Abstract
AIM
To evaluate the corneal cell morphology of new keratoconus patients wearing two different types of rigid gas-permeable (RGP) contact lenses for 1y.
METHODS
Thirty nine eyes of 39 new keratoconus patients were selected and randomly fitted with two types of RGP contact lenses. Group 1 had 21 eyes with regular rigid gas-permeable (RRGP) contact lens and rest 18 eyes were in group 2 with specially designed rigid gas-permeable (SRGP) contact lens. Corneal cell morphology was evaluated using a slit scanning confocal microscope at no-lens wear and after 1y of contact lens wearing.
RESULTS
After 1y of contact lens wearing in group 1, the mean anterior and posterior stromal keratocyte density were significantly less (P=0.006 and P=0.001, respectively) compared to no-lens wear. The mean cell area of anterior and posterior stromal keratocyte were also significantly different (P=0.005 and P=0.001) from no-lens wear. The anterior and posterior stromal haze increased by 18.74% and 23.81%, respectively after 1y of contact lens wearing. Whereas in group 2, statistically significant changes were observed only in cell density & area of anterior stroma (P=0.001 and P=0.001, respectively) after 1y. While, level of anterior and posterior stromal haze increased by 16.67% and 11.11% after 1y of contact lens wearing. Polymegathism and pleomorphism also increased after 1y of contact lens wearing in both the contact lens groups.
CONCLUSION
Confocal microscopy observation shows the significant alterations in corneal cell morphology of keratoconic corneas wearing contact lenses especially in group 1. The type of contact lens must be carefully selected to minimize changes in corneal cell morphology.
Keywords: keratoconus, contact lens, cornea, confocal microscopy
INTRODUCTION
Keratoconus is a progressive, non-inflammatory corneal dystrophy that causes thinning and protrusion of central and paracentral cornea. Ectatic cornea of keratoconus usually leads to high myopia and irregular astigmatism, which requires glasses, contact lenses or surgical intervention, depending on disease severity[1]–[2]. Visual correction of keratoconus can be achieved with any of several types of contact lenses, such as rigid gas-permeable (RGP), soft, hybrid, piggyback, or scleral[3]–[4]. However, RGP contact lenses are the most common and successful treatment options especially in early to moderate keratoconus[5]. RGP contact lenses mask the irregularities of keratoconic cornea and provide superior vision with reduced higher order aberration[6].
In vivo slit scanning confocal microscopy can reveal minute cellular alterations in living human cornea[7]–[9]. Previous researchers have stated that prolonged use of contact lenses alters the underlying corneal cell morphology in both normal healthy cornea and keratoconic cornea[10]–[12]. However, few studies have been performed to evaluate the effects of different types of contact lenses on keratoconic cornea[13]–[16]. It has been reported that mechanical injury to the corneal epithelium caused by contact lenses on keratoconic corneas results in release of apoptotic cytokines, which play a role in the morphological changes observed in patients with keratoconic cornea who wear contact lenses[13],[17]. The aim of the present study was to assess the alterations in corneal cell morphology in new patients with keratoconus wearing two different types of RGP contact lenses for 1y.
SUBJECTS AND METHODS
Subjects
This was a prospective longitudinal study in which one eye of each 39 new patient with keratoconus (15 males and 24 females) aged 18 to 45 years were selected from the Ophthalmology Department of a public hospital. Exclusion criteria were presence of keratoconus with any other ocular disease, history of contact lens wearing or surgical interventions to the cornea, or presence of any systemic disease that could affect ocular health. In patients with bilateral keratoconus, one eye was randomly selected for the study. Approval was obtained from the Ethics Committee of University Kebangsaan Malaysia, which follows the tenets of the Declaration of Helsinki, and informed consent was obtained from all participants.
A comprehensive eye examination was carried out for all recruited patients. Standard clinical signs of keratoconus, such as scissors reflex with retinoscopy, central or paracentral thinning, Fleischer ring, Vogt's striae, Munson's sign seen with slit lamp biomicroscopy, and central or paracentral steepening of the cornea seen by topography (Pentacam, Oculus Optikgerate GmbH, Wetzlar, Germany), were used to diagnose keratoconus. Severity of keratoconus was classified as mild (<45.00 D), moderate (45.00 to 52.00 D) and severe (>52.00 D), as described by the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study[18].
All recruited patients were divided into two groups and were randomized to one of two different types of daily-wear RGP contact lenses: regular rigid gas-permeable (RRGP) and special design rigid gas-permeable (SRGP). Group 1 (RRGP) contained 21 patients (8 males, 13 females) and group 2 (SRGP) contained 18 patients (7 males, 11 females). The design and parameters of the two RGP contact lenses are outlined in Table 1. Contact lens fittings were performed following the guidelines set by the contact lens manufacturer. Contact lens care and maintenance were explained to the patients, and they were instructed to wear contact lenses for 8h per day, at least 6d per week.
Table 1. Characteristics of SRGP and RRGP contact lens.
| Characteristics | SRGP contact lens | RRGP contact lens |
| Materials, Dk (Oxygen permeability) | Enflufocon A, 18 | Enflufocon A, 18 |
| Parameters | Base curve: 4.30 mm to 8.59 mm Diameter: 7.90 mm to 10.40 mm Edge lift: Flexible edge lift |
Base curve: 5.10 mm to 8.0 mm Diameter: 8.70 mm to 9.50 mm Edge lift: Standard edge lift |
| Design | 1. Multi-curve and bi-toric design 2. Smaller aspheric back optic zone 3. Asymmetric Corneal Technology design |
1. Tri-curve design 2. Large spherical back optic zone |
| Diagrammatic illustration of contact lens interaction with keratoconic cornea | Small back optic zone covers the cone with very mild touch on the apex (feather touch)
|
Large back optic zone rests on the apex of the cone with inferior mid-peripheral touch in keratoconic cornea
|
Methods
In vivo slit scanning confocal microscopy was performed (ConfoScan4; Nidek Technologies Srl, Albignasego, Italy). A non-aplanatic water immersion 40× objective lens with a numerical aperture of 0.75 and corneal full thickness scan were used in the study. Standard operative procedures were strictly followed while performing confocal microscopy at time of recruitment (baseline) and after 1y of contact lens wearing. Follow-up visits were carried out to assess contact lens fitting and ocular health after 3 and 6mo of contact lens wearing.
Three sharp, clear images from each corneal layer were selected for analysis. Qualitative analysis was performed following the grading scale designed by Hollingsworth et al[19], which describes the level of haze and hyper-reflectivity of the corneal stroma when viewed under confocal microscopy. In the present study, the hyper-reflectivity and brightness of stromal layer was considered to be “stromal haze”. Quantitative analysis was performed using Nidek Advanced Visual Information System (NAVIS) software in a fixed frame of 600×600-µm2. The cells were counted manually using the “L” method to calculate the cell area and density. Using this method, cells within the fixed frame along with any incomplete cells on the left side and bottom border of each frame were counted, while cells that were located partly on the right side and top border of the frame were not counted. The anterior stroma was considered to be the first image seen immediately after Bowman's membrane, and the posterior stroma was considered to be the keratocyte nuclei images seen before the endothelium. One observer (Ghosh S), who was blinded to the group allocation, performed selection and analysis of all the images.
Statistical Analysis
All obtained data were analyzed using SPSS software (IMB SPSS 17; SPSS Inc., Chicago, IL, USA). The Shapiro-Wilks test was used to check the normality of the data distribution. Descriptive statistics and Chi-square test were used to analyze the qualitative data, and the paired samples t-test was used to compare the difference in means in each group. All the tests were two-tailed and P<0.05 was considered as statistically significant.
RESULTS
Mean ages of the patients were 27.28±6.57y and 30.44±6.56y for groups 1 and 2, respectively, and mean age and sex were not significantly different between the two groups (P=0.143 and 0.961, respectively). In group 1, the numbers and proportions of patients with mild, moderate, and severe keratoconus were 4 (19.05%), 10 (47.62%), and 7 (33.33%), respectively, while in group 2, these were 3 (16.67%), 8 (44.44%), and 7 (38.89%), respectively. Average diameters of the contact lenses fitted for the study were 8.99 mm and 9.25 mm for groups 1 (n=21) and 2 (n=18), respectively.
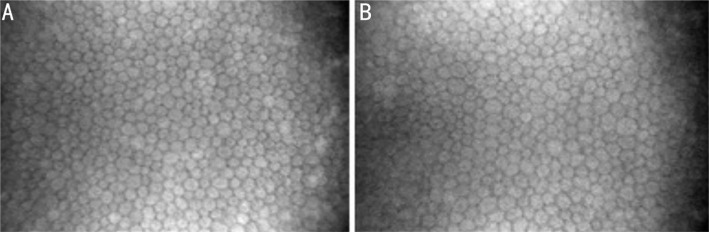
Qualitative analysis showed changes in stromal haze in both groups after 1y of contact lens wearing (Figure 1) compared with baseline observation. Anterior and posterior stromal haze were increased in 18.74% and 23.81% of eyes in group 1, and in 16.67% and 11.11% of eyes, in group 2. However, nonparametric χ2 statistical analysis showed no significant difference in anterior and posterior stromal haze in either group 1 (χ2=1.527; P>0.05 and χ2=3.436; P>0.05, respectively) or group 2 (χ2=1.003; P>0.05 and χ2=0.45; P>0.05, respectively) after 1y.
Figure 1. Comparison of stromal haze before and after 1y of group 1 (A) and group 2 (B).
ASH: Anterior stromal haze; PSH: Posterior stromal haze.
Quantitative analysis showed noticeable alterations in corneal cell morphology in patients with keratoconus after 1y in both groups. Table 2 shows the comparison of cell density and area of stroma and endothelium between the two groups at baseline and 1y. After 1y of contact lens wearing, mean anterior and posterior stromal keratocyte densities were significantly reduced (P=0.006 and P=0.001, respectively), and mean anterior and posterior stromal keratocyte areas were significantly increased (P=0.005 and P=0.001, respectively) in group 1 (Figure 2). In group 2, significant changes were observed in anterior stromal keratocyte density (P=0.001) and area (P=0.001) after 1y of contact lens wearing (Figure 3), whereas the posterior stroma did not show significant changes (P>0.05) after 1y of contact lens wearing. Using NAVIS Endo Cell Analysis software, polymegathism and pleomorphism were assessed in endothelium after 1y of contact lens wearing in 17 (80.95%) eyes in group 1 and 13 (72.22%) eyes in group 2, and found increased by 3.91% (P=0.288) and 14.85% (P=0.028), respectively, in group 1, and increased by 2.65% (P=0.586) and 14.79% (P=0.034), respectively, in group 2 (Figures 4 and 5). After 1y of contact lens wearing, endothelial cell density and area were not significantly (P>0.05) different from baseline observation in either of the contact lens groups.
Table 2. Comparison of the corneal cell morphology between group 1 and group 2 patients with keratoconus after 1y of contact lens wearing.
| Corneal layers | Group 1 (n=21) |
Group 2 (n=18) |
||||
| Baseline | 1a of contact lens wear | P | Baseline | 1a of contact lens wear | P | |
| ASKD (cells/mm2) | 880.84±48.21 | 844.70±34.50 | 0.006 | 897.91±43.27 | 847.87±25.73 | 0.001 |
| ASKA (µm2) | 1138.45±60.88 | 1187.48±46.19 | 0.005 | 1116.10±53.45 | 1180.47±36.74 | 0.001 |
| PSKD (cells/mm2) | 708.09±55.67 | 660.81±28.64 | 0.001 | 707.02±38.00 | 682.03±28.51 | 0.050 |
| PSKA (µm2) | 1420.70±114.11 | 1515.95±65.22 | 0.001 | 1418.50±81.49 | 1440.07±67.83 | 0.378 |
| ECD (cells/mm2) | 2802±191.21 | 2793±228.29 | 0.836 | 2849±208.49 | 2830±173.63 | 0.557 |
| ECA (µm2) | 354.37±17.06 |
359.64±30.82 |
0.369 |
352.78±25.46 |
354.77±22.12 |
0.617 |
|
n=17 |
n=13 |
|||||
| Polymegathism (%) | 43.47±13.82 | 47.38±8.25 | 0.288 | 42.71±15.79 | 45.36±8.27 | 0.586 |
| Pleomorphism (%) | 31.95±22.62 | 46.80±14.11 | 0.028 | 35.08±25.19 | 49.87±6.72 | 0.034 |
ASKD: Anterior stromal keratocyte density; PSKD: Posterior stromal keratocyte density; ASKA: Anterior stromal keratocyte cell area; PSKA: Posterior stromal keratocyte cell area; ECD: Endothelial cell density; ECA: Endothelial cell area.
x±s
Figure 2. Group 1 patient with keratoconus.
A: Anterior stroma before contact lens wearing; B: Anterior stroma after 1y of contact lens wearing.
Figure 3. Group 2 patient with keratoconus.
A: Anterior stroma before contact lens wearing; B: Anterior stroma after 1y of contact lens wearing.
Figure 4. Endothelium of a group 1 patient with keratoconus.
A: Before contact lens wearing; B: After 1y of contact lens wearing.
Figure 5. Endothelium of a group 2 patient with keratoconus.
A: Before contact lens wear; B: After 1y of contact lens wear.
Disease severity was also assessed for each contact lens group. In group 1, mean anterior stromal keratocyte densities pre and post contact lens wearing were 904.87±55.10 and 842.35±9.64 cells/mm2 for mild (n=4), 899.03±44.07 and 847.31±39.94 cells/mm2 for moderate (n=10), and 841.14±23.30 and 842.32±38.82 cells/mm2 for severe (n=7) keratoconus, while mean posterior stromal keratocyte densities pre and post contact lens wear were 742.22±63.13 and 671.32±45.93 cells/mm2 for mild, 705.54±35.20 and 653.84±20.52 cells/mm2 for moderate, and 692.22±73.46 and 664.78±29.56 cells/mm2 for severe keratoconus. Statistically significant differences in anterior (P=0.010) and posterior (P=0.003) keratocyte density were observed only in the moderate keratoconus group. In group 2, mean anterior stromal keratocyte densities pre and post contact lens wearing were 939.60±34.70 and 850.66±16.65 cells/mm2 for mild (n=3), 904.86±44.34 and 852.71±32.66 cells/mm2 for moderate (n=8), and 872.10±30.00 and 841.14±21.22 cells/mm2 for severe (n=7) keratoconus, while mean posterior stromal keratocyte densities pre and post contact lens wear were 728.33±25.50 and 656.06±9.64 cells/mm2 for mild, 700.52±17.81 and 698.46±31.43 cells/mm2 for moderate, and 705.31±56.73 and 674.40±18.82 cells/mm2 for severe keratoconus. Statistically significant differences were observed for anterior stroma in all subgroups (mild, P=0.047; moderate, P=0.006 and severe, P=0.032) and for posterior stroma in the mild group (P=0.023). There was no significant difference (P>0.05) in endothelium cell density for mild, moderate, or severe keratoconus in either of the two groups.
DISCUSSION
Contact lenses play an important role in management of keratoconus. However, corneal cell morphology in keratoconic cornea is affected by contact lens wearing[11]–[16]. In 2002, Erie et al[13] reported that RGP contact lenses caused more keratocyte cell death in keratoconic corneas compared with soft toric contact lenses, while in 2004, Edmonds et al[14] reported lower endothelial cell count in keratoconic cornea with use of aspheric RGP contact lenses compared with soft toric contact lenses. In 2012, Acar et al[15] found that piggyback has different levels of interleukin (IL)-6, IL-8 in tear and posterior stromal keratocyte density compared with Clearkone hybrid lenses, and the same year, Ohta et al[16] observed greater changes in keratocyte density with soft contact lenses than with RGP contact lenses. To our knowledge, the current study is the first longitudinal study to assess the long-term effects of two different types of RGP contact lenses on keratoconic cornea.
Hyper-reflectivity and brightness in stroma are common findings in patients with keratoconus[7],[20]. In the present study, increased stromal haze (anterior and posterior stroma) was observed after 1y of contact lens wearing in both groups, and there was no statistically significant difference between the groups, although group 1 showed relatively more stromal haze compared with group 2 after 1y of contact lens wearing. Disorganization and loss of collagen fibrils in stroma may be the causative factors for increased stromal haze[10],[19],[21]. Weed et al[21] reported that keratocytes and their regular distribution in stroma are responsible for the production and regular arrangement of collagen fibrils, which maintains corneal transparency. Kallinikos and Efron[22] observed irregular distribution of keratocytes across stroma in RGP contact lens wearers. Erie et al[13] and Lema et al[17] reported that apoptotic cytokines cause keratocyte death in keratoconic corneas in patients wearing contact lenses. In the present study, group 1 may have had more alteration in stromal keratocytes, which might have caused more stromal haze in group 1 compared with group 2.
Loss of stromal keratocyte density in patients with keratoconus wearing contact lenses has been reported in many studies[11]–[16]. In the present study, after 1y of contact lens wearing, significant loss of keratocyte density was seen in both anterior and posterior stroma in group 1, but only in anterior stroma in group 2. Statistical analysis of disease severity showed that after 1y of contact lens wearing, there was loss of keratocyte density in both anterior and posterior stroma in group 1, but significant loss only in the moderate keratoconus subgroup. By contrast, in group 2, after 1y of contact lens wearing, there was a significant reduction in keratocyte density in anterior stroma in all three subgroups (mild, moderate and severe) and in posterior stroma in the mild subgroup only. However, increasing number of patients in each subgroup may provide more appropriate result on post contact lens wearer's keratocyte loss depending on severity of keratoconus. Erie et al[13] and Lema et al[17] reported that mechanical injury to the epithelium due to contact lens wearing in patients with keratoconus releases apoptotic cytokines that cause keratocyte death. Another previous study also reported that this contact lens-induced keratocyte loss can be associated with three possible factors such as hypoxia, cytokine-mediated effects, and mechanically induced effects[22]. In the present study, the contact lens used for group 1 has a tri-curve design and a large back optic zone, and rests on the apex of the cone with inferior mid-peripheral contact on an irregular cornea. This might not have caused a smooth interaction with the corneal surface, and thus could have triggered the epithelial injury. By contrast, the contact lens used for group 2 has a multi-curve design and a small aspheric back optic zone, and therefore covers the cone with light contact on the apex of irregular cornea. This might have caused a smoother interaction with the corneal surface, which could have produced lower epithelial injury. Therefore, mechanical injury and release of apoptotic cytokines could have been greater with the group 1 contact lens compared with group 2, resulting in the significant loss of keratocyte density in both anterior and posterior stroma seen in group 1.
In the present study, after 1y of contact lens wearing, a significantly greater mean keratocyte cell area was observed in both anterior and posterior stroma in group 1, but only in anterior stroma in group 2. Yeniad et al[11] reported greater mean keratocyte cell area in patients with keratoconus wearing contact lenses compared with patients who did not wear contact lenses. Studies have reported that the substance synthesized by keratocytes and a non-uniform distribution of proteoglycan macro-molecules are associated with changes in shape and size of keratocytes[23]–[24]; however, these changes in patients with keratoconus wearing contact lenses are not completely understood. In the present study, the findings indicated that the distribution proteoglycan molecules might have been more affected in group 1 compared with group 2.
The effects of contact lens wearing on the corneal endothelium are well documented[11]–[12],[25]. In the present study, average polymegathism and pleomorphism were increased after 1y of contact lens wearing in both contact lens groups; however, the changes were relatively greater in group 1 compared with group 2. Similarly, Yeniad et al[11] and Bitirgen et al[12] reported higher degrees of polymegathism and pleomorphism in patients with keratoconus wearing contact lenses. Studies have shown that changes in the shape and size of endothelial cells are caused by the hypoxia and mechanical stress due to contact lens wearing[26]–[27], while Kaldawy et al[28] reported that the increased polymegathism and pleomorphism in patients with keratoconus wearing contact lenses is indirectly related to keratocyte apoptosis. However, the etiology of the increased polymegathism and pleomorphism in seen in patients with keratoconus wearing contact lenses is not yet clear. In the present study, mechanical stress could have been the reason behind the greater changes in group 1 compared with group 2 as the two different designs of contact lens may have had different impacts on the keratoconic cornea.
We found in the present study that endothelial cell density and area were not significantly different after 1y of contact lens wearing in either group; similar results were found by Yeniad et al[11] and Bitirgen et al[12] in endothelium. However, there was a difference in loss of mean endothelial cell density between our two groups after 1y. Edmonds et al[14] reported variation in endothelial cell count in keratoconus with different types of contact lens. The reason behind this endothelial cell loss is not yet completely understood; however, Kaldawy et al[28] stated that apoptosis is the only process of cell death in keratoconus. Hence, mechanical stress due to the contact lens could have been the reason behind the greater changes seen in group 1 compared with group 2.
In the present study, relatively larger diameter contact lenses were fitted in group 2 compared with group 1, and group 1 showed more cellular alteration compared with group 2. A larger diameter lens usually has less contact with the sensitive cone area and less movement in the eye compared with a smaller diameter contact lens[29]–[30], thus, the larger lens may cause less mechanical injury to the epithelium compared with the smaller lens. Therefore, quite apart from the actual design, the diameter of the contact lens might be another causative factor for the cellular alteration in keratoconic corneas in patients wearing contact lenses. However, no previous study has reported about the effects of contact lens diameter on corneal cell morphology.
To date, the goal of fitting contact lenses in patients with keratoconus has been to achieve good vision and comfort, and the effects of different types of contact lenses on the cell morphology of keratoconic corneas is not well established. The present study shows that cellular alterations in keratoconic corneas vary with the type of contact lens. Therefore, contact lenses for the management of keratoconus must be selected based on the material, design, and parameters that result in a smooth interaction and less friction with the irregular keratoconic cornea, in order to minimize changes in corneal cell morphology. Henceforth, the goal of fitting contact lenses should be to achieve good vision and comfort without compromising ocular integrity.
A limitation of the present study was the difficulty we encountered in imaging the paracentral and peripheral cornea with the slit scanning confocal microscope. In keratoconus, the whole cornea may be affected to some degree, therefore, analysis of the paracentral and peripheral cornea along with central corneal area provides a better understanding of the morphological changes in keratoconus. Further studies including the paracentral and peripheral keratoconic cornea with different types of contact lenses are recommended.
Acknowledgments
Conflicts of Interest: Ghosh S, None; Mutalib HA, None; Sharanjeet-Kaur, None; Ghoshal R, None; Retnasabapathy S, None.
REFERENCES
- 1.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157–166. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Jhanji V, Sharma N, Vajpayee RB. Management of keratoconus: current scenario. Br J Ophthalmol. 2011;95(8):1044–1050. doi: 10.1136/bjo.2010.185868. [DOI] [PubMed] [Google Scholar]
- 3.Bilgin LK, Yilmaz Ş, Araz B, Yüksel SB, Sezen T. 30 years of contact lens prescribing for keratoconic patients in Turkey. Cont Lens Anterior Eye. 2009;32(1):16–21. doi: 10.1016/j.clae.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Gore DM, Shortt AJ, Allan BD. New clinical pathways for keratoconus. Eye(Lond) 2013;27(3):329–339. doi: 10.1038/eye.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett M, Mannis MJ. Contact lenses in the management of keratoconus. Cornea. 2011;30(12):1510–1516. doi: 10.1097/ICO.0b013e318211401f. [DOI] [PubMed] [Google Scholar]
- 6.Jinabhai A, Radhakrishnan H, Tromans C, O'Donnell C. Visual performance and optical quality with soft lenses in keratoconus patients. Ophthalmic Physiol Opt. 2012;32(2):100–116. doi: 10.1111/j.1475-1313.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 7.Efron N, Hollingsworth JG. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 2008;91(1):34–55. doi: 10.1111/j.1444-0938.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Bitirgen G, Ozkagnici A, Bozkurt B, Malik RA. In vivo corneal confocal microscopic analysis in patients with keratoconus. Int J Ophthalmol. 2015;8(3):534–539. doi: 10.3980/j.issn.2222-3959.2015.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozgurhan EB, Kara N, Yildirim A, Bozkurt E, Uslu H, Demirok A. Evaluation of corneal microstructure in keratoconus: a confocal microscopy study. Am J Ophthalmol. 2013;156(5):885–893.e2. doi: 10.1016/j.ajo.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26(4):398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Yeniad B, Yilmaz S, Bilgin LK. Evaluation of the microstructure of cornea by in vivo confocal microscopy in contact lens wearing and non-contact lens wearing keratoconus patients. Cont Lens Anterior Eye. 2010;33(4):167–170. doi: 10.1016/j.clae.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Bitirgen G, Ozkagnici A, Malik RA, Oltulu R. Evaluation of contact lens-induced changes in keratoconic corneas using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2013;54(8):5385–5391. doi: 10.1167/iovs.13-12437. [DOI] [PubMed] [Google Scholar]
- 13.Erie JC, Patel SV, McLaren JW, Nau CB, Hodge DO, Bourne WM. Keratocyte density in keratoconus. A confocal microscopy study (a) Am J Ophthalmol. 2002;134(5):689–695. doi: 10.1016/s0002-9394(02)01698-7. [DOI] [PubMed] [Google Scholar]
- 14.Edmonds CR, Wung SF, Husz MJ, Pemberton B. Corneal endothelial cell count in keratoconus patients after contact lens wear. Eye Contact Lens. 2004;30(1):54–58. doi: 10.1097/01.ICL.0000105561.89689.C8. [DOI] [PubMed] [Google Scholar]
- 15.Acar BT, Vural ET, Acar S. Effects of contact lenses on the ocular surface in patients with keratoconus: piggyback versus ClearKone hybrid lenses. Eye Contact Lens. 2012;38(1):43–48. doi: 10.1097/ICL.0b013e31823ff181. [DOI] [PubMed] [Google Scholar]
- 16.Ohta K, Shimamura I, Shiraishi A, Ohashi Y. Confocal microscopic observations of stromal keratocytes in soft and rigid contact lens wearers. Cornea. 2012;31(1):66–73. doi: 10.1097/ICO.0b013e31821b71ff. [DOI] [PubMed] [Google Scholar]
- 17.Lema I, Durán JA, Ruiz C, Díez-Feijoo E, Acera A, Merayo J. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008;27(7):758–763. doi: 10.1097/ICO.0b013e31816a3591. [DOI] [PubMed] [Google Scholar]
- 18.Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223–232. doi: 10.1016/j.clae.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingsworth JG, Efron N, Tullo AB. In vivo corneal confocal microscopy in keratoconus. Ophthalmic Physiol Opt. 2005;25(3):254–260. doi: 10.1111/j.1475-1313.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 20.Somodi S, Hahnel C, Slowik C, Richter A, Weiss DG, Guthoff R. Confocal in vivo microscopy and confocal laser-scanning fluorescence microscopy in keratoconus. Ger J Ophthalmol. 1996;5(6):518–525. [PubMed] [Google Scholar]
- 21.Weed KH, MacEwen CJ, Cox A, McGhee CN. Quantitative analysis of corneal microstructure in keratoconus utilising in vivo confocal microscopy. Eye (Lond) 2007;21(5):614–623. doi: 10.1038/sj.eye.6702286. [DOI] [PubMed] [Google Scholar]
- 22.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45(9):3011–3020. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 23.Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BY. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998;116(1):62–68. doi: 10.1001/archopht.116.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36(13):2557–2567. [PubMed] [Google Scholar]
- 25.Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43(4):995–1003. [PubMed] [Google Scholar]
- 26.McMonnies CW. Corneal endothelial assessment with special references to keratoconus. Optom Vis Sci. 2014;91(6):e124–e134. doi: 10.1097/OPX.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 27.Sturbaum CW, Peiffer RL., Jr Pathology of corneal endothelium in keratoconus. Ophthalmologica. 1993;206(4):192–208. doi: 10.1159/000310390. [DOI] [PubMed] [Google Scholar]
- 28.Kaldawy RM, Wagner J, Ching S, Seigel GM. Evidence of apoptotic cell death in keratoconus. Cornea. 2002;21(2):206–209. doi: 10.1097/00003226-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Rathi VM, Mandathara PS, Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol. 2013;61(8):410–415. doi: 10.4103/0301-4738.116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langis Michaud. Large Diameter Lenses: the Future of GPs? Contact Lens Spectrum. 2013;28:32–39. [Google Scholar]