a nationwide survey of asthmatic patients in Japan revealed that 67.3% of asthmatic patients had AR (5). Previous studies demonstrated that among patients with asthma and concomitant AR, those who received treatment for AR had a significantly lower risk of subsequent asthma-related events (emergency care visits/hospitalizations) than those who did not receive treatment (3,6). Notwithstanding the above facts, many reports have pointed out a divergence in the knowledge among physicians regarding asthma and AR and that the comprehensive treatments for both diseases were insufficient (1-3,5). It is possible that asthmatic patients with perennial rhinitis will fail to mention their rhinitis symptoms to their physician. Indeed, Verdiani et al. reported that perennial rhinitis is much more important than seasonal rhinitis as a risk factor for developing non-specific bronchial hyperresponsiveness in AR patients without asthma (7). To diagnose the existence of AR, we would need to examine asthmatic patients in greater detail.
The Self-assessment of Allergic Rhinitis and Asthma (SACRA) questionnaire, which was developed by Ohta et al. is used for determining and estimating the severity of both asthma and AR (5). Before developing the questionnaire, a cross-sectional multicenter study (State of the impact of allergic rhinitis on asthma control study [SACRA study]) was conducted throughout Japan to assess the prevalence, classification, and severity of rhinitis using the Allergic Rhinitis and its Impact on Asthma (ARIA) criteria in patients with diagnosed and treated asthma based on the Global Initiative for Asthma (GINA) diagnostic guidelines (5). The main finding of the SACRA study was that AR is a common comorbidity (67.3%) in asthma and that it impairs asthma control (5). In addition, the survey clearly showed the divergence between the knowledge of the “one airway, one disease” concept and the medical treatments of both diseases in Japan (5). After establishing the results, Ohta et al. produced the SACRA questionnaire. Although only the Japanese version's is named “SACRA,” the same questionnaire is available in several languages on the ARIA home page (8). The questionnaire is approved and recommended for use by ARIA and GINA. It enables physicians to diagnose AR easily and more accurately. In fact, by using the prototype questionnaire, 92.3% of the cases of AR were diagnosed by the symptoms that the patient's reported in the SACRA survey (5).
We hypothesized that the use of the SACRA questionnaire would allow respiratory physicians to more easily diagnose and treat asthmatic patients with AR. We examined whether asthma control improved after the treatment of AR based on the results of the SACRA questionnaire in stable (i.e. no asthma attack in previous 6 months) asthmatic patients with symptomatic AR.
Materials and Methods
Subjects and study design
This study was conducted in strict accordance with the ethical standards and human research, conformed to the provisions of the latest version of the World Medical Association Declaration of Helsinki and was approved by the Shinshu University's Institutional Review Board (Permission Number, 1786). Written informed consent was obtained from each patient before they were examined in the present study.
This prospective, multicenter study was performed in Nagano prefecture from September 2011 to March 2012. Twenty three respiratory physicians in 12 institutions agreed to participate in this study. Two hundred five stable asthmatic patients were enrolled. No physicians had previously participated in any studies involving the diagnosis of rhinitis. The diagnosis of asthma was made by each respiratory physician according to Asthma Prevention and Management Guidelines (JGL) 2009 Japan (9). All patients with asthma who were enrolled in the study were included with and without considering whether they had previously been diagnosed with rhinitis. The eligible subjects consisted of patients who were older than 18 years of age, who had been diagnosed with asthma, and who were constantly followed up by their respiratory physicians. The exclusion criteria (except age), were as follows: complication with another pulmonary disease including obvious chronic obstructive pulmonary disease, oral or intravenous corticosteroid therapy within 6 months before day 1 of the study, and patients who were judged as inadequate for recruitment by the participating physicians.
Pulmonary function tests, the asthma visual analogue scale (VAS) in the SACRA questionnaire, and an asthma control test (ACT) (10,11) were performed to evaluate the patients' asthma control. The pulmonary function tests were performed using a CHESTAC-8900 system (CHEST M.I., Inc., Tokyo, Japan). Short-acting β2-agonists were not used to relieve dyspnea on the day of the examination.
SACRA questionnaire
As mentioned, the SACRA questionnaire was translated into eight other languages, including English and can be found on the ARIA home page as “The One Airway Questionnaires” (8). The use of the SACRA questionnaire and the English translation (Supplemental Table 1) of the Japanese original version were approved by the GINA and ARIA Japan committee.
Table 1.
Demographic Characteristics of the Patients.
|
Analyzed subjects |
AR- group | AR+ group | |
|---|---|---|---|
| Number of patients | |||
| n | 157 | 90 | 67 |
| Age | |||
| years old | 59.6± 1.2 | 60.5± 1.7 | 60.7± 2.0 |
| Gender | |||
| male, n (%) | 64 (40.8%) | 36 (40.0%) | 28 (41.8%) |
| female, n (%) | 93 (59.2%) | 54 (60.0%) | 39 (58.2%) |
| Pulmonary function | |||
| FEV1 (L) | 2.18 ± 0.06 | 2.25 ± 0.08 | 2.07 ± 0.09 |
| FEV1/FVC, (%) | 74.6 ± 1.03 | 75.9 ± 1.35 | 73.0 ± 1.60 |
| GINA treatment step | |||
| Step2, n (%) | 53 (33.8%) | 39 (43.3%) | 14 (20.9%) ** |
| Step3, n (%) | 46 (29.3%) | 30 (33.3%) | 16 (23.9%) |
| Step4, n (%) | 54 (34.4%) | 19 (21.1%) | 35 (52.2%) *** |
| Step5, n (%) | 4 (2.5%) | 2 (2.2%) | 2 (3.0%) |
| Patients with previous LTRA treatment | |||
| n, (%) | 76 (48.4%) | 38 (42.2%) | 38 (56.7%) * |
AR: allergic rhinitis; Values are means ± standard error of the mean; ***p<0.001, ** p<0.01, * p<0.05 vs. AR- group.
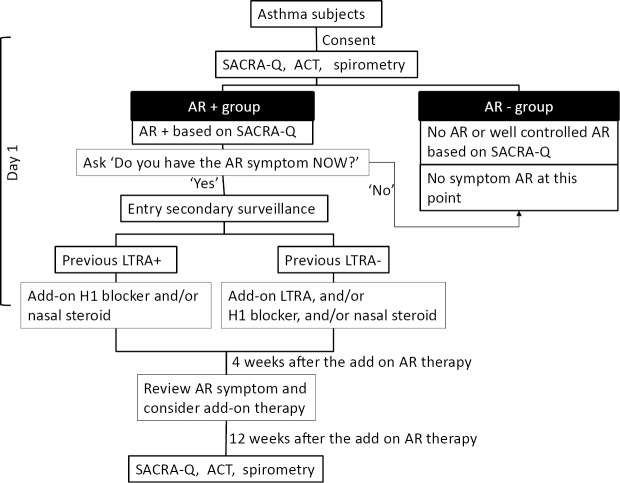
Participating respiratory physicians first explained the study objective and contents to their asthmatic patients. After obtaining written informed consent from the patients, they were requested to fill in the SACRA questionnaire and ACT in the presence of physician. Spirometry was also performed on the same day. The study design and flow are indicated in Fig. 1. Although the SACRA questionnaire can easily be used to diagnose AR based on the symptoms alone (5), the first part of the AR section in the Japanese version of this questionnaire does not necessarily ask the actual time of the AR symptoms ― it asks about the most symptomatic season for patients who demonstrate seasonal AR symptoms. Thus, if the patients checked “yes” at least once in the first part of the AR section of the SACRA questionnaire (same as part B-1 in Supplemental Table 1), then the physicians asked the patient, “Do you have that AR symptom now?”. If the patient answered, “yes”, they were classified into the “AR+ group” and the physician attempted to introduce add-on AR therapy. If they answered, “no”, then they were classified into the “AR- group,” which in principle contained patients without AR and those with asymptomatic, well-controlled AR at the time of the survey. If the “AR+ group” patients were currently treated with an leukotriene receptor antagonist (LTRA), the physicians added a histamine 1-receptor antagonist and/or nasal steroid sprays as an add-on therapy. If the “AR+ group” patients had not been treated with an LTRA, the physicians, at a minimum, administered an LTRA and were able to administer a histamine 1-receptor antagonist and/or nasal steroid sprays as an add-on treatment. After four weeks of the add-on treatment, the physicians asked the patients' (at the clinic) whether their subjective AR symptoms had improved. If patients still had the AR symptoms, the physicians could prescribe an additional add-on AR treatment (a histamine 1-receptor antagonist and/or a nasal steroid spray). Prior to the commencement of the study it was planned that the patients who received an add-on treatment, would again complete the SACRA questionnaire and that the ACT and spirometry would be performed again at 12 weeks after the first day of the add-on AR treatment (Fig. 1). If other anti-allergic agents had already been prescribed (e.g. sodium cromoglicate, splatast tosilate, or other herbal medicines), these agents were allowed to continue treatment under the same dose. There was no limitation on the use of anti-allergy eye drops.
Figure 1.
The study design. The study design and flow. Because leukotriene receptor antagonists (LTRAs) would have affected the AR pathophysiology, we divided the patients who received an add-on AR therapy into two groups (previous LTRA+and previous LTRA-). To estimate the precise effect of add-on AR therapy, we accepted the further administration of add-on AR therapies at one month after treatment of the initial add-on AR therapy. SACRA-Q: SACRA questionnaire, ACT: asthma control test, AR: allergic rhinitis, H1 blocker: histamine H1 receptor blocker
Statistical analysis
A statistical analysis was performed using the SPSS for Windows software program (version 14.0, SPSS Inc, Chicago, IL, USA). The values shown in the figures and tables represent the mean ± the standard error of the mean (SEM). The paired t-test was used to compare the results before and after AR treatment. For other comparisons, the distribution of the data for each variable was first assessed using Bartlett's test. When variables showed a normal distribution, the data were compared using the unpaired t-test. When variables did not show a normal distribution, the data were compared using Welch's method. p values of <0.05 were considered to indicate statistical significance.
Results
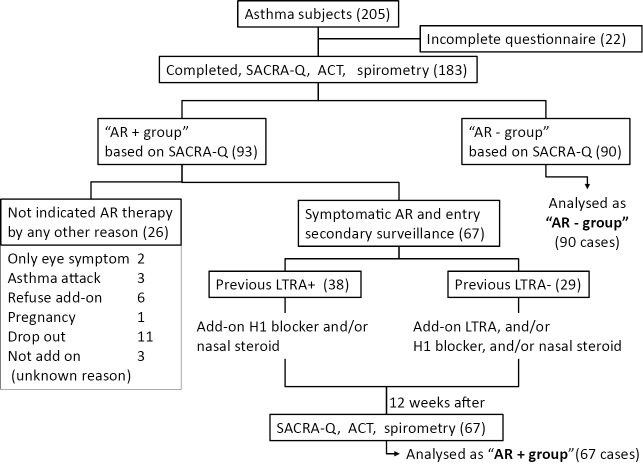
A flow diagram illustrating the study protocol and the characteristics of the patients are shown in Figure 1, 2, respectively. A total of 205 asthmatic patients were enrolled; completed data (no missing answers on the SACRA questionnaire or ACT, and pulmonary function tests) were obtained from 183 patients.
Figure 2.
A flow diagram of the study design and the numbers of patients in the present study. The number of patients is indicated in the flow diagram. Ninety-three of the 183 patients (50.8%) were classified into the “AR+group”. In the AR+group, 26 patients did not received an add-on AR therapy for several reasons (as indicated). Sixty seven symptomatic AR patients received an add-on AR therapy. We further divided the two groups based on whether they were treated with a leukotriene receptor antagonist. SACRA-Q: SACRA questionnaire, ACT: Asthma control test, AR: allergic rhinitis, LTRA: leukotriene receptor antagonist
Ninety-three of these 183 (50.8%) asthmatic patients were classified into the “AR + group” according to their SACRA questionnaire results at the time of the first survey. The remaining asthmatic patients were considered to be AR-negative or to have asymptomatic well-controlled AR (the AR- group) at the time of the first survey. The 93 patients in the “AR + group”, would be subsequently undergo a re-examination (which included pulmonary function tests, SACRA questionnaire and ACT) at three months after the first day of the add-on AR treatment. As a result, 67 of the 93 patients were re-examined. The remaining patients were excluded from this study for various reasons, including: refusing the add-on therapy (n=6), an asthma attack during the survey (n=3), only eye symptoms (n=2) and other reasons (Fig. 2). The demographic data of the 157 analyzed patients (90 AR - patients and 67 AR+ patients) are shown in Table 1. The 67 patients in the “AR + group” were further divided based on whether they previously been treated with an LTRA (Previous LTRA+ group, n=38) or if they had started an LTRA as an add-on AR treatment after the first survey (Previous LTRA- group, n=29). No serious adverse effects such as anaphylaxis, nasal deterioration or airway infection were observed during this study.
Add-on AR treatment improved asthma control in the asthmatic patients of the AR+ group
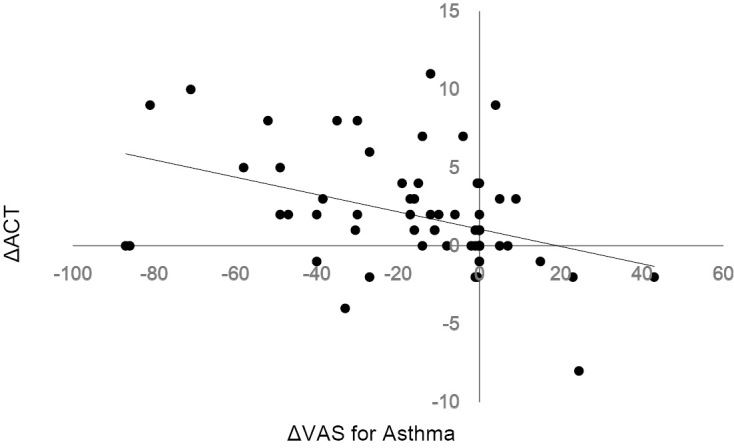
In the SACRA questionnaire, understandably, the VAS for rhinitis was significantly reduced after add-on AR treatment (p<0.001) (Table 2). A significant reduction of the VAS for asthma was also seen (p<0.001) (Table 2). The total score of ACT was significantly increased in the patients who received an add-on AR treatment (Table 2). Of the five queries in the ACT, queries #1, 3, 4 and 5 showed a significant improvement after the add-on AR treatment (Table 2), which indicated that daily work, night or early morning symptoms, inhaler use, and asthma control were improved. A significant correlation was observed between the variation of ACT score and the variation of the VAS for asthma before and after AR therapy (n=67) (r=-0.42, p<0.001 [Spearman's rank-order correlation]) (Fig. 3).
Table 2.
Results of VAS Scale in SACRA Questionnaire and ACT Score.
| AR- group (n=90) | AR+ group (n=67) | ||
|---|---|---|---|
| Before | After | ||
| VAS for AR, scores | 7.09±2.07 | 49.60±4.01*** | 18.49±2.85 ††† *** |
| VAS for Asthma, scores | 12.38±2.43 | 25.95±3.76 *** | 10.17±2.03 †† |
| ACT-total, scores | 22.70±0.33 | 20.79±0.48 ** | 22.68±0.35 †† |
| q1, scores | 4.74±0.07 | 4.50±0.09 * | 4.76±0.07 |
| q2, scores | 4.44±0.11 | 4.24±0.13 | 4.39±0.11 |
| q3, scores | 4.42±0.13 | 4.20±0.15 | 4.73±0.09 ††† |
| q4, scores | 4.76±0.08 | 4.18±0.16 ** | 4.58±0.11 †† |
| q5, scores | 4.34±0.10 | 3.67±0.13 *** | 4.23±0.11 ††† |
AR: allergic rhinitis, VAS: visual analogue scale, ACT: asthma control test; Values are means ± standard error of the mean; *** p<0.001, ** p<0.01, * p<0.05 vs. AR – group; †††p<0.001, ††p<0.01 vs. after the add-on AR therapy.
Figure 3.
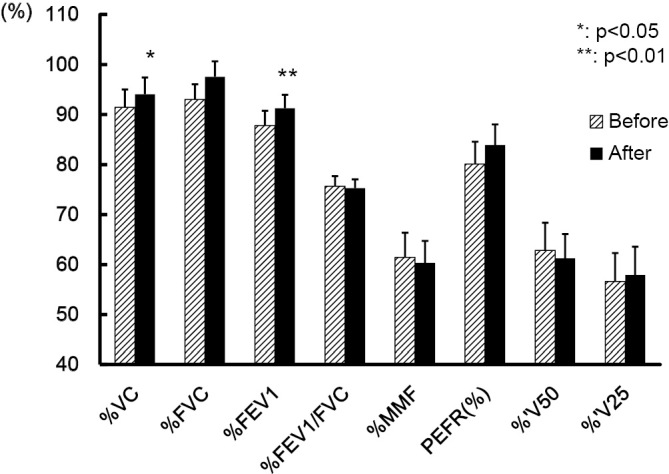
The pulmonary function test results before (tilted lined bar) and after (black bar) add-on AR therapy. A significant improvement was seen in the vital capacity and forced expiratory volume in 1 second. VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, %FEV1: percent predict of one second forced expiratory volume, MMF: maximum mid-expiratory flow, PEFR: peak expiratory flow rate
The pulmonary function tests, vital capacity and forced expiratory volume in one second were significantly improved in the AR+ group after the add-on AR treatment (Fig. 4).
Figure 4.
Add-on AR treatment improved several pulmonary functions in the AR+ group.
The effects of the add-on AR therapy on the AR-VAS, Asthma-VAS, ACT and pulmonary function test results in the AR+ group with and without previous LTRA treatment
Regardless of whether the patients had previously received an LTRA, the VASs for AR and asthma and the ACT score were significantly improved in the AR+ group. However, the vital capacity (VC), forced vital capacity (FVC) and forced expiratory volume (FEV1) were only significantly improved in the patients of the AR+ group who had previously undergone LTRA treatment (Table 3). The total ACT score of the previous LTRA+ group before the add-on AR treatment was significantly lower that of the previous LTRA- group. The patients of the previous LTRA+ group completely recovered from this lack of control to the same level of the total ACT score in the previous LTRA- group (Table 3).
Table 3.
Effects of the Add-on AR Therapy on AR-VAS, Asthma-VAS, ACT and Pulmonary Function Test in the AR+group with and without Previous LTRA Treatment.
| AR+ group (n=67) | |||||
|---|---|---|---|---|---|
| Previous LTRA- group (n=29) | Previous LTRA+ group (n=38) | ||||
| Before | After | Before | After | ||
| AR-VAS, scores | 46.40±5.17 | 18.90±4.28 *** | 48.10±5.17 | 23.90±4.16 *** | |
| Asthma-VAS, scores | 21.00±4.25 | 7.70±1.71 *** | 31.40±4.80 | 14.80±3.54 *** | |
| ACT-total, scores | 22.03±0.59 | 22.89±0.42 | 20.21±0.69 † | 22.82±0.53 *** | |
| Pulmonary function | |||||
| VC (L) | 3.15±0.19 | 3.17±0.20 | 2.64±0.20 | 2.71±0.20 | |
| VC (% pred) | 99.82±4.91 | 101.90±4.47 | 91.45±3.56 | 94.06±3.36 * | |
| FVC (L) | 3.09±0.16 | 3.03±0.18 | 2.75±0.19 | 2.84±0.18*** | |
| FVC(% pred) | 100.56±3.61 | 99.12±4.18 | 93.03±3.01 | 97.57±3.07 *** | |
| FEV1 (L) | 2.05±0.12 | 2.07±0.13 | 2.09±0.13 | 2.17±0.13* | |
| FEV1 (% pred) | 87.80±4.63 | 88.70±4.70 | 87.80±2.95 | 91.26±2.68 ** | |
| FEV1/FVC (%) | 69.49±2.45 | 71.41±2.16 | 75.65±2.03 | 75.27±1.77 | |
| MMF (L/sec) | 1.56±0.18 | 1.62±0.20 | 1.91±0.22 | 1.87±0.21 | |
| MMF (% pred) | 53.09±6.12 | 54.48±6.19 | 61.44±4.94 | 60.34±4.40 | |
| PEFR (L/sec) | 5.62±0.41 | 5.69±0.42 | 5.33±0.37 | 5.60±0.38 | |
| PEFR (% pred) | 82.64±5.20 | 82.58±4.83 | 80.09±4.49 | 83.91±4.13 | |
| V50 (L/sec) | 1.94±0.22 | 2.04±0.25 | 2.40±0.26 | 2.29±0.22 | |
| V50 (% pred) | 51.74±5.65 | 54.63±6.08 | 62.85±5.51 | 61.23±4.85 | |
| V25 (L/sec) | 0.58±0.08 | 0.64±0.08 | 0.83±0.12 | 0.82±0.12 | |
| V25 (% pred) | 40.98±4.70 | 45.24±4.56 | 56.59±5.70 † | 57.89±5.68 | |
AR: Allergic rhinitis, VAS: Visual Analogue Scale, ACT: Asthma control test, VC: Vital capacity, FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 second, MMF: Maximum mid-expiratory flow rate, PEFR: Peak expiratory flow rate; Values are means ± standard error of the mean; *** p<0.001, ** p<0.01, * p<0.05 vs. Before the add-on AR therapy in the Previous LTRA+ group; † p <0.05 vs. Before the add-on AR therapy in the Previous LTRA- group.
Discussion
In the present study, we showed that the administration of a SACRA questionnaire-based add-on AR treatment improved the asthma VAS, the ACT score and pulmonary function in symptomatic AR patients (AR+ group). We also showed that the SACRA questionnaire was useful, not only for detecting and assessing AR and asthma, but also for estimating the efficacy of the add-on therapy. These findings were based on the results of two sets of data (once before add-on AR treatment, and once after) which included the results of the SACRA questionnaire, an ACT, and pulmonary function tests, from the 67 patients in “AR+ group.” One set of data was collected from the 90 patients in the “AR- group”.
At present, we have a great deal of evidence to support the existence of a relationship between asthma and AR (1,5). However, it has also been reported that the knowledge about the link between asthma and AR does not always precisely reflect the clinical practice (2,5). From the otorhinolaryngological point of view, the under-treatment of rhinitis has been reported despite the availability of treatment guidelines (12,13). We need to treat AR more actively based on the guidelines (12). Recently, Ohta et al. produced the SACRA questionnaire based on the results of their nationwide survey (5). The usefulness of the SACRA questionnaire has also been reported (14) and the questionnaire is available online in other languages, including English (supplemental Table 1) (8). Ohta et al. reported that in 92.3% of cases, rhinitis was diagnosed based on its symptoms (5). The easier assessment of the existence and severity of AR could improve of asthma control via the administration of the add-on AR treatment.
Recently, Ohta et al. reported a nationwide study of asthmatic patients in Japan and validated the effectiveness of the VAS for asthma (the same as the VAS scale in the SACRA questionnaire) (15). The study revealed that the VAS for asthma severity was accurate in predicting GINA-defined asthma control (i.e. “controlled”, “partly controlled”, or “uncontrolled”). This study also indicated that 85% of patients with “controlled asthma” had VAS levels of <40 mm (15). In our study, 21 of the 67 patients in the “AR+ group” had a VAS for asthma of ≥40 mm before the add-on AR treatment; only 3 patients still had a VAS for asthma of ≥40 mm after the administration of the add-on AR treatment. We also used the ACT to estimate asthma control. We showed the improvement of both the VAS for asthma value (in the SACRA questionnaire) and the ACT score (Table 2, Fig. 3). Considering the improvement of asthma symptoms that was achieved with the add-on AR treatment, these simple questionnaires were useful for the improvement of asthma control.
Before the add-on AR treatment, asthma control, as assessed by the ACT was worse in the previous LTRA+ group than in the previous LTRA- group (Table 3). No difference was observed in the severity of AR, as estimated by the VAS for AR in the SACRA questionnaire (data not shown). The result seems somewhat paradoxical, since the administration of an LTRA should have beneficial effects for both asthma and AR. Since the ACT score in the previous LTRA+ group improved after the add-on AR treatment to almost the same as level as the previous LTRA- group, the administration of an LTRA was considered to be useful for treating more severe asthma rather than for the management of AR. Indeed, the administration of an LTRA might have been insufficient for the treatment of AR in the previous LTRA+ group, which included AR patients with more severe asthma - while their asthma control was improved by the add-on AR treatment. The fact that the under-treatment of rhinitis is common has also been mentioned another study (13). The presence of comorbidities has also been reported to increase the prescription of inhalants and oral steroids in the treatment of rhinitis in asthmatic patients (16).
We found observed the effects of LTRA treatment for asthma in the previous LTRA+ group. In the pulmonary function tests, the V25 value was higher in the previous LTRA+ group than in the previous LTRA- group (Table 3). The maximum mid-expiratory flow and V50 value also tended to be higher in the previous LTRA+ group (Table 3). These parameters reflect the function of the peripheral airway. Since LTRAs are orally administered, the agent should affect the whole lung. Yasui et al. reported that LTRA treatment increased the maximum mid-expiratory flow rate and decreased the peripheral airway/alveolar nitric oxide concentration (17). Kelly et al. revealed that LTRA treatment attenuated the increase in myofibroblasts after low-dose allergen challenge through the use of transbronchial biopsy specimens (18). Longer LTRA treatment might improve the function of the peripheral airway.
Stelmach et al. reported that add-on AR treatments did not affect asthma control in asthmatic patients (19). The prospective study divided patients with mild to moderate asthma into three groups (nasal steroid only, inhaled steroid only, and both). The clinical improvement was similar and parallel in the three groups. Their results differed from ours. First, we accepted patients with previous asthma and AR therapy. Second, LTRAs were the first choice of treatment for AR and further add-on AR treatments were accepted during the study period if the patients' AR symptoms did not improve. Finally, a recent systematic review with a meta-analysis showed the effects of nasal steroid spray on asthma outcomes (20). We attempted to analyze the effects of nasal steroid sprays for asthma control (Supplemental Table 2). Treatment with an LTRA and nasal steroid spray with or without a histamine 1-receptor antagonist significantly improved the asthma-VAS scores in the previous LTRA+ group. The effects of nasal steroid spray in the previous LTRA- group could not be assessed in the present study due to the small number of subjects (Supplemental Table 2). With regard to the pulmonary function, several functions were also significantly improved by treatment with an LTRA and nasal steroid in the previous LTRA+ group (Supplemental Table 3). These results were consistent with those of a previous analysis (20). Our protocol of study is closer to the clinical setting and we wish to advocate that the proper administration of add-on AR treatments further improves asthma control.
In Japan, Japanese cedar pollen-induced AR is a nationwide problem. The pollen may also induce asthma (21). In our prefecture, the dispersal season is in the latter half of March. Almost all of our study was performed outside of the pollen season. Recently, Hojo et al. reported a minute and critical analysis of the relationship between asthma control and Japanese cedar pollinosis (22). Their study suggested that there was no significant difference in asthma control level measured by the VAS (in the SACRA questionnaire) and the ACT score outside of the pollen season and that the control level worsened during the pollen season (22). It is considered that our study was performed outside of the pollen season. However, 50.8% of the patients in our study had symptomatic AR. It is considered that a number of patients with perennial AR would have been included in the symptomatic AR patient population. If patients have perennial AR, they may never complain about their AR symptoms to their respiratory physician. Perennial AR is reported to be a much more important risk factor for developing nonspecific bronchial hypersensitivity than seasonal AR (23). In this regard, a simple questionnaire for AR (like the SACRA questionnaire) is important for assessing the potential for AR. Our study showed the usefulness of the SACRA questionnaire for determining the therapeutic effect of AR treatment by asking “Do you have an AR symptom now?” if the patients had marked at least one answer in the AR part of the questionnaire.
The limitations of the present study
Not all of the participating respiratory physicians introduced all of their asthmatic patients. The enrolment of a small number of patients by a physician might cause a selection bias. We therefore analyzed patients from physicians who introduced ≥10 patients. As a result, 127 of the 157 patients (80.9%) were enrolled in the sub-analysis. The VAS of asthma, ACT score, and pulmonary function tests were almost the same results as the results that were obtained in the initial analysis (data not shown).
Hojo et al. reported that the sensitivity and specificity of SACRA for the diagnosis of AR were 92% and 66% respectively (14). Due to the lower specificity, some non-AR patients might have been included in our AR+ group. Indeed, the VAS for AR did not improve in 9 of the 67 (13.4%) patients who were included in the analysis of the add-on AR treatment in this study did not improve after three months of add-on AR treatment. Furthermore, only three of the nine patients showed improved or unchanged asthma control after three months of add-on AR treatment. The remaining six patients might not have had AR. It has been reported that the discrimination between AR and other forms of rhinitis is difficult, even for otorhinolaryngologists (24). This is further complicated by the fact that combined rhinitis (both allergic and from other causes) has also been reported to occur (24). Furthermore, both allergic and non-allergic rhinitis are associated with asthma (5). Considering the under-treatment of AR (5,13,16), it is important to diagnose AR easily and to strictly administer appropriate treatment. Patients who do not obtain a therapeutic effect after the add-on AR treatment, should consult an otorhynolaryngologist.
Conclusion
In conclusion, the diagnosis of AR and the administration of add-on AR treatments based on the SACRA questionnaire were effective for achieving the better control of asthma patients with AR. The use of the SACRA questionnaire allows respiratory physicians to diagnose symptomatic AR and introduce add-on AR therapies easier, faster, and more effectively.
The authors state that they have no Conflict of Interest (COI).
Cooperative facilities and physicians
Shinshu University School of Medicine: Masanori Yasuo, Tomonobu Koizumi, Masayuki Hanaoka, Hiroshi Yamamoto, Mie Hayashida
Suwa Toyoda Clinic: Yoshimichi Komatsu
Suwa Redcross Hospital: Yoshimichi Komatsu, Tsutomu Hachiya, Akane Kato
Okaya Municipal Hospital: Yoshiaki Kitaguchi
Nagano Municipal Hospital: Fumiaki Yoshiike, Takashi Ichiyama
Shinshu Ueda Medical Center: Masanori Yasuo, Toshihiko Agatsuma, Gen Ideura
Nagano Matsushiro General Hospital: Takanari Miyahara, Ryosuke Machida
Iiyama Redcross Hospital: Masanori Yasuo
Omachi General Hospital: Kayoko Ikegawa
Maruko Central Hospital: Yayoi Tokoro
Asama General Hospital: Mineyuki Hama
Yodakubo Hospital: Atsuhito Ushiki
Acknowledgement
The authors would like to thank Yunden Droma the Shinshu University School of Medicine for her valuable advice and for kindly checking the English language usage in our manuscript. The authors also thank Thomas Taylor, for kindly checking the English grammatical usage in our manuscript.
This study was partly supported by a grant to the Respiratory Failure Research Group from the Ministry of Health, Labor, and Welfare, Japan.
References
- 1. Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 26: 187-190, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braido F, Baiardini I, Brandi S, Porcu A, Canonica GW. Allergic rhinitis and asthma ad hoc survey: clinical and psychological perspectives. Clin Exp Allergy 37: 788-793, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol 109: 57-62, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Grossman J. One airway, one disease. Chest 111: 11S-16S, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Ohta K, Bousquet PJ, Aizawa H, et al. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy 66: 1287-1295, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: a case-control study. J Allergy Clin Immunol 113: 415-419, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Verdiani P, Di Carlo S, Baronti A. Different prevalence and degree of nonspecific bronchial hyperreactivity between seasonal and perennial rhinitis. J Allergy Clin Immunol 86: 576-582, 1990. [DOI] [PubMed] [Google Scholar]
- 8. Allergic Rhinitis and Its Impact on Asthma (ARIA) ARIA NEWS [Internet]. [cited October 2009]. Available from: http://www.whiar.org/ [Google Scholar]
- 9.Research Group for Asthma Prevention and Management Guidelines, supported by the Ministry of Health, Labor, and Welfare Japan. Asthma prevention and management guidelines, Japan (JGL2009). Ohta K, Akiyama K, Nishima S, Eds. 2009. (in Japanese). [Google Scholar]
- 10. Thomas M, Kay S, Pike J, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J 18: 41-49, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 117: 549-556, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Pawankar R, Bunnag C, Chen Y, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008)--western and Asian-Pacific perspective. Asian Pac J Allergy Immunol 27: 237-243, 2009. [PubMed] [Google Scholar]
- 13. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet 378: 2112-2122, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Hojo M, Ohta K, Iikura M, Mizutani T, Hirashima J, Sugiyama H. Clinical usefulness of a guideline-based screening tool for the diagnosis of allergic rhinitis in asthmatics: the SACRA (Self Assessment of Allergic Rhinitis and Asthma) Questionnaire. Respirology 18: 1016-1021, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Ohta K, Jean Bousquet P, Akiyama K, et al. Visual analog scale as a predictor of GINA-defined asthma control. The SACRA study in Japan J Asthma 50: 514-521, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Antonicelli L, Micucci C, Voltolini S, et al. Relationship between ARIA classification and drug treatment in allergic rhinitis and asthma. Allergy 62: 1064-1070, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Yasui H, Fujisawa T, Inui N, et al. Impact of add-on pranlukast in stable asthma; the additive effect on peripheral airway inflammation. Respir Med 106: 508-514, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Kelly MM, Chakir J, Vethanayagam D, et al. Montelukast treatment attenuates the increase in myofibroblasts following low-dose allergen challenge. Chest 130: 741-753, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Stelmach R, do Patrocínio T, Nunes M, Ribeiro M, Cukier A. Effect of treating allergic rhinitis with corticosteroids in patients with mild-to-moderate persistent asthma. Chest 128: 3140-3147, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy 68: 569-579, 2013. [DOI] [PubMed] [Google Scholar]
- 21. Maeda Y, Akiyama K, Shida T. A clinical study of Japanese cedar (Cryptomeria japonica) pollen-induced asthma. Allergol Int 57: 413-417, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Hojo M, Ohta K, Iikura M, Hirashima J, Sugiyama H, Takahashi K. The impact of co-existing seasonal allergic rhinitis caused by Japanese Cedar Pollinosis (SAR-JCP) upon asthma control status. Allergol Int 64: 150-155, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Verdiani P, Di Carlo S, Baronti A. Different prevalence and degree of nonspecific bronchial hyperreactivity between seasonal and perennial rhinitis. J Allergy Clin Immunol 86: 576-582, 1990. [DOI] [PubMed] [Google Scholar]
- 24. Bousquet J, Fokkens W, Burney P, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy 63: 842-853, 2008. [DOI] [PubMed] [Google Scholar]