Abstract
AIM
To investigate the intraocular pressure (IOP) of adult guinea pig eyes with rebound tonometry (RBT), and assess the effects of four distinctive topical IOP reducing medications including Carteolol, Brimonidine, Brinzolamide and Latanoprost.
METHODS
The IOPs of twenty-four 12-week-old guinea pigs (48 eyes) were measured every two hours in one day with RBT as baselines. All the animals were then divided into four groups (Carteolol, Brimonidine, Brinzolamide and Latanaprost groups, n=6). The IOPs were measured and compared to the baseline 1, 2, 3, 5, 7, 9, 15 and 24h after treatment.
RESULTS
The mean baseline IOP of 24 guinea pigs (48 eyes) was 10.3±0.36 mm Hg (6-13 mm Hg) and no binocular significant differences of IOPs were observed (t=1.76, P>0.05). No significant difference of IOP in Carteolol group at each time point was observed before and after treatment (t=1.48, P>0.05). In Brimonidine group, IOP was 2.2±1.9 mm Hg lower than the baseline after one hour (t=3.856, P=0.003) and lasted for one hour. In Brinzolamide group, IOP was 1.4±1.1 mm Hg lower than the baseline after one hour (t=4.53, P=0.001) and lasted for 7h and the IOP declined most at 3h. In Latanaprost group, IOP was 2.1±1.3 mm Hg lower than the baseline after one hour (t=6.11, P=0.001) and lasted for one hour.
CONCLUSION
The IOP of guinea pig eyes is relatively stable compared to human eyes. In four reducing IOP medications, no significant effect of Carteolol is observed. Brinzolamide has the longest duration, while the Brimonidine has the shortest duration and the maximum level of treatment.
Keywords: guinea pig, intraocular pressure, Carteolol, Brinzolamide, Brimonidine, Latanoprost
INTRODUCTION
Glaucoma is an optic neuropathy characterized by optic disk cupping and visual field loss[1]. The mechanism of raising intraocular pressure (IOP) in glaucoma is impaired resulting from abnormalities within the drainage system of the anterior chamber angle or impaired access of aqueous to the drainage system[2]. Treatment is directed toward reducing the IOP and correcting the underlying cause[3]. Until now, the experimental animals in glaucoma research are rabbit[4], monkey[5]–[6] and cat etc[7]. However, such animals all have the limitations of long growth cycle, difficult to acquire, or much expensive price. In recent years, the guinea pigs were introduced as a new animal model. Similar to the other rodents, guinea pigs owned the advantages including rapid growth, inexpensive price, breeding readily in captivity and being easily available[8]–[10]. However, the relationship between IOP and guinea pigs had not been adequately studied.
There is no known cure for glaucoma, but many forms of the disease are associated with pathological elevation of IOP, and elevated IOP is the most important risk factor associated with progression of the disease[11]. Tonometry had evolved greatly over decades. Some of which had proved useful for IOP assessment not only in human patients, but also in animal subjects for research purposes[11]–[12]. However, the tonometry which was used in humans is not appropriate to measure IOP like guinea pigs because the small eyeball size. The common tonometry which was used in rodent is Tonopen. Such an instrument is sometimes difficult to measure and susceptible to be influenced by manipulator[13]. In contrast, the rebound tonometry (RBT) has the obvious advantage to measure the IOP of small animals[14]–[15]. The device is simple, easy to use and portable, does not require eye drops and is particularly suitable for children and non-cooperative patients[16].
Until now, there are four kinds of glaucoma therapy drops which are commonly used including beta-adrenergic blocking agents, carbonic anhydrase inhibitors, alpha-adrenergic agonist and prostaglandin analogs. The mechanisms of these four reducing IOP medications are quite different, and therefore, more work is needed to make clear the effect of topical reducing IOP eye drops in guinea pigs eyes.
Therefore, in the current research, RBT was used to obtain the baseline IOP of guinea pigs. The effects in small animal eyes of four topical reducing IOP eye drops were investigated. We hoped to be able to gain more knowledge of using guinea pigs in glaucoma research.
MATERIALS AND METHODS
Animals
Twenty-four guinea pigs (English short hair stock, tricolor strain, 12 weeks of age) were obtained from the laboratory of Fudan University. The treatment and care of the animals were conducted according to the ARVO statement for the use of animals in Ophthalmic and Vision Research, and the animal research was approved by the Local Animal Care and Ethics Committee of Fudan University (Shanghai, China).
Experiment Instruments and Reagents
RBT (SW-500), which determined IOP by bouncing a tiny plastic tipped metal probe against the cornea was used in the current research. The device used an induction coil to magnetise the probe and fired it against the cornea. As the probe bounced against the cornea and back into the device, it created an induction current from which the IOP was calculated.
Finnpipette (labsystems, Inc.), 2% Carteolol eye drops (Mikelan, C14200168681), 0.15% Brimonidine eye drops (Alphagan, C1420060292), 1% Brinzolamide eye drops (Azopt, 014200220527), 0.005% Latanaprost eye drops (Xalatan, B01008361) were used in study.
Experimental Design
All measurements were performed by one researcher who was masked to the identity of the experimental groups. The guinea pig was placed on the table and kept quiet. Three repeated measurements were performed in the horizontal meridian. Animal were anaesthetised with one drop of 0.5% proparacaine hydrochloride (Alcon).
Baseline Intraocular Pressure Measurement
The IOPs of twenty-four guinea pigs (48 eyes) were measured every 2h at the beginning of 0:00, IOP of the whole days was recorded at each time point.
Intraocular Pressure Measurements of Four Treatment Groups
The IOP of four treatment groups were measured one week after the baseline measurements. All the guinea pigs were divided into four groups (Carteolol, Brimonidine, Brinzolamide and Latanaprost groups, n=6). All the animals were given the respective treatment 50 µL every drop with Finnpipette. The IOPs were measured 1, 2, 3, 5, 7, 9, 15 and 24h after treatment. In order to compare to the baseline, all the guinea pigs were raised without any treatment for one week, and the baseline at each specified time points were measured again.
Statistical Analysis
The raw data were processed using SPSS 20.0. The graph was created by Graphpad 5.0 software. The results were presented as means±standard deviation. Paired t-test was used to compare the difference between two eyes in one animal. The data at each time point were analyzed with analysis of variance (ANOVA). Paired t-test was also used to compare the difference before and after treatment in one animal. ANOVA was also applied to test the difference of IOP at each time point in one group. P<0.05 was considered significantly different.
RESULTS
Baseline Intraocular Pressure
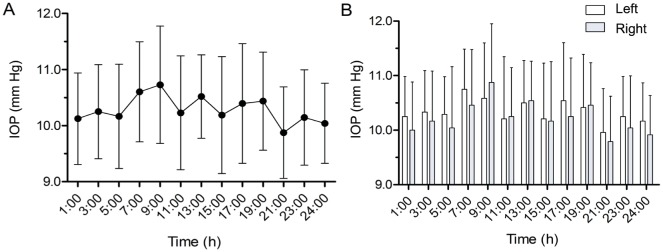
The mean twenty-four hours IOP of the whole 24 guinea pigs (48 eyes) was 10.3±0.36 mm Hg, which followed a normal distribution. No significant difference in IOP was found in 24 guinea pigs throughout 24h (ANOVA, F=3.49, P=0.76; Figure1A). The minimum and maximum values in one day were 9.88±0.82 mm Hg and 10.73±1.05 mm Hg relatively. The amplitude of IOP was 0.85±1.32 mm Hg. Meanwhile, no binocular difference in IOP was observed at each time point (Figure 1B).
Figure 1. Baseline IOP.
A: The baseline IOPs of adult guinea pigs at each time point during 24h. The minimum and maximum IOP values in one day appear at 21:00 and 9:00 relatively. B: No binocular difference of IOPs was observed at each time point, P>0.05.
Intraocular Pressure of Four Treatment Groups
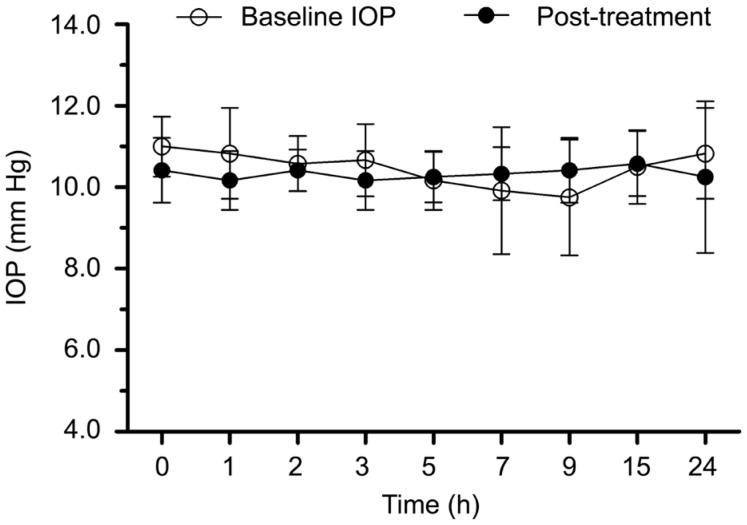
In Carteolol group, the IOPs after treatment during 24h were shown in Figure 2. No significant differences were observed at each time point compared to the baseline IOPs.
Figure 2. The IOPs before and after Carteolol treatment during 24h.
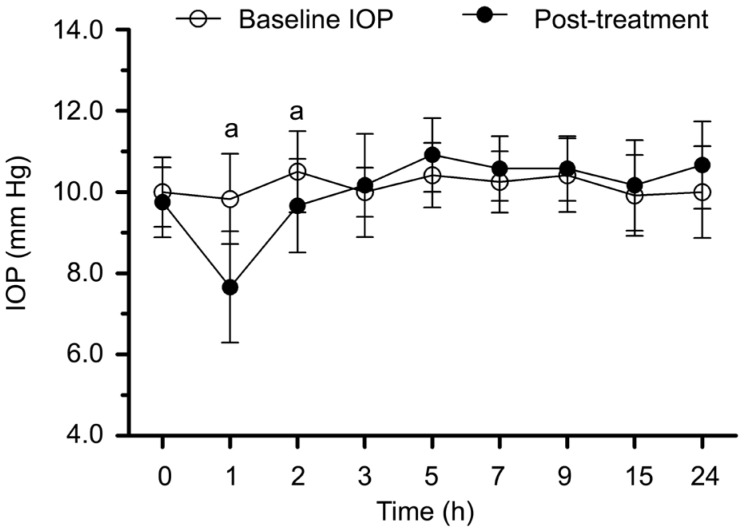
In Brimonidine group, the IOPs after using Brimonidine eye drops during 24h were shown in Figure 3. Significant difference was observed after one hour (t=3.856, P=0.003), the IOP was 2.2±1.9 mm Hg lower than baseline and the difference lasted for two hours.
Figure 3. The IOPs before and after Brimonidine treatment during 24h.
aP<0.05 between post-treatment IOP and baseline.
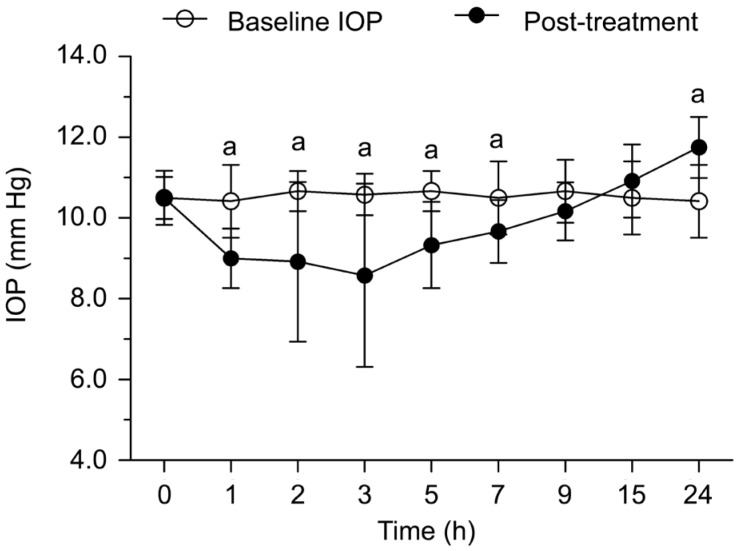
In Brinzolamide group, the IOPs after using eye drops during 24h were shown in Figure 4. Significant difference was found after one hour (t=4.53, P=0.001). The IOP after treatment was 1.4±1.1 mm Hg lower than baseline and the effect lasted for 7h. The decreased amplitude of IOP reached the maximum at 3h. The IOP was 2.0±2.29 mm Hg lower than baseline at 3h (t=3.02, P=0.012). Afterwards, the IOP increased. Significant difference was found at 24h (t=5.20, P=0.001). The IOP was 1.3±0.89 mm Hg higher than baseline IOP at the end of the experiment.
Figure 4. The IOPs before and after Brinzolamide treatment during 24h.
aP<0.05 between post-treatment IOP and baseline.
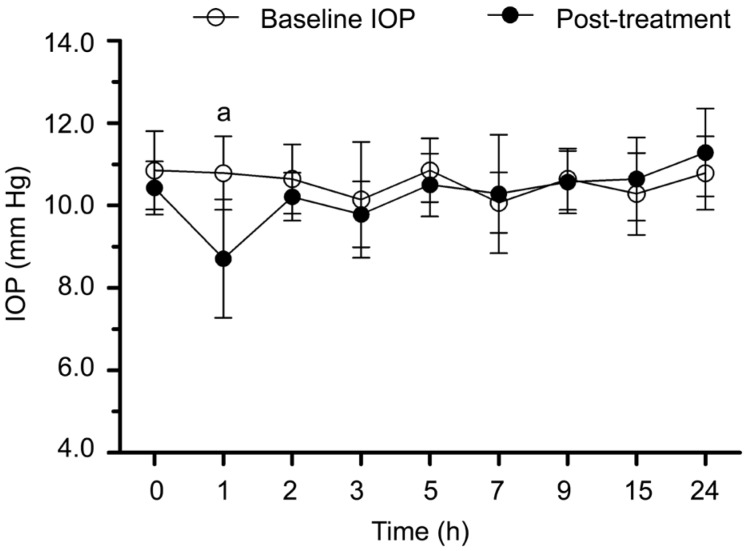
In Latanaprost group, the IOPs after taking the drug during 24h were shown in Figure 5. Significant difference was observed after one hour (t=6.11, P=0.001). The IOP was 2.1±1.3 mm Hg lower than baseline. The difference lasted for one hour.
Figure 5. The IOPs before and after Latanaprost treatment during 24h.
aP<0.05 between post-treatment IOP and baseline.
DISCUSSION
Our current research proved it was feasible to use RBT to measure the IOP of guinea pig. The guinea pigs were awake and little variation was found during measuring process. The result suggested the IOPs of guinea pigs in one day were normally distributed. The mean IOP was 10.3±0.36 mm Hg. In this respect, the mean IOP of adult guinea pigs by Coster et al[17] was 18.27±4.55 mm Hg. The tonometry we used was based on the rebound principle, while the research by Coster et al[17] used the applanation tonometer. It indicated the respective methods which were chosen would lead to a different result. The IOP by Goldmann applanation tonometer was reported 5 mm Hg higher than RBT in the same mice[18]–[20]. Therefore, our results was similar to the previous result despite different animals were selected. Moreover, our research observed the fluctuation range of IOP in guinea pigs was lower than that in humans. We speculated that it was related to the corneal thickness (CT). Research in humans by Rao et al[21] claimed the central CT in humans was 500 µm, while the CT in guinea pigs was 227.85±14.09 µm[22]. More work need to do to compare the difference between Goldmann applanation tonometer and RBT in guinea pigs.
The medical treatment of beta-adrenergic blocking agents mainly came from the suppression of aqueous production, Carteolol was the most widely used[23]. In our current research, no significant difference was observed before and after treatment in Carteolol group. It suggested beta-adrenergic blocking agents had a limited effect to reduce IOP in guinea pigs.
Brimonidine was an alpha-adrenergic agonist that primarily inhibited aqueous production and increased aqueous outflow further. In Brimonidine group, the IOP was significantly lower than baseline IOP after one hour, and the significant effect lasted for two hours, which was similar to the research of Akaishi et al[24].
The eyeball of guinea pigs was found sensitive to Brinzolamide. Our current research observed the IOP in Brinzolamide group was decreased one hour after treatment, and lasted the longest compared to the other groups. In this respect, inhibition of carbonic anhydrase in the ciliary processes of the eye decreased aqueous humor secretion and lowered the IOP in the anterior chamber, presumably by reducing the rate of formation of bicarbonate ions with subsequent reduction in sodium and fluid transport[25]. Our research was similar to the results of Toris et al[26], they reported a much decreased IOP in rabbit by Brinzolamide. However, Gray et al[27] reported no reducing IOP effects were found after 1% Brinzolamide every 12h in normal cats. Such result indicated reducing IOP effects varied with species. In addition, we observed a rising IOP compared to baseline IOP in spite of the drug effects began to disappear. The IOP was 1.3±0.89 mm Hg higher than baseline IOP at the end of the experiment. In this point, one reasonable explanation was the Brinzolamide could decrease aqueous humor secretion and thus lower the IOP in the anterior chamber, the ciliary body might increase the aqueous humor secretion through compensatory mechanism accompanied by the attenuated drug effect. Therefore, the IOP could increase in a short time, but the detailed mechanism still needs to be further studied.
Latanoprost is one kind of prostaglandin analogs which could increase uveoscleral outflow of aqueous. It was reported that Latanaprost drops could strikingly decrease the IOP of human[28] and monkey[29]. However, no obvious effect was found in rabbit[30] and cat[31]. In the current study, Latanaprost drops could decrease the IOP of guinea pig significantly. In the study by McDonald et al[32] in cats, no significant difference in IOP was found after 3wk of Latanoprost drops, but the outflow of aqueous was observed increased. Such results suggested a similar mechanism between human and small animals like guinea pig.
The shortcoming of this research was, only RBT was used, and the relationship between CT and IOP was still unclear. Thus, our further work will focus on the difference between Goldmann applanation tonometer and RBT in guinea pigs, and the CT of guinea pigs by using optical coherence tomography. The individual difference of each animal also required more attention.
In summary, our work represented an attempt to expand our knowledge of baseline IOPs of guinea pigs. We found a different effect on the IOP by using four reducing IOP agents. Studies of this sort might reveal some useful features of animal eyeball, and it would also make the guinea pig a promising animal model in future studies in glaucoma.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81400428); Science and Technology Commission of Shanghai Municipality (No.134119b1600).
Conflicts of Interest: Di Y, None; Luo XM, None; Qiao T, None; Lu N, None.
REFERENCES
- 1.Panarelli JF, Nayak NV, Sidoti PA. Postoperative management of trabeculectomy and glaucoma drainage implant surgery. Curr Opin Ophthalmol. 2016;27(2):170–176. doi: 10.1097/ICU.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 2.Durairaj C, Shen J, Cherukury M. Mechanism - based translational pharmacokinetic - pharmacodynamic model to predict intraocular pressure lowering effect of drugs in patients with glaucoma or ocular hypertension. Pharm Res. 2014;31(8):2095–2106. doi: 10.1007/s11095-014-1311-9. [DOI] [PubMed] [Google Scholar]
- 3.Wostyn P, De Groot V, Audenaert K, De Deyn PP. A new look at glaucoma. J Ophthalmic Vis Res. 2015;10(4):502–503. doi: 10.4103/2008-322X.176901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarchahi AA, Bozorgi H. Effect of tetracaine on intraocular pressure in normal and hypertensive rabbit eyes. J Ophthalmic Vis Res. 2012;7(1):29–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Ren R, Lockwood H, Williams G, Libertiaux V, Downs C, Gardiner SK, Burgoyne CF. The connective tissue components of optic nerve head cupping in monkey experimental glaucoma part 1: Global change. Invest Ophthalmol Vis Sci. 2015;56(13):7661–7678. doi: 10.1167/iovs.15-17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng S, Wang M, Yan Z, Tian Z, Chen H, Yang X, Zhuo Y. Autophagy in retinal ganglion cells in a rhesus monkey chronic hypertensive glaucoma model. PLoS One. 2013;8(10):e77100. doi: 10.1371/journal.pone.0077100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira LB, Buhr KA, Bowie O, Duke FD, Nork TM, Dubielzig RR, McLellan GJ. Quantifying optic nerve axons in a cat glaucoma model by a semi-automated targeted counting method. Mol Vis. 2014;20:376–385. [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Su D, O'Rourke B, Pogwizd SM, Zhou L. Mitochondria-derived ROS bursts disturb Ca2+ cycling and induce abnormal automaticity in guinea pig cardiomyocytes: a theoretical study. Am J Physiol Heart Circ Physiol. 2015;308(6):H623–H636. doi: 10.1152/ajpheart.00493.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui XG, Liu CY, Wei B, Zhao WJ, Zhang WF. Whole mitochondrial genome sequence for an osteoarthritis model of Guinea pig (Caviidae; Cavia) Mitochondrial DNA A DNA Mapp Seq Anal. 2016;27(6):3915–3916. doi: 10.3109/19401736.2014.987263. [DOI] [PubMed] [Google Scholar]
- 10.Gotzsche PC. “Human guinea pig” asks for animal studies. BMJ. 2014;349:g6714. doi: 10.1136/bmj.g6714. [DOI] [PubMed] [Google Scholar]
- 11.Marjanovic I, Martinez A, Marjanovic M, Kontic D, Hentova-Sencanic P, Markovic V, Bozic M. Changes in the retrobulbar arterial circulation after decrease of the elevated intraocular pressure in men and women with primary open angle glaucoma. Srp Arh Celok Lek. 2013;141(11-12):728–731. doi: 10.2298/sarh1312728m. [DOI] [PubMed] [Google Scholar]
- 12.Meidert AS, Huber W, Muller JN, Schofthaler M, Hapfelmeier A, Langwieser N, Wagner JY, Eyer F, Schmid RM, Saugel B. Radial artery applanation tonometry for continuous non-invasive arterial pressure monitoring in intensive care unit patients: Comparison with invasively assessed radial arterial pressure. Br J Anaesth. 2014;112(3):521–528. doi: 10.1093/bja/aet400. [DOI] [PubMed] [Google Scholar]
- 13.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34(2):363–369. [PubMed] [Google Scholar]
- 14.Dekking HM, Coster HD. Dynamic tonometry. Ophthalmologica. 1967;154(1):59–74. doi: 10.1159/000305149. [DOI] [PubMed] [Google Scholar]
- 15.Kontiola A. A new electromechanical method for measuring intraocular pressure. Doc Ophthalmol. 1996;93(3):265–276. doi: 10.1007/BF02569066. [DOI] [PubMed] [Google Scholar]
- 16.Feng CS, Jin KW, Yi K, Choi DG. Comparison of intraocular pressure measurements obtained by rebound, noncontact, and goldmann applanation tonometry in children. Am J Ophthalmol. 2015;160(5):937–943.e1. doi: 10.1016/j.ajo.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Coster ME, Stiles J, Krohne SG, Raskin RE. Results of diagnostic ophthalmic testing in healthy guinea pigs. J Am Vet Med Assoc. 2008;232(12):1825–1833. doi: 10.2460/javma.232.12.1825. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Resua C, Gonzalez-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. Other portable tonometers in healthy eyes. Optom Vis Sci. 2006;83(2):102–107. doi: 10.1097/01.opx.0000200673.96758.7b. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Darhad U, Tatsumi Y, Fujioka M, Kusuhara A, Maeda H, Negi A. Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006;142(2):332–334. doi: 10.1016/j.ajo.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Kim CY, Kuehn MH, Anderson MG, Kwon YH. Intraocular pressure measurement in mice: a comparison between Goldmann and rebound tonometry. Eye (Lond) 2007;21(9):1202–1209. doi: 10.1038/sj.eye.6702576. [DOI] [PubMed] [Google Scholar]
- 21.Rao A, Kumar M, Prakash B, Varshney G. Relationship of central corneal thickness and intraocular pressure by iCare rebound tonometer. J Glaucoma. 2014;23(6):380–384. doi: 10.1097/IJG.0b013e318279b819. [DOI] [PubMed] [Google Scholar]
- 22.Cafaro TA, Ortiz SG, Maldonado C, Esposito FA, Croxatto JO, Berra A, Ale OL, Torrealday JI, Urrets-Zavalia EA, Urrets-Zavalia JA, Serra HM. The cornea of guinea pig: structural and functional studies. Vet Ophthalmol. 2009;12(4):234–241. doi: 10.1111/j.1463-5224.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 23.Yao Q, Yu X, Zheng T, Liu H, Yang Y, Yi P. Spectroscopic studies on the interaction of Carteolol hydrochloride and urea-induced bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc. 2013;113:447–451. doi: 10.1016/j.saa.2013.04.129. [DOI] [PubMed] [Google Scholar]
- 24.Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009;25(5):401–408. doi: 10.1089/jop.2009.0006. [DOI] [PubMed] [Google Scholar]
- 25.Stavinohova R, Newton JR, Busse C. The effect of prophylactic topical carbonic anhydrase inhibitors in canine primary closed-angle glaucoma. J Small Anim Pract. 2015;56(11):662–666. doi: 10.1111/jsap.12393. [DOI] [PubMed] [Google Scholar]
- 26.Toris CB, Zhan GL, McLaughlin MA. Effects of brinzolamide on aqueous humor dynamics in monkeys and rabbits. J Ocul Pharmacol Ther. 2003;19(5):397–404. doi: 10.1089/108076803322472962. [DOI] [PubMed] [Google Scholar]
- 27.Gray HE, Willis AM, Morgan RV. Effects of topical administration of 1% brinzolamide on normal cat eyes. Vet Ophthalmol. 2003;6(4):285–290. doi: 10.1111/j.1463-5224.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 28.Painter SL, Mead AL. Patient experience of the transition from Xalatan to generic latanoprost. Eye (Lond) 2014;28(7):911. doi: 10.1038/eye.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takagi Y, Nakajima T, Shimazaki A, Kageyama M, Matsugi T, Matsumura Y, Gabelt BT, Kaufman PL, Hara H. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78(4):767–776. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Orihashi M, Shima Y, Tsuneki H, Kimura I. Potent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbits. Biol Pharm Bull. 2005;28(1):65–68. doi: 10.1248/bpb.28.65. [DOI] [PubMed] [Google Scholar]
- 31.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. Am J Vet Res. 2000;61(10):1220–1224. doi: 10.2460/ajvr.2000.61.1220. [DOI] [PubMed] [Google Scholar]
- 32.McDonald JE, Kiland JA, Kaufman PL, Bentley E, Ellinwood NM, McLellan GJ. Effect of topical latanoprost 0.005% on intraocular pressure and pupil diameter in normal and glaucomatous cats. Vet Ophthalmol. 2016;19(Suppl. 1):13–23. doi: 10.1111/vop.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]