Abstract
We studied the physiological mechanisms involved in the deleterious effects of a glyphosate-based herbicide (Factor® 540) on photosynthesis and related physiological processes of willow (Salix miyabeana cultivar SX64) plants. Sixty-day-old plants grown under greenhouse conditions were sprayed with different rates (0, 1.4, 2.1, and 2.8 kg a.e ha-1) of the commercial glyphosate formulated salt Factor® 540. Evaluations were performed at 0, 6, 24, 48, and 72 h after herbicide exposure. We established that the herbicide decreases chlorophyll, carotenoid and plastoquinone contents, and promotes changes in the photosynthetic apparatus leading to decreased photochemistry which results in hydrogen peroxide (H2O2) accumulation. H2O2 accumulation triggers proline production which can be associated with oxidative protection, NADP+ recovery and shikimate pathway stimulation. Ascorbate peroxidase and glutathione peroxidase appeared to be the main peroxidases involved in the H2O2 scavenging. In addition to promoting decreases of the activity of the antioxidant enzymes, the herbicide induced decreases in ascorbate pool. For the first time, a glyphosate-based herbicide mode of action interconnecting its effects on shikimate pathway, photosynthetic process and oxidative events in plants were presented.
Keywords: herbicide, oxidative stress, photosynthesis, proline, shikimate, willow
Introduction
Glyphosate [N-(phosphonomethyl)glycine)] is the most broadly used herbicide worldwide since the introduction of glyphosate-resistant (GR) plants (Coupe et al., 2012). Although it has been suggested as one of the least toxic pesticides to animals and humans (Williams et al., 2000; Cerdeira and Duke, 2006), the widespread use of glyphosate together with its great solubility trigger some concerns about its possible effects on the environment.
Glyphosate negative effects on non-target plants (Bott et al., 2011) and aquatic organisms (Vendrell et al., 2009; Inderjit and Kaushik, 2010) have been largely described. By inhibiting the EPSP synthase (EC 2.5.1.19), glyphosate-based herbicides prevent biosynthesis of aromatic amino acids (Siehl, 1997) leading to shikimic acid accumulation (Duke and Powles, 2008). The depletion of the aromatic amino acid pool leads to a reduction of protein synthesis necessary to growth maintenance (Siehl, 1997). On the other hand, in some plants, aromatic amino acid deficiencies upon glyphosate application has not been found, although deleterious effects of the herbicide have been observed (Lee, 1981; Wang, 2001; Serra et al., 2013). This indicates that glyphosate can affect other plant-physiological processes (Gomes et al., 2014b). Numerous studies demonstrated decreases in the photosynthetic rate of plants following treatment with glyphosate (Mateos-Naranjo et al., 2009; Yanniccari et al., 2012; Zobiole et al., 2012). In glyphosate-sensitive plants, the herbicide causes inhibition of CO2 assimilation (Diaz Vivancos et al., 2011) and depletion of intermediates of the photosynthetic carbon reduction cycle (Servaites et al., 1987) which could be linked to the unregulated flux of carbon into the shikimate pathway (Siehl, 1997). Moreover, glyphosate can indirectly affect photosynthesis by inhibiting chlorophyll biosynthesis (Fedtke and Duke, 2005) or inducing chlorophyll degradation (Gomes et al., 2016a), decreasing stomatal conductance (Yanniccari et al., 2012), and provoking nutritional disturbances (Cakmak et al., 2009; Su et al., 2009). Nowadays, a special attention has been giving to understand glyphosate-induced oxidative stress in plants (Gomes et al., 2014b).
Reactive oxygen species are essential in plant signaling; however, once accumulated, ROS become toxic, inducing irreversible changes in metabolism, cell cycle, and increase oxidative bursts (Gomes et al., 2014a). By interacting with biological molecules, ROS can induce destruction of DNA, lipids, and proteins (Foyer and Noctor, 2011). To avoid oxidative damage due to ROS accumulation, plants have developed enzymatic (e.g., SOD, CAT, APX, GPX, and GR) and non-enzymatic (e.g., ascorbate and glutathione) systems (Foyer and Noctor, 2011). The activity of antioxidant systems as well as the lipid peroxidation extent are oxidative stress markers which were shown to be modulated by glyphosate exposure (Ahsan et al., 2008; Moldes et al., 2008; Miteva et al., 2010).
Glyphosate effects on photosynthesis of non-resistant plants were associated to the herbicide induced decreases in the abundance of photosynthetic pathway proteins together with the oxidation of the major redox pools (Diaz Vivancos et al., 2011). However, it has been reported that glyphosate can also induce ROS accumulation (Ahsan et al., 2008; Moldes et al., 2008; Miteva et al., 2010; Gomes et al., 2016a) and glyphosate-resistance was related to the ability of plants to avoid oxidative bursts through activation of antioxidant systems (Maroli et al., 2015). Photosynthesis-targeting herbicides, such as atrazine, are known to induce oxidative stress by inhibiting Hill’s reactions (Fedtke and Duke, 2005). Plants exposed to these kinds of herbicides are not able to cope with the mass of triplet chlorophyll molecules produced due the blockage of the electron transport flow, resulting in cell oxidative bursts due to ROS accumulation. On the other hand, it is not clear how glyphosate can induce ROS accumulation in plants and if the oxidative stress induced by the herbicide could also be related to the observed decreases in photosynthesis. We hypothesized that the interference on shikimate pathway could induce ROS production and consequently affect photosynthesis of exposed plants. Therefore, in this study we accessed the physiological mechanisms involved in the deleterious effects of a glyphosate-based herbicide (Factor® 540) on photosynthesis of willow (Salix miyabeana cultivar SX64) plants. For the first time, a glyphosate-based herbicide mode of action interconnecting glyphosate effects on shikimate pathway, plant photosynthetic process and oxidative events were described.
Materials and Methods
Greenhouse Experiments
Salix miyabeana cultivar SX64 was chosen for this study due to its high tolerance to stress factors, fast growth and great biomass production (Labrecque and Teodorescu, 2005). Moreover, this species has been indicated for phytoremediation programs, particularly in the context of riparian buffer strips, to reclaim agricultural contaminants (Gomes et al., 2016b). Cuttings of S. miyabeana approximately 20 cm long were grown in plastic boxes (35 l) filled with distilled water amended with King Max nutrient solutions A (7% P2O5, 11% K2O, 1.5% Mg, 1.27% S, 0.07% B, 0.002% Mo, 0.12% Zn) and B (4% N, 1% NH4+, 3% NO3-2, 10% K2O, 2% Ca, 0.05% Fe, 0.05% Mn) (Montreal, QC, Canada), following the product’s instructions. The solutions were continuously aerated, and renewed every 15 days. The pH of the medium was checked and adjusted on a weekly basis to 6.5 ± 0.1. The greenhouse was maintained at 25/22°C (±3°C) day/night temperature with natural light supplemented by sodium vapor lamps to provide a 12 h photoperiod and an average photosynthetic active radiation of 825 μmol photons m-2 s-1. After an initial growth period (45 days), rooted, healthy (without leaf chlorotic spots) and uniform (similar height) plants were used in all treatments. A randomized block design with seven containers (corresponding to the replicates) per treatment, in a 4 (herbicide concentrations) × 4 (times of evaluation) factorial scheme was used. One hundred microliters of a freshly prepared herbicide solutions were hand-sprayed uniformly on each of the first three fully expanded leaves (corresponding to seventh to ninth leaves counting down from the shoot apex). This spray volume did not result in any runoff from the leaves. The herbicide (0, 56.15, 84.21, and 112.30 mM of glyphosate) applied concentrations were equivalent to field applications of 0, 1.4, 2.1, and 2.8 kg glyphosate ha-1, which represent scenarios of 50, 75, and 100% of the standard field herbicide concentration applied in agricultural areas in Quebec (Gomes et al., 2016a).
Photosynthetic (using chlorophyll fluorescence kinetic measurements) and biochemical evaluations were performed at 0, 6, 24, 48, and 72 h after the beginning of the treatments. The evaluations were stopped after 72 h of exposure as plants from the highest glyphosate treatment showed pronounced intoxication symptoms, including several necrotic spots and loss of leaves (data not shown). After photosynthetic and stomatal conductance evaluations, plants were harvested and thoroughly washed with distilled water. Samples of the seventh (first fully expanded leaf from the apex) to ninth leaves were immediately frozen in liquid nitrogen and stored in aluminum foil paper at -80°C until biochemical evaluations and oxidative damage evaluations.
Gas Exchange, Chlorophyll Fluorescence, and Pigment Concentrations
Gas exchange, chlorophyll fluorescence, and pigment contents were measured on samples from the first, second, and third fully expanded leaves (seventh–ninth leaves from the apex), which also received the herbicide, for a total of three measurements per plant. Measurements of stomatal conductance (gs) were performed using a leaf porometer (model SC-1, Decadon Devices Inc., Washington, DC, USA). Then, these leaves were dark-acclimated for 20 min and the chlorophyll fluorescence emission was assessed using a pulse-amplitude modulation (PAM) fluorometer (model PAM-2500, WALZ, Effeltrich, Germany). A RLC analysis was performed according to Juneau et al. (2015). An 11 steps RLC was performed. Saturating pulses were triggered at 0.8 min intervals with varying actinic light intensity for each step (0, 31, 48, 76, 117, 179, 253, 405, 586, 874, and 1326 μmol photons m-2 s-1). Using the RLC, the evaluation of the following parameters was performed: the ETR (Krall and Edwards, 1992), the qP (van Kooten and Snel, 1990), the UQFrel (Juneau et al., 2005), the NPQ (Redondo-Gómez et al., 2008), and the FV/FM (Kitajima and Butler, 1975). To compare treatments, fluorescence results from the 874 μmol photons m-2 s-1 (most similar irradiation in relation to light growth conditions) were used. Curves of ETR versus irradiance were also plotted and the ETRmax and the Ik were calculated according to Eilers and Peeters (1988).
For pigments evaluations, three foliar disks of approximately 5 mm in diameter were taken from each leaf, and after determining the fresh weight of the samples, their chlorophyll and carotenoid pigments were extracted in 80% acetone after macerating the disks with a mortar and pestle. The spectral absorption of the extracts (from 300 to 800 nm) was measured using a Varian Cary® 300 Bio UV-Vis spectrophotometer (Varian, USA). The concentrations (μg/g fresh leaf weight) of total chlorophylls and carotenoids were calculated using the equations described by Lichtenthaler and Wellburn (1983).
Biochemical Evaluations
Shikimate and proline concentrations were evaluated following the methods described in Bates et al. (1973) and Bijay and Dale (1998), respectively. To evaluate the pool of quinones in leaves, 0.1 g of fresh plant tissue was ground in liquid nitrogen, homogenized in 1000 μl of freeze-cold ethyl acetate and then centrifuged for 1 min at 6.590 × g (Kruk and Karpinski, 2006). The supernatant was then transferred to a collecting tube and the procedure was repeated twice (by adding 1000 μl of freeze-cold ethyl acetate to the pellet) to assure high extraction efficiency. Ten microliters of cold 1 M sodium borohydride (NaBH4) was added to the combined supernatant to convert quinone to its reduced form and then, samples were centrifuged for 2 min at 10.000 × g to remove impurities (Yoshida et al., 2010). The standard of plastoquinone (PQ-9, 1 mM) was acquired from the laboratory of J. Kruk (Jagiellonian University, Poland). After dilution in ethanol, the amount of 20 μl of cold 1 M NaBH4 was added to assure complete reduction of plastoquinone pool. The UHPLC (Agilent 1290 Infinity II LC, Wilmington, DE, USA) measurements were performed according to Yoshida et al. (2010), using UV-VIS detector, fluorescence detector, column (50 mm × 2.1 mm) isocratic solvent system (methanol/hexane, 340/20 vol/vol), flow rate of 0.31 ml/min, absorption detection wavelength at 255 nm, fluorescence excitation/emission detection at 290/330 nm, and injection volume of 1 μl.
To assess oxidative responses, H2O2, MDA contents and the activity of antioxidant systems were studied following the methods described by Gomes et al. (2014c). H2O2 was extracted in 2 ml of 0.1% trichloroacetic acid (TCA) and after centrifugation at 12000 × g for 15 min, 300 μl of the centrifuged supernatant was reacted with 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. Samples were read at 390 nm and H2O2 concentrations were determined using an extinction coefficient (𝜀) of 0.28 mM-1 cm-1. The estimation of lipid peroxidation was based on the production of 2-thiobarbituric acid reactive metabolites, particularly MDA. Samples containing 200 mg of leaf and root tissue were macerated in 5 mL of 0.1% TCA. After complete homogenization, 1.4 mL of the homogenate was transferred to an eppendorf tube and centrifuged at 10,000 rpm for 5 min. An aliquot of 0.5 mL of the supernatant was added to 2 mL 0.5% (v/v) TBA (thiobarbituric acid) in 20% TCA. The mixture was heated in a water bath at 95°C for 30 min and then ice-cooled for 10 min. Readings were taken using a spectrophotometer at 535 and 600 nm.
To study the antioxidant enzymes, 0.1 g of leaves were macerated in 800 μl of an extraction buffer containing 100 mM potassium buffer (pH 7.8), 100 mM EDTA, 1 mM L-ascorbic acid and 2% PVP (m/v). The protein contents of samples were determined using the Bradford method. Activities of SOD (EC 1.15.1.1), CAT (EC1.11.1.6), APX (EC 1.11.1.11), GPX (E.C. 1.11.1.9), and GR (E.C. 1.6.4.2) were assessed. To evaluate the ascorbate pool [total ascorbate (AsA + DHA), AsA and DHA], 0.2 g of frozen tissue were ground in liquid nitrogen in a mortar and pestle and homogenized with 5 ml of 6.5% (w/v) m-phosphoric acid containing 1 mM NaEDTA.
Statistical Analyses
Results were expressed as the average of three replicates. Statistical analyses were performed using JMP software 10.0 (SAS Institute Inc). Results were submitted to normality (Shapiro–Wilk) and homogeneity (Bartlett) tests and then statistically evaluated. MANOVA univariate repeated measures, with Time as the within-subject factor and the herbicide concentrations as the main effects, were used to analyze differences in the variables studied during exposure to the treatments. Glyphosate, Time, and the interaction between glyphosate and time were included within the model. The sphericity of the data was tested by the Mauchly’s criteria to determine whether the univariate F tests for the within-subject effects were valid. In cases of invalid F, the Greenhouse–Geisser test was used to estimate epsilon (𝜀). Contrast analysis was used when there were significant differences in the variables between treatments (Supplementary Tables 1S and 2S).
Results
Pigment Content, Gas Exchange, and Chlorophyll Fluorescence
Total chlorophyll and plastoquinone concentrations were decreased in leaves of plants by herbicide exposure and by treatment time (P > 0.001; Figure 1). The carotenoid concentration was greater in herbicide-treated plants at 6 h for all applied doses (Figure 1); then, carotenoid concentration was decreased in plants exposed for at least 24 h to herbicide concentrations (P < 0.0001). The stomatal conductance was decreased in herbicide-exposed plants for all the treatment times (P < 0.05; Figure 1). Similar effects were observed on the ETRmax, the Ik, and the qP, which were significantly reduced in treated plants (P < 0.0001; Figure 2). However, for the first evaluation (6 h), ETRmax, Ik, and qP were not decreased in plants treated with 1.4 kg a.e ha-1 (P > 0.05; Figure 2). The UQFrel increased in all treated plants (Figure 1). Concomitantly, the NPQ decreased in plants exposed for more than 24 h to the herbicide (P < 0.05; Figure 2). The maximal PSII photochemical efficiency (FV/FM) was decreased in herbicide-treated plants (P < 0.0001). Decreased FV/FM was seen in plants treated with 1.4 kg a.e ha-1 only after 72 h of herbicide exposure (P < 0.05; Figure 2). Plants exposed to 2.1 kg a.e ha-1 showed decreases in FV/FM at 48 and 72 h of exposure (P < 0.001). In contrast, in all the evaluations, plants exposed to 2.8 kg a.e ha-1 showed decreased FV/FM (P < 0.01; Figure 2).
FIGURE 1.
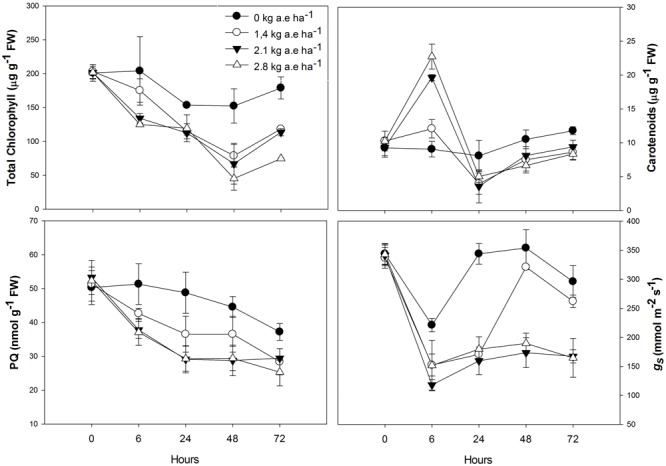
Time courses of pigment (total chlorophyl and carotenoids) concentrations, plastoquinone pool (PQ), and stomatal conductance (gs) in leaves of Salix miyabeana (cultivar SX64) plants spread with doses of increased (0, 1.4, 2.1, and 2.8 kg a.e ha-1) rates of the glyphosate based herbicide (Factor® 540). Values are means ± SE of three replicates.
FIGURE 2.
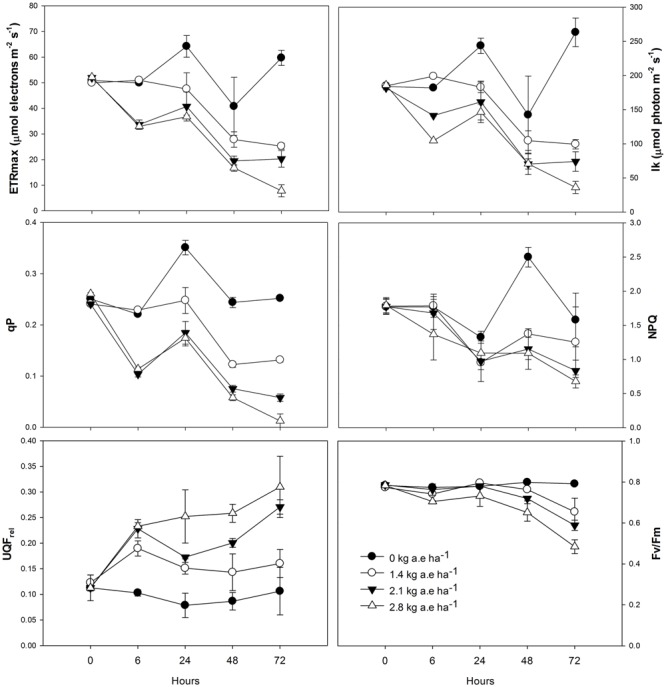
Time courses of photosynthesis-related measurements [maximum electron transport rate (ETRmax), minimum saturating irradiance (Ik), photochemical quenching (qP), non-photochemical quenching (NPQ), relative unquenched fluorescence (UQFrel), and maximal photochemical efficiency of PSII (FV/FM)] in leaves of Salix miyabeana (cultivar SX64) plants spread with doses of increased (0, 1.4, 2.1, and 2.8 kg a.e ha-1) rates of the glyphosate based herbicide (Factor® 540). Values are means ± SE of three replicates.
Shikimate and Proline Contents
The shikimate and proline concentrations in leaves of herbicide-treated plants were always higher than the control (P < 0.0001; Figure 3). In plants exposed to 2.1 and 2.8 kg a.e ha-1, an important shikimate accumulation was found after 72 h of herbicide-treatment (P < 0.0001).
FIGURE 3.
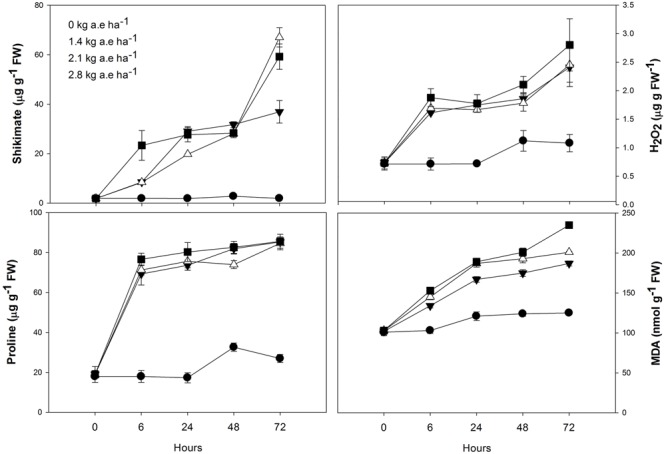
Time courses of shikimate, proline and hydrogen peroxide concentrations, and lipid peroxidation (MDA concentrations) in leaves of Salix miyabeana (cultivar SX64) plants spread with doses of increased (0, 1.4, 2.1, and 2.8 kg a.e ha-1) rates of the glyphosate based herbicide (Factor® 540). Values are means ± SE of three replicates.
H2O2 Contents and Lipid Peroxidation
Compared to control, H2O2 concentration was always higher in plants exposed to the herbicide (P < 0.001; Figure 3), and greatly increased in these plants after 72 h (P < 0.01). Similarly, lipid peroxidation (MDA concentration) was always higher in plants exposed to the herbicide (Supplementary Table 2S; Figure 3). In all plants, MDA content slightly increased at 24 h (P > 0.05). However, in plants treated with herbicide, a pronounced increase in MDA concentration was observed at 72 h (P < 0.05).
Antioxidant Responses
Plants treated with herbicide showed higher activity of all evaluated antioxidant enzymes after 6 h in relation to control (P < 0.05; Figure 4). We found that: (1) SOD and APX activities were higher in herbicide-treated plants up to 24 h (P < 0.0001), and then were reduced for the following exposure times (P < 0.0001); (2) CAT activity was always higher in plants treated with herbicide (P < 0.0001); (3) similar to SOD and APX, GPX activity was also reduced in herbicide treated plants at 48 and 72 h of exposure (P < 0.0001); (4) GR activity was higher in herbicide treated plants up to 48 h of exposure (P < 0.05). Regarding ascorbate pool (Figure 5) we found that, in relation to control: (1) total ascorbate concentrations (AsA + DHA) were higher in herbicide-treated plants up to 24 h of exposure, and then were reduced for the following exposure times (P < 0.0001); (2) the concentrations of the ascorbate reduced form (AsA) were greater in control plants up to 24 h, did not differ between treatments at 48 h and was increased in herbicide treated plants for 72 h (P < 0.0001); (3) the concentrations of oxidized form of ascorbate (DHA) were greater in herbicide treated plants up to 24 h and were reduced for the following exposure duration (P < 0.0001); (4) the AsA/DHA ratio was lower in 6 and 24 h treated plants compared to control, but was higher for the following treatment times (P < 0.0001).
FIGURE 4.
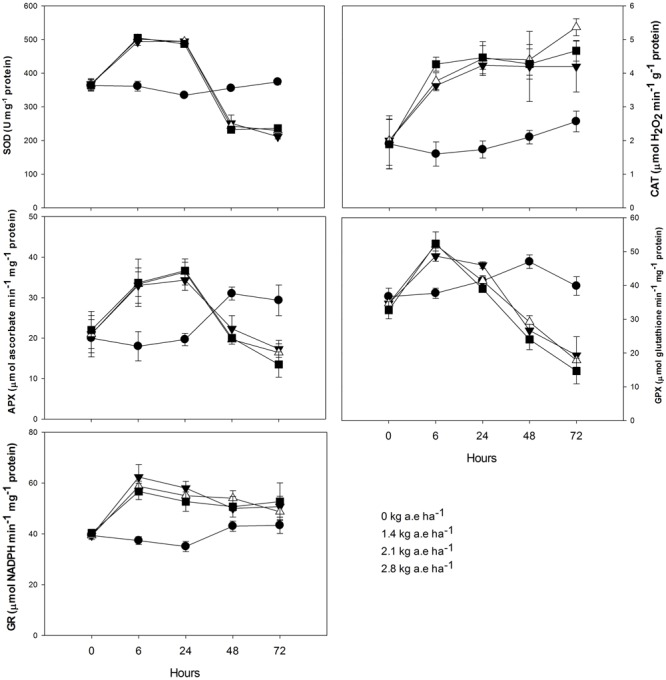
Time courses of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), and glutathione reductase (GR) activities in leaves of Salix miyabeana (cultivar SX64) plants spread with doses of increased (0, 1.4, 2.1, and 2.8 kg a.e ha-1) rates of the glyphosate based herbicide (Factor® 540). Values are means ± SE of three replicates.
FIGURE 5.
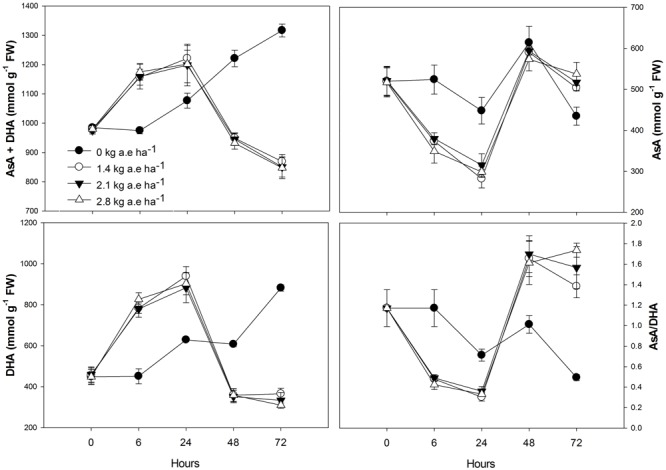
Time courses of total ascorbate (AsA + DHA), reduced ascorbate (AsA), oxidized ascorbate (DHA), and AsA/DHA ratio in leaves of Salix miyabeana (cultivar SX64) plants spread with doses of increased (0, 1.4, 2.1, and 2.8 kg a.e ha-1) rates of the glyphosate based herbicide (Factor® 540). Values are means ± SE of three replicates.
Discussion
In this study, for the first time, a wide investigation of the impacts of glyphosate-based herbicide on several physiological processes was done. We demonstrated that this type of herbicide affected not only the shikimate pathway, but several physiological processes in willow plants as previously reported by Gomes et al. (2016b). Figure 6 represents an integrative model interconnecting the studied physiological parameters (in particular, shikimate pathway, photosynthetic processes and oxidative events) affected by exposure to a glyphosate-based herbicide greater than 24 h (48 and 72 h). The various steps of this model are identified throughout the text as Figure 6, #1–19.
FIGURE 6.
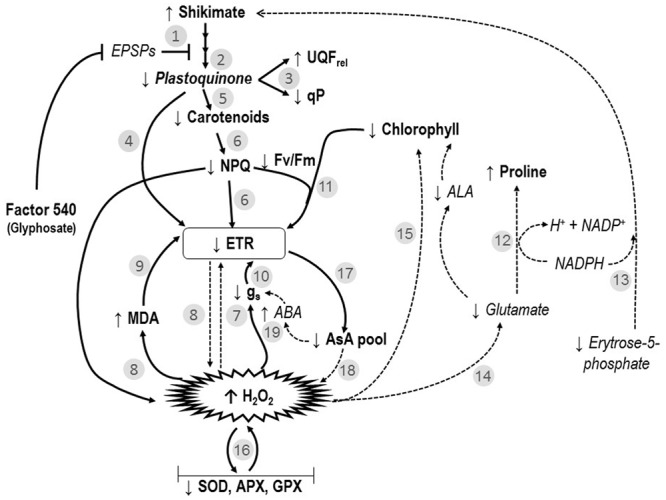
Interconnected model of the effects of the glyphosate-based-herbicide (Factor® 540) on shikimate pathway, photosynthesis and oxidative markers of willow plants. Numbers refer to the ones mentioned in the discussion. ABA, abscisic acid; ALA, δ-aminolevulinic acid; APX, ascorbate peroxidase; AsA, ascorbate; EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase; ETR, electron transport rate; FV/FM, maximal PSII photochemical efficiency; GPX, glutathione peroxidase; gs, stomatal conductance; H2O2, peroxide; Ik, minimum saturating irradiance; MDA, lipid peroxidation; NPQ, non-photochemical quenching; qP, photochemical quenching; SOD, superoxide dismutase; UQFrel, the relative unquenched fluorescence. Literature-based information in the models are expressed in italic words and in dotted arrows. While, observed data obtained in the present study are introduced in the model as bold words and non-dotted arrows.
The glyphosate-based herbicide clearly inhibited the shikimate pathway in willow plants, as demonstrated by the shikimate accumulation (Figure 3) and also reported by Huang et al. (2012) and Gomes et al. (2016b). By inhibiting the shikimate pathway (Figure 6, #1), the glyphosate-based herbicide may prevent the biosynthesis of several secondary plant compounds (Siehl, 1997), including plastoquinones (Figure 1; Figure 6, #2). It is known that UQFrel is an indicator of closed PSII reaction centers (RCs) present under continuous illumination (Juneau et al., 2005) and qP represents the proportion of open PSII RCs (Maxwell and Johnson, 2000). Therefore, the higher UQFrel and lower qP in treated plants indicate that the plastoquinone pool, and thus the PSII RCs, were in a more reduced state than in control plants, a consequence of a lower PQ content (Figure 6, #2 and 3) and/or less effective PSI. This may, together with the decrease in the Ik (for doses >1.4 kg a.e ha-1), have contributed to the observed lower ETR in treated plants (Figure 2; Figure 6, #4). Indeed, a lower ability for PSII to deliver electrons to the electron transport chain, leading to PSII saturation at low irradiance, may explain the observed decrease in photosynthesis observed here and in previous studies (Huang et al., 2012; Yanniccari et al., 2012; Gomes et al., 2016b). However, we demonstrated that other effects of the glyphosate-based herbicide have also caused the decrease in the ETR (see below).
The observed increase in the carotenoid concentration after 6 h in the herbicide-treated plants (Figure 1) could be related to the concomitant increase in H2O2 concentration, since it is known that ROS presence can induce carotenogenic responses (Fan et al., 1998). Indeed, by the activation of latent biosynthetic enzymes (such as glutathione transferase and glutathione reductase) or by the expression of genes coding for carotenogenic enzymes, ROS may regulate carotenoid concentration (Aniya and Anders, 1992; Bouvier et al., 1998). Since the maximal PSII photochemical yield (FV/FM) is a proxy of the PSII integrity (Walter et al., 2003), FV/FM up to 24 h in treated plants (with the exception of the highest dose; Figure 2) indicates that the glyphosate-based herbicide had no effect on the PSII integrity up to 24 h of exposure. Similarly, negative effects of glyphosate in FV/FM of Lolium perenne plants were only observed after 3 days of exposure (Yanniccari et al., 2012). This may be the consequence of the increased carotenoid concentration helping to prevent ROS-damages to PSII (Gomes et al., 2013b). Carotenoids are usually involved in the protection of the oxidative damage by the detoxification of oxygen singlets (1O2) produced by photosynthesis or by enzymatic conversion of other ROS to oxygen singlets (Boussiba, 2000). Although plants exposed to the highest herbicide doses contain high carotenoid concentration, this was not sufficient to prevent oxidative damages to PSII (since we observed lower FV/FM value). These plants also showed higher lipid peroxidation (Figure 3) indicating oxidative damages (Gunes et al., 2007), as it was also shown in maize (Sergiev et al., 2006) and rice (Ahsan et al., 2008). However, for exposure times longer than 24 h, plants treated with the glyphosate-based herbicide showed reduced carotenoid concentration (Figure 1). This can be a consequence of the inhibition of the shikimate pathway leading to decreased PQ concentration, since plastoquinone is a co-factor of the phytoene desaturase and ζ-carotene desaturase, enzymes involved in the carotenoid biosynthesis pathway (Sandmann et al., 2006). Therefore, decreased plastoquinone concentration will affect directly carotenoid biosynthesis (Figure 6, #5). In addition, the decrease in the non-photochemical energy dissipation (NPQ) [one of the mechanisms by which plants can dissipate excess light energy absorbed by PSII light-harvesting complexes in order to minimize the generation of the highly reactive 1O2 responsible for oxidative damages (Demming-Adams and Adams, 2000)], in plants treated with the glyphosate-based herbicide (Figure 2) can be related to the decreased biosynthesis of carotenoids. β-carotene is known to be the precursor of zeaxanthin, the first compound of xanthophyll cycle (Bouvier et al., 1996), and therefore, reduced carotenoid concentration could lead to a lower efficiency of the xanthophyll cycle, reducing plant capacity for photoprotection and thus, leading to increased PSII damages (as shown by reduced FV/FM) (Figure 6, #6). These decreases in the photosynthetic activity (shown by the decrease in ETR) and in the NPQ may also have contributed to a higher production of ROS (Sergiev et al., 2006; Ahsan et al., 2008; Gomes and Juneau, 2016; Gomes et al., 2016a) due to over-excitation of chlorophylls (Figure 6, #7).
As we found in the present study (Figure 3; Figure 6, #8), increased lipid peroxidation has been previously observed in glyphosate-exposed plants and was related to increased H2O2 content in plants (Moldes et al., 2008; Miteva et al., 2010; Gomes et al., 2016b). Lipid peroxidation resulting from increased levels of ROS (such as H2O2) has been shown to affect the integrity of the thylakoid membranes (Richter, 1992), contributing to the noted decrease in ETR (Figure 6, #9). Glyphosate was demonstrated to cause depletion of photosynthetic proteins leading to losses of photosynthetic capacity in plants (Diaz Vivancos et al., 2011). However, it has long been recognized that H2O2 is a potent inhibitor of photosynthesis, since, even at low concentrations, it can inhibit CO2 fixation by oxidizing the thiol groups of some essential enzymes of the Calvin cycle (Foyer and Noctor, 2011). We can therefore advance that the observed decrease in photosynthesis (ETR) in presence of glyphosate-based herbicide may also be directly linked to higher H2O2 concentration (Figure 5, #8). Carbon assimilation (and therefore photosynthesis) can also be negatively affected by the decreased stomatal conductance (gs) (Figure 1) in presence of herbicides (Zobiole et al., 2010). Reduced gs, as also previously reported in Hordeum vulgare (barley) and Lolium perenne plants exposed to glyphosate (Olesen and Cedergreen, 2010; Yanniccari et al., 2012), can limit photochemistry, resulting in decreased ETR (Figure 6, #10). The observed ETR reduction could also be due to the alteration of the integrity of PSII (lower FV/FM) (Figure 2; Figure 6, #11). In addition, the decrease in total chlorophyll concentration in presence of the glyphosate-based herbicide may be responsible for a lower light interception and thus, the noted lower electron transport rate (Figure 6, #11). Decreased chlorophyll contents when plants are exposed to herbicide application have been demonstrated previously and have been attributed to an increase chlorophyll degradation or to a decrease in chlorophyll synthesis (Cakmak et al., 2009; Mateos-Naranjo et al., 2009; Huang et al., 2012; Gomes et al., 2016a).
In order to better understand the processes involved in H2O2 accumulation (and herbicide-induced oxidative damage), we investigated the activity of antioxidant system in treated plants. Increases in proline synthesis is a common protective-response of plants to stress conditions (Hayat et al., 2012). It is important to note, however, that proline can also act as a significant signaling molecule in plant physiological processes, mainly under stress conditions (Hare and Cress, 1997). In the present study, we suggest that the observed proline accumulation in treated plants is associated to oxidative protection, NADP+ recovery and shikimate pathway stimulation (Figure 6, #12 and #13). As we also observed, proline biosynthesis is commonly stimulated by increased cellular-ROS concentration conditions (Soshinkova et al., 2013). Although proline can be synthesized from ornithine, metabolic labeling studies indicate that, under stress conditions, proline is mainly produced from glutamate (as reviewed by Hare and Cress, 1997). Therefore, the proline accumulation found in our study indicates that this pathway is highly activated (Figure 6, #12). A special function of proline in preventing oxidative damage and enhancing tolerance from abiotic oxidative stress has been proposed recently (Soshinkova et al., 2013) and proline accumulation in plants in response to glyphosate exposure was documented (Huang et al., 2012). Due to the loss of feedback control of the shikimate pathway by tyrosine (that regulates the activity of 3-deoxy-D-aravino-heptulosonate-7-phosphate synthase) (Crowley, 2006), the herbicide (glyphosate) led to an unregulated flux of carbon into the shikimate pathway (Siehl, 1997). As a result, there is an increased demand for erythrose-5-phosphate, the substrate of the first reaction of the shikimate pathway. Erythrose-5-phosphate is produced in the oxidative pentose phosphate pathway (OPPP), which is dependent on NAD(P)+ availability and inhibited by NADPH (Hare and Cress, 1997). During proline synthesis, NADPH is oxidized, therefore stimulating OPPP. Even a small change in the NAD(P)+/NADPH ratio may have a large effect on this redox-sensitive pathway (Hare and Cress, 1997). The oxidation of NADPH during proline synthesis, coupled with the reduction of NADP+ during the two oxidative steps of the OPPP, promotes a cycle of changes in NAD(P)+/NADPH ratio which stimulates proline biosynthesis, justifying its accumulation during stress (Hare and Cress, 1997). Therefore, upon the glyphosate-based herbicide exposure, the proline accumulation in willow plants could also be linked to the OPPP stimulation for the production of the erythrose-5-phosphate which will be used in shikimate pathway (Figure 6, #13). Supporting this hypothesis, Diaz Vivancos et al. (2011) observed decreased NADP/NADPH ratios in leaves of glyphosate-sensitive soybeans upon glyphosate treatment, as a result of the decreases in NADP+ pool. As mentioned previously, under stress conditions, proline is mainly produced from glutamate (Hare and Cress, 1997). Glutamate is also required during δ-aminolevulinic acid (ALA; a chlorophyll precursor) biosynthesis through ALA-synthetase and γ,δ-dioxivalerate cycles (Beale, 1978). Therefore, if glutamate was preferentially used for proline biosynthesis (as suggested by proline greater accumulation in treated plants in relation to control; Figure 3), a decrease in ALA biosynthesis may be obtained, therefore contributing to the decreased chlorophyll concentration observed in treated plants (Figure 6, #14). As suggested by Gomes et al. (2016a), the decrease in chlorophyll concentration may also be due to its degradation by increased ROS content (Figure 6, #15).
Even though treated plants showed increased activities of antioxidant enzymes after 6 h exposure, they were not able to prevent both peroxide accumulation and lipid peroxidation, indicating a clear deleterious effect of the glyphosate-based herbicide through oxidative burst. Moreover, the strong inhibition of SOD, APX, and GPX activities observed in plants exposed to herbicide after 48 h (Figure 4) can be related to the increased H2O2 and decreased ETR also observed in these plants. SOD is the first defense enzyme against oxidative stress (Pompeu et al., 2008) and is closely related to stress resistance in plants (Song et al., 2006). Indeed, this enzyme was involved in the PSII protection against the effects of prooxidant herbicides, limiting carbon dioxide and photoinhibitory conditions (Foyer et al., 1994; Arisi et al., 1998). The observed decrease in SOD activity (Figure 4) can therefore contribute to the herbicide-deleterious effects on photosynthesis in willow plants. We also demonstrated the key role of APX and GPX to prevent H2O2 accumulation in willow plants since: (1) decreased activities of both enzymes were related to increased H2O2 concentration in leaves; (2) even if treated plants shown higher CAT activity, it was not able to prevent H2O2 accumulation. The importance of APX and GR in avoiding oxidative stress has also been observed in metal(loid) treated plants (Chaoui et al., 1997; Gomes et al., 2013a,b) and the inactivation/degeneration of these enzymes has been related to increased H2O2 concentrations and oxidative damages to plants (Gomes et al., 2013b). When H2O2 accumulation exceeded the tolerance limit of plants, enzymatic systems are prone to protein carbonylation–an irreversible oxidative process in which the side chains of Lys, Arg, Pro, and Thr are converted to aldehyde or keto groups (Sohal et al., 2002), which may have been occurring in willow plants exposed to the studied herbicide (Figure 6, #16).
We also observed an interesting response of GR activity at 48 and 72 h, since its activity was not significantly decreased by the glyphosate-based herbicide exposure. GR is linked to APX and GPX activity by the glutathione-ascorbate cycle (Foyer and Noctor, 2011). However, as mentioned, the GR activity did not follow APX and GPX patterns upon the herbicide exposure. The maintenance of GR activity in treated plants indicates that APX and GPX activities were not limited by substrate availability, reinforcing that the proposed oxidative damage (protein carbonylation) of the enzymes could be responsible for their degeneration. We may hypothesize that, similarly to the proline production, the higher NADP(H)-dependent-GR activity can favor OPPP and contribute as a source of NADP+ for photochemistry.
In addition to being the substrate for APX, ascorbate is an important antioxidant component of the cellular redox potential and its activity is linked to ascorbate-glutathione metabolic cycle (Foyer and Noctor, 2011). In the present study, we found a link between the reduced form of ascorbate (AsA) and the APX activity. Indeed, up to 24 h, treated plants showed higher APX activity concomitantly to the reduced AsA concentration in their leaves; similarly, decreased APX activity for the following treatment periods was related to the increased AsA content. On the other hand, the contrary was observed for the oxidized form of ascorbate (DHA). The accumulation of AsA, as noted by the increased AsA/DHA ratio in treated plants, shows that the DHA has been effectively recycled to AsA by ascorbate-glutathione cycle. We also observed that total ascorbate concentrations (AsA + DHA) were reduced in herbicide treated plants (Figure 5). It is known that ascorbate concentration and ETR are closely linked, as the light-dependent stimulation of ascorbate biosynthesis requires photosynthetic electron transport activity (Yabuta et al., 2007). Thus, reduced ETR in treated plants could explain the observed reduction in ascorbate pool (Figure 6, #17). Low ascorbate pool favors the increase in both ROS (Figure 6, #18) and abscisic acid (ABA), leading to an increase in signal transduction through ROS-mediated and ABA-dependent signaling cascades (Foyer and Noctor, 2011). Among others, the interactive effect of ROS and ABA in stomatal movement is well studied, with increased ROS and ABA content inducing stomatal closure (Gomes et al., 2014a). This mechanism can also be related to the observed herbicide-induced decreases in gs (Figure 6, #19).
As expected, the primary target site of the studied glyphosate-based-herbicide (Factor® 540) on willow plants is the shikimate pathway. We demonstrated, for the first time, that on top of the alteration of this primary target site, this herbicide induces a series of interconnected events that leads to decreased photosynthetic activity in willow plants. Furthermore, we showed that the herbicide-deleterious effects on photosynthesis are strongly related to herbicide-induced oxidative stress, and that reduction of photosynthesis may amplify the observed effect by inducing ROS production. Our results evidenced that as for photosynthesis-target herbicides, which trigger ROS production and oxidative stress, glyphosate herbicidal effect may be related to induction of ROS accumulation. The inhibition of shikimate pathway may induce changes in redox status with important consequences in leaf metabolism, mainly on photosynthesis. Glyphosate tolerance in plants, for instance, have been related to the ability of plants to deal with ROS accumulation through the activation of antioxidant systems (Maroli et al., 2015). However, since photosynthetic processes of GR plants have been shown to be affected by glyphosate-based herbicides (Zobiole et al., 2010, 2011, 2012), glyphosate may target other cellular sites, inducing ROS formation, for example, mitochondrial electron chain, as proposed by Gomes and Juneau (2016). Although ROS formation may also be produced in the mitochondria, our model fits with several results presented in the literature about the effects of glyphosate in sensitive plants, highlighting the role of ROS induction in this herbicidal mechanism of action.
Author Contributions
MG, SL performed the experiments; MG, MLa, and PJ designed the experiments; MG and PJ wrote the paper; LH-E and MLu gave technical support and conceptual advice.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jerzy Kruk from the Department of Plant Physiology and Biochemistry, Jagiellonian University for providing us the plastoquinone standard.
Abbreviations
- APX
ascorbate peroxidase
- AsA
reduced ascorbate
- CAT
catalase
- EPSPS
5-enolpyruvylshikimate-3-phosphate synthase
- DHA
dehydroascorbate – oxidized ascorbate
- ETRmax
maximum electron transport rate
- FV/FM
maximal photochemical efficiency of PSII
- GPX
glutathione peroxidase
- GR
glutathione reductase
- gs
stomatal conductance
- H2O2
hydrogen peroxide
- Ik
minimum saturating irradiance
- MDA
malondialdehyde – lipid peroxidation
- NPQ
non-photochemical quenching
- PQ
plastoquinone
- PS
photosystem
- qP
photochemical quenching
- RLC
rapid light curve
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- UQFrel
relative unquenched fluorescence
Footnotes
Funding. This research was supported by the Natural Science and Engineering Research Council of Canada (NSERC) through a Strategic grant awarded to MLa, PJ, and MLu. MG received a Ph.D. scholarship from Fonds de Recherche du Québec–Nature et Technologies (FRQNT) and LH-E received a Ph.D. scholarship from the Natural Science and Engineering Research Council of Canada (NSERC).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00207/full#supplementary-material
References
- Ahsan N., Lee D.-G., Lee K.-W., Alam I., Lee S.-H., Bahk J. D., et al. (2008). Glyphosate-induced oxidative stress in rice leaves revealed by proteomic approach. Plant Physiol. Biochem. 46 1062–1070. 10.1016/j.plaphy.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Aniya Y., Anders M. W. (1992). Activation of rat liver microsomal glutathione S-transferase by hydrogen peroxide: role for protein-dimer formation. Arch. Biochem. Biophys. 296 611–616. 10.1016/0003-9861(92)90617-6 [DOI] [PubMed] [Google Scholar]
- Arisi A., Cornic G., Jouanin L., Foyer C. (1998). Overexpression of iron superoxide dismutase in transformed poplar modifies the regulation of photosynthesis at low CO2 partional pressures or following exposure to prooxidant methyl viologen. Plant Physiol. 117 565–574. 10.1104/pp.117.2.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L. S., Waldren R. P., Teare I. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39 205–208. 10.1016/j.dental.2010.07.006 [DOI] [Google Scholar]
- Beale S. (1978). S-aminolevulinic acid in plants: its biosynthesis, regulation, and role in plastid development. Annu. Rev. Plant Physiol. 29 95–120. 10.1146/annurev.pp.29.060178.000523 [DOI] [Google Scholar]
- Bijay K. S., Dale L. S. (1998). Rapid determination of glyphosate injury to plants and identification of glyphosate-resistant plants. Weed Sci. Soc. Am. 12 527–530. [Google Scholar]
- Bott S., Tesfamariam T., Kania A., Eman B., Aslan N., Römheld V., et al. (2011). Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilisation. Plant Soil 342 249–263. 10.1007/s11104-010-0689-3 [DOI] [Google Scholar]
- Boussiba S. (2000). Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol. Plant. 108 111–117. 10.1034/j.1399-3054.2000.108002111.x [DOI] [Google Scholar]
- Bouvier F., Backhaus R. A., Camara B., Chem J. B. (1998). Induction and control of chromoplast-specific carotenoid genes by oxidative stress induction and control of chromoplast-specific carotenoid genes by oxidative stress. J. Biol. Chem. 273 30651–30659. 10.1074/jbc.273.46.30651 [DOI] [PubMed] [Google Scholar]
- Bouvier F., D’Harlingues A., Hugueney P., Marin E., Marion-Poll A., Camara B. (1996). Xanthophyll biosynthesis. Cloning, expression, functional reconstitution, and regulation of beta -cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 271 28861–28867. 10.1074/jbc.271.46.28861 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Yazici A., Tutus Y., Ozturk L. (2009). Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. Eur. J. Agron. 31 114–119. 10.1016/j.eja.2009.07.001 [DOI] [Google Scholar]
- Cerdeira A. L., Duke S. O. (2006). The current status and environmental impacts of glyphosate-resistant crops: a review. J. Environ. Qual. 35 1633–1658. 10.2134/jeq2005.0378 [DOI] [PubMed] [Google Scholar]
- Chaoui A., Habib Ghorbal M., El Ferjani E. (1997). Effects of cadmium-zinc interactions on hydroponically grown bean (Phaseolus vulgaris L.). Plant Sci. 126 21–28. 10.1016/S0168-9452(97)00090-3 [DOI] [Google Scholar]
- Coupe R., Kalkhoff S., Capel P., Gregoire C. (2012). Factors affecting the fate and transport of glyphosate and AMPA into surface waters of agricultural watersheds in the United States and Europe. Geophys. Res. Abstr. 14 5877. [Google Scholar]
- Crowley V. (ed.) (2006). The Isozymes of 3-Deoxy-D-Arabino-Heptulosonate 7-Phosphate Synthase from Arabidopsis Perform Differential and Overlapping Roles in Vivo and May be Regulated by Tyrosine. Toronto: University of Toronto. [Google Scholar]
- Demming-Adams B., Adams W. (2000). Photosynthesis: harvesting sunlight safety. Nature 403 371–374. 10.1038/35000315 [DOI] [PubMed] [Google Scholar]
- Diaz Vivancos P., Driscoll S. P., Bulman C. A., Ying L., Emami K., Treumann A., et al. (2011). Perturbations of amino acid metabolism associated with glyphosate-dependent inhibition of shikimic acid metabolism affect cellular redox homeostasis and alter the abundance of proteins involved in photosynthesis and photorespiration. Plant Physiol. 157 256–268. 10.1104/pp.111.181024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. O., Powles S. B. (2008). Glyphosate: a once-in-a-century herbicide. Pest Manag. Sci. 64 319–325. 10.1002/ps.1518 [DOI] [PubMed] [Google Scholar]
- Eilers P. H. C., Peeters J. C. H. (1988). A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Modell. 42 199–215. 10.1016/0304-3800(88)90057-9 [DOI] [Google Scholar]
- Fan L., Vonshak A., Zarka A., Boussiba S. (1998). Does astaxanthin protect Haematococcus against light damage? Z. Naturforsch. C. 53 93–100. [DOI] [PubMed] [Google Scholar]
- Fedtke K., Duke S. (2005). “Herbicides,” in Plant Toxicology eds Hock B., Elstner E. (New York, NY: Marcel Dekker; ) 247–330. [Google Scholar]
- Foyer C. H., Leiandais M., Kunert K. J. (1994). Photooxidative stress in plants. Physiol. Plant. 92 696–717. 10.1111/j.1399-3054.1994.tb03042.x [DOI] [Google Scholar]
- Foyer C. H., Noctor G. (2011). Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155 2–18. 10.1104/pp.110.167569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M. P., Carvalho M., Carvalho G. S., Garcia Q. S., Guilherme L. R. G., Soares A. M. (2013a). Phosphorus improves arsenic phytoremediation by Anadenanthera peregrina by alleviating induced oxidative stress. Int. J. Phytoremediation 15 633–646. [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Duarte D. M., Carneiro M. M. L. C., Barreto L. C., Carvalho M., Soares A. M., et al. (2013b). Zinc tolerance modulation in Myracrodruon urundeuva plants. Plant Physiol. Biochem. 67 1–6. 10.1016/j.plaphy.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Juneau P. (2016). Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: Is the mitochondrial electron transport chain a target of this herbicide?. Environ. Pollut. 218 402–409. 10.1016/j.envpol.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Le Manac’h S. G., Maccario S., Labrecque M., Lucotte M., Juneau P. (2016a). Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic. Biochem. Physiol. 130 65–70. 10.1016/j.pestbp.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Le Manac’h S. G., Moingt M., Smedbol E., Paquet S., Labrecque M., et al. (2016b). Impact of phosphate on glyphosate uptake and toxicity in willow. J. Hazard. Mater. 304 269–279. 10.1016/j.jhazmat.2015.10.043 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Smedbol E., Carneiro M. M. L. C., Garcia Q. S., Juneau P. (2014a). “Reactive oxygen species and plant hormones,” in Oxidative Damage to Plants: Antioxidant Networks and Signaling ed. Ahmad P. (New York, NY: Academic Press; ) 65–81. [Google Scholar]
- Gomes M. P., Smedbol E., Chalifour A., Hénault-Ethier L., Labrecque M., Lepage L., et al. (2014b). Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid (AMPA), an overview. J. Exp. Bot. 65 4691–4703. 10.1093/jxb/eru269 [DOI] [PubMed] [Google Scholar]
- Gomes M. P., Soares A. M., Garcia Q. S. (2014c). Phosphorous and sulfur nutrition modulate antioxidant defenses in Myracrodruom urundeuva plants exposed to arsenic. J. Hazard. Mater. 276 97–104. 10.1016/j.jhazmat.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Gunes A., Inal A., Bagci E. G., Coban S., Pilbeam D. J. (2007). Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L.) grown under B toxicity. Sci. Hortic. 113 113–119. 10.1016/j.scienta.2007.03.009 [DOI] [Google Scholar]
- Hare P. D., Cress W. A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21 79–102. 10.1016/j.plaphy.2013.05.028 [DOI] [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M. N., Wani A. S., Pichtel J., Ahmad A. (2012). Role of proline under changing environments: a review. Plant Signal. Behav. 7 1456–1466. 10.4161/psb.21949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Silva E. N., Shen Z., Jiang B., Lu H. (2012). Effects of glyphosate on photosynthesis, chlorophyll fluorescence and physicochemical properties of cogongrass (Imperata cylindrical L.). Plant Omi. J. 5 177–183. [Google Scholar]
- Inderjit, Kaushik S. (2010). Effect of herbicides with different modes of action on physiological and cellular traits of Anabaena fertilissima. Paddy Water Environ. 8 277–282. 10.1007/s10333-010-0208-4 [DOI] [Google Scholar]
- Juneau P., Barnett A., Méléder V., Dupuy C., Lavaud J. (2015). Combined effect of high light and high salinity on the regulation of photosynthesis in three diatom species belonging to the main growth forms of intertidal flat inhabiting microphytobenthos. J. Exp. Mar. Biol. Ecol. 463 95–104. 10.1016/j.jembe.2014.11.003 [DOI] [Google Scholar]
- Juneau P., Green B. R., Harrison P. J. (2005). Simulation of Pulse-Amplitude-Modulated (PAM) fluorescence: limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 43 75–83. 10.1007/s11099-005-5083-7 [DOI] [Google Scholar]
- Kitajima M., Butler W. L. W. (1975). Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochem. Biophys. Acta 376 105–115. 10.1016/0005-2728(75)90209-1 [DOI] [PubMed] [Google Scholar]
- Krall J. P., Edwards G. E. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 86 180–187. 10.1111/j.1399-3054.1992.tb01328.x [DOI] [Google Scholar]
- Kruk J., Karpinski S. (2006). An HPLC-based method of estimation of the total redox state of plastoquinone in chloroplasts, the size of the photochemically active plastoquinone-pool and its redox state in thylakoids of Arabidopsis. Biochim. Biophys. Acta 1757 1669–1675. 10.1016/j.bbabio.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Labrecque M., Teodorescu T. I. (2005). Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 29 1–9. 10.1016/j.biombioe.2004.12.004 [DOI] [Google Scholar]
- Lee T. T. (1981). Effects of glyphosate on synthesis and degradation of chlorophyll in soybean and tobacco cells. Weed Res. 21 161–164. 10.1111/j.1365-3180.1981.tb00111.x [DOI] [Google Scholar]
- Lichtenthaler H. K., Wellburn A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11 591–592. 10.1042/bst0110591 [DOI] [Google Scholar]
- Maroli A. S., Nandula V. K., Dayan F. E., Duke S. O., Gerard P., Tharayil N. (2015). Metabolic profiling and enzyme analyses indicate a potential role of antioxidant systems in complementing glyphosate resistance in an Amaranthus palmeri Biotype. J. Agric. Food Chem. 63 9199–9209. 10.1021/acs.jafc.5b04223 [DOI] [PubMed] [Google Scholar]
- Mateos-Naranjo E., Redondo-Gómez S., Cox L., Cornejo J., Figueroa M. E. (2009). Effectiveness of glyphosate and imazamox on the control of the invasive cordgrass Spartina densiflora. Ecotoxicol. Environ. Saf. 72 1694–1700. 10.1016/j.ecoenv.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Maxwell K., Johnson G. N. (2000). Chlorophyll fluorescence–a practical guide. J. Exp. Bot. 51 659–668. 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- Miteva L.-E. L. P.-E., Ivanov S. V., Alexieva V. S. (2010). Alterations in glutathione pool and some related enzymes in leaves and roots of pea plants treated with the herbicide glyphosate. Russ. J. Plant Physiol. 57 131–136. 10.1134/S1021443710010188 [DOI] [Google Scholar]
- Moldes C. A., Medici L. O., Abrahão O. S., Tsai S. M., Azevedo R. A. (2008). Biochemical responses of glyphosate resistant and susceptible soybean plants exposed to glyphosate. Acta Physiol. Plant. 30 469–479. 10.1007/s11738-008-0144-8 [DOI] [Google Scholar]
- Olesen C. F., Cedergreen N. (2010). Glyphosate uncouples gas exchange and chlorophyll fluorescence. Pest Manag. Sci. 66 536–542. 10.1002/ps.1904 [DOI] [PubMed] [Google Scholar]
- Pompeu G. B., Gratão P. L., Vitorello V. A., Azevedo R. A. (2008). Antioxidant isoenzyme responses to nickel-induced stress in tobacco cell suspension culture. Sci. Agric. 65 548–552. 10.1590/S0103-90162008000500015 [DOI] [Google Scholar]
- Redondo-Gómez S., Mateos-Naranjo E., Cambrollé J., Luque T., Figueroa M. E., Davy A. J. (2008). Carry-over of differential salt tolerance in plants grown from dimorphic seeds of Suaeda splendens. Ann. Bot. 102 103–112. 10.1093/aob/mcn069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. (1992). Reactive oxygen and DNA damage in mitochondria. Mutat. Res. 275 249–255. 10.1016/0921-8734(92)90029-O [DOI] [PubMed] [Google Scholar]
- Sandmann G., Römer S., Fraser P. D. (2006). Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab. Eng. 8 291–302. 10.1016/j.ymben.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Sergiev I. G., Alexieva V. S., Ivanov S., Moskova I. I., Karanov E. N. (2006). The phenylurea cytokinin 4PU-30 protects maize plants against glyphosate action. Pestic. Biochem. Physiol. 85 139–146. 10.1016/j.pestbp.2006.01.001 [DOI] [Google Scholar]
- Serra A.-A., Nuttens A., Larvor V., Renault D., Couée I., Sulmon C., et al. (2013). Low environmentally relevant levels of bioactive xenobiotics and associated degradation products cause cryptic perturbations of metabolism and molecular stress responses in Arabidopsis thaliana. J. Exp. Bot. 64 2753–2766. 10.1093/jxb/ert119 [DOI] [PubMed] [Google Scholar]
- Servaites J. C., Tucci M. A., Geiger D. R. (1987). Glyphosate effects on carbon assimilation, ribulose bisphosphate carboxylase activity, and metabolite levels in sugar beet leaves. Plant Physiol. 85 370–374. 10.1104/pp.85.2.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl D. (1997). “Inhibitors of EPSPS synthase, glutamine synthetase and histidine synthesis,” in Herbicide Activity: Toxicology, Biochemistry and Molecular Biology eds Roe R., Burton J., Kuhr R. (Amsterdam: IOS Press; ) 37–67. [Google Scholar]
- Sohal R. S., Mockett R. J., Orr W. C. (2002). Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 33 575–586. 10.1016/S0891-5849(02)00886-9 [DOI] [PubMed] [Google Scholar]
- Song F. N., Yang C. P., Liu X. M., Liu G. B. (2006). Effect of salt stress on activity of superoxide dismutase (SOD) in Ulmus primula L. J. For. Res. 17 13–16. 10.1007/s11676-006-0003-7 [DOI] [Google Scholar]
- Soshinkova T. N., Radyukina N. L., Korolkova D. V., Nosov A. V. (2013). Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ. J. Plant Physiol. 60 41–54. 10.1134/S1021443713010093 [DOI] [Google Scholar]
- Su Y. S., Ozturk L., Cakmak I., Budak H. (2009). Turfgrass species response exposed to increasing rates of glyphosate application. Eur. J. Agron. 31 120–125. 10.1016/j.eja.2009.05.011 [DOI] [Google Scholar]
- van Kooten O., Snel J. (1990). The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25 147–150. 10.1007/BF00033156 [DOI] [PubMed] [Google Scholar]
- Vendrell E., Gómez de Barreda Ferraz D., Sabater C., Carrasco J. M. (2009). Glyphosate on growth of four freshwater species of phytoplankton: a microplate bioassay. Bull. Environ. Contam. Toxicol. 82 538–542. 10.1007/s00128-009-9674-z [DOI] [PubMed] [Google Scholar]
- Walter A., Rascher U., Osmond B. (2003). Transitions in photosynthetic parameters of midvein and interveinal regions of leaves and their importance during leaf growth and development. Plant Biol. (Stuttg) 6 184–191. 10.1055/s-2004-817828 [DOI] [PubMed] [Google Scholar]
- Wang C.-Y. (2001). Effect of glyphosate on aromatic amino acid metabolism in purple Nutsedge (Cyperus rotundus)1. Weed Technol. 15 628–635. 10.1614/0890-037X(2001)015[0628:EOGOAA]2.0.CO;2 [DOI] [Google Scholar]
- Williams G. M., Kroes R., Munro I. C. (2000). Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 31 117–165. 10.1006/rtph.1999.1371 [DOI] [PubMed] [Google Scholar]
- Yabuta Y., Mieda T., Rapolu M., Nakamura A., Motoki T., Maruta T., et al. (2007). Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 58 2661–2671. 10.1093/jxb/erm124 [DOI] [PubMed] [Google Scholar]
- Yanniccari M., Tambussi E., Istilart C., Castro A. M. (2012). Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 57 210–217. 10.1016/j.plaphy.2012.05.027 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Shibata M., Terashima I., Noguchi K. (2010). Simultaneous determination of in vivo plastoquinone and ubiquinone redox states by HPLC-based analysis. Plant Cell Physiol. 51 836–841. 10.1093/pcp/pcq044 [DOI] [PubMed] [Google Scholar]
- Zobiole L. H. S., de Oliveira R. S., Kremer R. J., Constantin J., Bonato C. M., Muniz A. S. (2010). Water use efficiency and photosynthesis of glyphosate-resistant soybean as affected by glyphosate. Pestic. Biochem. Physiol. 97 182–193. 10.1016/j.pestbp.2010.01.004 [DOI] [Google Scholar]
- Zobiole L. H. S., Kremer R. J., de Oliveira R. S., Jr., Constantin J. (2012). Glyphosate effects on photosynthesis, nutrient accumulation, and nodulation in glyphosate-resistant soybean. J. Plant Nutr. Soil Sci. 175 319–330. 10.1002/jpln.201000434 [DOI] [Google Scholar]
- Zobiole L. H. S. S., Kremer R. J., Oliveira R. S., Jr., Constantin J., Oliveira R. S. (2011). Glyphosate affects chlorophyll, nodulation and nutrient accumulation of “second generation” glyphosate-resistant soybean (Glycine max L.). Pestic. Biochem. Physiol. 99 53–60. 10.1016/j.pestbp.2010.10.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


