Abstract
Connections between the amygdala and medial prefrontal cortex (mPFC) are considered critical for the expression and regulation of emotional behavior. Abnormalities in frontoamygdala circuitry are reported across several internalizing conditions and associated risk factors (for example, childhood trauma), which may underlie the strong phenotypic overlap and co-occurrence of internalizing conditions. However, it is unclear if these findings converge on the same localized areas of mPFC or adjacent anterior cingulate cortex (ACC). Examining 46 resting-state functional connectivity magnetic resonance imaging studies of internalizing conditions or risk factors (for example, early adversity and family history), we conducted an activation likelihood estimation meta-analysis of frontoamygdala circuitry. We included all reported amygdala to frontal coordinate locations that fell within a liberal anatomically defined frontal mask. Peak effects across studies were centered in two focal subareas of the ACC: pregenual (pgACC) and subgenual (sgACC). Using publicly available maps and databases of healthy individuals, we found that observed subareas have unique connectivity profiles, patterns of neural co-activation across a range of neuropsychological tasks, and distribution of tasks spanning various behavioral domains within peak regions, also known as ‘functional fingerprints'. These results suggest disruptions in unique amygdala–ACC subcircuits across internalizing, genetic and environmental risk studies. Based on functional characterizations and the studies contributing to each peak, observed amygdala–ACC subcircuits may reflect separate transdiagnostic neural signatures. In particular, they may reflect common neurobiological substrates involved in developmental risk (sgACC), or the broad expression of emotional psychopathology (pgACC) across disease boundaries.
Introduction
The past few years have witnessed a paradigm shift in the characterization of neuropsychiatric disorders, away from categorical descriptions towards a dimensional view.1 This shift is due, in part, to the observations of common behavioral, neurobiological and genetic substrates shared across phenotypically related diagnoses. This is particularly true among the internalizing disorders (for example, anxiety, depression and posttraumatic stress disorder (PTSD)), which are highly comorbid and have common heritable and environmental influences.2, 3 These observations have prompted the search for potential transdiagnostic neural markers (for example, Goodkind et al.4), which may provide better understanding of the etiopathogenesis of internalizing psychopathology.
Central to the internalizing disorders is the altered expression and/or regulation of emotional behavior.5 As such, a core emotion circuitry comprised of amygdala and medial prefrontal cortex (mPFC) has become a prime translational target for understanding the neural substrates of internalizing conditions. Abnormal resting-state functional connectivity (FC) between amygdala and mPFC is repeatedly reported across studies of internalizing conditions (for example, Brown et al.,6 Roy et al.7 and Etkin et al.8) and associated risk factors, for example, family history9 and exposure to childhood adversity,10 suggesting that frontoamygdala FC may be a transdiagnostic marker of internalizing psychopathology.
Although the extant literature converges on frontoamygdala circuitry as a core neural substrate altered across internalizing, genetic and environmental risk studies, it is unclear if findings across these studies localize to the same areas of mPFC or adjacent anterior cingulate cortex (ACC). ACC and mPFC are large, heterogeneous regions. Focal subareas within these do not have uniform function,11, 12 cellular composition13 or position within neuroanatomic circuits.14, 15 ACC/mPFC subregions also have distinct and frequently opposing roles in emotion processing. In general, ventral regions subserve emotion regulation, whereas dorsal regions contribute to the appraisal, expression and facilitation of emotion (see Etkin et al.16). As such, abnormalities in amygdala connectivity with different ACC/mPFC subregions likely have distinct phenotypic consequences.
To test localization of findings across studies, we conducted a coordinate-based meta-analysis of neuroimaging studies reporting disruptions in resting-state FC of the amygdala with frontal regions. We used a data-driven approach to evaluate spatial localization in studies that report significant differences in frontoamygdala FC in patient or at-risk groups. We focused on resting-state FC because it is reproducible and robust to variation in experimental parameters.17, 18, 19 To better understand resulting meta-analytic peak effects, we evaluated their connectivity profiles in healthy individuals using FC mapping and publicly available task activation databases. We also used quantitative functional decoding to identify the distribution of tasks spanning various behavioral domains within each meta-analytic peak, also known as ‘functional fingerprints'.20 Finally, we assessed the studies contributing to each peak to look for common features (for example, age and diagnosis) that could inform whether the peak was a potential marker of premorbid risk vs expression of symptomology.
Materials and methods
Study selection
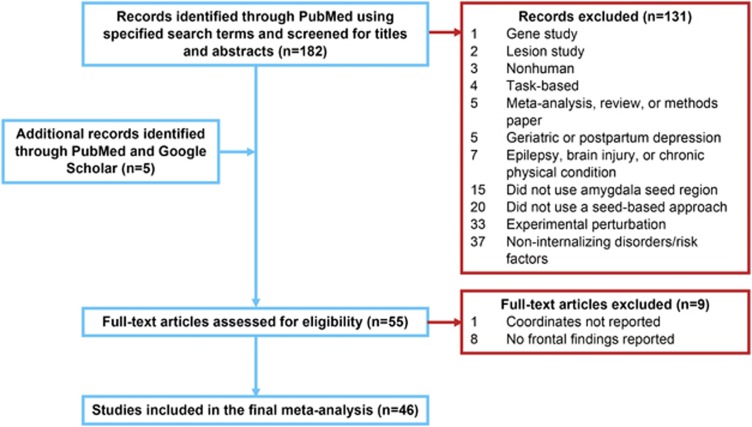
The selection process occurred in multiple stages. First, we searched PubMed (www.pubmed.gov) with the keywords ‘connectivity AND (‘resting-state' OR rest OR intrinsic) AND amygdala AND (*fMRI OR ‘functional MRI' OR ‘functional magnetic' OR fc-MRI OR fcMRI) AND (PTSD OR Borderline OR internalizing OR ‘behavioral inhibition' OR stress OR adversity OR abuse OR poverty OR maltreat* OR trauma OR depress* OR anxiety OR ‘negative affect' OR ‘reward sensitivity' OR anhedonia OR mood OR bipolar or dysthymia OR ‘negative emotionality' OR ‘neuroticism') AND (‘prefrontal' OR ‘*PFC' or cingulate OR ACC OR orbitofrontal)' for the time frame up to April 2016. This search identified 182 papers. We then refined our search from the 182 identified articles assessing from the title and abstract whether the studies: (1) investigated internalizing conditions (for example, anxiety or affective disorders) or associated risk factors (for example, family history, childhood adversity, trait anxiety and behavioral inhibition); (2) examined resting-state FC of the amygdala (that is, used a seed-based approach); (3) included a group comparison between patients/at-risk individuals and matched healthy control participants, or examined risk factors on a continuum (for example, behavioral inhibition); and (4) reported coordinates in a defined stereotaxic space (Talairach or Montreal Neurological Institute). Of note, coordinates reported in Talairach space were converted into Montreal Neurological Institute for the meta-analysis.21 When multiple patient groups were available, we included results comparing all patients vs healthy controls. Studies were excluded if they (1) used nonhuman animals, (2) used a non-seed-based approach (for example, independent components analysis), (3) seeded regions other than the amygdala, (4) examined externalizing conditions/risk factors (for example, substance use disorder, attention deficit hyperactivity disorder and aggression) or gene polymorphisms, or (5) examined FC following an experimental perturbation (for example, psychosocial stressor, intervention and mood induction). In addition, studies examining postpartum or geriatric depression were excluded, as well as studies with comorbid epilepsy, brain injury or chronic physical condition (for example, end-stage renal disease). Of note, five additional articles were later identified through PubMed and Google Scholar by assessing similar studies that met the initial inclusion/exclusion criteria. After applying outlined exclusion/inclusion criteria (see Figure 1), 55 studies were further assessed for eligibility by reviewing the full text of the article. Nine studies were excluded after full-text review because although these studies fulfilled our initial inclusion criteria, eight studies did not report significant findings in frontal regions and one study did not report coordinate locations (Figure 1; Supplementary Table S1). Thus, 46 studies with a total of 2401 participants (n=893 in patient/risk group) were ultimately included in the meta-analysis (Table 1). Twelve studies evaluated heritable or temperamental risk factors (for example, negative affect and family history), 9 included individuals with major depressive disorder (MDD), 7 with anxiety disorders, 6 with PTSD, 6 environmental risk studies (for example, adversity and stress), 4 with bipolar disorder, 1 with borderline personality disorder, and 1 examined PTSD and environmental risk (early stress) within the same study. Thirty-two studies included adults and 14 included youth ages 18 and under. The majority of included studies (38) contributed more than one foci, and in total, 206 experimental foci were analyzed.
Figure 1.
Study selection. Number of studies is given in bold letters.
Table 1. Included studies that examined internalizing conditions or risk factors and resting-state functional connectivity between amygdala and frontal regions.
| # | First author | Year | Journal | Participant ages (years) | Total number of participants | % Female | Groups or variable of interest | Length of scan (min) | Eyes closed or eyes open | n on medications | GSR (global signal regression)? | Scrubbing or regressing out affected volumes? | Amygdala seed definition | Whole-brain or ROI analysis | Number of frontal peaks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chai | 2016 | Biol Psychiatry | 8–14 | 43 | 49 | Children with familial risk of MDD vs HC | 6.2 | Eyes open, blank screen | Not specified | No | Yes—outlier volumes regressed out | AAL atlas | Whole brain | 2 |
| 2 | Zhang | 2016 | Prog Neuropsychopharmacol Biol Psychiatry | 38–62 | 66 | 58 | PTSD vs trauma-exposed controls | 8 | Eyes closed | Not specified | Yes | No | AAL atlas | Whole brain | 5 |
| 3 | Aghajani | 2016 | Hum Brain Mapp | 13–17 | 42 | 90 | Sexually abused adolescents with PTSD vs controls | 6 | Eyes closed | 3 | Yes | Yes | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 4 |
| 4 | Kim | 2016 | Neuropsychiatr Dis Treat | 12–16 | 44 | 32 | Adolescents with MDD and disruptive behaviors vs HC | 12 | Not specified | No current psychotropic use | No | No | AAL atlas | Whole brain | 2 |
| 5 | Barch | 2015 | Am J Psychiatry | 7–12 | 105 | 41 | Income-to-needs ratio in children | 6.8 | Eyes closed | Not specified | Yes | Yes | Subject-specific seeds derived from Freesurfer | Whole brain | 2 |
| 6 | Davey | 2015 | Psychol Med | 16.5±0.5 | 56 | 45 | Negative affect in adolescents | 11.9 | Eyes closed | 1 (fluoxetine) | Yes | No | AAL atlas | ROI: BA 25 (via WFU Pickatlas) | 1 |
| 7 | Liu | 2015 | Med Sci Monit | 13–18 | 46 | 59 | Adolescents with first-episode GAD vs HC | 8 | Eyes closed | All med free during 2 weeks before study | Yes | no | AAL atlas | Whole brain | 3 |
| 8 | Nicholson | 2015 | Neuropsychopharmacology | PTSD−DS: 37±12.9 PTSD+DS: 37±12.7 HC: 32.3±11.4 | 89 | 74 | PTSD±dissociative subtype (DS) vs HC | 6 | Eyes closed | No current psychotropic use | Not specified | Yes—outlier volumes regressed out | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 8 |
| 9 | Rohr | 2015 | Neuroimage | 25±2.37 | 43 | 53 | Negative affectivity and task interference (ability to inhibit negative information and negative affect) in healthy adults | 7.67 min & 15.33 min (1/2 of sample) | Not specified | Not specified | No | No | Harvard–Oxford atlas | Whole brain | 2 |
| 10 | Stoddard | 2015 | Psychiatry Res | 9–18.5 | 53 | 38 | Youth with BD vs vs severe mood dysregulation (SMD) vs HC | 6 | Not specified | 42 | No | Yes—outlier volumes regressed out | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 1 |
| 11 | Wang | 2015 | Behav Brain Res | MDD: 32.11±11.25 HC: 33.28±8.83 | 60 | 45 | MDD vs HC | 8 | Not specified | Not specified | Yes | No | 6 mm sphere around peak atrophy voxels (−16, −6 and −16) | Whole brain | 1 |
| 12 | Thomason | 2015 | Soc Cogn Affect Neurosci | 9–15 | 42 | 69 | Trauma-exposed youth vs controls | 6 | Eyes closed | 4 (3 trauma, 1 comparison) | No | Yes—scrubbing in secondary analysis | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain and ROI in vACC | 18 |
| 13 | Arnold Anteraper | 2014 | Brain Connect | SAD: 24.7±6.3 HC: 25±7.5 | 34 | 53 | SAD vs HC | 6.4 | Eyes open, fixation cross | Medication naive | No | No | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 2 |
| 14 | Baeken | 2014 | PLoS One | 21.7±2.5 | 56 | 100 | Harm avoidance (personality dimension) in healthy adults | 5 | Eyes closed | None used medications | No | No | −20, −4, -15 and 22, −2, 15 (Cisler et al.) | Whole brain | 10 |
| 15 | Birn | 2014 | Depress Anxiety | 22–31 | 27 | 0 | Childhood adversity and PTSD symptoms in veterans | 5.5 | Not specified | No current med use | No | Yes—despiking | 4mm spheres centered on coordinate centers provided by Talarach Daemon | Whole brain | 8 |
| 16 | Blackford | 2014 | Biol Psychol | 18–25 | 40 | 60 | Social inhibition in young adults (n=8 met criteria for 1 or more AD) | 7 | Eyes closed | No current psychotropic use | Yes | No | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 12 |
| 17 | Aghajani | 2014 | Cogn Affect Behav Neurosci | 40.51± 9.45 | 50 | 64 | Trait neuroticism in healthy adults | 7.67 | Eyes closed | No current med use | Yes | No | Harvard–Oxford atlas | Whole brain | 3 |
| 18 | Qin | 2014 | Biological Psychiatry | 7–9 | 76 | 50 | Anxiety scores in children | 8 | Eyes closed | No current psychotropic use | Yes | Yes—scrubbing in secondary analysis | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 6 |
| 19 | Brown | 2014 | Neuropsychopharmacology | PTSD: 44.1±11 Trauma-exposed controls: 40±8.9 | 42 | 24 | PTSD vs trauma-exposed controls (recent military veterans) | 6.3 | Eyes open, fixation cross | 14 | No | Yes—scrubbing in secondary analysis | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 2 |
| 20 | Coombs | 2014 | PLoS One | 19–53 | 38 | 29 | Negative affect in healthy adults | 6.2 | Eyes open, blank screen | No lifetime med use | Yes | No | Jeulich Histological atlas | Whole brain | 1 |
| 21 | Fan | 2014 | Hum Brain Mapp | 21–36 | 18 | 0 | Early-life stress exposure in adults | 8 | Eyes open, fixation cross | Not specified | No | No | AAL atlas and based on meta-analysis of emotion processing (Wager et al., 2012) | Whole brain | 15 |
| 22 | Golkar | 2014 | PLoS One | 19–46 | 93 | 57 | Work-related (perceived) chronic stress in adults | 8 | Eyes closed | All med free during 2 months before study (except contraceptives) | No | No | 5 mm spheres around (−22, −7, −18 and 22, −7, −19) | Whole brain | 5 |
| 23 | Hamm | 2014 | Biol Mood Anxiety Disord | AD: 13.9±3.1 HC: 14.6±3.9 | 55 | 64 | Pediatric AD (GAD, social phobia and SAD) vs HC | 8 | Eyes open, fixation cross | All med free at the time of scan | No | No | AAL atlas based on Talairach Daemon database | Partial brain mask of mPFC, ACC, PCC and insula (AAL atlas) | 5 |
| 24 | Jacobs | 2014 | PLoS One | 18–23 | 53 | 66 | Remitted MDD vs HC | 8 | Eyes open | All med free during 30 days prior to study | No | Yes—scrubbing in secondary analysis | ±23, −5, −19 (2.9 mm radius sphere) | Whole brain | 1 |
| 25 | Krause-Utz | 2014 | Psychol Med | 18–45 | 37 | 100 | Borderline personality disorder (with the history of interpersonal trauma) vs HC | 6.25 | Eyes closed | Free of medication within the past 14 days (28 on fluoxetine) | Yes (repeated analyses without) | No | ±23, −4, −19 (Veer et al., 2011; 4 mm radius sphere) | Whole brain | 2 |
| 26 | Liu | 2014 | Schizophr Bull | BD: 33±10.0 HC: 36.6±12 | 36 | 61 | BD (no comorbidities) vs HC | not specified | Eyes closed | 18 (bipolar patients) | Yes | No | Amunts 2005 (in SPM Anatomy toolbox) | Prefrontal mask (BA's 9–12, 24, 25, 32 and 44–47) | 8 |
| 27 | Pannekoek | 2014 | J Child Psychol Psychiatry | MDD: 15.4±1.5 HC: 14.7±1.5 | 52 | 88 | Youth with MDD vs HC | 6 | Eyes closed | None used medications | Yes | No | Harvard–Oxford Subcortical Structural Probability atlas (in FSL; ±22, −6, −16) | Whole brain | 3 |
| 28 | Ramasubbu | 2014 | Front Psychiatry | MDD: 36.5 ±10.41 HC: 32.89±9.97 | 74 | 59 | MDD vs HC | 7.67 | Eyes open, fixation cross | All med free at the time of scanning. Fifty-two MDD patients had been previously exposed to antidepressants | No | No | FSLView and registered to participants native fMRI image | Whole brain | 9 |
| 29 | Roy | 2014 | Biological Psychiatry | BI: 19.6±1 Non-BI: 19.5±0.94 | 38 | 53 | Young adults with childhood history of behavioral inhibition | 6 | Eyes closed | No current psychotropic use | Yes | Yes—despiking | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 4 |
| 30 | Singh | 2014 | Bipolar Disord | 8–17 | 49 | 63 | Youth with low vs high familial risk of BD | 7 | Eyes closed | None used medications | No | Yes—outlier volumes regressed out | Harvard–Oxford atlas | Whole brain | 1 |
| 31 | Zhang | 2014 | PLoS One | 18–24 | 67 | 52 | First-episode MDD vs HC | 5 | Eyes closed | Medication naive | Yes | No | AAL atlas | Whole brain | 1 |
| 32 | Anticevic | 2013 | Biological Psychiatry | Bipolar 1 psychosis hx: 34±10.8 Bipolar 1 no psychosis hx: 29.85±11.9 HC: 31.14±10.6 | 119 | 65 | BD (1/2 with the history of psychosis; 46% with comorbid AD, 57% alcohol use, and 43% drug use) vs HC | 5.25 | Eyes open | 57 | Yes | No | Freesurfer-based segmentation | Whole brain | 2 |
| 33 | Carlson | 2013 | Cortex | 19–23 | 15 | 60 | Attentional bias to threat | 5 | Eyes closed | Not specified | No | No | Harvard–Oxford atlas | ROI: 6 mm sphere at ±4, 46, −4 (ACC) | 8 |
| 34 | Herringa | 2013 | Proc Natl Acad Sci USA | 18.79±0.19 | 64 | 47 | Young adults with maltreatment during childhood | 7 | Eyes closed | Not specified | Yes | Yes—scrubbing | 4 mm rad spheres in amygdala defined by Talairach Daemon | Whole brain | 3 |
| 35 | Prater | 2013 | Depress Anxiety | gSAD: 25.95±5.39 HC: 25.71±7.15 | 37 | 57 | gSAD vs HC | 5 | Eyes open, fixation cross | 2 (gSAD patients; SSRIs) | Yes | No | All faces>shapes localizer confined within AAL-defined anatomical amygdala | Partial brain mask of ACC, mPFC, DLPFC and OFC (AAL atlas) | 2 |
| 36 | Roy | 2013 | J Am Acad Child Adolesc Psychiatry | 12–17 | 35 | 66 | Youth with GAD vs HC | 6 | Eyes open, fixation cross | No current or past use of psychotropic medication | Yes | No | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 3 |
| 37 | Tahmasian | 2013 | Front Human Neurosci | MDD: 51±15 HC: 49.6±13.9 | 41 | 54 | MDD vs HC | 10 | Eyes closed | 40 (5 with antidepressant mono-therapy, 10 with dual therapy and 5 with tri-therapy) | Yes | No | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 4 |
| 38 | Tang | 2013 | Psychol Med | MDD: 29.3±8.7 HC: 30.1± 8.4 | 58 | 53 | MDD vs HC | 6.67 | Eyes closed | Treatment naive | Yes | No | AAL atlas | Whole brain | 2 |
| 39 | Torrisi | 2013 | Bipolar Disord | BP1: 42.1±11.4 HC: 39.8±12.6 | 40 | 50 | BD (I) vs HC | 7 | Eyes closed | 17 (bipolar patients) | No | No | Talairach Daemen and Harvard–Oxford | Whole brain | 3 |
| 40 | van der Werff | 2013 | Psychol Med | CEM: 39±10.3 No CEM: 37.6±9.7 | 88 | 52 | Adults reporting childhood emotional maltreatment (CEM; before age 16; no physical or sexual abuse) vs non-CEM | 7.6 | Not specified | Not specified | Yes | No | Harvard–Oxford atlas | Whole brain | 5 |
| 41 | Sripada | 2012 | J Psychiatry Neurosci | 21–37 | 29 | 0 | PTSD vs combat-exposed controls | 10 | Eyes open, fixation cross | 1 (trazodone as sleep aid) | Yes | No | Amunts 2005 (in SPM Anatomy toolbox) | Whole brain | 3 |
| 42 | Hahn | 2011 | Neuroimage | GAD: 27.7±7.2 HC: 28.6±4.3 | 37 | 46 | AD (SAD, PD or both) vs HC | 6 | Eyes open, low-level illumination | All med free during 3 months before study | Yes | No | AAL atlas | Whole brain | 3 |
| 43 | Kim | 2011 | Cereb Cortex | 19.± −0.9 | 29 | 72 | Anxiety scores in healthy adults | 7 | Eyes open, ‘relax' on screen | None used medications | Yes | No | Harvard–Oxford atlas | Whole brain | 2 |
| 44 | Lui | 2011 | Am J Psychiatry | Nonrefractory MDD: 32±10 Refractory MDD: 33±11 HC: 35±12 | 108 | 35 | MDD (nonrefractory) vs HC | 6.7 | Eyes closed | 108 | No | No | AAL atlas | Whole brain | 3 |
| 45 | Rabinak | 2011 | Front Psychiatry | PTSD: 30.12±7.70 Combat-exposed controls: 33.71±9.12 | 34 | 0 | PTSD vs combat-exposed controls | 8 | Eyes open, fixation | All med free at time of scan. N=8 hx of psychotropic (n=8 SSRI and n=1 also taken NE-DA reuptake inhibitor, n=1 tricyclic, n=2 5-HT antagonist reuptake) | Yes | No | Walter 2003 (in MARINA software) | Whole brain | 1 |
| 46 | Liao | 2010 | PLoS One | SAD: 22.55±4.05 HC: 21.71±3.64 | 43 | 28 | SAD vs HC | 6.83 | Eyes closed | All med free at the time of scan | Yes | No | AAL atlas | Whole brain | 15 |
Abbreviations: AAL, Automated Anatomical Labeling; ACC, anterior cingulate cortex; AD, anxiety disorder; BD, bipolar disorder; DLPFC, dorsolateral prefrontal cortex; fMRI, functional magnetic resonance imaging; GAD, generalized anxiety disorder; gSAD, generalized social anxiety disorder; HC, healthy controls; med, medication; MDD, major depressive disorder; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; PD, panic disorder; PTSD, posttraumatic stress disorder; ROI, region of interest; SSRIs, selective serotonin reuptake inhibitors; SAD, social anxiety disorder.
We used a data-driven approach, and included all coordinate locations reported in eligible studies that fell within an anatomically defined frontal mask (Supplementary Figure S1) comprised of the 13 frontal areas defined by the Harvard–Oxford cortical atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html): frontal operculum cortex, frontal orbital cortex, cingulate gyrus (anterior division), paracingulate gyrus, subcallosal cortex, frontal medial cortex, precentral gyrus, inferior frontal gyrus (pars opercularis), inferior frontal gyrus (pars triangularis), middle frontal gyrus, superior frontal gyrus, insular cortex and frontal pole. The mask was dilated by one voxel in all directions. Studies could contribute more than one unique frontal peak.
ALE meta-analysis
We used the revised activation likelihood estimation (ALE) algorithm22, 23 to identify consistent patterns of amygdala resting-state FC changes with frontal regions. This algorithm aims to identify brain areas showing a convergence of reported coordinates across studies, which is higher than expected under a random spatial association. ALE treats reported peak coordinates, or ‘foci', as centers for three-dimensional Gaussian probability distributions that capture the spatial uncertainty associated with each focus. Width of the probability distribution is weighted based on sample size of the study from which foci were drawn, such that smaller distributions are used for larger samples and vice versa. Then, for each voxel, probabilities of all foci of a given study are aggregated to produce a modeled activation map.24 Modeled activation maps are combined to produce voxel-wise ALE scores, which reflect the convergence of results at each location of the brain.
Significance of convergence was assessed by comparison of ALE scores with a null distribution that includes the same number of peak foci distributed randomly throughout the brain's gray matter.23 Random-effects inference was applied. Resulting statistical maps show clusters where convergence between foci is greater than would be expected by chance. Statistical maps were thresholded using cluster-level family-wise error correction P<0.05 (cluster-forming threshold voxel-level P<0.001). When available, probabilistic cytoarchitectonic maps available through the SPM Anatomy toolbox25 were used to estimate spatial localization of results.
Functional characterization of meta-analytic peaks
To understand the functional significance of identified meta-analytic peaks, and to test whether these represent separate frontoamygdala subcircuits, we performed three complementary analyses in healthy adults to extrapolate what differences in amygdala FC might mean in patient or at-risk groups.
Resting-state FC profiles
First, we derived the pattern of whole-brain resting-state FC for each meta-analytic peak. This analysis was conducted in a sample of 1000 healthy adults via www.Neurosynth.org.26 Results are displayed at a false discovery rate-adjusted threshold of P<0.01.
Patterns of task-related co-activation
Next, we evaluated the pattern of task-based co-activation for each peak, using meta-analytic connectivity modeling.27 Spherical (6 mm radii) regions of interest (ROIs) were created for each meta-analytic frontal peak, and the BrainMap database (www.brainmap.org)28 was searched for all functional magnetic resonance imaging (fMRI) and positron emission tomographic (PET) experiments that activated each ROI. We only considered experiments reporting stereotaxic coordinates from normal mapping studies in healthy individuals. Thus, pharmacological interventions and group comparisons were excluded. First, three-dimensional peak coordinates from peak areas that co-activate with each ROI were pooled from retrieved studies. Then, ALE meta-analysis was used to test for spatial convergence in these co-activation peaks, using similar methods as described above. ALE statistical maps were again thresholded using cluster-level family-wise error correction, P<0.05 (cluster-forming threshold voxel-level P<0.001).
Behavioral domains associated with activation
To further characterize observed meta-analytic peaks, we tested the distribution of tasks spanning various behavioral domains within peak regions, also known as ‘functional fingerprints'.20 For each ROI, we evaluated the ‘behavioral domain' meta-data from the retrieved experiments in the BrainMap database that elicited activation in that ROI (above). Behavioral domains include cognition, action, perception, emotion and interception, as well as their related subcategories (see http://brainmap.org/scribe for more information on the BrainMap taxonomy). For each domain/subcategory, the number of experiments that reported activation in each ROI was calculated. Domains/subcategories with <25 corresponding experiments are not shown.
Results
Meta-analysis of frontoamygdala resting-state FC across internalizing conditions and risk factors
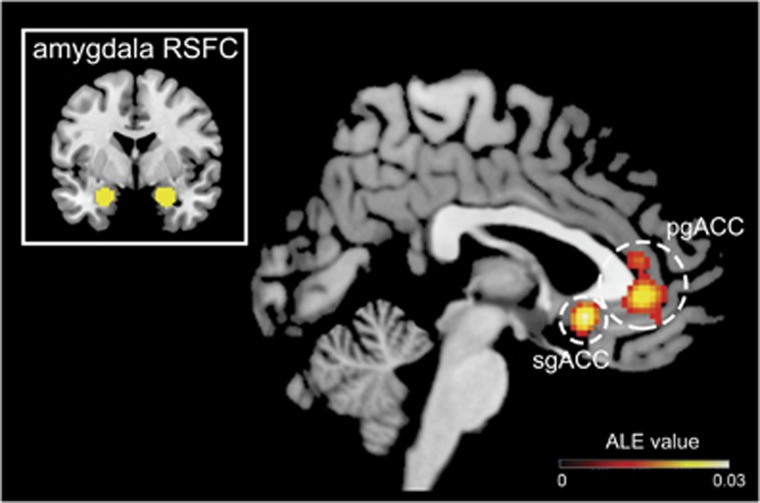
The coordinate-based meta-analysis revealed two frontal regions, or ‘clusters', where amygdala resting-state FC was reliably altered across studies (Figure 2; Supplementary Table S2). Notably, both clusters were centered in the ACC, with limited extension into mPFC. The largest cluster was centered in bilateral pregenual ACC (pgACC), and extended into both anterior dorsal and subgenual ACC (sgACC; 8% probability in s24; see Supplementary Table S2). Hereafter, we refer to this cluster as pgACC. The second cluster was more ventral and centered in right sgACC (72% probability in s24; Supplementary Table S2).
Figure 2.
Converging evidence of disrupted amygdala functional connectivity with two separate ACC subregions across 46 internalizing, genetic and environmental risk studies. Results of coordinate-based meta-analysis that included 2401 individuals. ACC, anterior cingulate cortex; ALE, activation likelihood estimation; pgACC, pregenual ACC; RSFC, resting-state functional connectivity; sgACC, subgenual ACC.
Next, we examined the studies contributing to each cluster (Supplementary Table S2) to look for commonalities across studies. We found that studies contributing to the sgACC cluster consisted predominantly of young people ages 20 and under, with varied environmental (for example, early stress exposure) and temperamental (for example, negative affect and behavioral inhibition) risk factors. χ2 analysis suggests that studies on youth and risk factors were over-represented in the sgACC peak relative to all studies included in the meta-analysis, χ2(1)=19.92, P<0.001. Further, re-running the meta-analysis with the 16 studies that consisted of young people ages 20 and under yielded significant convergence in the same sgACC area (x=4, y=18, z=−8, ALE=0.024). The pgACC cluster, in contrast, was observed broadly across studies of anxiety disorders, affective disorders and risk factors (for example, familial risk, social inhibition and childhood adversity). Notably, although ~20% of all studies included in the meta-analysis evaluated individuals with MDD, coordinates from those studies did not show significant spatial convergence.
Across all included foci, the pattern of change was inconsistent: amygdala–frontal FC was increased in patient or at-risk groups in 50% of reported foci and decreased in 45%. Directionality was not reported for 5% of foci. We also did not find consistent patterns of change within each meta-analytic cluster. One dimension that may contribute to inconsistent findings is the application of global signal regression (GSR; Table 1). GSR is a processing step used to reduce motion-related artifact and correct the global signal in fMRI time-series data, but may result in distortions within networks and across groups.29, 30 When only studies that did not apply GSR were considered, we found that FC was increased in 56% of reported foci and decreased in 37%. Directionality was not specified in 7% of foci. The pattern of FC change for foci contributing to each meta-analytic peak was not more consistent when considering only studies that did not apply GSR. In addition, because FC values are typically normalized within-study, it is unclear, for example, whether increased FC reflects increased positive vs reduced negative connectivity. Specific directionality of effects (for example, increased positive FC) was reported for only 38% of included peaks.
Medication use in the study sample was also considered (Table 1). For each peak, two to three of the contributing studies reported psychotropic use in a small number of study participants (one to four). There were one to two studies that contributed to each meta-analytic peak with substantial past and/or current medication use in the patient group. Thus, observed peaks are not likely driven by medication use. We also evaluated the use of a priori target ROIs, which may bias results.31 We re-ran the meta-analysis excluding the five studies utilizing these a priori target ROIs (Table 1). Results were consistent with findings reported here.
Laterality of the amygdala seed region was split across study foci, with 42% reporting effects with right amygdala, 38% with left amygdala and 19% with bilateral amygdala. Laterality was also split under each meta-analytic cluster: 6 of the 12 foci contributing to the pgACC peak reported effects with left amygdala, 5 with bilateral and one with right amygdala. Three of the five foci contributing to the sgACC peak reported effects with bilateral amygdala, one with right and one with left amygdala.
Functional characterization of ACC meta-analytic peaks
Next, we performed functional characterizations of the resulting ACC peaks in healthy individuals to infer what connectivity between amygdala and ACC may mean in patient or at-risk groups. Results may also inform whether the observed ACC peaks represent separate or overlapping brain circuits.
Resting-state FC profiles
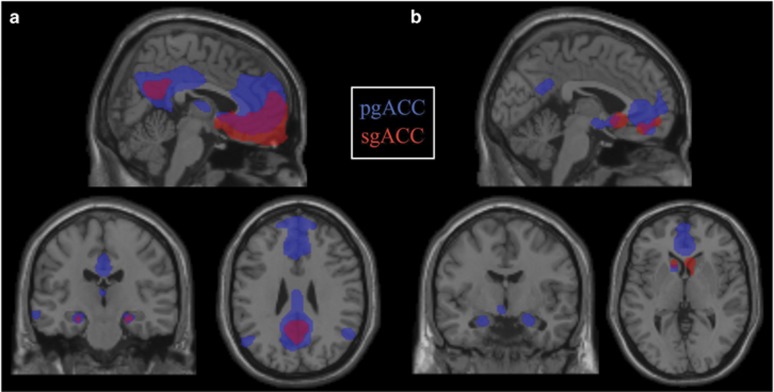
We first examined patterns of resting-state FC of each ACC peak in a sample of 1000 healthy individuals. As shown in Figure 3a, whole-brain FC patterns were unique for each peak. In brief, the activity in pgACC was correlated with the activity in precuneus and posterior cingulate cortex, resembling the canonical default mode network (DMN),32 as well as amygdala, insula and inferior frontal gyrus, involved in the salience network.33 sgACC correlations were observed in local ACC regions, extending into caudate, amygdala and hippocampus, and also in precuneus and posterior cingulate cortex (Supplementary Table S3).
Figure 3.
ACC meta-analytic peaks show unique patterns resting-state functional connectivity (FC) (a) and task-related co-activation (b), suggesting unique subcircuits. (a) Resting-state FC data in 1000 healthy individuals generated via www.Neurosynth.org, P<0.01 FDR corrected. (b) Coordinate-based meta-analysis of areas that co-activate with ACC meta-analytic peaks. A total of 971 healthy individuals contributed to pgACC and 493 to sgACC. Thresholded with cluster-level FWE correction P<0.05 and voxel-level, P<0.001. ACC, anterior cingulate cortex; FDR, false discovery rate; FWE, family-wise error; pgACC, pregenual ACC; sgACC, subgenual ACC.
Patterns of task-related neural co-activation
We quantitatively mapped task-based co-activations for each peak using the BrainMap database. Fifty-four and 29 experiments in the BrainMap database reported activations within pgACC and sgACC ROIs, respectively. These studies consisted of 971 and 493 healthy individuals, respectively (see Supplementary Table S5 for meta-data from retrieved experiments). Using meta-analytic connectivity modeling, we found distinct patterns of co-activation clusters for each ACC peak (Figure 3b; Supplementary Table S4). In brief, pgACC was associated with co-activation clusters in caudate, posterior cingulate/precuneus, amygdala and parahippocampal gyrus. sgACC was co-activated with caudate and orbitofrontal cortex.
Behavioral domains associated with activation
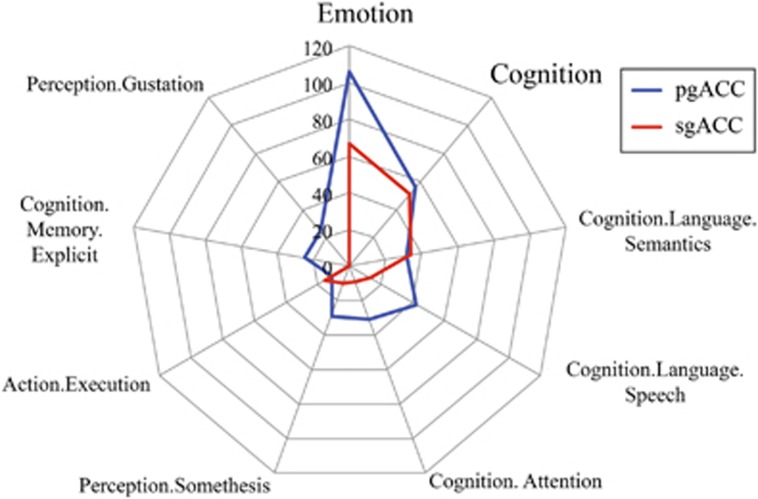
To outline the functional profiles of observed peaks, we performed a functional decoding analysis based on the BrainMap meta-data. We found that activation in both pgACC and sgACC was associated with the emotion domain and, to a lesser extent, cognition (Figure 4). Relative to sgACC, pgACC activation was more likely to be associated with perception, and language and memory subcategories of cognition.
Figure 4.
ACC meta-analytic peaks show unique functional fingerprints. Behavioral domains (number of studies) associated with activity in each ACC peak, according to studies in the www.BrainMap.org database (accessed on 2 May 2016). Behavioral domains with <25 corresponding studies are not included. A total of 971 healthy individuals contributed to pgACC and 493 to sgACC. ACC, anterior cingulate cortex; pgACC, pregenual ACC; sgACC, subgenual ACC.
Discussion
Altered connectivity between amygdala and frontal regions is commonly reported across a range of internalizing, genetic and environmental risk studies. Here we conducted a coordinate-based meta-analysis to test whether findings across studies localize to the same frontal subarea(s). Results converged on two focal subareas of the ACC, centered in pgACC and sgACC. Using FC analyses and publicly available databases of healthy individuals, we discovered that each peak has unique resting-state FC, functional co-activation profiles and ‘functional fingerprints'. These results suggest that observed peaks represent separate frontoamygdala subcircuits. Based on functional characterizations and the studies contributing to each peak, we assert that observed subcircuits reflect distinct transdiagnostic neural signatures. In particular, amygdala–pgACC disruptions were observed broadly in individuals across the internalizing spectrum and may thus reflect general emotional disturbance or specific symptoms that are shared across the internalizing conditions (for example, negative affect34). Altered amygdala–sgACC FC, in contrast, was observed almost exclusively in at-risk youth, implying a potential brain substrate of developmental vulnerability.
The largest meta-analytic cluster was centered in the pgACC, which is involved in automatic forms of emotion regulation, performing a generic negative emotion inhibitory function whenever there is a need for suppression of limbic reactivity.35 Explicit forms of emotion regulation occur by engaging this core circuitry (see Etkin et al.16), which is consistent with this peak's functional characterization under both cognition and emotion behavioral domains (Figure 4). Here we found that studies across the internalizing spectrum reported abnormalities in amygdala–pgACC circuitry. This raises the possibility that amygdala–pgACC circuitry is broadly involved in emotional psychopathology, or a construct that is shared across the internalizing conditions. For example, prominent models of core affect34, 36 emphasize that ‘loss' symptomology, or a general sense of negative affect or dysphoria (for example, feelings of sadness/withdrawal) is shared across internalizing disorders. Threat symptomology (for example, avoidance and hypervigilance), in contrast, is more specific to the anxiety disorders, and disruptions in positive affect (for example, reward deficits and anhedonia) are more specific to the mood disorders.34 In line with a general role of amygdala–pgACC circuitry in emotional psychopathology, reduced pgACC gray matter volume is consistently reported in meta-analyses of anxiety37 as well as affective disorders.38 Notably, FC and co-activation mapping in Figures 3 and 4 revealed strong connectivity and co-activation of the pgACC with core nodes of the DMN, including precuneus and posterior cingulate cortex. The tight coupling between pgACC and DMN may allow affective disruptions in amygdala–pgACC circuitry to integrate into self-referential processes supported by the DMN, thus propagating negative affect (see Hamilton et al.39). Connectivity was also observed between pgACC and fronto-insular regions, implicated in the salience network. Increased salience network response to negatively valenced stimuli is a consistently reported finding in MDD,40 suggesting a potential role for pgACC in negative emotion processing. Taken together, abnormalities in core emotion regulation amygdala–pgACC circuitry may underlie the generic negative affect dysregulation observed across internalizing conditions.
Studies contributing to the sgACC cluster consisted of environmental and temperamental risk studies (for example, childhood adversity, negative affect and behavioral inhibition) conducted predominantly in young people (ages 20 and under). Thus, disruptions in amygdala–sgACC circuitry might reflect a state of premorbid risk—a notion supported by prior research. For instance, longitudinal studies demonstrate that dysfunctional response in amygdala corresponds with genetic (that is, family history of depression) and environmental risk (that is, childhood emotional neglect41), and that response in sgACC predicts subsequent increases in depressive symptomology during adolescence.42 Broadly, sgACC is thought to subserve behavioral withdrawal and the promotion of safety behaviors.43, 44 Thus, early alterations in amygdala–sgACC circuitry may underlie early withdrawal behaviors that could lead to further development of internalizing symptomology. For instance, emergence of emotional psychopathology may depend on later changes in amygdala–pgACC circuitry.
Although the ALE meta-analysis identified significant spatial convergence in two areas of the cingulate cortex, a large portion (~70%) of studies did not contribute to observed meta-analytic peaks. Notably, there was particularly low spatial convergence in MDD. This is consistent with prior ALE meta-analyses in MDD and other psychiatric disorders. For example, one ALE meta-analysis in MDD45 reported consistent gray matter reductions (relative to healthy controls) in a similar bilateral pgACC region, with 40% of included studies contributing to this peak. In that study, and other ALE studies in psychiatric populations (for example, Chen et al.46), as low as 4% of included studies contribute to a single meta-analytic peak. Taken together, these findings suggest significant variability across studies, and particularly within MDD. Convergence within frontoamygdala circuitry might be achieved with the addition of more studies with specific patient subgroups (for example, early age of onset and recurrent). Signal dropout may also contribute to low convergence across studies, as amygdala and ventral frontal regions are highly susceptible to signal loss.47 Another possibility is that this variability reflects significant heterogeneity in network topology among patients. Balsters et al.48 suggest that conventional methods for generating seed regions may contribute to variable connectivity findings, as these methods do not account for heterogeneous network topology in patient groups.
Our meta-analytic results demonstrate the importance of improved anatomic specificity in reported findings. This point is not unique to the study of internalizing conditions, and there are several examples in the literature illustrating this.16, 49, 50 There are various means available for improving specificity in reported findings. One resource is cytoarchitectonic maps, including the widely used Brodmann areas and more recent three-dimensional multimodal brain atlases that allow registration of fMRI data into cyto-, myelo- and chemo-architectonic maps. For example, the Eickhoff–Zilles atlas distributed with SPM Anatomy toolbox25 and the Harvard–Oxford atlas51 distributed with the FSL software (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/)are increasingly used for fMRI processing and interpretation. As demonstrated here, publicly available tools and databases (for example, BrainMap and Neurosynth) can allow for better understanding of the functional profiles and circuitries in which resulting peak areas are embedded. It is encouraging that results derived from large databases (for example, coordinate-based meta-analysis and meta-analytic connectivity modeling) appear to recapitulate known cytoarchitectonic borders (see Fox et al.52).
Limitations of this work warrant mention. We focused on resting-state FC studies to circumvent variation in behavioral performance and differences in task parameters/paradigms across studies. However, there are still various experimental (for example, eyes open vs eyes closed) and analytic (for example, GSR and motion scrubbing) strategies that differ across FC studies that may have an impact on meta-analytic findings. Indeed, the field still lacks consensus on the best practices for collecting and processing resting-state FC data and, moreover, how these various approaches have an impact on observed findings. We attempted to address this by evaluating studies separately based on the use of GSR, which is known to alter resting-state FC correlations. We also provide key factors that vary between studies (Table 1), and suggest that there may be other factors contributing to variability across studies (for example, experimental or analytic methods, differences in sample characteristics, disease course and psychological state). Another consideration is that eight additional studies met criteria for inclusion, but did not report significant effects in frontal regions (Supplementary Table S1). Our goal was to examine spatial overlap in studies that do report findings in frontal regions. Future research should test the robustness of these effects using similar methodology. In addition, functional characterizations of observed meta-analytic peaks were conducted in healthy individuals, which allowed us to (1) evaluate whether peaks reflect unique brain areas that are embedded in unique circuitries, and (2) infer what behavioral consequences of altered connectivity in these areas might be. A comprehensive developmental and clinical characterization of these circuitries across ages and patient populations is warranted. Next, although we focus here on identifying focal subareas of frontal regions, there are also important subregions of the amygdala.53, 54 Seventeen percent of the experimental foci included in the meta-analysis reported effects of amygdala subregion(s): 17 in basolateral amygdala, 9 in centromedial and 8 in superficial. Further research is needed to understand contributions of amygdala subregion(s) to these findings, and advances in multiband and multiecho neuroimaging will make this all the more accessible.
Conclusions
The present meta-analysis indicates that findings across internalizing, genetic and environmental risk studies converge on two focal subareas of ACC. We demonstrate that these ACC subregions have unique patterns of resting-state FC, task-related co-activation and ‘functional fingerprints', suggesting that they represent distinct frontoamygdala subcircuitries. Based on these functional characterizations and the studies contributing to each meta-analytic peak, disruptions in frontoamygdala subcircuits might reflect separate transdiagnostic neural signatures involved in developmental risk (sgACC) or the broad expression of emotional psychopathology (pgACC).
Acknowledgments
Dr Marusak is supported by American Cancer Society award 129368-PF-16-057-01-PCSM. Dr Rabinak is supported by National Institutes of Health (NIH) National Institute of Mental Health award K01 MH101123. Dr Thomason is supported by NIH National Institute of Environmental Health Sciences awards P30 ES020957 and R21 ES026022, and NIH National Institute of Mental Health award R01 MH110793. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and design to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Cuthbert BN. Research domain criteria: toward future psychiatric nosologies. Dialogues Clin Neurosci 2015; 17: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HM, Gregory AM, Eley TC. The phenotypic and genetic structure of depression and anxiety disorder symptoms in childhood, adolescence, and young adulthood. JAMA Psychiatry 2014; 71: 905–916. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. Gene-environment interplay in internalizing disorders: consistent findings across six environmental risk factors. J Child Psychol Psychiatry 2009; 50: 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DP, Krueger RF, Andrews G, Hobbs MJ. Emotional disorders: cluster 4 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med 2009; 39: 2043–2059. [DOI] [PubMed] [Google Scholar]
- Brown VM, Labar KS, Haswell CC, Gold AL, Mid-Atlantic MW, Beall SK et al. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 2014; 39: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 2013; 52: 290–299.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009; 66: 1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Kelley RG, Saggar M, Reiss AL, Gotlib IH. Early signs of anomalous neural functional connectivity in healthy offspring of parents with bipolar disorder. Bipolar Disord 2014; 16: 678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR. Altered amygdala connectivity in urban youth exposed to trauma. Soc Cogn Affect Neurosci 2015; 10: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage 2011; 56: 2157–2172. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Henson RN, Simons JS. The scale of functional specialization within human prefrontal cortex. J Neurosci 2010; 30: 1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 1995; 359: 490–506. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 2009; 29: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 2007; 37: 579–588. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ et al. The resting brain: unconstrained yet reliable. Cereb Cortex 2009; 19: 2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C et al. Resting-state fMRI can reliably map neural networks in children. Neuroimage 2011; 55: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe AS, Jones CK, Joel SE, Muschelli J, Belegu V, Caffo BS et al. Reproducibility and temporal structure in weekly resting-state fmri over a period of 3.5 years. PloS One 2015; 10: e0140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci 2002; 3: 606–616. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007; 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30: 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012; 59: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 2012; 33: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 2005; 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011; 8: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K et al. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 2011; 57: 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M et al. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp 2005; 25: 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2012; 2: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 2009; 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings RG, Van Horn JD. Publication bias in neuroimaging research: implications for meta-analyses. Neuroinformatics 2012; 10: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008; 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991; 100: 316–336. [DOI] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci 2010; 14: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnorm Psychol 1988; 97: 346–353. [DOI] [PubMed] [Google Scholar]
- Shang J, Fu Y, Ren Z, Zhang T, Du M, Gong Q et al. The common traits of the ACC and PFC in anxiety disorders in the DSM-5: meta-analysis of voxel-based morphometry studies. PloS One 2014; 9: e93432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 2012; 138: 9–18. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry 2015; 78: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry 2015; 172: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Dev Psychopathol 2011; 23: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Arce E, Paulus MP. Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. Neuroreport 2005; 16: 755–760. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A et al. Adolescent subgenual anterior cingulate activity is related to harm avoidance. Neuroreport 2009; 20: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M-Y, Wu Q-Z, Yue Q, Li J, Liao Y, Kuang W-H et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2012; 36: 11–16. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord 2011; 13: 1–15. [DOI] [PubMed] [Google Scholar]
- Olman CA, Davachi L, Inati S. Distortion and signal loss in medial temporal lobe. PloS One 2009; 4: e8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Mantini D, Apps MA, Eickhoff SB, Wenderoth N. Connectivity-based parcellation increases network detection sensitivity in resting state fMRI: an investigation into the cingulate cortex in autism. NeuroImage Clin 2016; 11: 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage 2006; 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann NY Acad Sci 2012; 1251: E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, Laird AR, Eickhoff SB. Meta-analysis in human neuroimaging: computational modeling of large-scale databases. Ann Rev Neurosci 2014; 37: 409–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp 2013; 34: 3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009; 45: 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.