Abstract
AIM
To measure the retinal oxygen saturation in healthy subjects and early branch retinal vein occlusion (BRVO) in Chinese population.
METHODS
The retinal vessel oxygen saturation of the healthy subjects and BRVO patients were measured by a noninvasive retinal oximeter (Oxymap ehf., Reykjavik, Iceland).
RESULTS
The study included 22 patients with unilateral BRVO (mean age: 55.1±8.8y) in the study group and 91 healthy participants (mean age: 37.5±14.0y) in the control group. In the healthy individuals, mean arterial and venous oxygen saturation were significantly (P<0.001) higher in the superior nasal quadrant (98.5%±10.1% and 57.3%±8.7%, respectively) than in the inferior nasal quadrant (94.2%±9.0% and 54.1%±9.6%, respectively), followed by the superior temporal quadrant (89.1%±10.1% and 51.9%±8.9%, respectively) and the inferior temporal quadrant (86.4%±9.4% and 46.6%±9.6%, respectively). In patients with ischemic BRVO, arterial oxymetric values were significantly higher and venous measurements significantly lower for the affected vessel (107.5%±9.7% and 46.4%±14.2%, respectively) than the unaffected vessel in the same eye (99.2%±12.2% and 55.5%±7.9%, respectively) and as compared to the vessel in the unaffected fellow eye (93.1%±6.9% and 55.7%±6.8%) (P=0.005 and P=0.02, respectively). In the patients with non-ischemic BRVO, mean venous oxygen saturation was lower in the affected vein (39.8%±12.2%) than in the unaffected vessels of the same eye (50.8%±10.5%) and in the fellow eye (58.21%±5.7%) (P=0.03). Mean arterial oxygen saturation did not differ significantly (P=0.42) between all three groups.
CONCLUSION
In patients with BRVO, the venous oxygen saturation in the affected vessels is decreased potentially due to decreased blood velocity and flow. Interestingly, the arterial oxygen saturation in eyes with ischemic BRVO is increased in the affected arteries.
Keywords: ischemia, retinal oxygen saturation, branch retinal vein occlusion, retinal oximetry
INTRODUCTION
Retinal vein occlusions (RVOs) are the second most common retinal vascular disorder after diabetic retinopathy and are a significant cause of visual impairment[1]–[3]. Branch retinal vein occlusion (BRVO) is the most common form of RVOs, with a prevalence of approximately 0.5%-1.2% in a population aged 40+ years[1]–[2]. Although the pathogenesis of BRVOs has remained elusive yet, three pathomechanisms have been discussed: compression of the vein at an arteriovenous crossing in the retina, degenerative changes of the vessel wall, and abnormal hematological factors[4]–[6]. Complications of BRVOs include macular edema and decreased retinal perfusion. The retinal malperfusion affects predominantly the inner two-thirds of the retina since these retinal layers receive their blood and oxygen supply mainly from the retinal blood vessels[7]–[8]. The retinal malperfusion in BRVO is associated with a decrease in the partial oxygen pressure[9]–[10].
The retinal partial oxygen pressure can be measured with a recently developed new device, the retinal oximeter[11]–[15]. Since knowledge of the oxymetric status of the retina in eyes with BRVOs may be clinically valuable to differentiate between an ischemic type of BRVO and a non-ischemic type of BRVO, we conducted this study to measure the retinal vessel oxygen saturation by retinal oxymetry. Measurement of the retinal oxygenation in the early stage of BRVO may be of help to confirm the diagnosis and to assess the severity of the occlusion.
SUBJECTS AND METHODS
The hospital-based, observational, comparative study included normal individuals in Chinese population and a study group of patients with early BRVO. The study protocol was approved by the Medical Ethics Committee of the Beijing Tongren Hospital and written informed consent was obtained from all study participants. Inclusion criteria for the participants of the study group were occurrence of a unilateral BRVO within the last three months, and no history any other ocular disease and no history of any previous ocular therapy including retinal laser treatment and medical intravitreal anti-vascular endothelial growth factor therapy. BRVO was defined by ophthalmoscopical criteria including intraretinal, flame-shaped, dot-like or blot-shaped hemorrhages in a quadrant of the fundus, soft and hard exudates, retinal edema, dilatation and tortuosity of the related retinal veins, and by fluorescein angiographic characteristics such as macular edema and ischemia and delayed filling of the retinal veins. A capillary non-perfusion area >5 (disk diameters) DD upon fluorescein angiography was defined as ischemic BRVO and a non-perfusion area of the retinal capillary ≤5 DD was defined as non-ischemic BRVO. Inclusion criteria for the normal individuals were no history of any ocular disease, a normal appearance of the anterior segment and posterior segment of the eye, intraocular pressure within the normal range, best-corrected visual acuity of 20/20 or better, and an age ≥40y. For all study participants, the quality of the oxymetric fundus images had to be of sufficient quality for a quantitative assessment.
All study participants underwent an ophthalmic examination, including refractometry and measurement of best-corrected visual acuity, slit-lamp assisted biomicroscopic examination of the anterior and posterior segment of the eye, tonometry, fundus photography (Hybrid Digital Mydriatic Retinal Camera CX-1 Canon Inc., Tokyo, Japan), spectral-domain optical coherence tomography (Heidelberg Engineering Co, Heidelberg, Germany), and retinal oxymetry. Retinal oxymetry was performed using a retinal oximeter (Oxymap ehf., Reykjavik, Iceland), which is based on a fundus camera (Topcon Retinal Camera, TRC-50DX, Japan), two digital cameras, an optical adapter and a beam splitter. Since most of the oxygen in blood is carried bound to hemoglobin, SaO2 is a good measure of the amount of oxygen in blood. Oxygenated and deoxygenated hemoglobin have different colors. This means that blood with different oxygen saturation has different colors. Non-invasive oximetry is based on this color difference. Absorbance at wavelengths like 570 nm is not sensitive to oxygen saturation. Absorbance at 600 nm and most other wavelengths is sensitive to oxygen saturation. Oximetry with two wavelengths uses one oxygen sensitive wavelength and one oxygen insensitive wavelength. The device could automatically select measurement points on the oximetry images and calculate the optical density of retinal vessels at two wavelengths (600 nm and 570 nm)[12]–[15]. The final oxygen saturation result cannot be viewed as an absolute value. Factors such as vessel width and fundus pigmentation are likely to influence the results. After medical dilatation of the pupils using tropicamide eye drops (Mydrin-P; Santen Oy, Japan), the fundus images were taken in a dark room. For each study participant and eye, we took an image centered on the optic nerve head and an image centered on the macula (Figure 1). All of the fundus images were performed by the same photographer.
Figure 1. Fundus photograph showing the retinal vessels as imaged and measured by the oximeter.
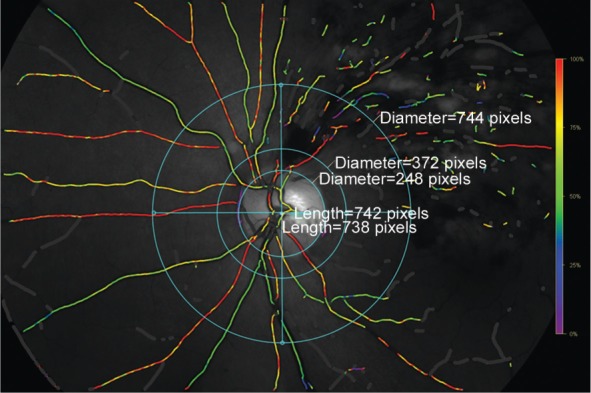
In the superior, inferior, nasal and temporal quadrant, we selected the thickest arteriole and thickest venule for each quadrant between the two circles outside of the optic disc border.
In the assessment of the fundus photographs, the retinal vessels were divided into three groups: 1) vessels affected by the occlusion in the eye with BRVO; 2) retinal vessels which were not affected by the occlusion in the eye with BRVO; 3) vessels in the fellow eye. The measured unaffected vessels were comparable in location to the affected vessels. For example, if a superotemporal venule was occluded, an inferotemporal venule was chosen for comparison in the same eye and a superotemporal venule in the fellow eye. We defined the affected vessels as the vessels that close to the occlusion and supplied the affected area at the greatest extent. In a second step of image assessment, we measured the vessels separately for the superior, inferior, nasal and temporal quadrants all eyes included into the study. For analyzing the quadrant, we selected the thickest arteriole and venule in each quadrant between two circles which had a distance to the optic disc border of 0.5 disc diameter and of 3 disc diameters, respectively (Figure 1).
Statistical Analysis
The statistical analysis was performed applying a statistical software program (SPSS for Mac, version 22, IBM/SPSS, Chicago, IL, USA). Friedman's test was used for the comparison between the BRVO eye and contralateral eye. A two-tailed P-value of <0.05 was considered to be statistically significant.
RESULTS
The control group included 91 healthy participants (37 men) with a mean age of 37.5±14.0y (range: 17 to 64y) (Table 1). The mean intraocular pressure was 14.5±3.5 mm Hg (range: 7.0 to 22.0 mm Hg) and mean refractive error (spherical equivalent) was -1.0±2.3 diopters (range: -7.8 to 2.5 diopters). The mean arterial oxygen saturation was significantly (P<0.001) higher in the superior nasal quadrant (98.5%±10.1%; range: 77.2%-126.8%) than in the inferior nasal quadrant (94.2%±9.0%; range: 72.9%-118.5%), followed by the superior temporal quadrant (89.1%±10.1%; range: 41.4%-119.6%) and finally the inferior temporal quadrant (86.4%±9.4%; range: 67.2%-119.7%) (Table 1). In a similar manner, the mean venous oxygen saturation was significantly (P<0.001) the highest in the superior nasal quadrant (57.3%±8.7%; range: 17.2% -71.8%), followed by the inferior nasal quadrant (54.1%±9.6%; range: 22.0%-76.1%), the superior temporal quadrant (51.9%±8.9%; range: 32.0%-88.3%), and finally the inferior temporal quadrant (46.6%±9.6%; range: 18.3%-69.2%) (Table 1).
Table 1. Characteristics in the healthy individuals of the control group.
| Parameters | Patients (n=91) |
| Age (a) | 37.5±14.0 |
| Women/Men | 54/37 (59.3%/40.7%) |
| Intraocular pressure (mm Hg) | 14.5±3.5 |
| Systolic pressure (mm Hg) | 116.3±13.4 |
| Diastolic pressure (mm Hg) | 76.6±10.3 |
| Refractive error (diopters) | -1.0±2.3 |
| Retinal arterial oxygen saturation (%) | |
| Superior nasal quadrant | 98.5±10.1 |
| Inferior nasal quadrant | 94.2±9.0 |
| Inferior temporal quadrant | 86.4±9.4 |
| Superior temporal quadrant | 89.1±10.1 |
| Retinal venous oxygen saturation (%) | |
| Superior nasal quadrant | 57.3±8.7 |
| Inferior nasal quadrant | 54.1±9.6 |
| Inferior temporal quadrant | 46.6±9.6 |
| Superior temporal quadrant | 51.9±8.9 |
x±s
The study group included 22 patients with unilateral BRVO (9 men) and with a mean age of 55.1±8.8y (range: 41 to 77y) (Table 2). An ischemic BRVO was present in 13 patients (age: 53.2±6.8y) and a non-ischemic BRVO was detected in 9 patients (age: 57.7±11.0y).
Table 2. Characteristics and measurements in the patients with a unilateral BRVO.
| Parameters | Patients (n=22) |
| Age (a) | 55.1±8.8 |
| Women/Men | 13/9 (59%/41%) |
| Intraocular pressure (mm Hg) | 16.2±3.7 |
| Systolic pressure (mm Hg) | 130±15.7 |
| Diastolic pressure (mm Hg) | 80±9.3 |
| Refractive error (diopters) | 0.16±2.9 |
| Retinal arterial oxygen saturation (%) | |
| Superior nasal quadrant | 99.6±9.0 |
| Inferior nasal quadrant | 97.5±8.3 |
| Inferior temporal quadrant | 93.1±9.6 |
| Superior temporal quadrant | 103.5±13.3 |
| Retinal venous oxygen saturation (%) | |
| Superior nasal quadrant | 57.9±12.3 |
| Inferior nasal quadrant | 55.2±8.5 |
| Inferior temporal quadrant | 46.3±13.1 |
| Superior temporal quadrant | 48.9±13.3 |
x±s
Comparing the retinal oxygen saturation measurements in the patients with an ischemic BRVO revealed significantly higher arterial oxymetric values for the affected artery (107.5%±9.7%) as compared to the unaffected artery in the same eye (99.2%±12.2%) and as compared to artery in the unaffected fellow eye (93.1%±6.9%) (P=0.005). It showed significantly lower values for the affected veins (46.4%±14.2%) as compared to the unaffected veins in the same eye (55.5%±7.9%) and as compared to the vein in the unaffected fellow eye (55.7%±6.8%) (P=0.02) (Table 3). In the non-ischemic BRVO group, the mean venous oxygen saturation was significantly lower in the affected vessels (39.8 %±12.2%) than in the unaffected vessels of the same eye (50.8%±10.5%) and the fellow eye (58.2%±5.7%) (P=0.03) (Table 3). The mean arterial oxygen saturation did not differ significantly (P=0.42) between all three groups.
Table 3. Retinal oxymetric measurements in the retinal vessels affected by a BRVO as compared with the unaffected vessels in the same eye and the vessels in the fellow eyes without BRVO.
| Groups | Ischemic BRVO (n=13 patients) |
Non-ischemic BRVO (n=9 patients) |
||||||
| Affected vessels | Unaffected vessels, same eye | Unaffected vessels, fellow eye | P | Affected vessels | Unaffected vessels, same eye | Unaffected vessels, fellow eye | P | |
| SaO2-A | 107.5±9.7 | 99.2±12.2 | 93.1±6.9 | 0.005 | 103.0±15.3 | 95.9±7.8 | 92.5±9.1 | 0.42 |
| SaO2-V | 46.4±14.2 | 55.5±7.9 | 55.7±6.8 | 0.02 | 39.8±12.2 | 50.8±10.5 | 58.2±5.7 | 0.03 |
%
DISCUSSION
The examinations showed for the healthy individuals of the control group that mean arterial and venous oxygen saturation were significantly (P<0.001) higher in the superior nasal quadrant than in the inferior nasal quadrant, followed by the superior temporal quadrant and the inferior temporal quadrant. In patients with ischemic BRVO, arterial oxymetric values were significantly higher and venous measurements were significantly lower for the affected vessel than for the unaffected vessels in the same eye and as compared to the vessels in the unaffected fellow eye. In the patients with non-ischemic BRVO, mean venous oxygen saturation was lower in the affected vein than in the unaffected vessels of the same eye and in the fellow eye.
The increased arterial oxygen saturation in the ischemic BRVO group in our study agreed with findings obtained in previous investigations[16]. In the majority of patients, BRVO may be due to several mechanisms: compression of the vein at the arteriovenous (A/V) crossing, degenerative changes of the vessel wall, and abnormal hematological factors[4]–[6]. Increased blood flow in the artery may result in venous endothelial injury by increase of hemodynamic stress at the arteriovenous crossing. Sclerosis of the retinal artery associated with systemic disorders may cause a compression of the vein, what may increase the endothelin-1 concentration and stimulate venous vasoconstriction[17]. Thus, the increased arterial oxymetric measurement in eyes with ischemic BRVO could be explained by several reasons. First, the perfusion in the occluded venules were decreased, which resulted in the reduction of oxygen consumption in the area, so that the oxygen saturation remained relatively high in the artery. Second, the association between BRVO and hyperviscosity of the blood caused by a high hematocrit have been reported in some studies[17]–[18]. Local blood viscosity increases parallel to an increase in the hematocrit under conditions of low blood flow and increased erythrocyte aggregation[19]. Locally increased blood viscosity and increased hematocrit may agree with an increased arterial oxymetric value.
The venous oxymetric values in the patients with an ischemic BRVO were lower in the occluded vessels than in the unaffected vessels and in the corresponding veins in the fellow eye. It may be explained by the compression of veins at arterio-venous crossing sites and potentially by a venous endothelial injury. Due to the decreased blood flow and velocity, the tissue in the capillary bed may extract oxygen more than normally out of the capillaries so that the oxygen saturation in the draining veins was reduced. The same may hold true for the patients with a non-ischemic BRVO, while in the same patients, the oxygen saturation did not differ for the arterial vessels.
Potential limitations of our study should be mentioned. First, the number of study participants was relatively small. Despite the small sample size, however, the differences between the subgroups were statistically significant so that this weakness of the study may only serve to strengthen the results and conclusions drawn. Second, the oximetry analysis of the oxygen saturation relied on reflected light, the fundus pigment, the diameter of the retinal vessels and on lens opacities, which might have influenced the measurements[12]–[15],[20].
In conclusion, the arterial and venous oxygen saturation in the healthy eyes was significantly higher in the superior nasal quadrant than in the inferior nasal quadrant, followed by the superior temporal quadrant and the inferior temporal quadrant. In patients with BRVO, the venous oxygen saturation in the affected vessels was decreased potentially due to decreased blood velocity and flow. Interestingly, the arterial oxygen saturation in eyes with ischemic BRVO was increased in the affected arteries.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81570891; No.81272981); the Beijing Municipal Administration of Hospitals' Ascent Plan (No.DFL20150201); Science & Technology Project of Beijing Municipal Science & Technology Commission (No.Z151100001615052); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No.ZYLX201307); Beijing Natural Science Foundation (No.7151003); Advanced Health Care Professionals Development Project of Beijing Municipal Health Bureau (No.2014-2-003).
Conflicts of Interest: Yang JY, None; You B, None; Wang Q, None; Chan SY, None; Jonas JB, None; Wei WB, None.
REFERENCES
- 1.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY. International Eye Disease Consortium. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–319. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W, Xu L, Jonas JB. Vein occlusions in Chinese subjects. Ophthalmology. 2007;114(9):1795–1796. doi: 10.1016/j.ophtha.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye (Lond) 2011;25(8):981–988.e1. doi: 10.1038/eye.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014;132(1):13–22. doi: 10.1001/jamaophthalmol.2013.5515. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24(4):493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106(11):2054–2062. doi: 10.1016/S0161-6420(99)90483-9. [DOI] [PubMed] [Google Scholar]
- 7.Lim HB, Kim MS, Jo YJ, Kim JY. Prediction of retinal ischemia in branch retinal vein occlusion: spectral-domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2015;56(11):6622–6629. doi: 10.1167/iovs.15-17678. [DOI] [PubMed] [Google Scholar]
- 8.Avila CP, Jr, Bartsch DU, Bitner DG, Cheng L, Mueller AJ, Karavellas MP, Freeman WR. Retinal blood flow measurements in branch retinal vein occlusion using scanning laser Doppler flowmetry. Am J Ophthalmol. 1998;126(5):683–690. doi: 10.1016/s0002-9394(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Pournaras CJ, Tsacopoulos M, Strommer K, Gilodi N, Leuenberger PM. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990;97(10):1321–1328. doi: 10.1016/s0161-6420(90)32415-6. [DOI] [PubMed] [Google Scholar]
- 10.Stefansson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31(2):284–289. [PubMed] [Google Scholar]
- 11.Harris A, Dinn RB, Kagemann L, Rechtman E. A review of methods for human retinal oximetry. Ophthalmic Surg Lasers Imaging. 2003;34(2):152–164. [PubMed] [Google Scholar]
- 12.Hardarson SH, Harris A, Karlsson RA, Halldorsson GH, Kagemann L, Rechtman E, Zoega GM, Eysteinsson T, Benediktsson JA, Thorsteinsson A, Jensen PK, Beach J, Stefansson E. Automatic retinal oximetry. Invest Ophthalmol Vis Sci. 2006;47(11):5011–5016. doi: 10.1167/iovs.06-0039. [DOI] [PubMed] [Google Scholar]
- 13.Olafsdottir OB, Vandewalle E, Abegao Pinto L, Geirsdottir A, De Clerck E, Stalmans P, Gottfredsdottir MS, Kristjansdottir JV, Van Calster J, Zeyen T, Stefansson E, Stalmans I. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br J Ophthalmol. 2014;98(3):329–333. doi: 10.1136/bjophthalmol-2013-303162. [DOI] [PubMed] [Google Scholar]
- 14.Geirsdottir A, Hardarson SH, Olafsdottir OB, Stefansson E. Retinal oxygen metabolism in exudative age-related macular degeneration. Acta Ophthalmol. 2014;92(1):27–33. doi: 10.1111/aos.12294. [DOI] [PubMed] [Google Scholar]
- 15.Goharian I, Iverson SM, Ruiz RC, Kishor K, Greenfield DS, Sehi M. Reproducibility of retinal oxygen saturation in normal and treated glaucomatous eyes. Br J Ophthalmol. 2015;99(3):318–322. doi: 10.1136/bjophthalmol-2014-305718. [DOI] [PubMed] [Google Scholar]
- 16.Hardarson SH, Stefansson E. Oxygen saturation in branch retinal vein occlusion. Acta Ophthalmol. 2012;90(5):466–470. doi: 10.1111/j.1755-3768.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 17.McGrath MA, Wechsler F, Hunyor AB, Penny R. Systemic factors contributory to retinal vein occlusion. Arch Intern Med. 1978;138(2):216–220. [PubMed] [Google Scholar]
- 18.Trope GE, Lowe GD, McArdle BM, Douglas JT, Forbes CD, Prentice CM, Foulds WS. Abnormal blood viscosity and haemostasis in long-standing retinal vein occlusion. Br J Ophthalmol. 1983;67(3):137–142. doi: 10.1136/bjo.67.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HC, Wiek J, Gupta A, Luckie A, Kohner EM. Effect of isovolaemic haemodilution on visual outcome in branch retinal vein occlusion. Br J Ophthalmol. 1998;82(2):162–167. doi: 10.1136/bjo.82.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell RA, Anderson AJ, Hosking SL, Batcha AH, Bui BV. Test-retest reliability of retinal oxygen saturation measurement. Optom Vis Sci. 2014;91(6):608–614. doi: 10.1097/OPX.0000000000000257. [DOI] [PubMed] [Google Scholar]


