Abstract
Brain-derived neurotrophic factor (BDNF) regulates the survival and growth of neurons, and influences synaptic efficiency and plasticity. The human BDNF gene consists of 11 exons, and distinct BDNF transcripts are produced through the use of alternative promoters and splicing events. The majority of the BDNF transcripts can be detected not only in the brain but also in the blood cells, although no study has yet investigated the differential expression of BDNF transcripts at the peripheral level. This review provides a description of the human BDNF gene structure as well as a summary of clinical and preclinical evidence supporting the role of BDNF in the pathogenesis of psychiatric disorders. We will discuss several mechanisms as possibly underlying BDNF modulation, including epigenetic mechanisms. We will also discuss the potential use of peripheral BDNF as a biomarker for psychiatric disorders, focusing on the factors that can influence BDNF gene expression and protein levels. Within this context, we have also characterized, for we believe the first time, the expression of BDNF transcripts in the blood, with the aim to provide novel insights into the molecular mechanisms and signaling that may regulate peripheral BDNF gene expression levels.
BDNF and the vulnerability to psychiatric disorders
The neurotrophin brain-derived neurotrophic factor (BDNF) is one of the most studied and characterized neurotrophin in the central nervous system, and it has received remarkable attention because of its importance in the development and maintenance of normal brain function. It has been established that BDNF mediates survival and differentiation of neurons by binding and activating the tropomycin receptor kinase B (TrkB), a member of the larger family of Trk receptors, localized both on the pre-synaptic and post-synaptic membranes. The binding of BDNF to TrkB leads to the dimerization and autophosphorylation of tyrosine residues in the intracellular domain of the receptor and subsequent activation of cytoplasmic signaling pathways including mitogen-activated protein kinase, phospholipase C-γ and phosphatidylinositol-3 kinase.1 In addition to neurotrophic effects, BDNF–TrkB signaling is involved in transcription, translation and trafficking of proteins during various phases of synaptic development and has been implicated in several forms of neuroplasticity in different brain areas. This is especially important, as growing evidence suggests a role for BDNF in the pathophysiology of brain-associated illnesses, including psychiatric disorders.2 Indeed, changes in BDNF expression have been extensively investigated in major depression, schizophrenia (SZ), bipolar and anxiety disorders, and a plethora of data demonstrate altered BDNF expression and impaired BDNF function.3, 4, 5, 6, 7 BDNF may also represent a shared risk factor for the vulnerability of these pathologies, by acting on biological mechanisms such as neuroplasticity, inflammation or hypothalamic–pituitary–adrenal axis functionality that are altered in all these psychiatric disorders.
BDNF and major depression
Reduced BDNF gene expression and protein levels have been reported in depressed patients, both in post-mortem brain tissues8, 9, 10 and in peripheral blood samples.3, 11, 12, 13, 14 Importantly, the investigation of animal models of depression has provided strong support to these clinical findings. Indeed, a variety of stress paradigms in rodents that are able to induce a depressive-like behavior, such as social defeat, maternal deprivation or prenatal stress exposure, are associated with a reduction of BDNF mRNA levels in different brain regions, including the hippocampus and prefrontal cortex.15, 16, 17 Conversely, antidepressant treatments are able to upregulate BDNF expression in animal models15, 18, 19 and also to normalize decreased BDNF blood levels in depressed subjects.11, 14
Emerging data suggest that electroconvulsive treatment in humans and in animal models may reduce depressive symptoms by increasing the expression of BDNF. In the systematic review and meta-analysis of preclinical and clinical data, Polyakova et al.20 tested the association between ECT (electroconvulsive stimulation in animals) and changes in BDNF concentrations and their effects on behavior. The authors found that in rodents electroconvulsive stimulation increased BDNF mRNA and protein concentration in the brain, with the largest effect size in the dental gyrus. The increase in BDNF was positively correlated with the number of treatments and negatively correlated with the time between the last ECT and BDNF measurement. Moreover, BDNF concentration did not increase in the course of treatment both in rodent and human serum samples, yet the levels were increased in human plasma. The authors concluded that electroconvulsive stimulation in rodents and ECT in humans can increase BDNF mRNA and protein levels, although this effect is not consistently associated with changes in behavior.
Consistent findings in clinical samples indicated the presence of low serum BDNF levels in depressed patients as compared with healthy subjects, suggesting that decreased peripheral levels of this gene could represent a ‘state' characteristic of depression. This has been supported by several studies,21, 22 including our study.13 Bus et al.21 performed a longitudinal study comparing serum BDNF levels at baseline and after 2 years in a large cohort of patients with persistent (n=310), remitted (n=420) or incident depression (n=153), as compared with non-depressed controls (n=868). They found a more profound decrease in serum BDNF levels in patients with persistent and remitted depression than in controls, whereas the incident depression group did not differ significantly from the non-depressed controls. On these bases, the authors suggested that the presence of reduced BDNF levels is a consequence of repeated episodes of depression rather than being the direct cause of depression development. Moreover, BDNF levels have been found even more reduced time after the onset of the illness, possibly reflecting the chronicity of the disorder.
In another interesting study, Molendijk et al.22 analyzed serum BDNF levels in 962 depressed patients, 700 fully remitted subjects (>6 months) and 382 healthy controls in order to identify whether (i) abnormalities in BDNF persisted beyond the clinical state of depression; (ii) BDNF levels were related to the clinical features of depression; (iii) distinct antidepressants equally affected BDNF levels. The authors found low serum BDNF levels in antidepressant-free depressed patients compared with healthy controls. Moreover, data showed that serum BDNF levels were lower in depressed patients who were not on antidepressant medication compared with antidepressant-free subjects who were in full remission and that BDNF levels of this latter group were comparable to those of controls.
These data suggest that low protein levels of BDNF in the blood can be considered as a state characteristic for depression, whose levels normalize during pharmacological treatment also in relation to an amelioration of clinical symptoms.
BDNF and SZ
The involvement of BDNF in SZ appears to be more complex. While it was originally found that BDNF expression is increased in the brain of patients affected by SZ, as compared with controls,23, 24 a large number of studies have shown that the expression of BDNF, and of its high-affinity receptor TrkB, is significantly downregulated in schizophrenic subjects.25, 26, 27 Several studies have also reported alterations in BDNF gene expression and protein levels in the blood of patients affected by SZ, although the results are quite heterogeneous.28, 29 Such heterogeneity can be due, at least in part, to the chronicity of the illness and also to the effects of antipsychotic therapies. Indeed, most of the data on BDNF in depression come from drug-free depressed patients and, thus, on patients at their first episode who have not started the pharmacological therapies yet. This is less frequent in SZ, in which BDNF levels have been primarily measured in patients with a long disease history, who have been treated with antipsychotic drugs for a long period of time, and thus in which the BDNF levels are the results of the chronicity of illness and of the long-term duration of pharmacological treatment. However, a recent meta-analysis has clearly shown that subgroup analyses performed in drug-naive and medicated patients with SZ reveal an association between reduced BDNF gene expression and protein levels and SZ, whereas the treatment with antipsychotics does not exert any significant effects on BDNF levels.30, 31
BDNF and bipolar disorder
Several studies on peripheral BDNF have been also conducted in bipolar disorder (BD), however, leading to discrepant results. Most of these studies have proposed a role for peripheral BDNF as a state marker also for BD, reporting decreased levels in both mania and depressive phases, returning to normal in euthymic state. Thus, it has been suggested that peripheral BDNF could have a role as a biomarker associated with a diagnosis of BD, in particular reflecting neuroprogressive changes in BD as a stage biomarker. Related to this, a recent meta-analysis conducted in 52 different studies by Fernandes et al.32 aimed to verify the properties of peripheral BDNF serum and plasma levels as a biomarker associated with disease activity in BD. In particular, the authors examined the association between BDNF protein levels with the duration of illness, in order to determine whether peripheral BDNF levels could represent a stage biomarker of illness. To assess whether BDNF levels could change during pharmacological treatment of an acute mood episode, the authors performed a series of meta-analyses of all cross-sectional studies comparing peripheral BDNF levels in BD versus healthy subjects. The same meta-analyses were also conducted, comparing studies before and after prescription of medication, to explore the relations with manic and depressive symptoms and response to treatment. The results indicated that BDNF protein levels were moderately decreased during the mania phase and largely decreased during the depressive phase. There were no alterations in peripheral BDNF levels in euthymia or in mixed states and no association between BDNF levels and duration of illness.
Several lines of evidence have also suggested that glucocorticoids, oxidative stress markers, inflammatory cytokines and neurotrophins may have important roles in BD.33, 34 Kauer-Sant'Anna33 found that BDNF levels were decreased in the late, but not in the early stage of BD when compared with controls and appeared to be related to the illness duration. On the other hand, tumor necrosis factor-alpha and interleukin (IL)-6 protein levels were increased in both early and late stages of BD, whereas the anti-inflammatory IL-10 was increased in the early stage of BD, but not at later stages. These data indicated that BD patients may be in a sort of pro-inflammatory state, which worsen during the later stages of illness, and also suggested that both BDNF and cytokines may have a key role in the pathophysiology of BD.
In another study, Kose Cinar et al.34 investigated the peripheral blood BDNF levels of adult male, of drug-free BD patients during manic and remission periods and of matched controls. They showed that BDNF levels, as well as those of molecules involved in BDNF processing (tissue-plasminogen activator), were reduced during manic episodes, as compared with controls, whereas an increased BDNF expression was found in remission, as compared with mania.
BDNF and anxiety disorder
Regarding anxiety disorder, Molendijk et al.35 tested possible changes in serum BDNF levels also in patients with social anxiety disorder, panic disorder, generalized anxiety disorder (GAD), agoraphobia and depressive disorders (that is, major depressive disorder, minor depressive disorder and dysthymia) as compared with healthy controls. They also examined the associations with gender and with other clinical features in these patients. The results showed no differences between patients and controls in serum BDNF levels. Additional analyses on gender differences revealed that female patients had lower serum BDNF levels as compared with female controls as well as with male patients. Moreover, BDNF levels in male patients were slightly higher as compared with male controls, whereas BDNF levels among female and male controls were similar. The authors also found that the difference between female and male patients could not be attributed to the presence of a specific subtype of anxiety as serum BDNF levels were similar across the subtypes, and thus peripheral BDNF measurements did not have the specificity to categorize anxiety disorders. Similarly, Carlino et al. 36 analyzed serum BDNF levels in adult subjects with different anxiety disorder subtypes such as social and specific phobias, GAD and panic disorder in order to test whether serum BDNF levels could be a potential biomarker for different subtypes of anxiety disorders. No significant correlation between serum BDNF levels and anxiety disorders was found as a whole or as a single diagnostic category. Moreover, no correlation between BDNF levels and severity of anxiety symptoms was found. However, when patients were stratified for both gender and disorder subtype, the authors found a significant decrease in serum BDNF levels, but only in GAD female patients. In another study, Uguz et al.37 investigated serum BDNF levels in the cord blood of newborn infants of mothers with or without GAD during pregnancy. They reported that newborns of healthy mothers had twofold higher BDNF levels compared with those of mothers with GAD, suggesting that GAD may alter neurotrophin levels, thus leading to impaired neurodevelopment.
As it has been suggested that the presence of low levels of BDNF could contribute also to the pathogenesis of obsessive-compulsive disorder also,38 Simsek et al.39 analyzed serum BDNF, and also cortisol and adrenocorticotropic hormone levels, in children and adolescents with obsessive-compulsive disorder prior to treatment, as compared to healthy controls. Serum BDNF, cortisol and adrenocorticotropic hormone levels were significantly higher in obsessive-compulsive disorder patients than in controls, suggesting that BDNF levels may increase during the early phase of the disease and that this increase may represent an adaptive response to preserve neuronal function in these patients.
The human BDNF gene: structure and transcript variants
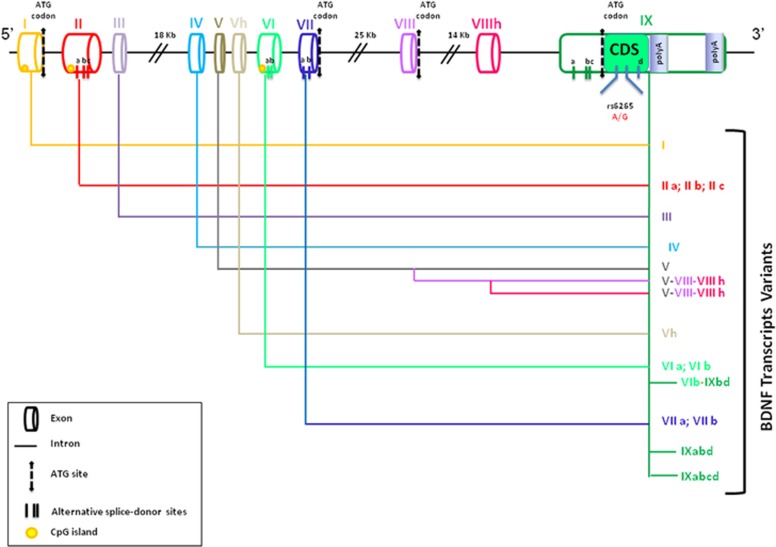
The human BDNF gene is located on chromosome 11, region p13–14 and it spans ∼70 kb. The gene has a complex structure as it consists of 11 exons (I–IX, plus Vh and VIIIh) in the 5′ end and of nine functional promoters that are used in tissue and brain regions specifically, namely exon I, II (with the transcripts IIa, IIb and IIc), III, IV, V (with the transcripts Va, Vb, Vc and V–VIII–VIIIh), VI (with the transcripts VIb, VIb–IXabd and VIb–IXbd), VII (with transcripts VIIa and VIIb) and IX (with transcripts IXabd and IXabcd; Figure 1).40 Although the BDNF gene comprises nine exons, the coding sequence resides in exon 9, with eight upstream exons that encode promoters regulating regional and cell-type-specific expression.41 Among these, exon IV has been the most extensively characterized, as this exon, containing promoter elements, regulates activity-dependent BDNF expression.
Figure 1.
Human BDNF gene structure. In the figure the structure of the human BDNF gene is shown; the gene contains 11 exons (I–IX, plus Vh and VIIIh), which combine to many different transcripts. Coding DNA sequence (CDS) is in exon IX. Alternative splice-donor sites and CpG islands are also reported. BDNF, brain-derived neurotrophic factor.
Each 5′ exon can generate, through alternative splicing, a specific transcript or isoform that are all characterized by the presence of a common coding region at the 3′ end. This 3′ coding region, located within exon IX, contains the common sequence that encodes for the pro-BDNF protein. Accordingly, through the use of alternative promoters and splicing mechanisms, different BDNF transcripts can be generated, with all of them encoding for the same pro-BDNF protein product.
Indeed, BDNF is initially synthesized in the endoplasmic reticulum as a precursor protein (pre-pro-BDNF; ~27 kDa);42 the signal peptide is then cleaved to produce pro-BDNF, the precursor form of the neurotrophin consisting of a pro-domain of 129 amino acids and a mature domain of 118 amino acids,2 which is then transported into the Golgi complex where it is sorted into either the constitutive or the regulated secretory pathways. Vesicular pro-BDNF is either proteolytically cleaved intracellularly to generate mature BDNF (mBDNF) or it is secreted as pro-BDNF and then cleaved at the extracellular level to generate mBDNF. Vesicular pro-BDNF can be converted intracellularly into mBDNF by endoproteases, such as furin, or within secretory granules by pro-protein convertases. Alternatively, pro-BDNF can be cleaved extracellularly by other factors such as plasmin and matrix metalloproteinases. Vesicular secretion can involve both pro-BDNF and mBDNF, with the amount of secreted mBDNF depending on the type and activity of the convertases.43
The efficiency of cleavage, and hence the ratio of pro-BDNF to mBDNF, is different at distinct developmental stages and during postnatal life; specifically, in the neonatal and adolescent stages, both pro-BDNF and mBDNF are detectable, whereas in adulthood mBDNF predominates.2 The precursor form or proneurotrophin, including pro-BDNF, activates the p75 receptor, rather than TrkB that is activated by mBDNF. The p75 receptor is a member of the tumor necrosis factor family of receptors that encode a cytoplasmic death domain and can initiate apoptosis following ligand binding.44
A pro-BDNF knock-in mouse was generated to examine the effects of pro-BDNF under the control of its endogenous promoter and other regulatory elements.45 In this in vivo model, pro-BDNF expression negatively regulates hippocampal dendritic complexity and spine density. This finding suggests that pro-BDNF acts in vivo as a biologically active factor that regulates hippocampal structure, synaptic transmission and synaptic plasticity—effects that are distinctive from the mBDNF. These results, coupled with the higher levels of expression of pro-BDNF in the developing postnatal brain, suggest that this ligand may be a key regulator in shaping neural circuitry and synaptic plasticity in adolescence—effects that may be maintained also through adulthood.45
Another level of complexity in the BDNF structure is because of the presence of two alternative polyadenylated transcription stop sites located within exon IX, that generate two distinct populations of mRNA with either short (~0.35 kb) or long (~2.85 kb) 3′ untranslated regions (3′ UTRs).2 The diversity of these two clusters of transcripts is associated with a different neuronal distribution. Indeed, the short 3′ UTR BDNF mRNA variant is restricted to the cell body in hippocampal neurons, whereas the long 3′ UTR mRNAs are also observed in dendrites,46 indicating a specific dendritic transport system for the long 3′ UTR BDNF mRNA and local dendritic translation.
BDNF transcripts IV and IX as the most abundant in human blood samples
BDNF is not only expressed in the brain, but can also be detected in peripheral blood cells besides other tissues.47 In the blood, especially in the context of psychiatric disorders, where blood has been suggested to be an alternative and useful peripheral tissue to assess biomarkers of illness, BDNF gene expression studies offer the opportunity to investigate not only the levels of total BDNF but also the modulation of different transcripts. The analyses of the BDNF transcripts may provide novel insights into the mechanisms regulating BDNF expression and may reveal more specific alterations related to a specific psychiatric condition. Indeed, although a reduction of total BDNF mRNA is shared by several psychiatric disorders, including depression and SZ,13, 26, 30 the modulation of specific BDNF transcripts may be disease-specific or, more importantly, may represent a better predictor in term of treatment response. However, up to now, no data are available on the expression of the different BDNF transcripts in the human blood as well as on BDNF transcript alterations in the contest of psychiatric conditions.
BDNF transcript expression in human peripheral blood and hippocampus
We decided to characterize the expression of BDNF transcripts in peripheral blood cells as compared with brain samples. To this purpose, we have investigated, using real-time PCR, the mRNA levels of the main BDNF isoforms (transcripts I, IV, Va, Vb, Vh, V–VIII–VIIIh, VIb, VIb–IXabd, VIb–IXbd, VIIb, IXabd and IXabcd) in human blood cells as well as in human hippocampus. RNA from human hippocampus was obtained from Clontech (Diatech Lab Line, Milan, Italy), whereas human blood samples were collected in PaxGene tubes from four control volunteers who signed an informed consent approved by our Local Ethical Committee. RNA from peripheral blood cells was isolated by using the PAXgene miRNA Kit (Qiagen, Milan, Italy) and a pool of RNA samples was made for qualitative analyses of BDNF transcript expression.
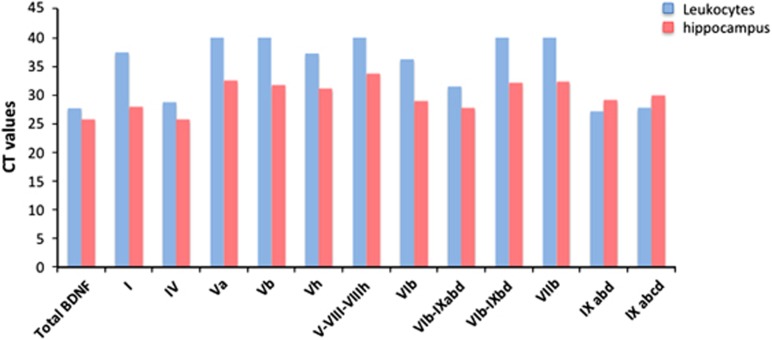
Diluted RNA samples were then run using Biorad qPCR Mix (Bio-Rad) on a 384-well Real-Time PCR System (Bio-Rad). As shown in Figure 2, our results indicate that exons IV and IX (namely transcripts IV, IXabd and IXabcd) are expressed at the highest expression levels in both tissues, as indicated by low Ct values. Conversely, the other transcripts, namely Va, Vb, V–VIII–VIIIh, VIb–IXabd and VIIb, are expressed at low levels in peripheral blood, as suggested by higher Ct values (>40 cycles of PCR), as compared with brain samples, where their expression is higher (all the Ct values are <38 cycles of PCR).
Figure 2.
Expression analysis of BDNF splicing variants in human hippocampus and peripheral blood. The graph shows the Ct values obtained with real-time PCR for the total BDNF mRNA and for the different BDNF splicing variants, as measured in human blood cells (blue bars) or in the human hippocampus (red bars). BDNF, brain-derived neurotrophic factor.
To the best of our knowledge, these data provide the first evidence that BDNF exons IV and IX may directly contribute to the modulation of total BDNF expression at the peripheral level. These transcripts could contribute to BDNF alterations that are reported in several psychiatric conditions and could also be modulated by successful pharmacological treatments.11, 13, 14, 31 The high expression of BDNF exon IV is particularly intriguing, as its promoter region is well investigated in term of epigenetic regulation, which may sustain long-lasting changes in its expression (see the below paragraph for details on BDNF DNA methylation).
Epigenetic regulation of BDNF: DNA methylation and microRNA
DNA methylation
The exact mechanisms that may lead to BDNF alterations in psychiatric disorders are still largely unknown. Epigenetic changes at the BDNF gene locus may provide a link between genes and environment and may also explain the long-lasting nature of the changes in BDNF expression and function. Epigenetics refers to alterations in gene expression and in a specific phenotype without involving changes within the DNA sequence. Among the epigenetic mechanisms, DNA methylation probably represents the process that has been investigated more in detail. In mammals, DNA methylation occurs largely in association with CpG dinucleotides, also called CpG islands, where methyl groups are added to cytosine residues.48, 49 As CpG islands tend to cluster within or close to promoter regions, methylation may represent one of the mechanisms regulating the transcription of selective BDNF promoters under different conditions. BDNF promoter IV represents the best investigated promoter in the contest of DNA methylation changes associated with alterations in BDNF expression.50
Up to now, limited knowledge is available on methylation levels within BDNF exon IV in the context of psychiatric conditions and most of the available data have been obtained in brain samples. For example, when compared with controls, a significant increase in DNA methylation at BDNF promoter IV was found in the Wernicke area from suicide subjects and, interestingly, this higher methylation pattern correlated with lower mRNA levels for BDNF transcript IV.51 An increase in DNA methylation levels of this BDNF promoter region was reported in association with lower BDNF protein levels in the prefrontal cortex of elderly people.52 Furthermore, higher DNA methylation levels were also found in the prefrontal cortex of valine/valine homozygous subjects, as compared with metionine (Met) carriers, suggesting an association between BDNF genotype and DNA methylation.53 The notion that epigenetic changes are associated with variations in DNA sequence may underlie, at least in part, the inconsistencies of genetic-association studies in psychiatric disorders. Indeed, polymorphisms can exert an effect on gene function via epigenetic processes, suggesting a common pathway beyond genetic and environmental effects as well as a potential mechanism for gene–environment interaction. This suggests that an integration between epigenetic and gene association data could stratify the effect of candidate polymorphisms and haplotypes on disease vulnerability and may allow the identification of vulnerability signatures associated with psychiatric disorders.
In addition to DNA methylation, histone modifications, which influence the condensation of the DNA around histone proteins and regulate the accessibility of functional regions to transcriptional regulators, represent an interesting epigenetic mechanism. There are few studies examining the role of histone modifications at the BDNF gene promoter in humans. In post-mortem brain tissue, the transcriptional upregulation of BDNF, which occurs from fetal to childhood and/or young adult stages, was shown to be accompanied by increases in histone methyltransferase H3K4 trimethylation, a mark of active chromatin, at promoters I and IV.54 In depressed patients, two studies suggest that antidepressants may regulate BDNF expression through alterations in the H3K27 methylation state at promoter IV; for example, H3K27 methylation was reduced in post-mortem brain tissue of patients who had used antidepressants, compared with patients without a history of antidepressant consumption.55 A prospective study conducted in blood cells of treatment-naive depressed subjects revealed a decrease in H3K27 methylation in responders, but not in non-responders after 8 weeks of citalopram treatment.56 Interestingly, H3K27 methylation was inversely correlated with serum BDNF levels and treatment efficacy. Only subjects with positive therapeutic responses to citalopram exhibited higher BDNF and lower H3K27 methylation, although H3K27 methylation did not differ between future responders and non-responders before the treatment period.56
All these studies suggest that DNA methylation at the BDNF promoter might predict a possible antidepressant response.
DNA methylation analyses of BDNF exon IV promoter region
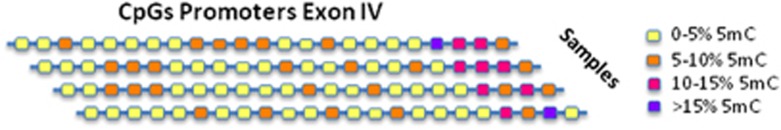
Here, in our review, we have also provided a DNA methylation profile of the promoter region of exon IV, one of the most expressed exons in peripheral blood and the most investigated BDNF region in term of epigenetic regulation. We used a bisulphite pyrosequencing approach, including 23 CpG sites, from nucleotide (nt) 27723095 to nt 27723658 of the BDNF gene, spanning the transcriptional start site. DNA samples were obtained from the same control subjects that we used for the expression of BDNF transcripts, and DNA was isolated by using the Gentra Puregene Blood kit (Qiagen) according to the manufacturer′s instructions. Genomic DNA was treated with the EZ DNA Methylation-Gold Kit (Zymo Research) and the bisulfite-treated DNA was PCR-pyrosequenced using the PSQ HS96 Pyrosequencing System. The assays were designed to cover the greatest number of CpG sites possible, taking into account the limitations of the method (PCR amplicon ⩽350 bp, primers that avoided CpGs, target sequence ⩽40 bp). We then expressed the degree of methylation as percentage of methylated cytosines (% 5-methyl-cytosine). Our data indicated that the BDNF exon IV region was methylated at low levels in blood cells. Indeed, as we can observe in Figure 3, 15 CpG sites had less than 5% 5 mC; five CpG sites had 5–10% 5 mC; and only three CpG sites showed more than 10% 5mC. Interestingly, the three CpG sites with the highest level of methylation status were those located at greatest distance from the transcriptional start site.
Figure 3.
DNA methylation profile of exon IV in control subjects. The figure shows the methylation profile of 23 CpG sites within the promoter region of exon IV. The degree of methylation has been expressed as percentage of cytosines that were methylated (% 5-methyl-Cytosine). Fifteen CpG sites had <5% 5 mC, five CpG sites from 5 to 10% 5 mC and only three CpG sites >10% 5 mC.
MicroRNAs targeting BDNF
Another biological mechanism that has emerged as an important epigenetic process is represented by microRNAs (miRNAs). MiRNAs are endogenous small non-coding RNAs that regulate the expression of different genes at the post-transcriptional level, by promoting the cleavage of target mRNAs or by repressing their translation. MiRNAs have been shown to regulate a variety of biological processes, including development, cell proliferation, fate specification, growth control and apoptosis.57, 58 A fine regulation of miRNA expression is required for normal brain development,59, 60 whereas changes in the expression and/or function of specific miRNAs have been associated with different mental diseases, such as SZ,61 major depressive disorder,62, 63 BD64, 65 and autism spectrum disorder.66 Recent studies have demonstrated that also BDNF expression and function may be directly regulated through miRNAs. BDNF is potentially regulated by several hundreds of miRNAs, primarily via its 3′ UTR region, and in Supplementary Table 1and 2 we have reported a list of miRNAs that are predicted or that have been validated (according to miRWalk Database, http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/) to target the human or rodent BDNF gene. Although several miRNAs are predicted to target BDNF, only few miRNAs have been investigated experimentally. Recently, using in silico approaches, such as reporter systems and analysis of endogenous BDNF, it has been shown that miR-1, miR-10b, miR-155 and miR-191 directly repress BDNF gene expression by binding to specific predicted sites within BDNF 3′ UTR.67 Furthermore, an overexpression of miR-1 and miR-10b suppressed endogenous BDNF protein levels, whereas the silencing of endogenous miR-10b increased BDNF mRNA and protein levels. The suppression of BDNF via miR-10b occurred via targeting both the long and short 3′ UTRs of BDNF, as mutating the putative miR-10b-binding site within the long 3′ UTR abolished BDNF suppression.67 The same study showed that miR-155 and miR-191 specifically reduced the expression of a luciferase construct containing the long, but not the short, 3′ UTR of the neurotrophin. The authors also measured BDNF levels after transfecting neural cell lines with pre-miRNAs, showing that the majority of BDNF transcripts containing the short 3′ UTR did not respond to miR-155 or miR-191. Consistently with this notion, the expression of BDNF mRNA isoforms carrying the long 3′ UTR was reduced following treatment with miR-155 and miR-191 precursors, although total BDNF mRNA and BDNF protein levels were unaffected.67
The effect of miR-1 on BDNF expression was also investigated in a recent study where its overexpression in human primary glioblastoma (U87) cells caused a decrease in BDNF protein levels, suggesting a negative effect of this miR-1 on BDNF protein levels.68 Similarly to miR-1, miR-206 shares the same predicted binding site within the 3′ UTR of the BDNF mRNA, and different studies have shown that miR-206 suppresses BDNF expression via its 3′ UTR region.69, 70, 71 For example, Lee et al.70 examined miRNA expression in transgenic Alzheimer‘s disease mice and human Alzheimer‘s disease brain samples, showing that miR-206 regulates BDNF transcription and that the antagomir AM206, a specific inhibitor of miR-206, enhances BDNF gene expression levels, synaptogenesis and neurogenesis. However, other studies reported that BDNF mRNA levels are not regulated by miR-206,72, 73 although endogenous BDNF expression is reduced after treating C2C12 myoblasts with miR-206.72
Recently, Imam et al.74 examined the role of miR-204 in regulating BDNF expression reporting that endogenous BDNF mRNA and protein levels were reduced after miR-204 overexpression, whereas an increased neurotrophin expression was found after inhibition of endogenous miR-204. Furthermore, this miRNA suppressed luciferase expression via the full-length BDNF 3′ UTR, an effect that was abolished after mutating the predicted binding site of miR-204. Furthermore, overexpression of another miRNA, namely miR-210, in HEK-293 cells leads to a downregulation of BDNF, whereas an upregulation of the neurotrophin was observed after transfection of anti-miR-210 oligonucleotide.75
A number of other miRNAs, including miR-15a, miR-22, miR-26a and miR-26b, miR-30a, miR-124, miR-132, miR-182, miR-195 and miR-376b-5p, have been shown to regulate BDNF expression. For example, Mellios et al.54 have found that miR-30a-5p and miR-195 target the 3′ UTR of BDNF demonstrating that miR-30a-5p overexpression in rat forebrain neurons markedly reduces BDNF protein. Recently, a marked reduction in BDNF mRNA levels was found in a mouse paradigm that mimics binge alcohol drinking in humans, an effect that was associated with an increased expression of several miRNAs, including miR-30a-5p, in the medial prefrontal cortex.76 The authors demonstrated that miR-30a-5p binds the 3′ UTR region of BDNF, and that overexpression of this miRNA in the medial prefrontal cortex decreased BDNF expression. Conversely, inhibition of miR-30a-5p, by using a locked nucleic acid sequence targeting miR-30a-5p, restored BDNF levels and decreased excessive alcohol intake.76 These studies provide evidence for a role of miRNA in selectively regulating the expression of the pool of BDNF transcripts, which undergo dendritic targeting and may contribute to local, activity-dependent, neurotrophin synthesis.15, 77, 78
On the basis of the regulation of BDNF by different miRNAs, it may be inferred that these epigenetic regulators could contribute to the development of psychiatric disorders in which BDNF is having a key role. Accordingly, Li et al.79 showed that the reduction of serum BDNF levels in patients affected by depression is paralleled by an upregulation of miR-132 and miR-182, suggesting that miRNA dysregulation may contribute to the changes of neurotrophin gene expression levels in depressed subjects. A recent report has shown that miR-16 has an important role in the negative modulation of hippocampal neurogenesis and depression-like behaviors.80 Interestingly, an upregulation of miR-16 and a downregulation of BDNF mRNA expression was found in the hippocampus of maternally deprived rats characterized by depression-like behaviors, suggesting a potential link between miR-16 and BDNF.81
Furthermore, it has been demonstrated that BDNF Val66Met is associated with an altered expression of a specific subsets of miRNAs and of their downstream targets. Indeed, Hsu et al.82 suggest that Val66Met can affect miRNA levels by modulating the affinity of the miRNA on the target-binding region on BDNF gene according to the presence of the Val or Met allele. These results suggest that BDNF Val66Met may mediate its effects on psychiatric and cognitive phenotypes in part because of miRNA-dependent effects on gene expression.
BDNF as peripheral biomarker for psychiatric disorders
Disease biomarkers can be defined as biological alterations of specific molecules that either have diagnostic or predictive value in relation to an illness, allowing an estimation of a patient's responsiveness to a specific treatment.83, 84 Biomarkers can be potentially found in any biomedical context, helping in the differential diagnosis of psychiatric disorders, which usually show an overlapping symptomatology.11 Toward this goal, identification of biological markers could improve and facilitate the classification of disease subtypes, as well as could control the response to medication, allowing a stratification of the patients into more homogeneous, clinically distinct subpopulations.85
Up to now, genome-wide association studies have not identified any significant association between common variants within BDNF gene and psychiatric disorders. This could be because of the evidence that psychiatric disorders are complex diseases involving several common variants within different vulnerability genes, each contributing with a small effect size. Moreover, these disorders are also characterized by a complex interplay between genetic and environmental factors. In line with this, several findings have now reported an interaction between the Val66Met SNP with environmental factors, and in particular childhood adversities, in influencing the formation of hippocampus,86, 87 or in leading to an enhanced vulnerability to suicide 88 in depressed patients. In addition, genetic epistasis between BDNF Val66Met with Serotonin Transporter 1 or Catechol-O-methyltransferase functional SNPs in moderating depression vulnerability has been reported in several studies.89, 90, 91
On the basis of the association between BDNF and several psychiatric disorders, particularly depression,11, 12, 13, 92, 93 this neurotrophin has been suggested to represent a valuable biomarker for a specific psychiatric condition as well as for treatment response.
Unfortunately, cerebrospinal fluid levels of BDNF are already at the limit of detection in healthy individuals, despite the sensitivity of analytic methodologies.94 Moreover, the analysis of BDNF in brain tissues is restricted to autoptic examination, which represents a big limitation as studies on post-mortem tissues are subjected to several artifacts, related to medication, cause of death, agonal state and post-mortem interval.95, 96 Hence, in order to have the possibility to investigate BDNF in living individuals, during the last decade a number of studies have investigated the gene expression and protein levels of the neurotrophin in the peripheral blood, which may provide significant advantages, being readily available in large quantities with minimally invasive techniques.11, 97
Interestingly, when BDNF was first measured in the peripheral blood of depressed patients and controls, Karege et al. reported that BDNF serum and plasma levels were reduced in drug-free depressed patients, as compared with controls.98, 99 These data have been subsequently replicated by different groups, including our own group,13, 100 which consistently demonstrate that plasma or serum BDNF levels are reduced in depressed patients and may be normalized following effective antidepressant treatments.3, 11, 12, 101 Interestingly, in a recent meta-analysis, Polyakova et al.3 showed a reduction of serum and plasma BDNF levels not only in depressed patients, but also in bipolar subjects, as compared with healthy ones. Indeed, alterations of circulating BDNF levels are not specific for major depression, as they have been found also in other psychiatric disorders.30, 84 Another recent meta-analysis has demonstrated that serum BDNF levels are reduced in chronic schizophrenic patients31, 102 as well as in first-episode psychotic subjects.103 These results may not be surprising, given that stress, which represents a risk factor not only for depression, but also for SZ and other psychiatric disorders, is able to reduce BDNF levels.47 Thus, the reduction in peripheral BDNF may underlie common pathophysiological mechanisms, characterized by reduced neuronal plasticity, which may be shared by several mental diseases.
Given that brain BDNF crosses the blood–brain barrier,104 it is reasonable to assume that blood BDNF gene expression and protein levels resemble those in the brain. This hypothesis has been upheld by data showing a positive correlation between serum BDNF and cortical levels of the neurotrophin in rats during neurodevelopment98 and by a positive association between BDNF serum content and cortical levels of N-acetyl aspartate, a marker of neuronal integrity.105 However, the high concentration of BDNF in the blood may not only originate from the brain, but also from other peripheral sources. Within blood, BDNF is mainly stored, but not synthesized, in platelets/thrombocytes.98, 99, 106, 107 This may explain the ∼200-fold difference between BDNF levels determined in serum versus plasma, probably because of the release of BDNF from platelets into serum during coagulation.99 However, platelets may serve as storage compartment for BDNF deriving from different sources, thus representing active factors in the regulation of BDNF homeostasis.106 Interestingly, a deficit in the BDNF release from platelets has been shown to occur in depressed patients.99
BDNF mRNA levels are also significantly reduced in the leukocytes of depressed patients, as compared with controls.100 Our group has confirmed these results, showing decreased BDNF mRNA expression and reduced serum BDNF levels in the blood of depressed patients, as compared with a group of controls.14 Importantly, we have also reported that antidepressant treatments are able to restore BDNF levels to control values and, more importantly, that a correlation between increased BDNF levels and symptomatology improvement does occur, suggesting that the modulation of BDNF may be required in order to achieve a positive clinical outcome.14 Moreover, we have also found a correlation between BDNF mRNA levels in leukocytes and BDNF protein levels in the serum both at baseline and during antidepressant treatment, suggesting that the changes in BDNF protein levels found in depressed patients may be, at least in part, related to the synthesis and secretion of BDNF from white blood cells rather than from platelets only.99, 108
Importantly, BDNF mRNA expression in peripheral blood may reflect more accurately the central mechanism of BDNF physiology, as compared with protein levels.47 In support of this hypothesis, the mRNA profile of peripheral blood cells shows an overlap with those observed in different brain regions,109, 110, 111 suggesting that blood cells may provide valuable information on the pathogenesis of different brain disorders, including psychiatric diseases, and may lead to the identification of innovative biomarkers for diagnostic assessment and personalized treatments.112, 113
Although peripheral BDNF gene expression and protein levels have been often suggested as biomarkers for several psychiatric disorders and in particular for depression, limitations exist and should be discussed. Unfortunately, the majority of the published studies are very heterogeneous, showing a very small effect size. Moreover, according to many authors, peripheral BDNF alterations are not specific enough for a given psychiatric disorder. Future studies should be designed following a multidisciplinary approach, thus taking into account BDNF expression and protein levels with methylation.
In order to examine the potential determinants (sampling characteristics, sociodemographic variables, lifestyle indicators and chronic diseases) influencing serum BDNF levels, Bus et al.114 conducted a study in a large and well-defined cohort of people without current psychiatric or neurologic diseases. The authors identified eight independent determinants of serum BDNF levels: (1) time of blood withdrawal, (2) time of storage, (3) food intake before sampling, (4) urbanicity, (5) age, (6) sex, (7) smoking status and (8) drinking behavior. According to these findings, the authors suggest that future studies on serum BDNF levels in humans should take into account and correct for all these variables. Although effect sizes are generally small and clinical relevance needs to be tested in subsequent clinical samples, the authors also suggested the importance of excluding smokers, excessive alcohol drinkers and subjects who did not adhere to the pretest fasting protocol from clinical studies and to keep storage time limited.
Another interesting confounder associated with BDNF changes, shown by Molendijk et al.,115 is the pronounced seasonal variation in serum BDNF concentrations of both depressed patients and healthy controls: an increasing BDNF concentration has been found over the course of the spring and summer, whereas decreasing BDNF levels have been identified over the course of the autumn and winter. As reported also by the authors, these results provide strong evidence that serum BDNF concentrations systematically vary over the year and should be taken into account when designing and interpreting studies on BDNF.
Conclusions
Several lines of evidence suggest that BDNF may represent a key factor in the pathogenesis of different psychiatric disorders. Although the exact mechanisms underlying this relationship remain to be fully established, epigenetic factors, such as DNA methylation and miRNAs, may be involved. Moreover, BDNF exons IV and IX represent the most abundant transcripts in peripheral blood, suggesting their key role in BDNF regulation at the peripheral level. On these bases, the analysis of these transcripts may provide novel insights into the mechanisms contributing to BDNF modulation under pathologic conditions as well as during pharmacological treatments.
Importantly, as we have addressed in this review, peripheral BDNF gene expression and protein levels in serum or plasma have been consistently found dysregulated in several psychiatric disorders, including major depressive disorder, SZ, BD and anxiety disorders. Thus, peripheral BDNF levels cannot be considered as a biomarker for specific clinical manifestations and psychiatric symptoms. BDNF splice variants, in turn, could represent more specific peripheral biomarkers for a differential diagnosis of psychiatric disorders. Indeed, specific BDNF isoforms are modulated by specific factors and are involved in differential pathways; thus, they could reflect alterations in specific signals and could represent possible useful biomarkers associated with a differential diagnosis, but this needs further investigation. However, our main hypothesis is that the presence of reduced BDNF levels, observed across several psychiatric disorders, could represent a transdiagnostic biomarker, indicator of reduced neural plasticity and ability in stress-related coping mechanisms, which are features reported to be altered in all these psychiatric disorders.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 2010; 70: 304–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. Brain-derived neurotrophic factor: three ligands, many actions. Trans Am Clin Clim Assoc 2015; 126: 9–19. [PMC free article] [PubMed] [Google Scholar]
- Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 2015; 174: 432–440. [DOI] [PubMed] [Google Scholar]
- Mitchelmore C, Gede L. Brain derived neurotrophic factor: epigenetic regulation in psychiatric disorders. Brain Res 2014; 1586: 162–172. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 2012; 64: 238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res 2010; 1314: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology 2013; 38: 312–330. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804–815. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol Neurobiol 2004; 29: 289–302. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol 2008; 11: 1047–1061. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 2008; 64: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 2010; 11: 763–773. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Bocchio-Chiavetto L, Zanardini R, Milanesi E, Placentino A, Gennarelli M. Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol 2010; 13: 103–108. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 2013; 38: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoni A, Macchi F, Papp M, Molteni R, Riva MA. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int J Neuropsychopharmacol 2015; 18: pii: pyu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, van der Doelen RH, Guidotti G, Racagni G, Kozicz T, Homberg JR et al. Exposure to early life stress regulates Bdnf expression in SERT mutant rats in an anatomically selective fashion. J Neurochem 2015; 132: 146–154. [DOI] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry 2004; 55: 708–714. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, Gennarelli M et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol 2010; 77: 846–853. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 2007; 18: 391–418. [DOI] [PubMed] [Google Scholar]
- Polyakova M, Schroeter ML, Elzinga BM, Holiga S, Schoenknecht P, de Kloet ER et al. Brain-derived neurotrophic factor and antidepressive effect of electroconvulsive therapy: systematic review and meta-analyses of the preclinical and clinical literature. PLoS One 2015; 10: e0141564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus BA, Molendijk ML, Tendolkar I, Penninx BW, Prickaerts J, Elzinga BM et al. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol Psychiatry 2015; 20: 602–608. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 2011; 16: 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 2000; 5: 293–300. [DOI] [PubMed] [Google Scholar]
- Iritani S, Niizato K, Nawa H, Ikeda K, Emson PC. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Progr Neuropsychopharmacol Biol Psychiatry 2003; 27: 801–807. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizoph Res 2001; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003; 8: 592–610. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 2005; 25: 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Dhandapani KM, Pillai BA, Terry AV Jr., Mahadik SP. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology 2008; 33: 1942–1951. [DOI] [PubMed] [Google Scholar]
- Durany N, Thome J. Neurotrophic factors and the pathophysiology of schizophrenic psychoses. Eur Psychiatry 2004; 19: 326–337. [DOI] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry 2011; 16: 960–972. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry 2015; 20: 1108–1119. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Molendijk ML, Kohler CA, Soares JC, Leite CM, Machado-Vieira R et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med 2015; 13: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer-Sant'Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol 2009; 12: 447–458. [DOI] [PubMed] [Google Scholar]
- Kose Cinar R, Sonmez MB, Gorgulu Y. Peripheral blood mRNA expressions of stress biomarkers in manic episode and subsequent remission. Psychoneuroendocrinology 2016; 70: 10–16. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Prickaerts J, Oude Voshaar RC et al. Gender specific associations of serum levels of brain-derived neurotrophic factor in anxiety. World J Biol Psychiatry 2012; 13: 535–543. [DOI] [PubMed] [Google Scholar]
- Carlino D, Francavilla R, Baj G, Kulak K, d'Adamo P, Ulivi S et al. Brain-derived neurotrophic factor serum levels in genetically isolated populations: gender-specific association with anxiety disorder subtypes but not with anxiety levels or Val66Met polymorphism. PeerJ 2015; 3: e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguz F, Sonmez EO, Sahingoz M, Gokmen Z, Basaran M, Gezginc K et al. Maternal generalized anxiety disorder during pregnancy and fetal brain development: a comparative study on cord blood brain-derived neurotrophic factor levels. J Psychosom Res 2013; 75: 346–350. [DOI] [PubMed] [Google Scholar]
- Hemmings SM, Kinnear CJ, Van der Merwe L, Lochner C, Corfield VA, Moolman-Smook JC et al. Investigating the role of the brain-derived neurotrophic factor (BDNF) val66met variant in obsessive-compulsive disorder (OCD). World J Biol Psychiatry 2008; 9: 126–134. [DOI] [PubMed] [Google Scholar]
- Simsek S, Gencoglan S, Yuksel T, Kaplan I, Alaca R. Cortisol and brain-derived neurotrophic factor levels prior to treatment in children with obsessive-compulsive disorder. J Clin Psychiatry 2016; 77: e855–e859. [DOI] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007; 90: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J Biol Chem 2014; 5: 409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 2009; 65: 11–22. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 2001; 276: 12660–12666. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science 2001; 294: 1945–1948. [DOI] [PubMed] [Google Scholar]
- Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q et al. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep 2014; 7: 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 2008; 60: 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Hellweg R. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker for affective disorders? Int J Neuropsychopharmacol 2010; 13: 1–4. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Can we observe epigenetic effects on human brain function? Trends Cogn Sci 2015; 19: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D et al. The epigenetics of aging and neurodegeneration. Progr Neurobiol 2015; 131: 21–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 2007; 8: 355–367. [DOI] [PubMed] [Google Scholar]
- Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V et al. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry 2010; 67: 258–267. [DOI] [PubMed] [Google Scholar]
- Keleshian VL, Modi HR, Rapoport SI, Rao JS. Aging is associated with altered inflammatory, arachidonic acid cascade, and synaptic markers, influenced by epigenetic modifications, in the human frontal cortex. J Neurochem 2013; 125: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 2008; 82: 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet 2008; 17: 3030–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ES, Ernst C, Turecki G. The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression. Int J Neuropsychopharmacol 2011; 14: 427–429. [DOI] [PubMed] [Google Scholar]
- Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT et al. Epigenetic regulation of BDNF expression according to antidepressant response. Mol Psychiatry 2013; 18: 398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MF, Shi Y. Dynamic Roles of microRNAs in Neurogenesis. Front Neurosci 2012; 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E, Shi Y. MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp Neurol 2015; 268: 46–53. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci 2006; 7: 911–920. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S et al. MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci 2010; 30: 14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 2009; 65: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Pathogenetic and therapeutic applications of microRNAs in major depressive disorder. Progr Neuropsychopharmacol Biol Psychiatry 2016; 64: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Zhao G, Sun R, Mao Y, Li G, Chen X et al. Genetic variants in the promoters of let-7 family are associated with an increased risk of major depressive disorder. J Affect Disord 2015; 183: 295–299. [DOI] [PubMed] [Google Scholar]
- Walker RM, Rybka J, Anderson SM, Torrance HS, Boxall R, Sussmann JE et al. Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J Psychiatr Res 2015; 62: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Chibane FL, Elkahloun AG, Henderson R, Singh R, Lawson J et al. Novel integrative genomic tool for interrogating lithium response in bipolar disorder. Transl Psychiatry 2015; 5: e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry 2012; 3: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varendi K, Matlik K, Andressoo JO. From microRNA target validation to therapy: lessons learned from studies on BDNF. Cell Mol Life Sci 2015; 72: 1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburger T, Grievink H, Heinen N, Barthel F, Huhn R, Stachuletz F et al. Effects of remote ischemic preconditioning and myocardial ischemia on microRNA-1 expression in the rat heart in vivo. Shock 2014; 42: 234–238. [DOI] [PubMed] [Google Scholar]
- Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K et al. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 2011; 31: 15407–15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim JH, Huh JY, Yoon H et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol 2012; 72: 269–277. [DOI] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A et al. MicroRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci 2014; 34: 4581–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 2006; 174: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura P, Amirouche A, Clow C, Belanger G, Jasmin BJ. Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. J Neurochem 2012; 120: 230–238. [DOI] [PubMed] [Google Scholar]
- Imam JS, Plyler JR, Bansal H, Prajapati S, Bansal S, Rebeles J et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS One 2012; 7: e52397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem 2009; 284: 35134–35143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D. MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry 2015; 20: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AG, Irier HA, Gu J, Tian D, Ku L, Liu G et al. Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc Natl Acad Sci USA 2010; 107: 15945–15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Richetto J, Racagni G, Feldon J, Meyer U, Riva MA. Effects of withdrawal from repeated amphetamine exposure in peri-puberty on neuroplasticity-related genes in mice. Neuroscience 2013; 250: 222–231. [DOI] [PubMed] [Google Scholar]
- Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang YX et al. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One 2013; 8: e63648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Mouillet-Richard S, Baudry A, Pietri M, Kellermann O. Raphe-mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl Psychiatry 2011; 1: e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Zhu X, Zhang Y, Zhang S, Zhang L, Xue L et al. Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS One 2012; 7: e46921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SD, Huang HY, Chou CH, Sun YM, Hsu MT, Tsou AP. Integrated analyses to reconstruct microRNA-mediated regulatory networks in mouse liver using high-throughput profiling. BMC Genomics 2015; 16(Suppl 2): S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Maes M, Andreazza A, McGrath JJ, Tye SJ, Berk M. Towards a classification of biomarkers of neuropsychiatric disease: from encompass to compass. Mol Psychiatry 2015; 20: 152–153. [DOI] [PubMed] [Google Scholar]
- Pillai A, Buckley PF. Reliable biomarkers and predictors of schizophrenia and its treatment. Psychiatr Clin N Am 2012; 35: 645–659. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011; 36: 2375–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N, Frey EM, Morris D, Gill M, Carballedo A. BDNF Val66Met genotype interacts with childhood adversity and influences the formation of hippocampal subfields. Hum Brain Mapp 2014; 35: 5776–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballedo A, Morris D, Zill P, Fahey C, Reinhold E, Meisenzahl E et al. Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Mel Genet B 2013; 162B: 183–190. [DOI] [PubMed] [Google Scholar]
- Pregelj P, Nedic G, Paska AV, Zupanc T, Nikolac M, Balazic J et al. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. J Affect Disord 2011; 128: 287–290. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Schwahn C, Mahler J, Appel K, Schulz A, Spitzer C et al. Genetic epistasis between the brain-derived neurotrophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Progr Neuropsychopharmacol Biol Psychiatry 2012; 36: 264–270. [DOI] [PubMed] [Google Scholar]
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med 2009; 39: 1425–1432. [DOI] [PubMed] [Google Scholar]
- Savitz JB, van der Merwe L, Newman TK, Solms M, Stein DJ, Ramesar RS. The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? Int J Neuropsychopharmacol 2008; 11: 149–161. [DOI] [PubMed] [Google Scholar]
- Altar CA. Neurotrophins and depression. Trends Pharmacol Sci 1999; 20: 59–61. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54: 597–606. [DOI] [PubMed] [Google Scholar]
- Burbach GJ, Hellweg R, Haas CA, Del Turco D, Deicke U, Abramowski D et al. Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J Neurosci 2004; 24: 2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasinghe N, Tooney PA, Schall U. Finding the needle in the haystack: a review of microarray gene expression research into schizophrenia. Aust N Z J Psychiatry 2012; 46: 598–610. [DOI] [PubMed] [Google Scholar]
- Mistry M, Gillis J, Pavlidis P. Genome-wide expression profiling of schizophrenia using a large combined cohort. Mol Psychiatry 2013; 18: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol 2008; 11: 1169–1180. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109: 143–148. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 2005; 57: 1068–1072. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Progr Neuropsychopharmacol Biol Psychiatry 2010; 34: 645–651. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 2014; 19: 791–800. [DOI] [PubMed] [Google Scholar]
- Ahmed AO, Mantini AM, Fridberg DJ, Buckley PF. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: a meta-analysis. Psychiatry Res 2015; 226: 1–13. [DOI] [PubMed] [Google Scholar]
- Toll A, Mane A. Brain-derived neurotrophic factor levels in first episode of psychosis: a systematic review. World J Psychiatry 2015; 5: 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998; 37: 1553–1561. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Seifert F, Schubert F, Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biological psychiatry 2007; 62: 530–535. [DOI] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemostasis 2002; 87: 728–734. [PubMed] [Google Scholar]
- Pliego-Rivero FB, Bayatti N, Giannakoulopoulos X, Glover V, Bradford HF, Stern G et al. Brain-derived neurotrophic factor in human platelets. Biochem Pharmacol 1997; 54: 207–209. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Progr Neuropsychopharmacol Biol Psychiatry 2009; 33: 849–853. [DOI] [PubMed] [Google Scholar]
- Rollins B, Martin MV, Morgan L, Vawter MP. Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet B 2010; 153B: 919–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B 2006; 141B: 261–268. [DOI] [PubMed] [Google Scholar]
- Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: a review and evaluation of the comparability of blood and brain ‘-omes'. Am J Med Genet B 2013; 162B: 595–603. [DOI] [PubMed] [Google Scholar]
- Gardiner EJ, Cairns MJ, Liu B, Beveridge NJ, Carr V, Kelly B et al. Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res 2013; 47: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Stone WS, Nossova N, Liew CC, Seidman LJ, Tsuang MT. Similarities and differences in peripheral blood gene-expression signatures of individuals with schizophrenia and their first-degree biological relatives. Am J Med Genet B 2011; 156B: 869–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 2011; 36: 228–239. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Haffmans JP, Bus BA, Spinhoven P, Penninx BW, Prickaerts J et al. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS One 2012; 7: e48046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.