Abstract
Purpose
This study aimed to investigate the relationship between the SUVmax of primary breast cancer lesions and the molecular subtypes based on the recommendations of the St. Gallen consensus meeting 2013.
Methods
Clinical records of patients who underwent F-18 FDG PET/CT for initial staging of invasive ductal carcinoma (IDC) of the breast were reviewed. A total of 183 patients were included. SUVmax was correlated with the molecular subtypes defined by the St. Gallen Consensus Meeting 2013, i.e., luminal A-like (LA), luminal B-like HER2 negative (LBHER2-), luminal B-like HER2 positive (LBHER2+), HER2 positive (HER2+), and triple negative (TN), and with the clinicohistopathologic characteristics.
Results
The molecular subtype was LA in 38 patients, LBHER2- in 72, LBHER2+ in 21, HER2+ in 30, and TN in 22. The mean SUVmax in the LA, LBHER2-, LBHER2+, HER2+, and TN groups were 4.5 ± 2.3, 7.2 ± 4.9, 7.2 ± 4.3, 10.2 ± 5.5, and 8.8 ± 7.1, respectively. Although SUVmax differed significantly among these subtypes (p < 0.001), the values showed a wide overlap. Optimal cut-off SUVmax to differentiate LA from LBHER2-, LBHER2+, HER2+ and TN were 5.9, 5.8, 7.5, and 10.2 respectively, with area under curve (AUC) of 0.648, 0.709, 0.833, and 0.697 respectively. The cut-off value of 5.9 yielded the highest accuracy for differentiation between the LA and non-LA subtypes, with sensitivity, specificity, and AUC of 79.4 %, 57.9 %, and 0.704 respectively.
Conclusion
The SUVmax showed a significant correlation with the molecular subtype. Although SUVmax measurements could be used along with immunohistochemical analysis for differentiating between molecular subtypes, its application to individual patients may be limited due to the wide overlaps in SUVmax.
Keywords: Breast cancer, F-18 FDG PET/CT, Invasive ductal carcinoma, Molecular subtype, SUVmax
Introduction
Breast cancer is a heterogeneous tumor with distinct molecular subtypes and characteristics. Histopathologically, breast cancer can be divided into several subtypes. Among them, invasive ductal carcinoma (IDC) is most common. On the basis of gene expression profiling, breast cancer can be divided into five subtypes—luminal A, luminal B, human epidermal growth factor receptor type 2 (HER2)-enriched, basal cell-like, and normal breast like [1]. However, due to complexity, costs, and technical feasibility, application of gene expression profiling to general clinical practice has been limited. Therefore, classification based on immunohistochemistry is widely used since it is inexpensive, popular, and technically simple.
According to the recommendations of the St. Gallen consensus meeting 2013, breast cancer can be categorized into five subtypes, namely, luminal A-like (LA), luminal B-like HER2 negative (LBHER2-), luminal B-like HER2 positive (LBHER2+), HER2 positive (HER2+), and triple negative (TN) based on the expression status of estrogen receptor (ER), progesterone receptor (PgR), HER2, and Ki-67. Moreover, the method and outcome of treatment in breast cancer is defined by the molecular subtype. For example, unlike other subtypes, LA has good prognosis without chemotherapy [2]. The St. Gallen consensus meeting held in 2013 proposed some changes to the classification of molecular subtypes that were absent in the recommendations of the 2011 consensus meeting, e.g., PgR positivity defined by a cut-off value of 20 % was added to distinguish between LA and LB subtype, the number of patients classified as LA subtype was reduced, and the number for whom cytotoxic therapy is generally recommended was increased [2].
F-18 FDG PET, which evaluates glucose metabolism of tumors, is a widely used technique for diagnosing tumors, assessing treatment response, and patient follow-up. In various tumors including breast cancer, it is well known that maximum standardized uptake value (SUVmax) reflecting glucose metabolism of tumor is an independent prognostic factor [3, 4].
The correlation between SUVmax of the primary tumor and histopathologic factors in breast cancer has been assessed in many studies previously. Most of the previous studies mainly assessed the correlation between each histopathologic factor and SUVmax [5–12]. Garcia et al. [13] reported that semiquantitative parameters of F-18 FDG PET/CT showed statistically significant differences among the molecular subtypes according to the recommendations of St. Gallen consensus meeting 2011. However, there is no study on the relationship between molecular subtypes and SUVmax based on the recommendations of the St. Gallen consensus meeting 2013. A few studies reported that status of hormonal receptors can be changed after chemotherapy, so they recommended retesting the receptor status of the residual tumor [14]. Ascertainment for the relationship between molecular subtypes and SUVmax might not only give basic data for other clinical studies, but also help to make a diagnostic or therapeutic plan for patients with recurrent breast cancer where a biopsy is difficult to obtain. Therefore, we intended to investigate the relationship between the molecular subtypes proposed by the St. Gallen consensus meeting 2013 and SUVmax of primary breast cancer lesion. Because the pathological and clinical features and FDG avidity of IDC differ from those of invasive lobular carcinoma (ILC) [15], our study specifically enrolled patients with IDC to perform investigations in a homogeneous patients’ group.
Material and Methods
Patients
A total of 183 female patients with IDC of breast who underwent F-18 FDG PET/CT for staging between April 2011 to December 2013 were included in the study. The following patients were excluded from the study: patients who received operation or chemotherapy before F-18 FDG PET/CT; patients with tumor size of less than 1 cm in order to avoid the partial volume effect. This retrospective study was approved by the institutional review board of our hospital.
FDG PET/CT Image Acquisition
In all patients enrolled for the study, oral intake and intravenous glucose injection were prohibited for at least 6 h before the PET/CT scan. Before injection of F-18 FDG, blood glucose levels of the patients were checked. PET/CT scans were obtained when the blood glucose level was less than 200 mg/dl (Most of the patients had glucose level lower than 150 mg/dl, only six patients had higher glucose level than 150 mg/dl). A whole-body scan from head to thigh was acquired 60 min following intravenous injection of approximately 370 MBq of F-18 FDG. PET/CT examinations were performed using a PET/CT scanner (Discovery STE; GE Healthcare, Milwaukee, WI, USA). The CT images were acquired using a multidetector CT equipment with the standard protocol that consists of 140 kV, 60–80 mA, a tube rotation time of 0.4 s per rotation, a pitch of 0.984, and a section thickness 3.75 mm. Emission PET data were acquired for 2 min per bed. PET images were reconstructed using an ordered-subset expectation maximization iterative reconstruction algorithm with three iterations, 18 subsets, matrix size of 256x256, 50 cm transaxial field-of-view (FOV). PET images were then fused with CT images.
Image Analysis
Semi-quantitative analysis was performed independently by two experienced nuclear medicine physicians who were aware of the patients’ clinical history based on the results of other imaging studies and also by the information provided to them by the referring physician. For semi-quantitative analysis, a three-dimensional volumes of interest (3D VOI) was drawn on the primary tumor by referring to the finding of breast sonography and MRI, and then SUVmax which was defined as the maximum SUV in the pixel within the 3D VOI was calculated. Clinical stages were determined according to the American Joint Committee on Cancer manual, 7th edition [16].
Histopathological Analysis
The histopathological analysis was performed on needle biopsy sections for pathological diagnosis, or on representative sections of surgical specimens from patients who did not receive neo-adjuvant chemotherapy. Histological grade was also classified according to the modified Bloom-Richardson classification: well differentiated (grade 1), moderately differentiated (grade 2), and poorly differentiated (grade 3) [17]. Immunohistochemistry was done using paraffin-embedded tissue sections to examine the expression status of ER, PgR, HER2, and Ki-67.
ER and PgR expression levels were evaluated according to the sum of intensity score (0, weak: 1, moderate: 2, strong: 3) and proportion score (0, <1 %: 1, 1∼10 %: 2, 10∼33 %: 3, 34∼66 %: 4, 67∼100 %: 5). Tumors with 1 % or more nuclear expression were considered positive for ER and PgR expression. Expression of PgR in less than 20 % of tumor cells with less than moderate intensity was defined as low PgR expression [18]. According to the guidelines of the American Society of Clinical Oncology (ASCO), tumors were considered HER2 positive only if they were either scored 3+ by IHC or 2+ by IHC and also HER2 amplified (ratio > 2.0) on the basis of fluorescence in situ hybridization (FISH) [19]. The Ki-67 labeling index was recorded as low or high according to the percentage of nuclear expression of Ki67 in 3 high power fields using a cutoff vale of 14 % [20].
Molecular Classification
According to the recommendations of St. Gallen Consensus Meeting 2013, molecular subtypes were categorized as follows [2].
LA: ER and/or PgR positive, HER2 negative, Ki-67 ‘low’ (<14 %), recurrence risk ‘low’ based on multi-gene-expression assay
LBHER2-: ER positive, HER2 negative and at least one of the following: Ki-67 ‘high’, PgR ‘negative or low’ (<20 %), Recurrence risk ‘high’ based on multi-gene-expression assay
LBHER2+: ER positive, HER2 over-expressed or amplified, Any Ki-67, Any PgR
HER2+: HER2 over-expressed or amplified, ER and PgR absent
TN: ER and PgR absent, HER2 negative
Statistical Analysis
Medcalc Statistical Software version 14.12.0 (Medcalc Software bvba, Ostend, Belgium; 2014) was used for all analyses. Pearson correlation was used to evaluate the correlation between tumor size and SUVmax. Kruskal-Wallis test was used to compare the SUVmax among molecular subtypes. Mann–Whitney U test was used to compare the mean SUVmax according to histopathologic factors. Receiver operating characteristic (ROC) curve analysis was performed to examine the diagnostic performance of F-18 FDG PET/CT to differentiate LA subtype from others; p < 0.05 was considered statistically significant.
Result
Characteristics of the Study Population
Characteristics of the patients included in the study can be seen in Table 1. The mean age of study population was 54 ± 10.0 years, and the mean size of the primary tumor was 2.1 ± 1.6 cm. The mean SUVmax of the primary tumor was 7.4 ± 5.2. There was no definite correlation between SUVmax and tumor size (rho = 0.09, p = 0.224).
Table 1.
Patient’s characteristics and relationship between SUVmax and clinicopathological parameters
| Number (%) | SUVmax (mean ± SD) | p value | |
|---|---|---|---|
| Age (yr) mean ± SD | 54.0 ± 10.0 | 7.4 ± 5.2 | |
| Size (cm) | 2.1 ± 1.6 | ||
| Histologic grade | |||
| Well differentiated | 42 (23.0) | 4.3 ± 3.1 | <0.001 |
| Moderate differentiated | 82 (44.8) | 7.5 ± 5.0 | |
| Poorlydifferentiated | 59 (32.2) | 9.3 ± 5.6 | |
| Stage | |||
| I | 80 (43.7) | 7.4 ± 5.9 | 0.527 |
| II | 77 (42.1) | 7.2 ± 4.3 | |
| III | 20 (10.9) | 7.5 ± 5.0 | |
| IV | 6 (3.3) | 9.9 ± 6.2 | |
SUVmax maximum standardized uptake value
Relationship Between SUVmax and Clinicopathological Parameters
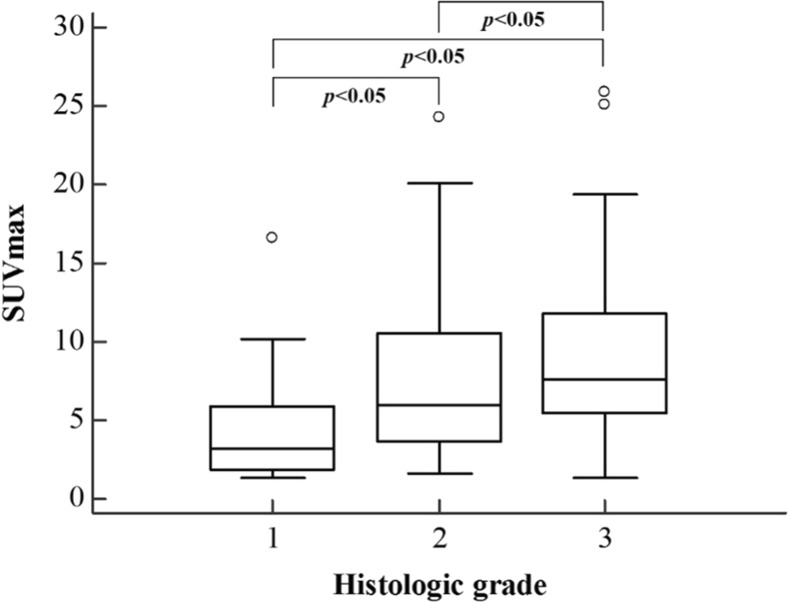
SUVmax was 4.3 ± 3.1 in the well-differentiated group (grade 1), 7.5 ± 4.9 in the moderately differentiated group, and 9.3 ± 5.6 in the poorly differentiated group. There were significant differences in SUVmax among the three grades (p < 0.001). Post-hoc analysis using Bonferroni correction revealed significant differences in SUVmax between the well differentiated and moderately differentiated groups (p < 0.05), the well differentiated and poorly differentiated groups (p < 0.05), and the moderately differentiated and poorly differentiated groups (p < 0.05) (Fig. 1). In terms of clinical stage, there was no significant difference in SUVmax among different stages.
Fig. 1.
SUVmax according to histologic grade. There was significant differences in SUVmax among histologic grade (p < 0.001). Boxplots show interquartile range (box), median (black line within interquartile range), data range (vertical lines), and outliers (open dots). SUVmax maximum standardized uptake value
Relationship Between SUVmax and Status of ER, PR, HER2, and Ki-67
The ER(−) group showed significantly higher SUVmax than ER(+) group (9.4 vs. 6.3, p < 0.001). In the case of the PgR(−) group, SUVmax of the primary tumor was significantly higher in comparison to that in the PgR(+) group (8.9 vs. 6.1, p < 0.001). The SUVmax of the HER2(+) group was significantly higher than the HER2(−) group (8.9 vs. 6.7, p = 0.002), and the group with a Ki-67 ≥ 14 % showed significantly higher SUVmax than the group with Ki-67 < 14 % (8.7 vs. 4.6, p < 0.001). These results are summarized in Table 2.
Table 2.
Relationship between SUVmax and status of ER, PgR, HER2, and Ki-67
| Number (%) | SUVmax (mean ± SD) | p value | |
|---|---|---|---|
| ER status | |||
| Negative | 62 (33.9) | 9.4 ± 6.1 | <0.001 |
| Positive | 121 (66.1) | 6.3 ± 4.2 | |
| PgR status | |||
| Negative | 80 (43.7) | 8.9 ± 5.8 | <0.001 |
| Positive | 103 (56.3) | 6.1 ± 4.2 | |
| HER2 status | |||
| Negative | 132 (72.1) | 6.7 ± 5.0 | 0.002 |
| Positive | 51 (27.9) | 8.9 ± 5.2 | |
| Ki-67 index | |||
| Low | 62 (33.9) | 4.6 ± 2.9 | <0.001 |
| High | 121 (66.1) | 8.7 ± 5.5 | |
ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, SUVmax maximum standardized uptake value
Relationship Between SUVmax and Molecular Subtypes
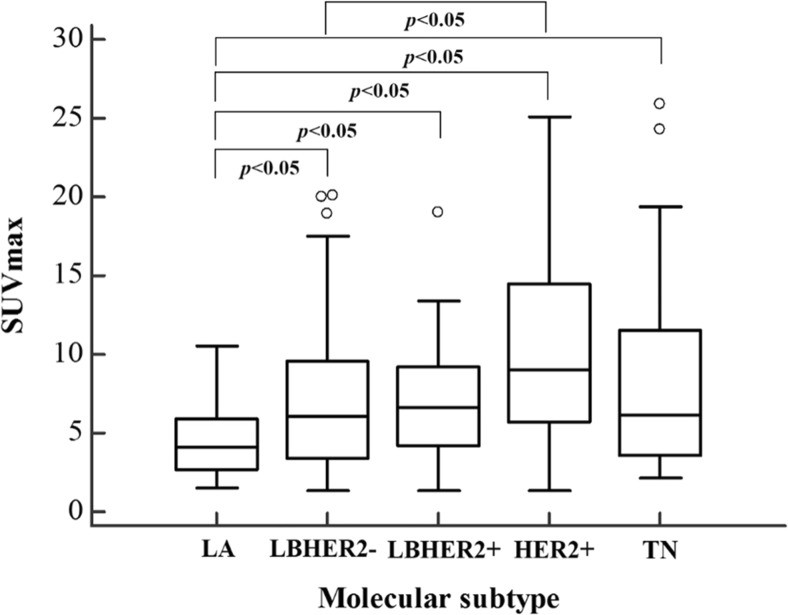
Among the 183 patients included in the study, the molecular subtype was LA in 38 patients, LBHER2- in 72 patients, LBHER2+ in 21 patients, HER2+ in 30 patients, and TN in 22 patients. The mean SUVmax of these subtypes was 4.5 ± 2.3, 7.2 ± 4.9, 7.2 ± 4.3, 10.2 ± 5.5, and 8.8 ± 7.1 respectively (Table 3). There were significant differences in SUVmax among the five molecular subtypes (p < 0.001). Post-hoc analysis using Bonferroni correction was performed to identify the subtypes that were different. There were significant differences in SUVmax between the group with LA and the rest of the molecular subtypes (p < 0.05), i.e., the SUVmax of LA was significantly lower than that of others. The patient group with the LBHER2 subtype showed significantly lower SUVmax than the patient group with HER2+ tumors (p < 0.05) (Fig. 2).
Table 3.
SUVmax of each molecular subtype according to the recommendations of the St. Gallen consensus meeting 2013
| Subtype | Number (%) | SUVmax (mean ± SD) |
|---|---|---|
| LA | 38 (20.8) | 4.5 ± 2.3 |
| LBHER2- | 72 (39.3) | 7.2 ± 4.9 |
| LBHER2+ | 21 (11.5) | 7.2 ± 4.3 |
| HER2+ | 30 (16.4) | 10.2 ± 5.5 |
| TN | 22 (12.0) | 8.8 ± 7.1 |
| Total | 183 | 7.4 ± 5.2 |
LA luminal A-like, LBHER2- luminal B-like HER2 negative, LBHER2+ luminal B-like HER2 positive, HER2+ HER2 positive, TN triple negative, SUVmax maximum standardized uptake value
Fig. 2.
SUVmax according to molecular subtype. There was significant differences in SUVmax among molecular subtypes (p < 0.001). Boxplots show interquartile range (box), median (black line within interquartile range), data range (vertical lines), and outliers (open dots). LA luminal A-like, LBHER2- luminal B-like HER2 negative, LBHER2+ luminal B-like HER2 positive, HER2+ HER2 positive, TN triple negative, SUVmax maximum standardized uptake value
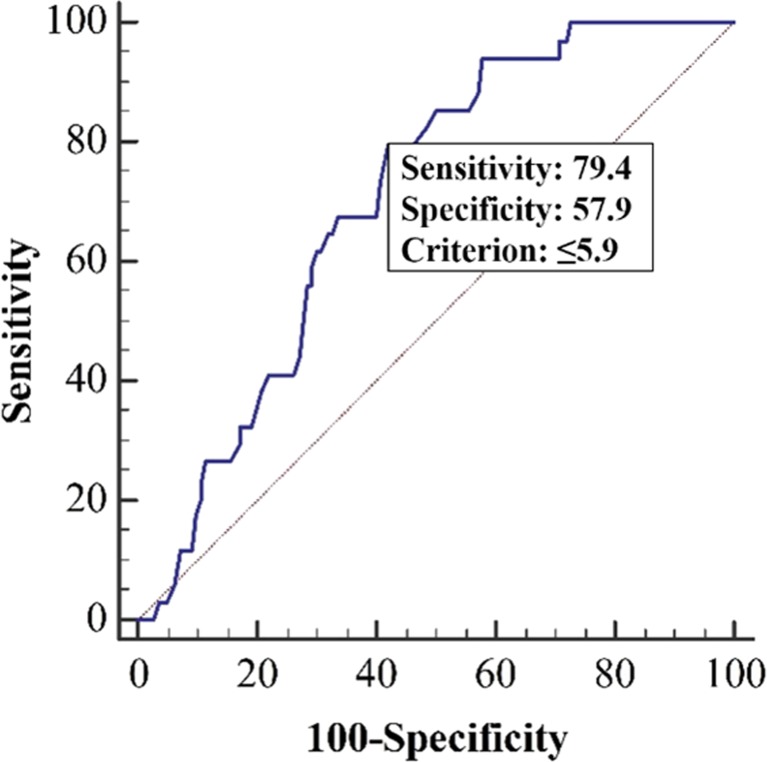
In the ROC curve analysis for differentiation of LA from non-LA subtype, a cut-off SUVmax of 5.9 yielded the highest accuracy (sensitivity of 79.4 %, specificity of 57.9 %, and AUC of 0.704) (Fig. 3).
Fig. 3.
ROC curve of SUVmax in distinguishing LA from non-LA group. The cut-off value of SUVmax was 5.9 (AUC 0.704). LA luminal A-like, SUVmax maximum standardized uptake value, AUC area under curve
Discussion
Our study shows significant correlation between status of hormonal receptors and SUVmax of primary lesion of IDC, and difference of FDG uptake according to molecular subtype based on the recommendation of St. Gallen consensus meeting 2013.
The correlation between the expression of hormone receptor and F-18 FDG uptake has been controversial and unclear. While some studies have shown no correlation between the expression of hormone receptor and F-18 FDG uptake [5–7, 21, 22], some recent studies have revealed that the SUVmax of the ER(−) group is significantly higher than that of the ER(+) group [8, 9, 23]. In corroboration to the results of latter groups, we found the ER(−) group to display significantly higher SUVmax than the ER(+) group (9.4 vs. 6.3, p < 0.001). Laudanski et al. [24] have reported a significant correlation between ER-α and glucose transporter-1 (GLUT-1) in breast cancer. According to them, of the total GLUT-1 positive tumors, 45.3 % were also ER-α positive, whereas 81.3 % of ER-α negative tumors were GLUT-1 positive. In view of the fact that GLUT protein and hexokinase are important factors in F-18 FDG uptake by tumor [25], the relationship between expression of ER and GLUT could be a reason for the SUVmax to be higher in the ER(−) group vis-á-vis the ER(+) group.
In our study, the SUVmax of the PgR(−) group was significantly higher than that of the PgR(+) group (8.9 vs. 6.1, p < 0.001). Contrary to our result, Mavi et al. [23] reported that PgR status alone had no effect on F-18 FDG uptake. This difference might result from the different criteria for the positivity of hormonal receptor in their study. Mavi et al. considered tissues with less than 5 % nuclear labeling as negative. Meanwhile, Prat et al. [26] categorized luminal group by PgR positivity using a cutoff value of 20 % and reported that the PgR g 20 % group showed good prognosis as compared to the PgR < 20 % group. Considering that F-18 FDG uptake is associated with prognosis, the relationship between F-18 FDG uptake and Prat et al.’s classification can be worth evaluating. Our study is, however, based on retrospective medical records, so we cannot focus on this question. We think that further study is needed to determine the correlation between PgR positivity categorized by the 20 % cut-off value and glucose metabolism.
Over-expression of the C-erbB2 oncogene is associated with poor response to tamoxifen and other hormonal therapies, and hence, is known as a poor prognostic factor [27]. While our results revealed that the HER2(+) group has significantly higher SUVmax than the HER2(−) group (8.9 vs. 6.7 p = 0.002), many studies reported that there was no correlation between SUVmax and HER2 positivity [3, 8–10, 22, 23]. It is not clear why our result is different from those of previous studies, but we cautiously suggest that the histopathology of our study population could be one of the reasons for it. Unlike other studies, all patients in our study had IDC. ILC has a low histological grade, low mitotic index, and is generally hormone receptor positive and HER2, p53, and basal marker negative [28–30].
The expression of Ki-67 represents proliferative activity of a malignant tumor, so the histopathologic grade and the expression of Ki-67 in the breast cancer is an important prognostic factor [31, 32], and the correlation between histopathologic grade and Ki-67 is well known [33]. Akin to others [4, 6, 8, 10, 34], our study revealed that primary malignant lesions with higher histopathologic grade and expression of Ki-67 showed significantly higher SUVmax (4.3 vs. 7.5 vs. 9.3; 4.6 vs. 8.7, respectively p < 0.001).
In terms of the difference in FDG uptake among molecular subtypes, our study showed statistical differences between the LA group and every other group. This is in agreement with the observations of Garcia et al. In their study, greatest differences were observed for LA and basal subtype (TNBC). Although our study showed that the LA group had the lowest mean SUVmax among molecular subtypes, the greatest difference was shown for the LA and HER2+ groups. Although Garcia et al. [13] reported discrimination of SUVmax between the LA and TN groups, our results showed substantial overlapping of range of SUVmax among each subtype, even for the LA and HER2+ subtypes. In terms of HER2 expression, this is a predictive marker for treatment with trastuzumab [35], so molecular imaging-aided differentiation between these subtypes can be valuable. But this substantial overlap of SUVmax in each subtype (LA vs. LBHER2+, and LBHER2- vs. LBHER2+) suggests its limited role in clinical application.
In LA group, hormonal therapy is the mainstay of treatment. Unlike non-LA group, cytotoxic therapy is used in only a limited number of patients [2]. Therefore, it is important to distinguish between the LA and non-LA group to decide the treatment plan. Mean SUVmax of LA group was significantly lower than that of non-LA group (4.5 vs. 8.1, p < 0.001), and the optimal cut-off value of SUVmax to distinguish between LA and non-LA group was 5.9 (sensitivity: 79.4 %, specificity: 57.9 %, AUC: 0.704). However, there was a wide overlap in SUVmax between the two groups. So, the use of SUVmax to differentiate between LA and non-LA group may also be limited in individual patient.
Our study may suffer from certain limitations. First, because a diagnostic core needle biopsy was done before F-18 FDG PET/CT in all patients, it is likely that the inflammatory reaction in response to the needle biopsy may affect the F-18 FDG uptake. Most patients, however, underwent F-18 FDG PET/CT at least 1 week after the biopsy procedure, and SUVmax for IDC is usually higher than for minor inflammatory reaction following biopsy. So, we think that the SUVmax of breast lesions represents glucose metabolism of malignant lesions. Second, the correlation between F-18 FDG uptake and molecular subtype was evaluated by using only SUVmax. Further investigations with other parameters such as SUVmean, SUVpeak, or tumor heterogeneity measured on F-18 FDG PET/CT are warranted [36, 37].
Conclusion
SUVmax using F-18 FDG PET/CT could be used as an adjunct method to immunohistochemical analysis for differentiating between molecular subtypes based on recommendation of the St. Gallen consensus meeting 2013 in patients with IDC of the breast. However, application to individual patient may be limited due to wide overlap of the SUVmax of each subtype.
Compliance with Ethical Standards
Conflict of Interest
Sun Seong Lee, Sang Kyun Bae, Yun Soo Park, Ji SUN Park, Tae Hyun Kim, Hye Kyoung Yoon, Hyo Jung Ahn and Seok Mo Lee declare that they have no conflict of interest.
Ethical statement
The study was approved by the Institutional Review Board of Inje University Busan Paik Hospital (IRB No. 15–0050) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Institutional review board waived the need to obtain informed consent for this retrospective study.
References
- 1.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–23. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8. doi: 10.1093/jjco/hyn019. [DOI] [PubMed] [Google Scholar]
- 4.Berriolo-Riedinger A, Touzery C, Riedinger JM, Toubeau M, Coudert B, Arnould L, et al. 18F]FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–24. doi: 10.1007/s00259-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98:267–74. doi: 10.1007/s10549-006-9159-2. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer. 2007;14:260–8. doi: 10.2325/jbcs.14.260. [DOI] [PubMed] [Google Scholar]
- 7.Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 8.Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96:166–70. doi: 10.1002/bjs.6459. [DOI] [PubMed] [Google Scholar]
- 9.Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: Microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50. doi: 10.2967/jnumed.108.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 11.Koo HR, Park JS, Kang KW, Cho N, Chang JM, Bae MS, et al. 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol. 2014;24:610–8. doi: 10.1007/s00330-013-3037-1. [DOI] [PubMed] [Google Scholar]
- 12.Miyake KK, Nakamoto Y, Kanao S, Tanaka S, Sugie T, Mikami Y, et al. Diagnostic value of (18)F-FDG PET/CT and MRI in predicting the clinicopathologic subtypes of invasive breast cancer. AJR Am J Roentgenol. 2014;203:272–9. doi: 10.2214/AJR.13.11971. [DOI] [PubMed] [Google Scholar]
- 13.García Vicente AM, Soriano Castrejón Á, León Martín A, Chacón López-Muñiz I, Muñoz Madero V, Muñoz Sánchez Mdel M, et al. Molecular subtypes of breast cancer: Metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11. [DOI] [PubMed]
- 14.van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–30. doi: 10.1016/j.ctrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Adachi Y, Ishiguro J, Kotani H, Hisada T, Ichikawa M, Gondo N, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. doi: 10.1186/s12885-016-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 18.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 20.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: Recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehdashti F, Mortimer JE, Siegel BA, Griffeth LK, Bonasera TJ, Fusselman MJ, et al. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36:1766–74. [PubMed] [Google Scholar]
- 22.Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 23.Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48:1266–72. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 24.Laudanski P, Koda M, Kozlowski L, Swiatecka J, Wojtukiewicz M, Sulkowski S, et al. Expression of glucose transporter GLUT-1 and estrogen receptors ER-alpha and ER-beta in human breast cancer. Neoplasma. 2004;51:164–8. [PubMed] [Google Scholar]
- 25.Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med. 2004;45:930–2. [PubMed] [Google Scholar]
- 26.Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–9. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–7. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khilko N, Wang J, Wei B, Hicks DG, Tang P. Invasive lobular carcinomas do not express basal cytokeratin markers CK5/6, CK14 and CK17. Breast Cancer (Auckl) 2010;4:49–55. doi: 10.4137/BCBCR.S5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27:49–61. doi: 10.1053/j.semdp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Le Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, Brunet M. Prognostic value of histologic grade nuclear components of scarff-bloom-richardson (SBR). an improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer. 1989;64:1914–21. doi: 10.1002/1097-0142(19891101)64:9<1914::AID-CNCR2820640926>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Denkert C, Loibl S, Muller BM, Eidtmann H, Schmitt WD, Eiermann W, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–93. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 33.Weidner N, Moore DH, 2nd, Vartanian R. Correlation of ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum Pathol. 1994;25:337–42. doi: 10.1016/0046-8177(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 34.Ikenaga N, Otomo N, Toyofuku A, Ueda Y, Toyoda K, Hayashi T, et al. Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg. 2007;73:1151–7. [PubMed] [Google Scholar]
- 35.Duffy MJ. Predictive markers in breast and other cancers: A review. Clin Chem. 2005;51:494–503. doi: 10.1373/clinchem.2004.046227. [DOI] [PubMed] [Google Scholar]
- 36.Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, et al. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0094017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlier T, Bailly C. State-of-the-art and recent advances in quantification for therapeutic follow-up in oncology using PET. Front MED (Lausanne) 2015;2:18. doi: 10.3389/fmed.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]