Abstract
AIM
To assess the levels of the neutrophil to lymphocyte ratio (N/L) and the platelet to lymphocyte ratio (P/L) in patients with idiopathic acute anterior uveitis (AAU) and to compare with healthy controls.
METHODS
Thirty-six male patients with idiopathic AAU and 36 male healthy subjects were enrolled in this retrospective study. Complete ophthalmological examination and complete blood count measurements results of all subjects were evaluated.
RESULTS
There was a significant difference in N/L and P/L between idiopathic AAU and control groups (P=0.006, P=0.022). Also, correlation analysis revealed a significant correlation between C-reactive protein (CRP) and N/L (P=0.002; r=0.461).
CONCLUSION
Our study for the first time provides evidence of N/L and P/L may be useful biomarkers in patients with idiopathic AAU. N/L is correlated with CRP, so it can be a useful biomarker to predict the prognosis in idiopathic AAU.
Keywords: C-reactive protein, inflammation, neutrophil, lymphocyte, platelet, uveitis
INTRODUCTION
The term “uveitis” identifies all inflammatory diseases of intraocular structures. And it is the fifth leading cause of functional visual loss and blindness in western countries[1]. The classification of uveitis, may be based on anatomical involvement or etiology of the intraocular inflammation. Anatomical classification includes anterior, intermediate, posterior and panuveitis. Intraocular inflammation may also be classified as the etiology, such as, infections, autoimmune and/or immunity mediated systemic diseases, traumatic, drug induced and lens induced uveitis. The most common type of uveitis in general population is acute anterior uveitis (AAU), which refers to intraocular inflammation affects the anterior portion of the eye. The most common identifiable etiology is human leukocyte antigen (HLA) B27-related AAU and the etiology of AAU is unclear approximately 50% of cases, and these cases constitute the idiopathic AAU subgroup[2]–[3].
The relationship between uveitis and inflammatory processes are being studied for a long time. Earlier studies demonstrated that Th1 mediated autoimmune responses play a pivotal role in this disease[4]. Various cytokines in the sera of the patients with uveitis have been identified, including tumor necrosis factor (TNF)-α, IFN-γ, IL-2, IL-5, IL-8, IL-10[5]–[6]. Also, among uveitis patients, several genes including, IL-19, IL-20, IL-22 and IL-25/IL-17E, were found to be highly expressed compared to the normal individuals[7]. Additionally, C-reactive protein (CRP) is a reliable marker of inflammatory conditions and previous studies revealed high serum CRP levels were found in patients with uveitis[8]–[9].
The neutrophil to lymphocyte ratio (N/L) and platelet to lymphocyte ratio (P/L) are easy to analyze inflammation biomarkers derived from complete blood count (CBC) which have emerged as useful indicator markers of subclinical systemic inflammation[10]–[11]. Also these biomarkers have usually been investigated as predictor of prognosis of several cancers, cardiovascular, metabolic and inflammatory diseases in recent studies[12]–[15]. Although papers from the ophthalmology clinics related to N/L and P/L have a trend of increase and they have rose to prominence as an early biomarker of some ocular diseases like age-related macular degeneration (AMD), retinal vein occlusion (RVO), keratoconus and pseudoexfoliation syndrome (PEX), studies investigating the predictive value of these markers, as indicator of patients with idiopathic AAU are limited[16]–[20].
The purpose of the present study is to assess the levels of N/L and P/L in patients with idiopathic AAU, to investigate the value of N/L and P/L levels in indicating the diagnosis of patients with idiopathic AAU and to compare the N/L and P/L results with healthy controls and CRP levels of the patients.
SUBJECTS AND METHODS
This retrospective study was approved by the Institutional Review Board of Gulhane School of Medicine, which was conducted according to the Declaration of Helsinki. The data were collected from patients' medical records between January 2009 and December 2014. We reviewed 145 patients' files with the diagnosis of idiopathic uveitis. After the initial assessment of the records we excluded 109 patients regarding the different types of idiopathic uveitis such as, intermediate, posterior, panuveitis and chronic form of uveitis. Thirty-six male patients with idiopathic AAU and 36 age matched male control subjects were included in the study.
All subjects underwent an ophthalmological assessment including best-corrected visual acuity, intraocular pressure measurement, fundoscopy with indirect ophthalmoscopy, and slit-lamp biomicroscopy to determine the posterior segment including pars plana findings. Fluorescein angiography, indocyanine green angiography and optical coherence tomography were performed in presence of complications. CBC, erythrocyte sedimentation rate, CRP, liver and renal function tests, and serum protein electrophoresis were performed in all patients. Additional tests including HLA class I and II phenotypes, serum C3 and C4 levels, anti-nuclear antibodies, anti-double-stranded DNA, rheumatoid factor, anti-thyroglobulin, anti-thyroperoxidase, and anti-neutrophil cytoplasmic antibodies with cytoplasmic (c)- or perinuclear (p)-staining. Bacterial, viral, fungal, and protozoal infections were also excluded as baseline investigations in all patients by disease specific laboratory tests.
The diagnosis of idiopathic AAU was made if ophthalmological examination revealed anterior chamber cells with or without flare, without vitreous cells, indicating iritis, iridocyclitis, and anterior cyclitis and none of the infectious etiologies, including tuberculosis, toxoplasmosis, syphilis, toxocariasis, borreliosis, rickettsiosis, herpes virus, human immunodeficiency virus and rubella were exist.
The control group consisted of randomly selected subjects with no evidence of intraocular inflammation and history of ocular disease (except for refractive error).
Exclusion criteria were: history of ocular surgery, ocular trauma, ocular manifestations other than uveitis, major systemic diseases including diabetes, renal failure, hepatic disease, hematologic and autoimmune diseases, arteriosclerotic/cardiovascular disease that could probably affect the N/L and P/L levels, and any history of drug usage such as, iron preparations, chemotherapy, vitamin and corticosteroid preparates that might influence the CBC measurements.
On admission, venous blood samples from the antecubital vein were obtained and CBC measurements were performed within 1h following blood collection with an automated blood cell counter (ABX Pentra 120, Horiba, Japan). Levels of neutrophils, lymphocytes, platelets, red blood cells, and white blood cell (WBC) were measured as part of the automated CBC. The N/L and P/L were calculated as the ratio of the neutrophils to lymphocytes, and platelets to lymphocytes.
Statistical Analysis
The statistical analyses were conducted by using the Statistical Package for Social Science v16.0 software (SPSS, Chicago, IL, USA). Demographic, clinical and biochemical variables were classified as categorical variables or continuous variables. Student's t-test or Mann-Whitney U test were used to compare the groups as appropriate. The χ2 test was used to compare categorical variables between groups. Pearson's correlations were performed for correlation analyses. P-values less than 0.05 were considered to be statistically significant. Receiver operating characteristic (ROC) analysis was also performed to identify the cut-off threshold and quantify the accuracy of N/L and P/L. Sensitivity, specificity and the area under the ROC curve were used for an overall estimation of the accuracy of the classifier.
RESULTS
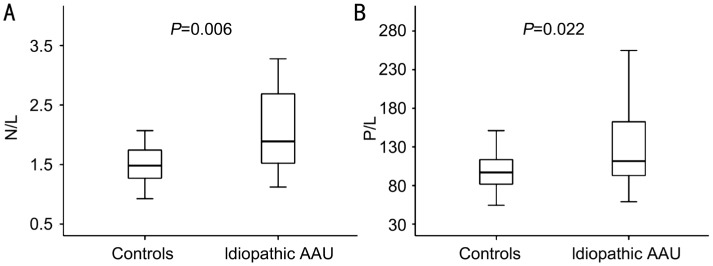
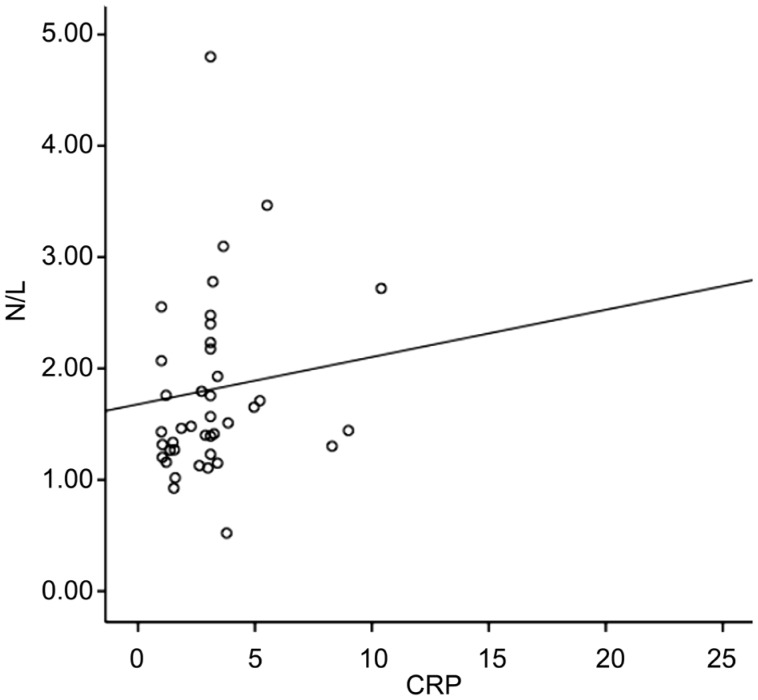
Comparison of demographic, clinical and biochemical variables of patients with idiopathic AAU and control groups are shown in Table 1. The mean age was 22±1y in the control group (n=36) while 22±1y in the AAU group (n=36) (P>0.05). Median CRP was 2.72 (3.43) in control group and 3.1 (11.65) in AAU group (P=0.046). In terms of N/L values, there was a statistically significant difference between control and AAU groups (P= 0.006) (Figure 1A). And also, a statistically significant difference was found in P/L between AAU and control groups (P=0.022) (Figure 1B). In addition, correlation analysis showed a significant correlation between CRP and N/L (P=0.002; r=0.461) as shown in Figure 2.
Table 1. Comparison of demographic and laboratory parameters between two groups.
| Variable | Control (n=36) | AAU (n=36) | P |
| Age (a) | 22±1 | 22±1 | 0.182 |
| CRP (µg/mL) | 2.72 (3.43) | 3.1 (11.65) | 0.046 |
| Sedim (mm/h) | 5 (6) | 6 (14) | 0.829 |
| WBC (103/µL) | 7.54±2.24 | 7.67±1.91 | 0.494 |
| Neutrophil (103/µL) | 3.79±1.26 | 4.69±1.87 | 0.054 |
| Lymphocyte (103/µL) | 2.43±0.86 | 2.14±0.67 | 0.207 |
| Platelet (103/µL) | 226±46 | 255±62 | 0.071 |
| N/L | 1.49 (0.52) | 1.89 (1.22) | 0.006 |
| P/L | 96.8 (32.9) | 111.7 (74) | 0.022 |
Data are expressed as the mean±SD, median (interquartile range) or number of cases as appropriate. P values were calculated using Student's t-test and Mann-Whitney U test as appropriate. n: Number of patients; CRP: C-reactive protein; WBC: White blood cells; N/L: Neutrophil to lymphocyte ratio; P/L: Platelet to lymphocyte ratio; AAU: Idiopathic acute anterior uveitis.
Figure 1. Boxplot showing N/L and P/L values.
A: Comparison of two groups in terms of N/L: P=0.006; B: Comparison of two groups in terms of P/L: P=0.022. AAU: Acute anterior uveitis; N/L: Neutrophil to lymphocyte ratio; P/L: Platelet to lymphocyte ratio.
Figure 2. Correlation between N/L and CRP (P=0.002; r=0.461).
N/L: Neutrophil to lymphocyte ratio; CRP: C-reactive protein.
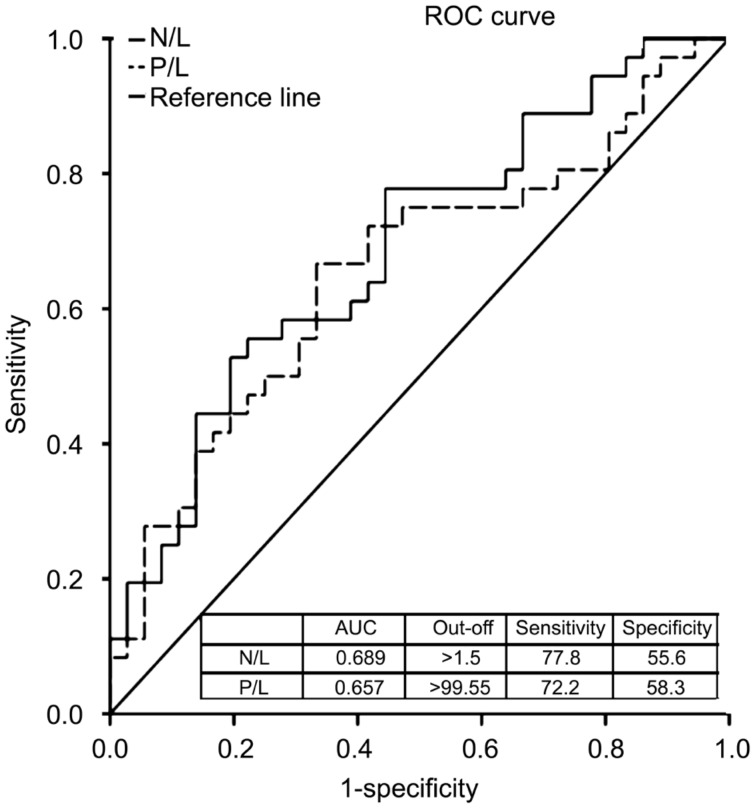
The receiver-operating characteristic (ROC) analysis of the studied variables are shown in Figure 3. According to this, the area under the ROC (AUROC) value of the N/L to distinguish patients and controls was found to be 0.689. The best cut-off value was 1.51 with a sensitivity of 77.8% and a specificity of 55.6% (Figure 3). On the other hand, the AUROC value of the P/L to distinguish patients and controls were 0.657, with the cut-off value 99.55, sensitivity 72.2% and specificity 58.3% (Figure 3).
Figure 3. ROC curve analysis for discrimination between controls and idiopathic AAU.
AAU: Acute anterior uveitis; N/L: Neutrophil to lymphocyte ratio; P/L: Platelet to lymphocyte ratio.
DISCUSSION
In this study, we investigated N/L and P/L in patients with idiopathic AAU and in control subjects. To the best of our knowledge, the current study showed for the first time that patients with idiopathic AAU have increased N/L and P/L levels compared to controls. In addition, correlation results suggest that there was a positive correlation between serum CRP levels, an indicator of systemic inflammation, and N/L. According to ROC analysis, a cut-off value, 1.51, for N/L was determined to distinguish between idiopathic AAU patients and controls while the cut-off value for P/L was 99.55.
While the inflammatory responses are well established, the studies to uncover the exact mechanisms underlying the pathogenesis of idiopathic AAU are still ongoing. In a study by Deschenes et al[21], it was reported that lymphocyte activity measured by expression of IL-2 receptors was positively correlated with clinical uveitis activity, suggesting that abnormal systemic immune activation in the cases with idiopathic uveitis. Conversely, a study aimed to find out differences in the immune cell types and cytokines within the aqueous humor in patients with uveitis associated and not associated with a systemic disease by Calder et al[22] stated that the cell types within the aqueous humor are significantly differ. They speculated that in uveitis patients without association with systemic disease, either anterior or posterior, the immune responses within the eye were too localized to be detected in the peripheral blood samples. The authors concluded that sera of these patients might not be the best appropriate site for detecting immunity abnormalities in anterior and/or posterior uveitis patients due to the limited reflection of the immune processes occuring within the eye[22]. In another study to quantify the neutrophil gelatinase-associated lipocalin (NGAL) levels in the aqueous humor of patients with idiopathic AAU, the authors found out the level of NGAL in the aqueous humor of idiopathic AAU patients was higher and they stated that aqueous humor NGAL level has a regulatory role in inflammation in the eyes of idiopathic AAU patients and might be a useful biomarker of intraocular imlammation and response to the treatment for immunomodulation[23]. As indicated in a study aimed to evaluate the systemic inflammatory status and native immune responsiveness in currently normal subjects with a history of AAU, the authors concluded that high native immune responsiveness might have a key role in the development of intraocular inflammation[8].
As discussed above, alteration of different immune mediators measured in blood or aqueous humor supports abnormal activity of the immune system in idiopathic AAU. Therefore, the presence and persistence of inflammation is effective on prognosis, and specific inflammatory markers that possible to use widely, would be beneficial to follow up of these patients. As given in a recent study, evaluating the predictive value of N/L in identifying risk for RVO, N/L was found to be significantly higher in RVO patients compared to the controls[16]. In another study evaluating N/L in dry and wet AMD, patients with AMD were determined to have higher N/L values compared with controls, and N/L was correlated with disease severity[17]. In keratoconus patients which local inflammatory cascades are more prominent, N/L had significantly higher levels in patients with progressive keratoconus compared to the non-progressive keratoconus patients and control group[18]. In addition, according to a study performed in the patients with diabetic retinopathy (DR), N/L values were found to be correlated with the presence of DR and DR grades[19]. In another study to determine the levels of N/L and P/L in patients with PEX, the authors concluded that P/L and N/L might be a predictive biomarker of the prognosis of PEX patients and progression to PEX glaucoma[20]. All these findings prove that the prognostic value of N/L in ocular diseases that thought both local and systemic inflammatory cascades have critical roles in the pathophysiology. However, as in other system inflammation processes, the lack of specific inflammatory biomarkers creates a major shortcoming in ocular inflammatory pathologies. But, as we demonstrated in our study, >1.51 cut-off value for N/L distinguishes the 68.9% of patients with 77.8% sensitivity in monitoring disease development. At the same time, especially during the treatment process, we think this cut-off value may play a crucial role in determining the limitation of inflammation. Nevertheless, when we evaluated P/L to distinguish AAU and control groups, P/L cut-off value (>99.55) was found with a sensitivity of 72% and the specificity of 58%, which were lower than the specificity of N/L. The similar sensitivity levels of N/L and P/L cut-off values to distinguish idiopathic AAU patients from controls prove the discriminative and prognostic power of these biomarkers once again. This also indicates that, N/L and P/L have equivalent value to distinguish AAU group from controls.
CRP is a reliable marker for systemic inflammatory conditions and studies uncovered that serum CRP levels are higher than the healthy subjects, suggesting the continued systemic inflammation[8]. In addition, our study showed the patients with AAU have significantly higher serum CRP levels compared to controls. Also, we found a positive correlation between serum CRP levels and N/L. This finding suggests that N/L could be a useful biomarker, like CRP, indicates that inflammatory status in patients with AAU. Due to N/L is more significant than P/L to distinguish the patients from controls and correlated with CRP, it could be a useful biomarker predicting the prognosis in idiopathic AAU patients.
The main limitation of our study is the relatively small sample size. The present data should therefore be interpreted with caution and need reconfirmation in a larger cohort. Another limitation is the retrospective nature of the study. Our findings need to be confirmed with prospective controlled clinical trials. Also, as a result of being a military hospital, our patient population exists predominantly by males.
In conclusion, considering the limited numbers of studies investigating the association between N/L, P/L and ocular diseases in the literature and despite the limitations indicated above, this is the first study evaluating N/L and P/L in patients with idiopathic AAU, and this makes the results of our study more meaningful. This is the first study provides the evidence of N/L and P/L might be a biomarker for prediction of the prognosis of idiopathic AAU patients. On the other hand, further studies investigating mechanisms playing role in chronic low-grade inflammation in patients with AAU are needed.
Acknowledgments
Conflicts of Interest: Ozgonul C, None; Sertoglu E, None; Ayyildiz O, None; Mumcuoglu T, None; Kucukevcilioglu M, None; Gokce G, None; Durukan AH, None.
REFERENCES
- 1.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, Thorne JE. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linssen A, Rothova A, Valkenburg HA, Dekker-Saeys AJ, Luyendijk L, Kijlstra A, Feltkamp TE. The lifetime cumulative incidence of acute anterior uveitis in a normal population and its relation to ankylosing spondylitis and histocompatibility antigen HLA-B27. Invest Ophthalmol Vis Sci. 1991;32(9):2568–2578. [PubMed] [Google Scholar]
- 3.Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. 2005;50(4):364–388. doi: 10.1016/j.survophthal.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21(2-3):197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- 5.Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, Omar M. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33(5):251–255. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 6.Takase H, Futagami Y, Yoshida T, Kamoi K, Sugita S, Imai Y, Mochizuki M. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47(4):1557–1561. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Liu B, Maminishkis A, Mahesh SP, Yeh S, Lew J, Lim WK, Sen HN, Clarke G, Buggage R, Miller SS, Nussenblatt RB. Gene expression profiling in autoimmune noninfectious uveitis disease. J Immunol. 2008;181(7):5147–5157. doi: 10.4049/jimmunol.181.7.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huhtinen M, Repo H, Laasila K, Jansson SE, Kautiainen H, Karma A, Leirisalo-Repo M. Systemic inflammation and innate immune response in patients with previous anterior uveitis. Br J Ophthalmol. 2002;86(4):412–417. doi: 10.1136/bjo.86.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tervo T, van Setten GB, Hovi M, Pakarinen M, Tarkkanen A, Valtonen V. C-reactive protein serum levels in patients with ocular disease. Acta Ophthalmol (Copenh) 1994;72(1):110–113. doi: 10.1111/j.1755-3768.1994.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozgonul C, Sertoglu E. Accurate use of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. 2015;24(3):359–360. doi: 10.3109/09273948.2014.970281. [DOI] [PubMed] [Google Scholar]
- 11.Sertoglu E, Uyanik M. Accurate use of neutrophil/lymphocyte ratio from the perspective of laboratory experts. Vasc Health Risk Manag. 2014;10:13–14. doi: 10.2147/VHRM.S56906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltran BE, Aguilar C, Quinones P, Morales D, Chavez JC, Sotomayor EM, Castillo JJ. The neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leuk Lymphoma. 2016;57(1):58–62. doi: 10.3109/10428194.2015.1045897. [DOI] [PubMed] [Google Scholar]
- 13.Bozbay M, Ugur M, Uyarel H, Cicek G, Koroglu B, Tusun E, Sunbul M, Murat A, Sari I, Eren M. Neutrophil-to-lymphocyte ratio as a prognostic marker in infective endocarditis: in-hospital and long-term clinical results. J Heart Valve Dis. 2014;23(5):617–623. [PubMed] [Google Scholar]
- 14.Kara M, Dogru T, Genc H, Sertoglu E, Celebi G, Gurel H, Kayadibi H, Cicek AF, Ercin CN, Sonmez A. Neutrophil-to-lymphocyte ratio is not a predictor of liver histology in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27(10):1144–1148. doi: 10.1097/MEG.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 15.Spolverato G, Maqsood H, Kim Y, Margonis G, Luo T, Ejaz A, Pawlik TM. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111(7):868–874. doi: 10.1002/jso.23900. [DOI] [PubMed] [Google Scholar]
- 16.Dursun A, Ozturk S, Yucel H, Ozec AV, Dursun FG, Toker MI, Erdogan H, Arici MK, Topalkara A. Association of neutrophil/lymphocyte ratio and retinal vein occlusion. Eur J Ophthalmol. 2015;25(4):343–346. doi: 10.5301/ejo.5000570. [DOI] [PubMed] [Google Scholar]
- 17.Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H, Keskin U. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. 2014:1–4. doi: 10.3109/09273948.2014.921715. [DOI] [PubMed] [Google Scholar]
- 18.Karaca EE, Ozmen MC, Ekici F, Yuksel E, Turkoglu Z. Neutrophil-to-lymphocyte ratio may predict progression in patients with keratoconus. Cornea. 2014;33(11):1168–1173. doi: 10.1097/ICO.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 19.Wang RT, Zhang JR, Li Y, Liu T, Yu KJ. Neutrophil-Lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complicat. 2015;29(2):245–249. doi: 10.1016/j.jdiacomp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Ozgonul C, Sertoglu E, Mumcuoglu T, Ozge G, Gokce G. Prediction of pseudoexfoliation syndrome and pseudoexfoliation glaucoma by using neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. Ocul Immunol Inflamm. 2016;24(6):665–670. doi: 10.3109/09273948.2015.1063671. [DOI] [PubMed] [Google Scholar]
- 21.Deschenes J, Char DH, Kaleta S. Activated T lymphocytes in uveitis. Br J Ophthalmol. 1988;72(2):83–87. doi: 10.1136/bjo.72.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calder VL, Shaer B, Muhaya M, Mclachlan M, Pearson RV, Jolly G, Towler HM, Lightman S. Increased CD4+ expression and decreased IL-10 in the anterior chamber in idiopathic uveitis. Invest Ophthalmol Vis Sci. 1999;40(9):2019–2024. [PubMed] [Google Scholar]
- 23.Salom D, Sanz-Marco E, Mullor JL, Lopez-Prats MJ, Garcia-Delpech S, Udaondo P, Millan JM, Arevalo JF, Diaz-Llopis M. Aqueous humor neutrophil gelatinase-associated lipocalin levels in patients with idiopathic acute anterior uveitis. Mol Vis. 2010;16:1448–1452. [PMC free article] [PubMed] [Google Scholar]