Abstract
Objective
To evaluate the spontaneous brain activity alterations in liver transplantation (LT) recipients using resting-state functional MRI.
Materials and Methods
Twenty cirrhotic patients as transplant candidates and 25 healthy controls (HCs) were included in this study. All patients repeated the MRI study one month after LT. Amplitude of low-frequency fluctuation (ALFF) values were compared between cirrhotic patients (both pre- and post-LT) and HCs as well as between the pre- and post-LT groups. The relationship between ALFF changes and venous blood ammonia levels and neuropsychological tests were investigated using Pearson's correlation analysis.
Results
In the cirrhotic patients, decreased ALFF in the vision-related regions (left lingual gyrus and calcarine), sensorimotor-related regions (left postcentral gyrus and middle cingulate cortex), and the default-mode network (bilateral precuneus and left inferior parietal lobule) were restored, and the increased ALFF in the temporal and frontal lobe improved in the early period after LT. The ALFF decreases persisted in the right supplementary motor area, inferior parietal lobule, and calcarine. The ALFF changes in the right precuneus were negatively correlated with changes in number connection test-A scores (r = 0.507, p < 0.05).
Conclusion
LT improved spontaneous brain activity and the results for associated cognition tests. However, decreased ALFF in some areas persisted, and new-onset abnormal ALFF were possible, indicating that complete cognitive function recovery may need more time.
Keywords: Liver transplantation, Cirrhosis, Hepatic encephalopathy, Resting state, Functional magnetic resonance imaging, Amplitude of low-frequency fluctuation, Brain activity change
INTRODUCTION
Hepatic encephalopathy (HE), one of the serious complications in patients with end-stage liver cirrhosis, is caused by accumulation in the bloodstream of ammonia and other endogenous substances deriving from hepatic metabolism. The spectrum of the syndrome ranges from psychometric performance alterations (minimal hepatic encephalopathy [MHE]) to stupor and coma (1). Evidence has shown that neurocognitive dysfunction in cirrhotic patients is associated with deterioration in daily functioning, poorer quality of life, and mortality (2). Liver transplantation (LT) is an effective treatment that can improve life quality and prolong survival (3); impaired cognitive function can also be greatly corrected after LT (4,5,6). However, the mechanisms underlying the neurological changes in patients who undergo LT remain largely unclear. By understanding this, better therapeutic methods for cirrhosis-related cognitive dysfunctions may be developed.
Recently, resting-state functional MRI (rs-fMRI) plays an important role in investigating the neural mechanisms of various mental disorders (7,8,9). This approach is relatively economical and easy to implement in clinical studies, and it can also overcome potential limitations in task-based fMRI studies (10). In recent years, some groups have studied the changes in cerebral activity in cirrhotic patients using rs-fMRI (11,12,13,14,15). These studies indicated that the impairments and reorganization of brain function are dynamic processes and that these changes can both exist in every stage of HE from simple cirrhosis (without HE) to MHE and, ultimately, overt HE. Using resting-state functional connectivity, Lin et al. (16) found dynamic disruptions and reconstruction of intrinsic large-scale networks approximately one year after LT accompanied by cognitive deficits and recovery. Moreover, Zhang et al. (17) found improved long- and short-range functional connectivity density one month after LT and persistence of posterior cingulate cortex/precuneus (PCu) functional connectivity disturbance. However, these functional connectivity studies reveal the abnormal brain connections only between two remote regions, not from the perspective of local brain activity. Although aberrant brain connectivity between the two remote regions is integrative and comprehensive, no definite conclusion can be reached about which one is abnormal (8).
To overcome these limitations, amplitude of low-frequency fluctuation (ALFF) (18), a newly developed approach to quantitatively measure the amplitude of spontaneous brain activity, is recommended. This method can provide us with regional spontaneous brain activity information, which is important for completely understanding a disease. Thus, the goals of this study were to 1) assess local brain activity alterations after LT using ALFF and 2) examine the relationship between ALFF alterations and the changes in the neuropsychological test scores following LT.
MATERIALS AND METHODS
Subjects
We recruited a consecutive series of cirrhotic patients who were scheduled for LT from December 2013 to October 2015. The diagnosis of liver cirrhosis was made by clinical history and imaging (abdominal computed tomography and ultrasound findings). We excluded subjects if they had: 1) history of drug abuse; 2) psychiatric or neurologic illness; 3) any serious complications after LT such as rejection, severe biliary stenosis, liver failure, or any central nerve system complications; or 4) poor image quality with a head motion more than 2.0 mm/degree. In all, we ultimately included 20 cirrhotic patients (13 men and 7 women; mean age: 51.9 ± 6.9 years) who received successful LT operations in our hospital. Among them, 13 patients had been previously included in another prospective study of ours that was already published (17). All patients underwent MRI scan both before and one month after LT. The etiologies of the liver cirrhosis included type C hepatitis, type B hepatitis, cryptogenic cirrhosis, and primary biliary cirrhosis (n = 8, 7, 3, 2 for each). All patients received laboratory tests, including prothrombin time and total bilirubin, albumin, and venous blood ammonia values, within one week before the MRI scans; we also used Child-Pugh classification to grade hepatic function. We recruited 25 healthy controls (HCs; 18 men and 7 women; mean age: 49.6 ± 8.3 years) who were frequency matched in age, gender, and education using advertisements within our hospital. The control subjects had no history of neurologic, psychiatric, or traumatic diseases that could have affected brain function. All controls also had no liver or other systemic diseases.
This study was approved by the Ethics Committee of Tianjin First Central Hospital, and we conducted all experiments in compliance with relevant guidelines and regulations. All participants provided written informed consent prior to the study.
Cognitive Assessment
The psychometric test battery comprised the number connection test-A (NCT-A) and digit-symbol test (DST) (19), which we used to evaluate the cognitive impairment in cirrhotic patients; we performed these tests for all subjects just before their MRI scans. The NCT-A tests psychomotor function, whereas the DST tests attention and processing speed. Lower scores are considered to reflect poor cognitive abilities (1). Cirrhotic patients were diagnosed as MHE if they showed no symptoms of overt encephalopathy but their scores were abnormal on at least one neuropsychological test (beyond 2 standard deviations of the mean value for the HCs; 19).
MRI Data Acquisition
We acquired the MRI data using a clinical 3 Tesla whole body MR imager (TIM-Trio; Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil; the head coil was fitted with foam padding and headphones to minimize head motion and reduce scanner noise. During scanning, each subject was asked to hold the head still, relax with eyes closed, and not to think of anything in particular. rs-fMRI images were obtained using a rapid gradient echo-planar sequence (200 volumes; repetition time = 2500 ms; echo time = 30 ms; field of view = 220 × 220 mm2; flip angle = 90°; section thickness = 3 mm; acquisition matrix = 96 × 96; a total of 40 slices covering the whole brain).
Data Preprocessing
We conducted functional imaging data analysis using the Data Processing Assistant for Resting-State fMRI software (http://www.restfmri.net/forum/DPARSF) toolbox. Briefly, we discarded the first 10 time points for each subject due to the signal stabilization and subject adaptation to the scanning noise. The remaining 190 time points were left for correcting the acquisition delays between slices and the head motion. We excluded data from subjects with head motion of more than 2.0 mm translation in any direction and more than 2.0° rotation in each axis.
Subsequently, we spatially normalized the realigned images according to the standard Montreal Neurological Institute template and resampled them into a voxel size of 3 × 3 × 3mm3. After this, we performed spatial smoothing by means of convolution with a Gaussian kernel of 4 mm.
ALFF Calculation
Before we calculated ALFF, in order to reduce the effects of very-low-frequency drift and very-high-frequency noises, we removed the linear trends and performed temporal filtering (band-pass, 0.01–0.08 Hz). Then, using a fast Fourier transformation, we converted the time course for each voxel into the frequency domain. Then, each frequency of the power spectrum was square root transformed and averaged across 0.01–0.08 Hz, and we took the averaged square root as the ALFF measurement. For standardization purposes, we divided the ALFF of each given voxel by the global mean ALFF of the whole brain.
Statistics Analysis
We used SPSS (version 17.0; SPSS Inc., Chicago, IL, USA) to analyze the demographic data and SPM8 for the rs-fMRI data. We conducted two-sample t tests to assess the differences in age, education level, and clinical scores. We also used two-tailed chi-square tests examine the differences by gender between the patients and the HCs. We also performed paired t tests to evaluate the changes in clinical scores after LT, second-level random-effect two-sample t tests to characterize the ALFF differences between the cirrhotic patients (pre- and post-LT) and HCs, and paired t tests to compare the differences between pre- and post-LT patients. We considered age, sex, and education years as no-interest covariates, and corrected the results using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf); an overall false positive p < 0.05 was achieved by combining an individual voxel threshold of p < 0.01 and a minimum cluster volume threshold of 729 mm3.
Furthermore, we selected the regions that showed significantly altered ALFF in pre- and post-LT comparisons as a mask and extracted the mean ALFFs for each patient in these masks. We calculated the ΔALFF, Δammonia, and ΔNCT-A/ΔDST, which reflected the changes in ALFF and the ammonia and neuropsychological tests before and after LT. Subsequently, we used Pearson's correlation analysis to study the relationship between ΔALFF in these brain regions and Δammonia or ΔNCT-A/ΔDST. The statistical threshold for significant difference was p < 0.05 for these analyses.
RESULTS
Demographics and Clinical Data
The demographic and clinical data of the cirrhotic patients and HCs are shown in Table 1. There were no significant differences in the data including age, gender, and education years between the cirrhotic patients and HCs (all p > 0.05). The Child-Pugh classifications revealed 17 patients as class C and 3 patients as class B. Thirteen patients had histories of overt HE episodes, 5 patients with abnormal neuropsychological results were diagnosed as MHE, and the remaining 2 patients with normal neuropsychological results were diagnosed as non-HE.
Table 1. Demographics and Clinical Data of Pre- and Post-LT Groups and HCs.
| Parameter | HCs (n = 25) | Pre-LT (n = 20) | Post-LT (n = 20) | P | ||
|---|---|---|---|---|---|---|
| Pre-LT/HCs | Post-LT/HCs | Post-/Pre-LT | ||||
| Sex (M/F) | 18/7 | 13/7 | 13/7 | 0.614* | 0.614* | - |
| Age (years) | 49.6 ± 8.3 | 51.9 ± 6.9 | 51.9 ± 6.9 | 0.316† | 0.316† | - |
| Education (years) | 12.6 ± 3.0 | 12.1 ± 4.1 | 12.1 ± 4.1 | 0.642† | 0.642† | - |
| NCT-A (seconds) | 44.1 ± 10.8 | 78.6 ± 33.8 | 59.2 ± 30.8 | 0.000† | 0.028† | 0.000‡ |
| DST (score) | 48.6 ± 10.4 | 27.8 ± 12.8 | 35.0 ± 14.0 | 0.000† | 0.001† | 0.000‡ |
| Biochemical parameters | ||||||
| Prothrombin time (seconds) | - | 17.9 ± 4.3 | 12.1 ± 2.6 | - | - | 0.000‡ |
| Albumin (mg/dL) | - | 29.6 ± 6.2 | 38.0 ± 5.8 | - | - | 0.000‡ |
| Total bilirubin (mg/dL) | - | 68.6 ± 54.9 | 21.7 ± 13.2 | - | - | 0.001‡ |
| Venous ammonia (μmol/L) | - | 71.6 ± 29.2 | 42.2 ± 13.8 | - | - | 0.000‡ |
| Child-Pugh B/C | - | 3/17 | - | - | - | - |
| No-HE/MHE/HE | - | 2/5/13 | - | - | - | - |
*Chi-square test, †Two-sample t test, ‡Paired two-sample t test. DST = digit-symbol test, HCs = healthy controls, HE = hepatic encephalopathy, LT = liver transplantation, MHE = minimal hepatic encephalopathy, NCT-A = number connection test-A
The cirrhotic patients performed worse on cognitive tests than did the HCs before LT (p < 0.01). They needed more time for the NCT-A and got lower DST scores. After LT, both the NCT-A and DST performances improved (p < 0.01), although they were still worse the HCs' scores (p < 0.05). The prothrombin time and albumin, total bilirubin, and venous ammonia concentrations of the pre-LT patients were abnormal. After LT, all the test indexes improved significantly (All p < 0.005).
Group Differences in ALFF
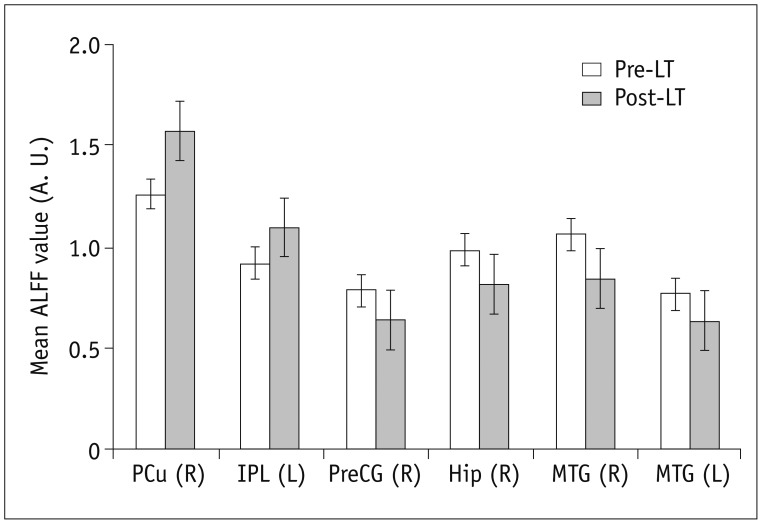
The ALFF differences between pre- and post-LT group and the HCs and between the post- and pre-LT groups are displayed in Tables 2,3,4 and Figures 1,2,3. Compared with the HCs, the pre-LT patients showed significantly decreased ALFF in the bilateral calcarine, inferior parietal lobe (IPL), PCu, left lingual gyrus, postcentral gyrus (PoCG), middle cingulate cortex, and right supplementary motor area (SMA) and increased ALFF in the bilateral lateral temporal cortex, parahippocampal gyrus (PHG), right hippocampus (Hip), and superior frontal gyrus (SFG). The post-LT patients displayed ALFF decreases in the right IPL, calcarine, and SMA and increases in the right SFG, inferior frontal gyrus (IFG), left PHG, and left middle frontal gyrus (MFG). Interestingly, compared with the pre-LT group, the post-LT patients demonstrated significant ALFF increases in the left IPL and right Pcu and ALFF decreases in the bilateral middle temporal gyrus, right precentral gyrus (PreCG), and Hip (Fig. 4).
Table 2. Differences of ALFF between Pre-LT Patients and HCs.
| Brain Regions | Brodmann's Area | MNI Coordinates | Cluster Size | Peak t Value |
|---|---|---|---|---|
| Left LG | 19 | −15, −45, −9 | 28 | −3.74 |
| Right calcarine | 18 | 24, −60, 18 | 77 | −3.65 |
| Left calcarine | 18 | −18, −60, 12 | 53 | −4.04 |
| Left PoCG | 2/3 | −63, −15, 12 | 82 | −4.90 |
| Right PCu | 7 | 12, −76, 44 | 48 | −4.21 |
| Left PCu | 7 | −6, −66, 48 | 334 | −5.93 |
| Right MCC | 24 | 3, 21, 30 | 73 | −4.53 |
| Right IPL | 40 | 48, −48, 54 | 124 | −4.30 |
| Left IPL | 40 | −45, −45, 51 | 108 | −4.20 |
| Right SMA | 6 | −3, 12, 51 | 201 | −4.75 |
| Right LTC | 21/22 | 46, 11, −25 | 215 | 5.79 |
| Left LTC | 21/22 | −45, 0, −24 | 218 | 5.51 |
| Right Hip | 20 | 25, −4, −18 | 47 | 3.78 |
| Right PHG | 36 | 30, −6, −36 | 28 | 3.29 |
| Left PHG | 36 | −21, −6, −30 | 86 | 4.69 |
| Right SFG | 10 | 15, 60, 3 | 27 | 3.61 |
p<0.05, AlphaSim corrected. Negative t value represents decrease, and positive t value represents increase. ALFF = amplitude of low-frequency fluctuation, HCs = healthy controls, Hip = hippocampus, IPL = inferior parietal lobule, LG = lingual gyrus, LT = liver transplantation, LTC = lateral temporal cortex, MCC = middle cingulate cortex, MNI = Montreal Neurological Institute, PCu = precuneus, PHG = parahippocampal gyrus, PoCG = postcentral gyrus, SFG = superior frontal gyrus, SMA = supplementary motor area
Table 3. Differences of ALFF between Post-LT Patients and HCs.
| Brain Regions | Brodmann’s Area | MNI Coordinates | Cluster Size | Peak t Value |
|---|---|---|---|---|
| Right calcarine | 18 | 24, −60, 18 | 27 | −3.41 |
| Right IPL | 40 | 48, −51, 54 | 30 | −3.74 |
| Right SMA | 6 | 3, −18, 60 | 56 | −3.51 |
| Left PHG | 36 | −18, 0, −33 | 28 | 3.95 |
| Left MFG | 10/46 | −42, 42, 18 | 33 | 4.39 |
| Right SFG | 10 | 18, 57, 0 | 72 | 3.70 |
| Right IFG | 9 | 51, 33, 6 | 37 | 3.86 |
p< 0.05, AlphaSim corrected. Negative t value represents decrease, and positive t value represents increase. ALFF = amplitude of low-frequency fluctuation, HCs = healthy controls, IFG = inferior frontal gyrus, IPL = inferior parietal lobule, LT = liver transplantation, MFG = middle frontal gyrus, MNI = Montreal Neurological Institute, PHG = parahippocampal gyrus, SFG = superior frontal gyrus, SMA = supplementary motor area
Table 4. Differences of ALFF between Post- and Pre-LT Patients.
| Brain Regions | Brodmann's Area | MNI Coordinates | Cluster Size | Peak t Value |
|---|---|---|---|---|
| Right MTG | 21 | 56, 2, −25 | 66 | −4,85 |
| Left MTG | 21 | −60, −15, −21 | 73 | −4.75 |
| Right Hip | 20 | 33, −6, −18 | 46 | −5.66 |
| Right PreCG | 4 | 30, −18, 51 | 45 | −4.98 |
| Left IPL | 40 | −45, −45, 48 | 28 | 4.61 |
| Right PCu | 7 | 12, −72, 45 | 30 | 4.15 |
p< 0.05, AlphaSim corrected. Negative t value represents decrease, and positive t value represents increase. ALFF = amplitude of low-frequency fluctuation, Hip = hippocampus, IPL = inferior parietal lobule, LT = liver transplantation, MNI = Montreal Neurological Institute, MTG = middle temporal gyrus, PCu = precuneus, PreCG = precentral gyrus
Fig. 1. ALFF maps show differences between pre-LT patients and HCs (p < 0.05, corrected by AlphaSim).
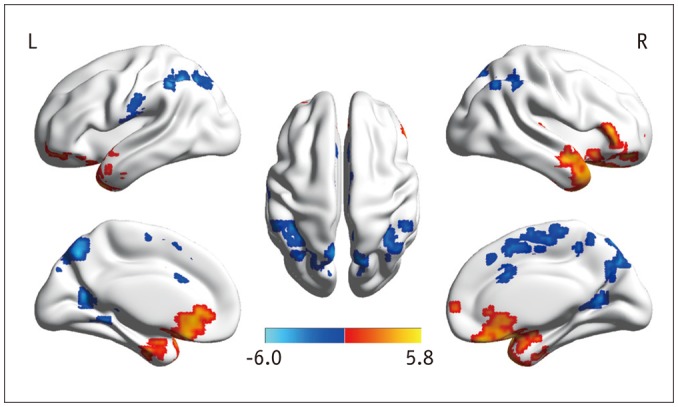
Diffused ALFF decreases in bilateral calcarine, IPL, LG, PCu, PoCG, MCC, and right SMA and increases in bilateral LTC, PHG, right Hip, and SFG are observed in pre-LT patients relative to controls. ALFF = amplitude of low-frequency fluctuation, HCs = healthy controls, Hip = hippocampus, IPL = inferior parietal lobule, LG = lingual gyrus, LT = liver transplantation, LTC = lateral temporal cortex, MCC = middle cingulate cortex, PCu = precuneus, PHG = parahippocampal gyrus, PoCG = postcentral gyrus, SFG = superior frontal gyrus, SMA = supplementary motor area
Fig. 2. ALFF maps show differences between post-LT patients and HCs (p < 0.05, corrected by AlphaSim).
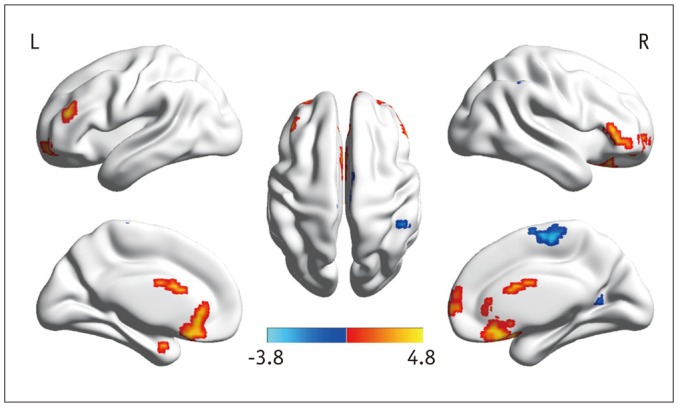
ALFF decreases in right IPL, calcarine, and SMA and increases in right SFG, IFG, left PHG, and MFG are detected in post-LT patients. ALFF = amplitude of low-frequency fluctuation, HCs = healthy controls, IFG = inferior frontal gyrus, IPL = inferior parietal lobule, LT = liver transplantation, MFG = middle frontal gyrus, PHG = parahippocampal gyrus, SFG = superior frontal gyrus, SMA = supplementary motor area
Fig. 3. ALFF maps show differences between post- and pre-LT patients (p < 0.05, corrected by AlphaSim).
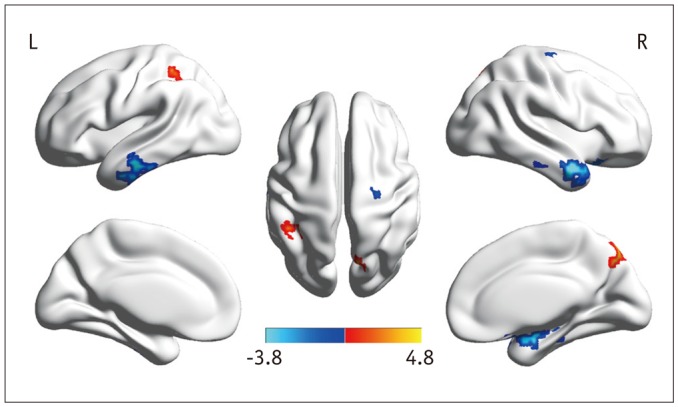
Paired t tests reveal ALFF increases in left IPL and right PCu and decreases in bilateral MTG, right PreCG, and Hip after LT. ALFF = amplitude of low-frequency fluctuation, Hip = hippocampus, IPL = inferior parietal lobule, LT = liver transplantation, MTG = middle temporal gyrus, PCu = precuneus, PreCG = precentral gyrus
Fig. 4. Six brain regions were altered significantly in post-LT patients (p < 0.05, corrected by AlphaSim).
A. U. = arbitrary unit, ALFF = amplitude of low-frequency fluctuation, Hip = hippocampus, IPL = inferior parietal lobule, LT = liver transplantation, MTG = middle temporal gyrus, PCu = precuneus, PreCG = precentral gyrus
Figure 5 illustrates the Pearson's correlation analysis results between the clinical data and the brain regions with altered ALFF, which revealed negative correlations between ΔALFF in the right PCu and ΔNCT-A (r = 0.507, p < 0.05). There were no correlations between ΔALFF and ΔDST or Δammonia (p > 0.05).
Fig. 5. Correlations between brain regions with altered ALFF and neuropsychological tests.
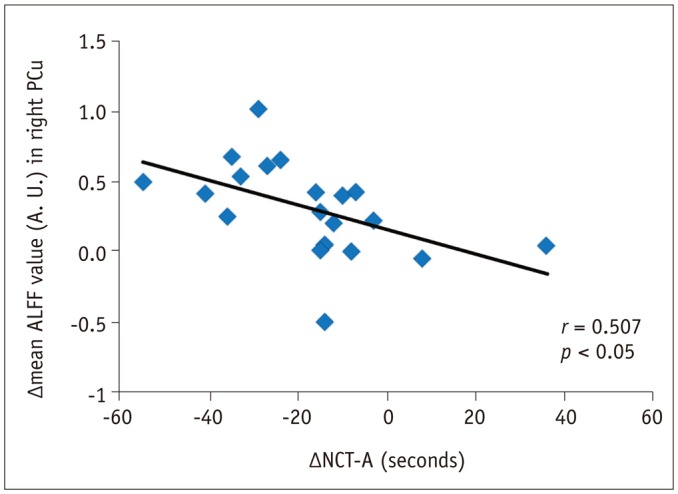
ΔALFF in right PCu was negatively correlated with ΔNCT-A (r = 0.507, p < 0.05). Δrepresents differences before and after LT. A. U. = arbitrary unit, ALFF = amplitude of low-frequency fluctuation, LT = liver transplantation, NCT-A = number connection test-A, PCu = precuneus
DISCUSSION
In this rs-fMRI study, we found that most of the brain regions with altered ALFF were restored one month after LT, whereas the reduced ALFF in some regions such as the right SMA, IPL, and calcarine persisted one month after LT. Furthermore, there were new-onset ALFF decreases in the right PreCG and increases in the left MFG and right IFG one month after LT. These findings suggest that partial renormalization of spontaneous brain activity and complete cognitive function recovery may need more time. The ALFF had practical value in detecting the brain changes after LT.
Although the exact biologic mechanisms of ALFF are still unclear, many studies have suggested that altered ALFF is associated with abnormal regional neuronal activity (13,20). Many fMRI algorithms have been developed such as independent component analysis, seed correlation analysis, and regional homogeneity (14,18,21). Different analysis methods can reflect different aspects of integrated human brain function; compared with other methods, the strength of ALFF lies in its directly reflecting the amplitude of spontaneous brain activity (13). ALFF has been used widely in studies of neuropsychological diseases including HE (13,22,23). In the present study, abnormal ALFF in pre-LT patients indicated neural function impairment in specific brain areas, consistent with previous studies (22,23). In addition, ALFF algorithms have also been used in detecting the brain functional alterations of cirrhotic patients after treatment, such as transjugular intrahepatic portosystemic shunt (24). Thus, ALFF is potentially valuable for uncovering the mechanism of how transplantation affects the brain function in the early postoperative period.
Our study found that one month after LT, most of the brain regions with decreased ALFF before LT had reversed. The lingual gyrus and calcarine, the key components of the visual cortex, are the vision center in the human brain. These areas play important roles in visual attention, visual discrimination, and color perception (25). As the region of the primary somatosensory cortex, the PoCG initially participates in processing information relevant to tactile awareness (23). The normalization of ALFF in these vision-related and sensorimotor-related regions in post-LT patients may contribute greatly to improving visual information processing and motor control. The PCu is an important part of the default mode network (DMN) and is primarily recruited for highly integrated work including episodic memory retrieval, visuo-spatial attention, and self-processing operations (26). The IPL is a major posterior component of the DMN that may participate in memory function (27). The restoration of ALFF values in these regions may be at least in part involved in the improved cognitive function. Additionally, prior to transplant, some brain areas (mainly located in the frontal and temporal lobes) demonstrated increased ALFF compared with the HCs, which was regarded as a functional process to compensate for the reduced ALFF in the parietal and occipital lobes (12). After LT, these regions were also back to normal or improved. These findings suggest that LT can improve brain function in the early post-LT period. Additionally, the changes in brain activity were accompanied by significant improvement in cognitive function. After LT, both the neuropsychological tests improved obviously, and we also identified a correlation between ΔALFF in the right PCu with the improved neuropsychological test scores (ΔNCT-A). The ALFF changes in this area may be used as a marker to reflect the degree of cognitive recovery in cirrhotic patients pre- and post-LT.
An important finding in this study was the irreversibility of abnormal brain activity in some regions, for instance, the right SMA. SMA plays a crucial role in movement planning and initiation, especially self-initiated movements (28). Although the neurotoxic factors reduced clearly after LT, brain cellular damage caused by previous metabolic dysfunction can only be partially fixed. Garcia-Martinez et al. (29) reported that post-LT neurologic outcomes were associated with HE episodes prior to LT. Previous studies have also shown a degree of irreversibility of neurological change even after LT (4,30,31). In the present study, the results of neuropsychological tests for post-LT patients improved more clearly than did those of the pre-LT, although they were still worse than those of the HCs. Thus, the present results are consistent with previous findings.
Interestingly, we found reduced ALFF in the right PreCG in the post-LT group in comparison with the pre-LT group. However, compared with the HCs, neither the pre- nor post-LT patients showed abnormal ALFF in this region. The PreCG is intimately related to motor control (23); thus, we speculate that the reduced ALFF in the PreCG might imply new-onset motor function impairment, which might be explained by dynamic reorganization under the effect of LT; more research is needed to identify the exact mechanisms. The reasons for this alteration might be mixed, including at least the influence of the operation and the immunosuppressive agents taken after LT.
The current study has a number of limitations. First, our results were derived from a relatively small sample size, which may have affected the statistical results. Second, we did not completely exclude the effects of pre-LT HE episodes, and thus additional studies should be considered. Third, the potential effects of the specific type of cirrhosis on brain function could not be excluded. Fourth, only the patients underwent neuropsychological test twice; therefore, improved performance might be at least partly attributable to learning effects. In a future study, we will perform the neuropsychological test in controls with the same time interval to correct this confounding factor. Finally, we followed up at only one time point with the post-LT patients, and thus longer-term and multiple time point follow-ups may be more valuable for investigating the recovery process. Our future study will focus on this domain.
In summary, we found that decreased intrinsic brain activity in the vision-related and motor-related regions and the DMN can be reversed one month after LT, indicating that LT can improve brain function. However, the reversibility is not complete in that abnormality persists in some brain regions in post-LT patients, such as in the SMA. rs-fMRI with the ALFF algorithm had practical value in detecting the brain function change after LT.
Acknowledgments
We acknowledge Dr. Tian-Yi Qian from Siemens Ltd. China for his help in data analysis.
Footnotes
This work was supported by National Natural Science Foundation of China (No. 81601482 to Y. C. and No. 61503278 to G. Y. Z)
References
- 1.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–2021. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 2.Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030–1040. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412–421. doi: 10.1136/gut.2009.179937. [DOI] [PubMed] [Google Scholar]
- 4.Mechtcheriakov S, Graziadei IW, Mattedi M, Bodner T, Kugener A, Hinterhuber HH, et al. Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl. 2004;10:77–83. doi: 10.1002/lt.20009. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz M, Córdoba J, Jacas C, Flavià M, Esteban R, Guardia J. Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol. 2006;44:104–110. doi: 10.1016/j.jhep.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Pujol A, Graus F, Rimola A, Beltrán J, Garcia-Valdecasas JC, Navasa M, et al. Predictive factors of in-hospital CNS complications following liver transplantation. Neurology. 1994;44:1226–1230. doi: 10.1212/wnl.44.7.1226. [DOI] [PubMed] [Google Scholar]
- 7.Guo WB, Liu F, Xue ZM, Yu Y, Ma CQ, Tan CL, et al. Abnormal neural activities in first-episode, treatment-naïve, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2011;135:326–331. doi: 10.1016/j.jad.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Hu M, Wang S, Guo W, Zhao J, Li J, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:326–331. doi: 10.1016/j.pnpbp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013;70:845–851. doi: 10.1001/jamaneurol.2013.38. [DOI] [PubMed] [Google Scholar]
- 10.Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272:29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- 11.Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Wu X, et al. Brain regional homogeneity changes following transjugular intrahepatic portosystemic shunt in cirrhotic patients support cerebral adaptability theory--a resting-state functional MRI study. Eur J Radiol. 2014;83:578–583. doi: 10.1016/j.ejrad.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Zhang Z, et al. Altered regional homogeneity in the development of minimal hepatic encephalopathy: a resting-state functional MRI study. PLoS One. 2012;7:e42016. doi: 10.1371/journal.pone.0042016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi R, Zhang L, Wu S, Zhong J, Zhang Z, Zhong Y, et al. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology. 2012;264:187–195. doi: 10.1148/radiol.12111429. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Qi R, Wu S, Zhong J, Zhong Y, Zhang Z, et al. Brain default-mode network abnormalities in hepatic encephalopathy: a resting-state functional MRI study. Hum Brain Mapp. 2012;33:1384–1392. doi: 10.1002/hbm.21295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LJ, Zheng G, Zhang L, Zhong J, Wu S, Qi R, et al. Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology. 2012;265:528–536. doi: 10.1148/radiol.12120185. [DOI] [PubMed] [Google Scholar]
- 16.Lin WC, Hsu TW, Chen CL, Lu CH, Chen HL, Cheng YF, et al. Reestablishing brain networks in patients without overt hepatic encephalopathy after liver transplantation. J Cereb Blood Flow Metab. 2014;34:1877–1886. doi: 10.1038/jcbfm.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XD, Cheng Y, Poon CS, Qi R, Xu Q, Chen HJ, et al. Longand short-range functional connectivity density alteration in non-alcoholic cirrhotic patients one month after liver transplantation: a resting-state fMRI study. Brain Res. 2015;1620:177–187. doi: 10.1016/j.brainres.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010;31:1851–1861. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Chen HJ, Zhu XQ, Jiao Y, Li PC, Wang Y, Teng GJ. Abnormal baseline brain activity in low-grade hepatic encephalopathy: a resting-state fMRI study. J Neurol Sci. 2012;318:140–145. doi: 10.1016/j.jns.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Lv XF, Ye M, Han LJ, Zhang XL, Cai PQ, Jiang GH, et al. Abnormal baseline brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy revealed by resting-state functional MRI. Metab Brain Dis. 2013;28:485–492. doi: 10.1007/s11011-013-9420-4. [DOI] [PubMed] [Google Scholar]
- 24.Qi R, Zhang LJ, Zhong J, Wu S, Zhang Z, Zhong Y, et al. Dynamic changes of intrinsic brain activity in cirrhotic patients after transjugular intrahepatic portosystemic shunt: a resting-state fMRI study. PLoS One. 2012;7:e46681. doi: 10.1371/journal.pone.0046681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HW, Hong SB, Seo DW, Tae WS, Hong SC. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000;54:849–854. doi: 10.1212/wnl.54.4.849. [DOI] [PubMed] [Google Scholar]
- 26.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 27.Luo S, Qi RF, Wen JQ, Zhong JH, Kong X, Liang X, et al. Abnormal intrinsic brain activity patterns in patients with end-stage renal disease undergoing peritoneal dialysis: a resting-state functional MR imaging study. Radiology. 2016;278:181–189. doi: 10.1148/radiol.2015141913. [DOI] [PubMed] [Google Scholar]
- 28.Joebges EM, Heidemann M, Schimke N, Hecker H, Ennen JC, Weissenborn K. Bradykinesia in minimal hepatic encephalopathy is due to disturbances in movement initiation. J Hepatol. 2003;38:273–280. doi: 10.1016/s0168-8278(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simón-Talero M, Chavarria L, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17:38–46. doi: 10.1002/lt.22197. [DOI] [PubMed] [Google Scholar]
- 30.Lin WC, Chou KH, Chen CL, Chen HL, Lu CH, Li SH, et al. Longitudinal brain white matter alterations in minimal hepatic encephalopathy before and after liver transplantation. PLoS One. 2014;9:e105887. doi: 10.1371/journal.pone.0105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattarozzi K, Cretella L, Guarino M, Stracciari A. Minimal hepatic encephalopathy: follow-up 10 years after successful liver transplantation. Transplantation. 2012;93:639–643. doi: 10.1097/TP.0b013e318244f734. [DOI] [PubMed] [Google Scholar]