Abstract
Axonal injury is a common cause of neurological dysfunction. Unfortunately, in contrast to axons from the peripheral nervous system, the limited capacity of regeneration of central nervous system (CNS) axons is a major obstacle for functional recovery in patients suffering neurological diseases that involve the subcortical white matter. Urokinase-type plasminogen activator (uPA) is a serine proteinase that upon binding to the urokinase-type plasminogen activator receptor (uPAR) catalyzes the conversion of plasminogen into plasmin on the cell surface. uPAR expression increases after an injury, and signaling through uPAR promotes tissue remodeling. However, it is yet unknown whether uPA binding to uPAR has an effect on axonal recovery in the CNS. Here, we used in vitro and in vivo models of CNS axonal injury to test the hypothesis that uPA binding to uPAR promotes axonal regeneration in the CNS. We found that newly formed growth cones from axons re-emerging from an axonal injury express uPAR and that binding of uPA to this uPAR promotes axonal recovery by a mechanism that does not require the generation of plasmin. Our data indicate that the binding of recombinant uPA or endogenous uPA to uPAR induces membrane recruitment and activation of β1 integrin via the low density lipoprotein receptor-related protein-1 (LRP1), which leads to activation of the Rho family small GTPase Rac1 and Rac1-induced axonal regeneration. Our results show that the uPA/uPAR/LRP1 system is a potential target for the development of therapeutic strategies to promote axonal recovery following a CNS injury.
Keywords: integrin, plasmin, plasminogen, Ras-related C3 botulinum toxin substrate 1 (Rac1), urokinase receptor, Rac
Introduction
Axons are highly dynamic structures that play a pivotal role in neuronal repair following a CNS injury (1). Paradoxically, because of the expression of inhibitory molecules at the injured site and to a low intrinsic capacity of CNS neurons for axon growth, the ability of CNS axons to regrow after a lesion is restricted (2). This limited capacity of regeneration constitutes a major hurdle for functional recovery in patients suffering neurological diseases, such as trauma and ischemic stroke, that frequently involve the subcortical white matter (3).
The regeneration of an injured axon involves a sequence of events that is similar to that followed by the growth of axons in the developing CNS and that requires the establishment of a polarized extension guided by a newly formed growth cone (4). Structurally, the growth cone can be divided in three well defined areas as follows: a peripheral domain that contains F-actin bundles that form finger-like filopodium- and lamellipodium-like veils; a central domain that harbors microtubules that enter the growth cone from the axon shaft; and a transition zone between the peripheral and central domains that contain actomyosin contractile structures (5).
The outgrowth of an axon follows three phases that begin at the growth cone: protrusion, engorgement, and consolidation (6). During the protrusion phase, the distal end of the growth cone links its actin cytoskeleton to an adhesive substrate in the extracellular matrix (ECM),2 allowing the filopodium- and lamellipodium-like veils of the peripheral domain to move forward as F-actin assembles. This is followed by an engorgement phase characterized by the advancement of microtubules from the central domain into the newly formed filopodia and a consolidation stage in which the growth cone compacts at its neck to form a new segment of axon shaft (7).
UPA is a serine proteinase that upon binding to the uPAR is cleaved by plasmin and other proteases to generate an active two-chain form that catalyzes the conversion of plasminogen into plasmin. uPAR expression increases after an injury (8), and signaling through uPAR promotes tissue remodeling, inflammation, chemotaxis, cell proliferation, adhesion, and migration (9) via activation of the Ras-MAPK pathway, the Tyr kinases focal adhesion kinase and Src, and the Rho family small GTPase Rac (10). In line with these observations, recent evidence indicates that uPA binding to uPAR plays a central role in CNS repair after an ischemic stroke by promoting the reorganization of the actin cytoskeleton in dendritic spines that surround the ischemic area (11).
Because uPAR is a glycosylphosphatidylinositol (GPI)-anchored glycoprotein, it needs transmembrane co-receptors such as integrins (12) and LRP1 (13, 14) to activate intracellular cell signaling pathways. Integrins are a large family of transmembrane receptors assembled by α and β subunits that bind to ligands in the ECM such as laminin, collagen, fibronectin, vitronectin, and tenascin (15) and that in the CNS play a central role in axon growth, guidance, and regeneration (16). Importantly, several studies have shown that uPAR associates with integrins (12) and that this association confers specificity to the signaling output of uPAR (10).
LRP1 is a member of the LDL receptor gene family assembled by a 515-kDa heavy chain non-covalently bound to an 85-kDa light chain containing a transmembrane and a cytoplasmic domain (17). LRP1 regulates the cell surface expression of uPAR by endocytosis of a complex formed by uPA, uPAR, and the plasminogen activator inhibitor-I (PAI-I) (13, 14). However, besides its role as an endocytic receptor, LRP1 also activates cell-signaling pathways (18). Accordingly, several lines of experimental evidence indicate that LRP1 regulates Rac1 activation (19). Significantly, although the importance of LRP1 on peripheral nerve injury has been established (20–22), a role for LRP1 on CNS axonal function is yet unclear.
The in vitro and in vivo work presented here shows that uPA binding to uPAR promotes axonal recovery in the CNS. We show that an injury to mature axons induces the expression of uPAR in the filopodia and transition zone of nascent growth cones, and the binding of uPA to uPAR promotes LRP1-mediated membrane recruitment and activation of β1 integrin, followed by β1 integrin-mediated Rac1 activation and Rac1-induced axonal regeneration. In summary, our data indicate that uPA/uPAR are potential therapeutic targets to promote axonal recovery following a CNS injury.
Results
Axonal Expression of uPAR in Cerebral Cortical Neurons
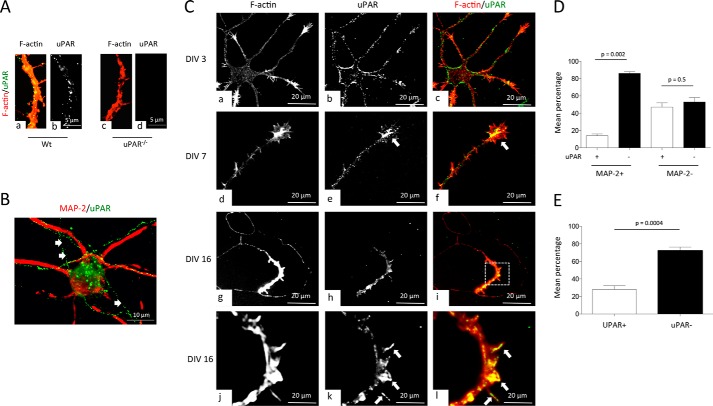
First our immunocytochemical studies with WT and uPAR−/− cerebral cortical neurons showed that our antibody is specific for uPAR (Fig. 1A). Then we used these antibodies to study the expression of uPAR in DIV 16 WT cerebral cortical neurons. We found that uPAR has a predominantly axonal distribution (Fig. 1B). To further characterize these findings, we stained with phalloidin and anti-uPAR antibodies WT cerebral cortical neurons at different stages of development. Our data indicate that while at DIV 3, uPAR is abundantly found throughout the cell body and neurites (Fig. 1C, panels a–c), and at later time points it is expressed mainly in axons. Interestingly, while at DIV 7, uPAR is detected along the axon shaft and growth cones (Fig. 1C, panels d–f), and at DIV 16, its expression is restricted to axons and few growth cones (Fig. 1C, panels g–l). More specifically, at DIV 16, 14 ± 2.08% of the dendrites, 47 ± 5% of the axons, and 28 ± 4.3% of the growth cones were immunoreactive for uPAR (Fig. 1, D and E).
FIGURE 1.
Axonal expression of uPAR. A, representative micrographs of F-actin and uPAR staining in axons of WT (panels a and b) and uPAR−/− (panels c and d) neurons. Magnification ×40. Red, F-actin; green and white, uPAR. B, representative micrograph of MAP-2 (red) and uPAR (green) staining in a DIV 16 cerebral cortical neuron. Arrows denote uPA-positive axons (MAP-2 negative). Magnification ×40. C, representative micrographs of DIV 3 (panels a–c), DIV 7 (panels d–f), and DIV 16 (panels g–l) WT axons stained with phalloidin (red) and antibodies against uPAR (green). Arrows in panels e and f and panels k and l depict uPAR staining in growth cones of DIV 7 and DIV 16 axons, respectively. Magnification ×40 in panels a–i and ×60 in panels j–l. D, mean percentage of uPAR+ and uPAR− dendrites (MAP-2+) and axons (MAP-2−) in 100 DIV 16 WT neurons. Bars denote S.E. E, mean percentage of uPAR+ and uPAR− growth cones in 200 DIV 16 WT neurons. Bars denote S.E.
Axonal Injury Induces the Expression of uPAR in Growth Cones
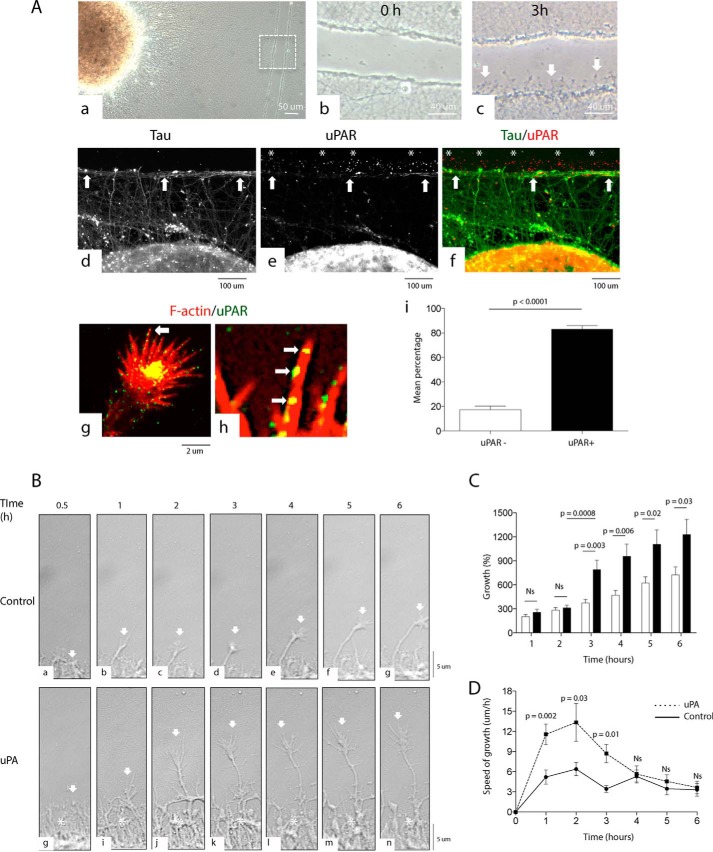
Because it has been shown in in vivo and in vitro systems that the expression of uPAR increases after different forms of injury (8), we decided to investigate its expression following an axonal wound. Neurosphere-like aggregates (NLA) prepared from WT neurons as described under “Experimental Procedures” were plated on a mixture of poly-l-lysine, and either laminin, vitronectin, fibronectin, or collagen and allowed to develop a surrounding mantle of axons. We found that at day 7, NLA plated on fibronectin, but not on vitronectin, laminin, or collagen, developed a dense blanket of surrounding axons. Then, we performed a wound injury across the axonal mantle of WT NLA plated on fibronectin (Fig. 2A, panel a) and monitored the injured area 0–3 h later. We found that newly formed axons emerge from the wounded area within 30 min after the cut and are readily noticeable 3 h later (Fig. 2A, panels b and c). Furthermore, our immunocytochemical studies show that most of the newly formed growth cones express uPAR (Fig. 2A, panels d–h). More specifically, we found that in the border of the wound injury 82.93 ± 3.1% of the growth cones express uPAR in their growing filopodia (Fig. 2A, panel i).
FIGURE 2.
uPA induces axonal recovery. A, panel a, representative micrograph of NLA and its surrounding axonal mantle. The dashed square denotes the area of the wound injury magnified in panels b and c. Magnification ×10 in panels b and c. Representative micrographs of the area are depicted by the dashed square in panel a; panel b is 0 h; and panel c is 3 h after a wound injury. Arrows in panel c point to axons emerging from the border of the injury. Magnification ×60 in panels d–f. Representative micrographs of Tau (green) and uPAR (red) staining in the axonal mantle of NLA after a wound injury. Arrows in panels d–f depict the border of the injury. Asterisks in panels e and f denote examples of uPAR-positive growth cones. Magnification ×20 in panels g and h. Representative micrographs of a growth cone from an axon emerging from the border of the wound injury and stained with phalloidin (red) and antibodies against uPAR (green). Arrows denote uPAR-positive filopodia associated with F-actin bundles. Magnification ×60 in panel j. Mean percentage of uPAR− (white bar) and uPAR+ (black bar) growth cones 3 h after a wound injury to the axonal mantle of WT NLAs. n = 162 growth cones. Lines denote S.E. B, representative micrographs from continuous live confocal microscopy imaging 0.5–6 h after a wound injury to the axonal mantle of WT NLA and treatment with vehicle (control; panels a–g) or 5 nm uPA (panels h–n). Arrows depict a growth cone from an axon emerging from the border of the wound. Asterisks in panels h–n depict the formation of a dense mesh of axons in the border of the injury following treatment with uPA. C, mean percentage growth of 120 axons monitored during 6 h following a wound injury and treatment with vehicle (control; white bars) or 5 nm uPA (black bars). Lines depict S.E. Values are given as a percentage relative to each axon's length 30 min after the injury. D, mean speed of growth in the axons analyzed in C. Lines denote S.E. NS, non-significant.
Effect of uPA on Axonal Recovery
Our immunocytochemical studies suggest that uPAR may play a role in the regeneration of injured axons. Because uPA is the ligand for uPAR, to test this hypothesis we decided to use live confocal microscopy to monitor the emergence of new axons following a lesion in the axonal mantle of WT NLA treated immediately after the injury with 5 nm uPA or a comparable volume of vehicle (control). We found that, compared with controls (Fig. 2B, panels a–g), treatment with uPA induces the formation of a denser mesh of axons in the border of the injury (asterisks in Fig. 2B, panels h–n) and the emergence from this area of longer and more branched axons (compare growth cones depicted by arrows in Fig. 2B, panels a–g, with arrows in panels h–n), which were already evident 30 min after the wound. More specifically, compared with their own length 30 min after the injury, 3–6 h after the wound and treatment with vehicle (control) axons grew 372.6 ± 46.6%, 470 ± 60%, 621.9 ± 78.67%, and 722.6 ± 101.7%, respectively. In contrast, at the same time points axons treated with uPA grew 789.5 ± 117.5% (p = 0.003 compared with control-treated axons), 976 ± 151.7% (p = 0.006), 1106 ± 179.7% (p = 0.02), and 1226 ± 192.3% (p = 0.03), respectively (Fig. 2C). Furthermore, our data indicate that within the first 3 h of treatment axons incubated with uPA grew faster than those treated with vehicle (controls; Fig. 2D).
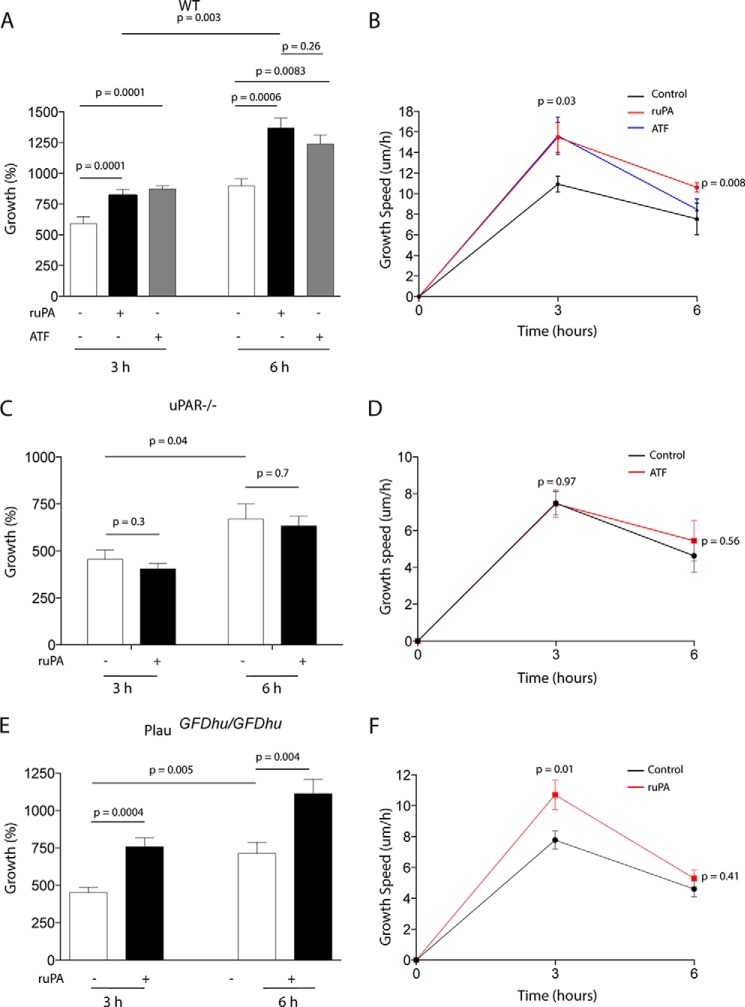
Binding of uPA to uPAR Induces Axonal Regeneration
To better characterize these observations, we quantified axonal growth and the speed of growth in the axonal mantle of either WT or uPAR−/− NLA 0–6 h after a wound injury and treatment with 5 nm uPA or its N-terminal fragment (ATF; contains uPAR's growth factor-like and kringle but not its proteolytic domain). Our data show that uPA promotes axonal growth by a mechanism that does not need the conversion of plasminogen into plasmin (Fig. 3, A and B) but requires uPA binding to uPAR (Fig. 3, C and D). Importantly, compared with vehicle (control)-treated WT axons, uPAR−/− axons had a slower growth (7.45 ± 0.74 μm/h in uPAR−/− axons versus 10.93 ± 0.77 μm/h in WT axons; p = 0.03), and less axonal growth 3 h (455.6 ± 49.25% in uPAR−/− axons versus 591 ± 55.37% in WT axons; p = 0.04) and 6 h (669.2 ± 80.89% in uPAR−/− axons versus 896.7 ± 60.62% in WT axons; p = 0.03) after the injury.
FIGURE 3.
uPAR mediates the effect of uPA on axonal recovery. Mean percentage of axonal growth (A, C, and E) and mean speed of growth (B, D, and F) in the axonal mantle of WT (A and B), uPAR−/− (C and D), and PlauGFDhu/GFDhu (E and F) NLAs, 3 and 6 h after a wound injury and treatment with either vehicle (control; white bars and black line) or 5 nm uPA (black bars and red line), or 5 nm uPA's ATF (gray bars and blue line). n = 960 axons per condition in A and B, 600 axons per condition in C and D, and 1000 axons per condition in E and F. Lines depict S.E.
To investigate whether the binding of endogenous uPA to uPAR also promotes axonal regeneration, we performed similar experiments in the axonal mantle of PlauGFDhu/GFDhu NLA, in which the binding of endogenous uPA to uPAR is precluded by a 4-amino acid substitution into uPA's growth factor (23). We found that vehicle (control)-treated PlauGFDhu/GFDhu axons had a slower growth (7.79 ± 0.6 μm/h) than WT controls (10.93 ± 0.77 μm/h; p = 0.004) and less axonal growth 3 h (450.5 ± 37.02% in PlauGFDhu/GFDhu versus 591 ± 55.37% in WT axons; p = 0.04) and 6 h (714.1 ± 73.54% in PlauGFDhu/GFDhu versus 896.7 ± 60.62% in WT axons; p = 0.04) after the injury. In contrast, we failed to detect a statistically significant difference in the growth and speed of growth between control-treated uPAR−/− and PlauGFDhu/GFDhu axons. However, in sharp difference with uPAR−/− axons, PlauGFDhu/GFDhu showed increased growth following treatment with ruPA (Fig. 3, E and F).
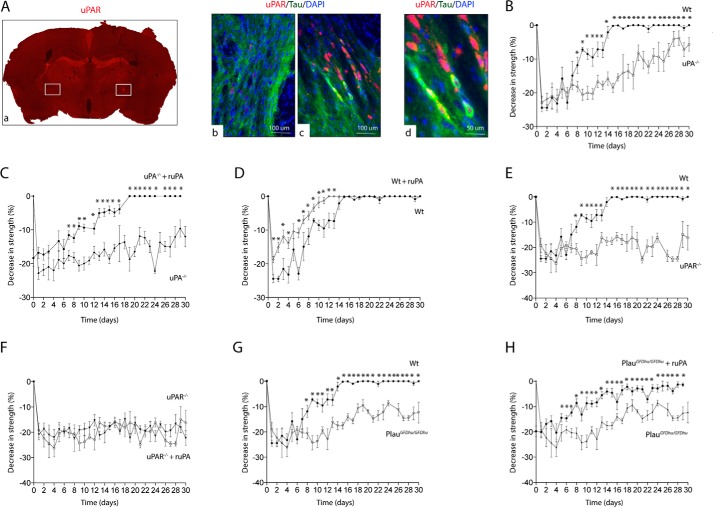
uPA/uPAR Binding Induces Neurological Recovery in Vivo
The internal capsule (IC) is a subcortical structure formed by axons of pyramidal neurons that project to the spinal cord and that are frequently affected in ischemic stroke patients (3). Thus, to determine the in vivo significance of our in vitro findings, we studied the expression of uPAR 24 h after the induction of an ischemic lesion in the IC of WT mice (Fig. 4A, panel a). We found that compared with the contralateral non-ischemic IC (Fig. 4A, panel b), the ischemic injury induces the expression of uPAR in axons of neurons that surround the lesion (Fig. 4A, panels c–e). Because the main clinical manifestation following an IC stroke is contralateral hemiparesis, we measured the strength in the contralateral forelimb of WT, uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu mice before and 0–30 days after the induction of an IC stroke and treatment with either 0.1 mg/kg/i.v. ruPA or a comparable volume of saline solution. Our data show that the four strains of mice experience a comparable decrease in the strength of their contralateral forelimb following an IC stroke. However, although all WT mice recovered completely by day 15 after the injury (Fig. 4, B and G), uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu animals failed to recover completely by the end of the study (30 days after IC stroke; Fig. 4, C and E–H). Remarkably, treatment with ruPA improved neurological outcome in WT, uPA−/−, and PlauGFDhu/GFDhu animals but not in uPAR−/− mice (Fig. 4, C, D, F, and H). Together, these data indicate that the binding of both endogenous and recombinant uPA to uPAR induce axonal recovery in vivo and that this is associated with improvement in neurological function following a CNS axonal lesion.
FIGURE 4.
uPA/uPAR binding in vivo improves functional neurological outcome following an ischemic lesion to subcortical axons. A, panel a, representative micrograph of a uPAR-stained brain section 24 h after the injection of endothelin-1 into the posterior limb of the IC. Squares denote the ET-injected (Inj) and contralateral non-injected (Con) IC. Panels b and c correspond to a staining for uPAR (red), Tau (green), and DAPI (blue) in the non-injected (panel b; control) and injected (panel c) IC. Magnification ×20, Panel d corresponds to a magnification of the micrograph depicted in panel c denoting the presence of uPAR-positive axons. Magnification ×40. B–H, mean decrease in the strength of the forelimb contralateral to the injected site in WT (B, D, E, and G), uPA−/− (B and C), uPAR−/− (E and F), and PlauGFDhu/GFDhu (G and H) mice, 0–30 days after the induction of an ischemic lesion in the posterior limb of their IC followed by treatment with saline solution (C, D, F, and H) or recombinant uPA (D, E, G, and H). Lines denote S.E. *, p < 0.05 compared with the strength of the animals presented in each graph and examined at similar time points. n = 15 mice per experimental condition at each time point.
LRP1 Mediates uPA/uPAR-induced Axonal Recovery
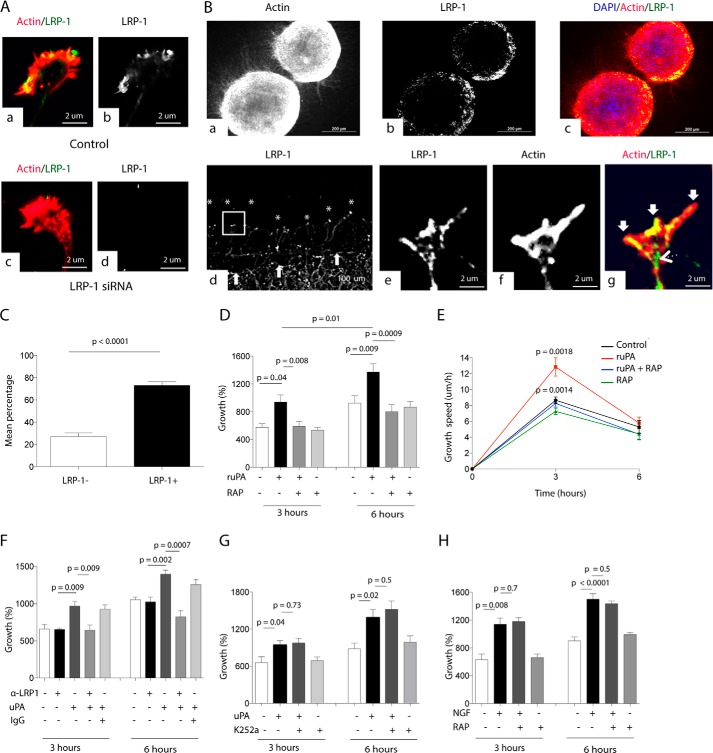
Because the association of uPA/uPAR with LRP1 leads to clathrin-dependent endocytosis of the complex (13, 14), we reasoned that abrogation of LRP1-mediated uPAR endocytosis with the receptor-associated protein (RAP; an inhibitor of the binding of LRP1 to its ligands (24)) would increase uPAR's beneficial effect on axonal growth. To test this hypothesis, first we studied the expression of LRP1 in the model of axonal injury used in this study with a previously described mouse monoclonal antibody directed against the last 13 amino acids of the intracellular domain of LRP1 (11H4) (25). Our immunostaining studies with neurons treated with siRNA for LRP1 or non-targeting siRNA indicate that this antibody is specific for LRP1 (Fig. 5A). We found that LRP1 is abundantly expressed in the growing border of the axonal mantle of WT NLA (Fig. 5B, panels a–c) and that following an axonal injury LRP1 is expressed in 73 ± 3.42% of the growth cones that emerge from the border of the injury (Fig. 5, B, panels d–g, and C). Then we measured axonal growth and speed of growth 0–6 h after a wound injury to the axonal mantle of WT NLA and treatment with either 5 nm uPA or a combination of uPA and 125 nm RAP, or with RAP alone, or with vehicle (control). Surprisingly, our data indicate that RAP abrogates the effect of uPA on axonal growth (Fig. 5, D and E) suggesting that in this model of axonal injury LRP1 mediates uPAR-induced cell signaling instead of uPAR endocytosis. To ensure that LRP1 instead of other members of the LDL receptor gene family mediates the effect of uPA on axonal growth, we performed similar observations with WT NLA treated with 5 nm uPA alone or in the presence of 40 μg/ml of an antibody that recognizes multiple epitopes in the ligand-binding region of LRP1's extracellular domain (R2629). This antibody has been previously shown to effectively block the binding of LRP1 to its ligands (26, 27). Our data indicate that LRP1 is the member of the LDL receptor gene family that mediates the effect of uPA on axonal regeneration (Fig. 5F). Because several studies indicate that TrkA transactivation mediates LRP1-induced growth of peripheral nerve axons (22), we decided to investigate the effect of uPA on axonal regeneration in the presence of the TrkA inhibitor K252a. We found that TrkA inhibition does not block the effect of uPA on axonal regeneration (Fig. 5G). Finally, to further study the specificity of the uPA-LRP1 pathway in axonal regeneration, we quantified axonal growth in WT NLA incubated with nerve growth factor (NGF), a known inductor of axonal growth via activation of multiple cell signaling pathways (28), either alone or in the presence of RAP. Our results indicate that LRP1 mediates the regenerative effect of uPA but not of NGF (Fig. 5H).
FIGURE 5.
LRP1 mediates the effect of uPA on axonal regeneration. A, representative micrograph of a growth cone of a WT cerebral cortical neuron treated with LRP1 siRNA or with siRNA control and stained with phalloidin (red) and antibodies against LRP1 (green and white). B, panels a–c, representative micrographs of two NLA and their surrounding axonal mantle, plated on fibronectin, and stained with phalloidin (red), DAPI (blue), and antibodies against LRP1 (white in panel b and green in panel c). Panel d, axonal mantle of NLA was stained with anti-LRP1 antibodies following a wound injury. Arrows denote the border of the injury. Asterisks depict examples of LRP1-positive growth cones from axons re-emerging from the border of the wound. Magnification ×20 in panels e–g, immunocytochemical staining for phalloidin (red) and LRP1 (green) in the growth cone depicted with the square in panel d. Arrows in panel g denote LRP1-positive filopodia, and arrowhead depicts LRP1 expression in the transition zone and newly formed axon shaft. Magnification ×100 in C. Mean percentage of LRP1− (white bar) and LRP1+ (black bar) growth cones in the border of a wound injury to the axonal mantle of WT NLAs. n = 200 growth cones. D and E, mean percentage axonal growth (B) and mean speed of growth (C) 3 and 6 h after a wound injury and treatment with 5 nm uPA alone or in combination with 125 nm RAP. n = 1200 axons examined at each time point per experimental condition. Lines depict S.E. F, mean percentage axonal growth 3 and 6 h after a wound injury and treatment with 5 nm uPA alone or in combination with 40 μg/ml anti-LRP1 blocking antibodies, or with 40 μg/ml of an IgG isotyped control. n = 250 axons examined. Lines denote S.E. G, mean percentage axonal growth 3 and 6 h after a wound injury and treatment with uPA alone or in combination with 10 μm of the TrkA inhibitor K252a or with K252a alone. n = 300 axons examined per experimental condition. Lines denote S.E. H, mean percentage axonal growth 3 and 6 h after a wound injury and treatment with 10 μm NGF alone or in combination with 125 nm RAP or with RAP alone. n = 400 axons examined per experimental condition. Lines denote S.E.
uPA Induces LRP1-mediated Membrane Recruitment of Active β1 Integrin
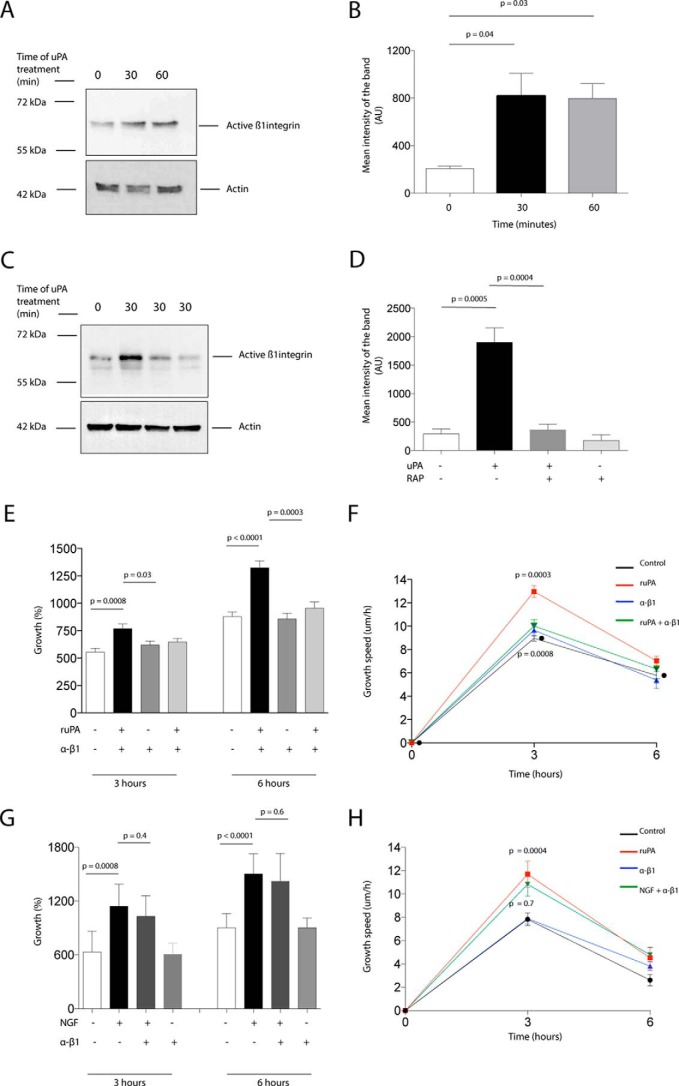
Our studies indicate that fibronectin is required for axonal growth in our in vitro model of axonal injury. Because integrin co-receptors mediate uPAR signaling (8) and β1 integrin is a fibronectin receptor that is known to associate with uPAR (29), we then decided to use biotin labeling to assess β1 integrin levels on the plasma membrane of WT neurons treated 0–60 min with 5 nm uPA. Our data indicate that uPA induces membrane recruitment of β1 integrin (Fig. 6, A and B) and that this effect is prevented by co-treatment with RAP (Fig. 6, C and D). The functional importance of these observations was underscored by the finding that treatment with anti-β1 integrin blocking antibodies abrogated the effect of uPA on axonal regeneration following a wound injury (Fig. 6, E and F). Furthermore, the specificity of this pathway was indicated by the fact that anti-β1 integrin blocking antibodies did not block the effect of NGF on axonal growth (Fig. 6, G and H).
FIGURE 6.
LRP1-mediated membrane recruitment and activation of β1 integrin mediates uPA-induced axonal regeneration. A–D, representative Western blotting analysis and quantification of the mean intensity of the band of active β1 integrin expression in the plasma membrane of WT cerebral cortical neurons. Biotin labeling was used to assess active β1 integrin levels following 0–60 min of incubation with 5 nm uPA alone (A and B) or in the presence of 125 nm RAP (C and D). E and F, mean percentage axonal growth (G) and mean speed of growth (H) 3 and 6 h after a wound injury and treatment with 5 nm uPA, alone or in combination with anti-β1 integrin neutralizing antibodies. n = 600 axons per experimental condition. p in H = 0.0003 when controls are compared with ruPA-treated axons and p in H = 0.0008 when ruPA-treated axons are compared with axons treated with a combination of ruPA and RAP. Lines depict S.E. G and H, mean percentage axonal growth 3 and 6 h after a wound injury and treatment with 10 μm NGF alone or in combination with anti-β1 integrin neutralizing antibodies. n = 400 axons examined per experimental condition. Lines denote S.E.
Effect of uPA on Axonal Recovery Is Mediated by β1 Integrin-mediated Rac1 Activation
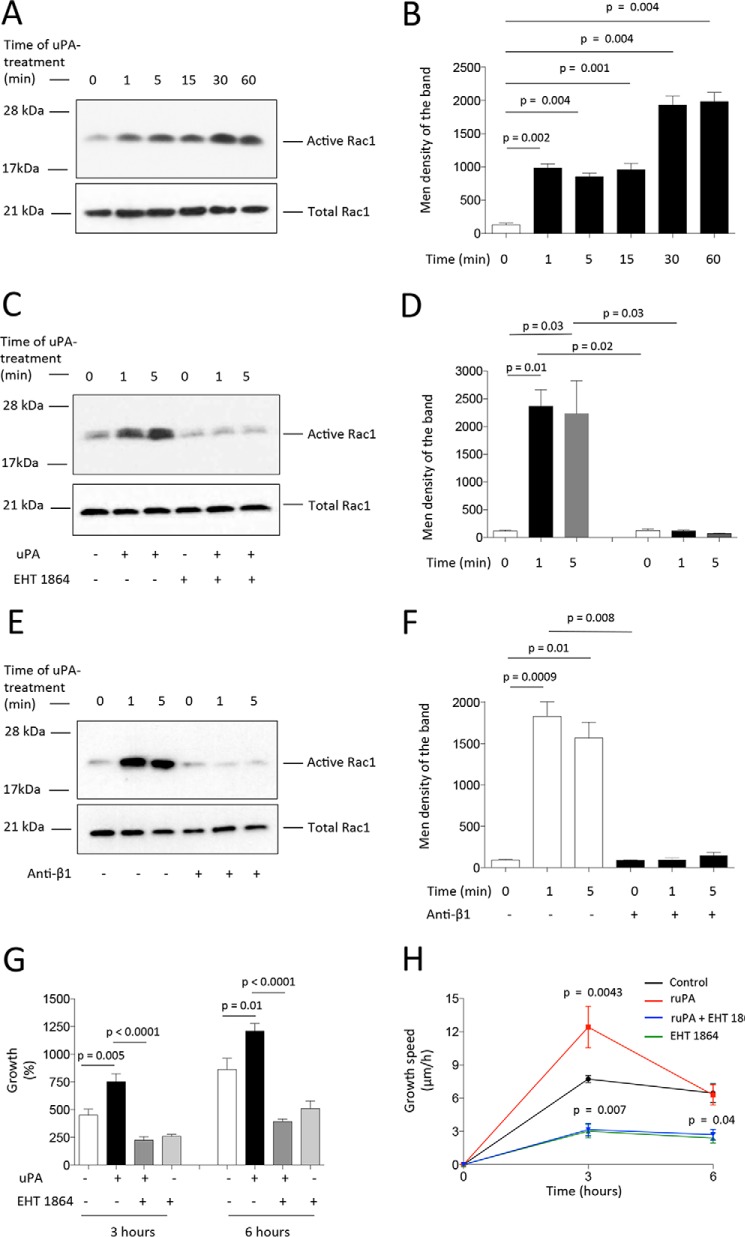
Because uPA/uPAR-induced activation of the Rho GTPase Rac1 leads to F-actin assembly and directional migration by a mechanism that does not require plasmin generation (30), we decided to investigate whether Rac1 activation mediates the effect of uPA on axonal regeneration. First, we studied the effect of treatment with uPA on Rac1 activation. We found that uPA induces activation of Rac1 (Fig. 7, A and B) and that this effect is blocked by the Rac1 inhibitor EHT 1864 (Fig. 7, C and D) and by anti-β1 integrin blocking antibodies (Fig. 7, E and F). Then, we quantified axonal growth following a wound injury and treatment with uPA alone or in the presence of the Rac1 inhibitor EHT 1864. Our data indicate that Rac1 inhibition abrogates uPA-induced axonal regeneration (Fig. 7, G and H).
FIGURE 7.
β1 integrin-mediated Rac1 activation mediates uPA-induced axonal regeneration. A–F, representative immunoblots (A, C, and E) and corresponding quantification of the mean intensity of the band (B, D, and F) for Rac1 activation in WT cerebral cortical neurons incubated during the indicated times with 5 nm uPA alone (A and B) or in combination with either 5 μm Rac1 inhibitor EHT 1864 (C and D) or anti-β1 integrin neutralizing antibodies (E and F). G and H, mean percentage axonal growth (G) and speed of growth (H) 3 and 6 h after a wound injury and treatment with 5 nm uPA alone or in combination with 5 μm Rac1 inhibitor EHT 1864. n = 450 axons at each time point per experimental condition. p in F = 0.0043 when recombinant uPA-treated axons are compared with axons treated with a combination of recombinant uPA and EHT 1864. Lines depict S.E.
Discussion
It has been long recognized that the binding of uPA to its receptor uPAR plays a pivotal role in tissue repair and remodeling (10). However, although it was initially believed that the mechanism underlying this effect is uPA/uPAR-mediated localization of plasmin's proteolytic activity to the leading edge of the cell (31), it was soon evident that uPA/uPAR interaction with other molecules and receptors on the cell surface and ECM also activates cell signaling pathways that promote tissue remodeling, cell motility, invasion, proliferation, and survival by a variety of mechanisms that not always require the generation of plasmin (11, 32). The in vitro and in vivo data presented here show that the binding of uPA to uPAR induces axonal regeneration in the CNS by a plasminogen-independent mechanism.
The presence of a long axon and several dendrites bestows the structural and functional polarization on mature neurons that is required for a directional flow of action potentials from dendrites to axons (33). However, a substantial amount of experimental data indicates that the conduction of an action potential is not the only role of axons, and instead they are pivotal for neuronal metabolism, cell-to-cell interaction, and the response to neuronal injury (34–36). Indeed, axon elongation underlies not only the formation of neuronal circuits during development but also nerve regeneration after an injury. This process begins at the growth cone, a highly dynamic structure located at the tip of each axon and formed by a peripheral domain containing F-actin-rich filopodium- and lamellipodium-like veils, a transition zone with actomyosin contractile structures known as actin arcs, and a central area with microtubules that enter the growth cone from the axon shaft (5).
Our studies show that during the early stages of neuronal development, uPAR is abundantly expressed in the cell soma and throughout immature neurite extensions that exhibit intense filopodial and lamellipodial protrusive activity. However, when the symmetry of these extensions is broken by the emergence of a longer neurite that becomes an axon, the expression of uPAR disappears from the smaller extensions and persists in the shaft and growth cone of the newly formed axon. Remarkably, as the neuron reaches maturity, the axonal expression of uPAR decreases to remain in few growth cones. Our immunocytochemical studies show that newly formed growth cones following an injury to mature axons express uPAR in their transition and peripheral zones, recapitulating the expression pattern observed during earlier developmental stages. These results and our live confocal microscopy studies and in vivo observations not only agree with previous reports indicating that uPAR expression increases after an injury (11) but also indicate that uPA binding to uPAR plays a central role in axonal regeneration after a CNS injury.
In most neurons, axonal elongation is limited to few days during development after which the molecular processes required for growth are inactivated. Yet, under some conditions mature neurons may regenerate after an injury by reactivating some of these early growth-related mechanisms (37). Based on the in vitro and in vivo findings presented here, we postulate that uPA/uPAR binding is one of these mechanisms and that its activation promotes axonal regeneration and functional improvement in the injured CNS.
It has been shown that the secretion of glia-derived inhibitory molecules in the injured area precludes axonal regeneration following a CNS lesion (4). However, our data with an in vitro system assembled with mature CNS axons grown in the presence of glia and an in vivo model of axonal injury strongly suggest that the binding of uPA to uPAR activates a regenerating mechanism capable of overcoming the unwanted effect of this inhibitory microenvironment on axonal repair. Still, an important caveat for the interpretation of these data is that our in vitro model lacks cells other than neurons and astroglia that in an in vivo system may become an as yet unidentified source of growth inhibitory molecules. However, we believe that our results with an in vivo system of axonal injury indicate that this is not the case and instead that uPA/uPAR binding activates a cell signaling pathway that enables axonal regeneration in an unfavorable microenvironment. Moreover, our in vivo system also indicates that treatment with ruPA is a potential therapeutic approach to promote regeneration and functional improvement following an axonal injury to the CNS.
Our live confocal microscopy experiments show that the speed of growth of axons emerging from the wounded area increases within the first 3 h of treatment with uPA and that this leads to a statistically significant increase in axonal length 3 h later. Despite the importance of these observations, we still do not know why the speed of growth decreases after 3 h. Furthermore, although the role of the binding of endogenous uPA to uPAR has remained elusive, our data with PlauGFDhu/GFDhu axons, in which endogenous uPA does not bind to uPAR, indicate that endogenous uPA/uPAR binding is pivotal for axonal growth and regeneration. These observations were further confirmed by our data showing that in contrast to endogenous uPA, treatment with ruPA induces regeneration in PlauGFDhu/GFDhu axons.
Because uPAR is bound to the external plasma membrane by a GPI anchor, it needs transmembrane receptors to activate intracellular signaling pathways (8). In line with these observations, our data show that a member of the LDL receptor gene family, most likely LRP1, mediates the effect of uPA/uPAR binding on axonal regeneration and that in injured axons LRP1 does not act as an endocytic receptor but instead it becomes a signaling receptor that activates an intracellular cell signaling pathway that promotes axonal repair. Moreover, our results indicate that a pharmacological approach to increase LRP1 expression on the membrane of growth cones may be a potentially effective therapeutic tool to promote axonal repair in the injured CNS. Importantly, in contrast with observations by others with axons from dorsal root ganglion neurons (22), our data indicate that TrkA does not mediate the effect of uPA/LRP1 on CNS axonal growth. We believe that this apparent discrepancy can be explained by the fact that different pathways may mediate the effect of LRP1 on the growth of peripheral and CNS axons. Likewise, our data indicate that in CNS axons LRP1 mediates the effect of uPA but not of other known inductors of axonal growth such as NGF.
Our observation that fibronectin is required for optimal axonal growth suggests that β1 integrin acts as a transmembrane co-receptor that mediates the effect of uPA/uPAR binding on axonal growth. This hypothesis is supported by our data indicating that uPA induces membrane recruitment of active β1 integrin by an LRP1-mediated mechanism and that this is required for uPA/uPAR-induced axonal repair. Furthermore, the specificity of β1 integrin for this pathway was indicated by our observation that anti-β1 integrin blocking antibodies did not prevent NGF-induced axonal grow.
Rac1 plays an essential role in axon growth in the CNS (38), and experimental work with murine embryonic fibroblasts indicates that LRP1 is a major regulator of Rac1 activation by a mechanism that requires uPAR (19). In agreement with these observations, the work presented here shows that uPA induces β1 integrin-mediated Rac1 activation and that this effect is required for uPA/uPAR-induced axonal repair.
In summary, based on the data presented here, we propose a model in which the peripheral and transition areas of growth cones of injured CNS axons express uPAR. We found that the binding of uPA to this uPAR leads to LRP1-mediated membrane recruitment of active β1 integrin, β1 integrin-mediated Rac1 activation, and Rac1-dependent axonal repair. These data indicate that the uPA/uPAR/LRP1 system is a potential target for the development of therapeutic strategies to promote axonal repair in the injured CNS.
Experimental Procedures
Animals and Reagents
Animal strains were 8–12-week-old uPA-deficient (uPA−/−) and uPAR-deficient (uPAR−/−) mice on a C57BL/6J background and their wild-type (WT) littermate controls (a generous gift from Dr. Thomas H. Bugge (Oral and Pharyngeal Cancer Branch, NIDCR, National Institutes of Health, Bethesda)). We also used a mouse strain developed by Dr. Bugge on a C57BL/6 background (PlauGFDhu/GFDhu) (23), in which a 4-amino acid substitution into the growth factor domain of uPA abrogates its binding to uPAR while preserving other functions of the protease and its receptor. Experiments were approved by the Institutional Animal Care and Use Committee of Emory University, Atlanta, GA. Recombinant murine uPA and uPA's ATF were purchased from Molecular Innovations (Novi, MI). The RAP and antibodies against LRP1, directed to either the last 13 amino acids of its intracellular domain (monoclonal 11H4) or to its extracellular ligand-binding domains (polyclonal R2629), were kind gifts from Dr. Dudley K. Strickland (University of Maryland; Baltimore). Other materials were as follows: Rac1 inhibitor EHT 1864 (Tocris Bioscience; Bristol, UK); Pierce cell surface protein extraction kit, phalloidin, and DAPI (Thermo Fisher; Rockford, IL); nerve growth factor (NGF) and antibodies against uPAR (R&D Systems; Minneapolis, MN); Tau (Millipore; Billerica, MA); Rac1 and β1 integrin blocking antibodies (BD Biosciences); microtubule-associated protein-2 (MAP-2) and activated β1 integrin (EMD Millipore; Billerica, MA). Endothelin-1 (ET-1), digitonin, vitronectin, fibronectin, laminin, collagen, and antibodies against β-actin were obtained from Sigma; the TrkA inhibitor K252a was from Calbiochem; the Rac1 activity assay was from Abcam (Cambridge, MA); and LRP1 siRNA and non-targeting control siRNA were from Dharmacon (Lafayette, CO).
Neuronal Cultures and Preparation of NLA
Cerebral cortical neurons were cultured from E16 to E18 WT, uPAR−/−, and PlauGFDhu/GFDhu mice as described elsewhere (39). Briefly, the cerebral cortex was dissected, transferred into Hanks' balanced salt solution containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 mm HEPES, and incubated in trypsin containing 0.02% DNase at 37 °C for 15 min. Tissue was triturated, and the supernatant was resuspended in GS21-supplemented neurobasal medium containing 2 mm l-glutamine and plated onto 0.1 mg/ml poly-l-lysine-coated wells. NLA were prepared as described elsewhere (40) with few modifications. Briefly, dissociated cortical neurons cultured from E17 to E18 embryos as described above were allowed to aggregate during 48 h as hanging drops (4000 cells/drop) containing 0.2% Methocel and 10% horse serum in Neurobasal/B27 media. Then they were plated on glass coverslips previously treated with a combination of poly-l-lysine and either 10 μg/ml fibronectin or 10 μg/ml vitronectin or 20 μg/ml laminin or 10 μg/ml collagen, in the presence of a co-culture of astrocytes and 10 μm AraC to prevent glial proliferation on the coverslip. Experiments were performed 7 days later when NLA were surrounded by a dense mantle of axons.
Quantification of Axonal Growth and Speed of Growth
For the live experiments, a cut was performed at 250 μm of the edge of WT NLAs with a micropipette tip followed by treatment with 5 nm uPA or a comparable volume of vehicle (control). Then, a previously selected region in the wounded area was continuously imaged during 6 h with a Nikon TE2000 inverted confocal microscope, at ×60 magnification and 1.4 numerical aperture. Images were captured by a Nikon A1R camera. Acquisition software was Nikon Element 4. Image sequences were transformed to stacks, and the length of each axon and the speed of growth were quantified every hour with ImageJ (National Institutes of Health). A total of 120 axons were examined per experimental condition. For the remaining experiments, the mantle of axons of WT, uPAR−/−, and PlauGFDhu/GFDhu mice were wounded as described above and treated with either 5 nm uPA or 5 nm UPA's ATF or with 125 nm RAP or 40 mg/ml anti-β1-integrin blocking antibodies, or 5 μm Rac1 inhibitor EHT 1864, or 10 μm nerve growth factor, or 10 μm TrkA inhibitor K252a, or with a comparable volume of vehicle (control). Pictures from the wounded area were taken every hour at ×10 magnification during 6 h, and axonal growth and the speed of growth were measured with ImageJ. The total number of axons examined per condition was 960 for WT, 600 for uPAR−/−, and 1000 for PlauGFDhu/GFDhu NLA.
siRNA Preparations and Immunostainings
For siRNA preparations, DIV 3 WT cerebral cortical neurons were incubated with 3 μm LRP1 siRNA or with non-targeting siRNA following the manufacturer's instructions, and 4 days later they were stained with a monoclonal antibody directed against LRP1's intracellular domain (11H4). For immunostaining, DIV 3, DIV 7, and DIV 16 WT cerebral cortical neurons and WT NLA were fixed with 4% paraformaldehyde, washed three times in TBS, and incubated during 30 min in a blocking solution containing 1 ml of 0.2 m glycine, 20 μl/ml casein, and 5 μl/ml donkey serum. Then samples were kept overnight on a solution containing anti-uPAR (1:1000) and anti-Tau (1:1000) antibodies, followed by incubation with an Alexa Fluor 488 donkey secondary anti-goat antibody (1:2500) during 30 min and the addition of digitonin 10 μl/ml and phalloidin (1:2000). The specificity of the anti-uPAR antibody was confirmed performing similar studies in WT and uPAR−/− neurons. A sub-set of NLA was stained following a similar procedure with phalloidin, the nuclear marker DAPI, and anti-LRP1 antibodies (1:5000). The brains of WT mice (n = 3) were harvested and cut into 20-μm sections 24 h after the injection of ET-1 into the posterior limb of their internal capsule. Antigen retrieval was performed with sodium citrate, pH 8.5, during 30 min, followed by incubation in 0.5% Triton X-100, and a blocking solution containing 1 ml of 0.2 m glycine, 20 μl/ml casein, and 5 μl/ml fish gelatin. The samples were stained with antibodies against Tau (1:1000) and uPAR (1:1000) followed by the addition of a donkey anti-goat Alexa 488 antibody (1:500) and a donkey anti-rabbit Alexa 594 antibody (1:500).
Cell Surface Biotinylation Assay
WT cerebral cortical neurons were incubated 0–60 min with 5 nm uPA alone or in the presence of 125 nm RAP. At the end of each time point, cells were washed, incubated with biotin solution (Sulfo-NHS-SS-Biotin), and centrifuged at 500 × g for 3 min. Pellets were resuspended in 5 ml of TBS, centrifuged at 500 × g for 3 min, lysed, sonicated on ice, and centrifuged at 10,000 × g for 2 min at 4 °C. The supernatant was added to the gel in columns previously prepared with NeutrAvidin-agarose, washed three times, and centrifuged for 1 min at 1000 × g. Then, 400 μl of dithiothreitol-containing Sample Buffer was added to the columns and centrifuged for 2 min at 1000 × g, and bromphenol blue was added, and the samples were immunoblotted with active anti-β1 integrin antibodies.
Animal Model of White Matter Stroke
WT, uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu 8–10-week-old male mice (n = 30 per strain) were intraperitoneally anesthetized with 400 mg/kg 4% chloral hydrate and placed on a stereotaxic frame. A burr hole was opened at bregma −1.22 mm, lateral 1.8 mm, and ventral 4.5 mm (41), and 0.25 μl of ET-1 were injected over 30 min into the posterior limb of the internal capsule with a Hamilton syringe attached to a microinfusion pump. The needle was left in place for 20 min to avoid reflow. Then, mice were intravenously treated with either 0.1 mg/kg ruPA (n = 15 per strain) or a comparable volume of saline solution (n = 15 per strain). The forelimb grip strength was measured before the injection and 0–30 days thereafter as described elsewhere (42) using a grip strength meter (Bioseb). Briefly, mice were allowed to grasp a smooth metal triangular pull bar with their right forelimb and then pulled backward in the horizontal plane. The force applied to the bar at the moment when the grasp was released was recorded as the peak tension (in newtons). Each test was repeated five times, and the mean of all trials was recorded as the mean grip strength for that animal. For both tests, values are given as a percentage compared with results obtained in the baseline evaluation.
Rac1 Activation Assay
WT cerebral cortical neurons were incubated 0–60 min with 5 nm uPA or 0–5 min with uPA alone or in combination with 20 mg/ml β1 integrin blocking antibodies. Then, cells were recollected on 1 ml of ice-cold lysis solution, and lysates were centrifuged for 15 min at 12,000 × g and incubated for 30 min with glutathione-Sepharose (1:1), run on a 4–20% precast linear gradient polyacrylamide gel, and immunoblotted with antibodies against Rac1. Each observation was repeated three times.
Statistical Analysis
Statistical analysis was performed with two-tailed t test and two-way analysis of variance with Greenhouse-Geisser correction, as appropriate. p values of <0.05 were consider as significant.
Author Contributions
P. M., A. D., V. J., F. W., E. T., and L. C. performed the experiments; M. Y. designed the experiments and wrote the manuscript.
This work was supported in part by National Institutes of Health Grants NS-079331 (to M. Y.) and NS-091201 (to M. Y.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ECM
- extracellular matrix
- uPA
- urokinase-type plasminogen activator
- uPAR
- urokinase-type plasminogen activator receptor
- GPI
- glycosylphosphatidylinositol
- ATF
- amino-terminal fragment
- RAP
- receptor-associated protein
- NLA
- neurosphere-like aggregate
- DIV
- day in vitro
- ruPA
- recombinant uPA
- IC
- internal capsule.
References
- 1. Hur E. M., Saijilafu, and Zhou F. Q. (2012) Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 35, 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu K., Tedeschi A., Park K. K., and He Z. (2011) Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 34, 131–152 [DOI] [PubMed] [Google Scholar]
- 3. Fries W., Danek A., Scheidtmann K., and Hamburger C. (1993) Motor recovery following capsular stroke: role of descending pathways from multiple motor areas. Brain 116, 369–382 [DOI] [PubMed] [Google Scholar]
- 4. He Z., and Jin Y. (2016) Intrinsic control of axon regeneration. Neuron 90, 437–451 [DOI] [PubMed] [Google Scholar]
- 5. Lowery L. A., and Van Vactor D. (2009) The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10, 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dent E. W., and Gertler F. B. (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209–227 [DOI] [PubMed] [Google Scholar]
- 7. Goldberg D. J., and Burmeister D. W. (1986) Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J. Cell Biol. 103, 1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blasi F., and Carmeliet P. (2002) uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 3, 932–943 [DOI] [PubMed] [Google Scholar]
- 9. Alfano D., Franco P., Vocca I., Gambi N., Pisa V., Mancini A., Caputi M., Carriero M. V., Iaccarino I., and Stoppelli M. P. (2005) The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb. Haemost. 93, 205–211 [DOI] [PubMed] [Google Scholar]
- 10. Smith H. W., and Marshall C. J. (2010) Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36 [DOI] [PubMed] [Google Scholar]
- 11. Wu F., Catano M., Echeverry R., Torre E., Haile W. B., An J., Chen C., Cheng L., Nicholson A., Tong F. C., Park J., and Yepes M. (2014) Urokinase-type plasminogen activator promotes dendritic spine recovery and improves neurological outcome following ischemic stroke. J. Neurosci. 34, 14219–14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei Y., Lukashev M., Simon D. I., Bodary S. C., Rosenberg S., Doyle M. V., and Chapman H. A. (1996) Regulation of integrin function by the urokinase receptor. Science 273, 1551–1555 [DOI] [PubMed] [Google Scholar]
- 13. Nykjaer A., Conese M., Christensen E. I., Olson D., Cremona O., Gliemann J., and Blasi F. (1997) Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 16, 2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nykjaer A., Petersen C. M., Møller B., Jensen P. H., Moestrup S. K., Holtet T. L., Etzerodt M., Thøgersen H. C., Munch M., and Andreasen P. A. (1992) Purified α2-macroglobulin receptor/LDL receptor-related protein binds urokinase·plasminogen activator inhibitor type-1 complex: evidence that the α2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J. Biol. Chem. 267, 14543–14546 [PubMed] [Google Scholar]
- 15. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 16. Myers J. P., Santiago-Medina M., and Gomez T. M. (2011) Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev. Neurobiol. 71, 901–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herz J., and Strickland D. K. (2001) LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108, 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herz J. (2001) The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron 29, 571–581 [DOI] [PubMed] [Google Scholar]
- 19. Ma Z., Thomas K. S., Webb D. J., Moravec R., Salicioni A. M., Mars W. M., and Gonias S. L. (2002) Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J. Cell Biol. 159, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campana W. M., Li X., Dragojlovic N., Janes J., Gaultier A., and Gonias S. L. (2006) The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J. Neurosci. 26, 11197–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaultier A., Arandjelovic S., Li X., Janes J., Dragojlovic N., Zhou G. P., Dolkas J., Myers R. R., Gonias S. L., and Campana W. M. (2008) A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J. Clin. Invest. 118, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon C., Van Niekerk E. A., Henry K., Ishikawa T., Orita S., Tuszynski M. H., and Campana W. M. (2013) Low-density lipoprotein receptor-related protein 1 (LRP1)-dependent cell signaling promotes axonal regeneration. J. Biol. Chem. 288, 26557–26568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Connolly B. M., Choi E. Y., Gårdsvoll H., Bey A. L., Currie B. M., Chavakis T., Liu S., Molinolo A., Ploug M., Leppla S. H., and Bugge T. H. (2010) Selective abrogation of the uPA-uPAR interaction in vivo reveals a novel role in suppression of fibrin-associated inflammation. Blood 116, 1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., and Argraves W. S. (1990) Sequence identity between the α2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J. Biol. Chem. 265, 17401–17404 [PubMed] [Google Scholar]
- 25. Kowal R. C., Herz J., Goldstein J. L., Esser V., and Brown M. S. (1989) Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 86, 5810–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yepes M., Sandkvist M., Moore E. G., Bugge T. H., Strickland D. K., and Lawrence D. A. (2003) Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orr A. W., Pedraza C. E., Pallero M. A., Elzie C. A., Goicoechea S., Strickland D. K., and Murphy-Ullrich J. E. (2003) Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 161, 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sofroniew M. V., Howe C. L., and Mobley W. C. (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24, 1217–1281 [DOI] [PubMed] [Google Scholar]
- 29. Wei Y., Eble J. A., Wang Z., Kreidberg J. A., and Chapman H. A. (2001) Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol. Biol. Cell 12, 2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sturge J., Wienke D., East L., Jones G. E., and Isacke C. M. (2003) GPI-anchored uPAR requires Endo180 for rapid directional sensing during chemotaxis. J. Cell Biol. 162, 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collen D. (1999) The plasminogen (fibrinolytic) system. Thromb. Haemost. 82, 259–270 [PubMed] [Google Scholar]
- 32. Kjøller L., and Hall A. (2001) Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J. Cell Biol. 152, 1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasband M. N. (2010) The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, 552–562 [DOI] [PubMed] [Google Scholar]
- 34. Kukley M., Capetillo-Zarate E., and Dietrich D. (2007) Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 [DOI] [PubMed] [Google Scholar]
- 35. Stys P. K. (2011) The axo-myelinic synapse. Trends Neurosci. 34, 393–400 [DOI] [PubMed] [Google Scholar]
- 36. Hinman J. D. (2014) The back and forth of axonal injury and repair after stroke. Curr. Opin. Neurol. 27, 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skene J. H. (1989) Axonal growth-associated proteins. Annu. Rev. Neurosci. 12, 127–156 [DOI] [PubMed] [Google Scholar]
- 38. Hua Z. L., Emiliani F. E., and Nathans J. (2015) Rac1 plays an essential role in axon growth and guidance and in neuronal survival in the central and peripheral nervous systems. Neural Dev. 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Echeverry R., Wu J., Haile W. B., Guzman J., and Yepes M. (2010) Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J. Clin. Invest. 120, 2194–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torre E. R., Gutekunst C. A., and Gross R. E. (2010) Expression by midbrain dopamine neurons of Sema3A and 3F receptors is associated with chemorepulsion in vitro but a mild in vivo phenotype. Mol. Cell. Neurosci. 44, 135–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paxinos G., and Franklin K. B. (2001) The Mouse Brain in Stereotaxic Coordinates, Graph 41, Academic Press Inc., San Diego [Google Scholar]
- 42. Wu F., Wu J., Nicholson A. D., Echeverry R., Haile W. B., Catano M., An J., Lee A. K., Duong D., Dammer E. B., Seyfried N. T., Tong F. C., Votaw J. R., Medcalf R. L., and Yepes M. (2012) Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J. Neurosci. 32, 9848–9858 [DOI] [PMC free article] [PubMed] [Google Scholar]