Abstract
Anal intraepithelial neoplasia (AIN) is a premalignant lesion of the anal mucosa that is a precursor to anal cancer. Although anal cancer is relatively uncommon, rates of this malignancy are steadily rising in the United States, and among certain high risk populations the incidence of anal cancer may exceed that of colon cancer. Risk factors for AIN and anal cancer consist of clinical factors and behaviors that are associated with the acquisition and persistence of human papilloma virus (HPV) infection. The strongest HPV-associated risk factors are HIV infection, receptive anal intercourse, and high risk sexual behavior. A history of HPV-mediated genital cancer, which suggests infection with an oncogenic HPV strain, is another risk factor for AIN/anal cancer. Because progression of AIN to anal cancer is known to occur in some individuals over several years, screening for AIN and early anal cancer, as well as treatment of advanced AIN lesions, is reasonable in certain high-risk populations. Although randomized controlled trials evaluating screening and treatment outcomes are lacking, experts support routine screening for AIN in high risk populations. Screening is performed using anal cytological exams, similar to those performed in cervical cancer screening programs, along with direct tissue evaluation and biopsy via high resolution anoscopy. AIN can be treated using topical therapies such as imiquimod, 5-flurouracil, and trichloroacetic acid, as well as ablative therapies such as electrocautery and laser therapy. Reductions in AIN and anal cancer rates have been shown in studies where high-risk populations were vaccinated against the oncogenic strains of HPV. Currently, the CDC recommends both high-risk and average-risk populations be vaccinated against HPV infection using the quadrivalent or nonavalent vaccines. It is important for clinicians to be familiar with AIN and the role of HPV vaccination, particularly in high risk populations.
Keywords: Anal cancer, Anal intraepithelial neoplasia, Anal squamous cell carcinoma, Human papillomavirus vaccine, Human papillomavirus
Core tip: Anal intraepithelial neoplasia (AIN) is the precursor lesion to anal squamous cell carcinoma. AIN incidence is low in the general population, but rivals colon cancer in high risk groups, particularly those with human immunodeficiency virus infection and men who have sex with men. Thus, screening for AIN and early anal cancer and treatment of these lesions at expert centers should be considered in high risk populations. Screening is performed using anal cytology and high resolution anoscopy, and treatment consists of either topical or ablative therapies. Finally, human papillomavirus vaccination appears to reduce the rate of AIN and possibly anal cancer.
INTRODUCTION
Anal intraepithelial neoplasia (AIN) is a clinically important premalignant lesion which can progress to squamous cell carcinoma of the anus. Despite this, AIN is frequently underappreciated by most gastroenterologists and other health care providers. With a predicted incidence of 8080 cases in the United States in 2016, equating to 1.8 cases per 100000 individuals, the incidence of anal cancer is dwarfed by colorectal cancer, which is predicted to affect roughly 135000 people in the United States in 2016[1,2]. Despite the lower rate of anal cancer, it is associated with significant morbidity and mortality, and has been steadily increasing in incidence, nearly doubling in the last 25 years[1]. The purpose of this review is to outline the burden of disease, risk factors, progression rates, and clinical consequences of AIN. We also address treatment and surveillance guidelines pertinent to practicing clinicians. Finally, special attention is given to the role of human papilloma virus (HPV) vaccination.
DEFINITIONS
Anal cancer is defined as cancer arising from the squamous epithelium of the anus, making it distinct from colorectal cancer. The anal canal consists of stratified squamous epithelium originating outside the body and extending into the anus up to the dentate line, the point where it intersects the columnar epithelium of the rectum. Thus, the vast majority of anal cancer is squamous cell carcinoma (SCC), with a small minority consisting of adenocarcinoma or skin cancer variants[3]. The term “anal cancer” commonly refers to SCC and given the predominance of SCC, the two terms will be used interchangeably in this review.
Anal cancer is preceded by intraepithelial neoplasia[4,5], a premalignant lesion which has been described by a variety of different classification schemes throughout the literature owing to the evolving understanding of its underlying pathophysiology. Dysplastic lesions in the anal region were initially reported as “mild”, “moderate”, and “severe” by pathologists. However, as the connection between anal dysplasia and the HPV was established, new criteria borrowing from the cervical pathology classification system were developed, using the Bethesda System terminology AIN I, II, and III[6,7]. Unfortunately this led to uncertainty regarding the significance of AIN II lesions, which have been associated with poor interobserver agreement among pathologists, leaving clinicians unsure which lesions required close follow up and which could be followed expectantly[8]. Further clarification of the relationship of AIN with HPV, and the discovery that the oncogenic pathways of anal and genital cancers are closely related, has recently led to a simpler system consisting of a two-tiered approach of “low grade” and “high grade squamous intraepithelial lesions” (LSIL and HSIL, respectively; Table 1)[9]. Under this system, AIN I corresponds to LSIL and AIN II/III to HSIL. HSIL lesions are considered premalignant, whereas LSIL lesions are not felt to be premalignant, but do have the potential to progress to HSIL. Cytology reports will occasionally include the term “ASCUS”, or “atypical squamous cells of undetermined significance”, which can be generally included in the LSIL category.
Table 1.
Unification of terms relating to anal intraepithelial neoplasia
| Normal |
LSIL |
HSIL |
||
| Condyloma or ASCUS | AIN grade I | AIN grade II | AIN grade III | |
| Mild dysplasia | Moderate dysplasia | Severe dysplasia | ||
ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; AIN: Anal intraepithelial neoplasia.
EPIDEMIOLOGY AND RISK FACTORS
Due to the low incidence of anal cancer, the changing nomenclature, the difficulties with validated testing, and the absence of large, population-based screening programs, it is difficult to estimate the true burden of AIN in the general population. However, as certain high risk groups have been identified and subsequently studied, it is possible to comment on disease prevalence within these groups. Table 2 summarizes the estimated risks for anal cancer among various populations, which will be explored in more detail below.
Table 2.
Rates of anal cancer among various populations compiled from various sources
| Anal cancer rates among select populations, per 100000 person-years | |
| General population | 2[1] |
| General population, female | 0.55-2.4[13] |
| HIV positive women | 3.9-30[13] |
| HIV negative MSM | 5.1[12] |
| Solid organ transplant | 10-15[66] |
| Prior HPV related malignancy | 0.8-63.8[13] |
| HIV positive MSM | 49.5[12] |
| Colon cancer in general population | 41[2] |
HIV: Human immunodeficiency virus; MSM: Sex with men; HPV: Human papilloma virus.
High-risk sexual behavior
High-risk sexual behavior, most commonly defined as men who have sex with men (MSM), receptive anal intercourse, or history of multiple sexual partners has been shown to be associated with higher rates of HSIL. One study of 1262 human immunodeficiency virus (HIV) negative MSM revealed 15% prevalence of LSIL and 5% for HSIL[10]. Another recent study of 203 individuals revealed elevated rates of HSIL, finding a prevalence of 20.9% of HIV negative MSM[11]. The largest meta-analysis to evaluate HIV negative MSM included 53 studies and determined a HSIL prevalence of 21.5%, and was able to provide an estimated anal cancer rate of 5 per 100000[12]. Less data is available regarding the rate of AIN in women; however, in a study of 251 HIV+ and 68 HIV- women, Holly et al[13] found rates of 8% for any type of AIN, and 2% for HSIL in HIV negative women. Receptive anal intercourse and concomitant abnormal cervical cytology were found to be statistically significant risk factors for AIN.
HIV infection
The first clue that the HIV population was at particular risk for AIN came with the appreciation that anal cancer rates have notably increased in the HIV era as compared to previous eras[1]. Infection with HIV is associated with an increased risk for AIN in all infected persons, and the risk appears especially high in MSM populations. In the previously mentioned study by Holly et al[13] regarding risks of AIN in women, 26% and 6% of HIV positive women had any type of AIN or HSIL, respectively. Similarly, the aforementioned meta-analysis revealed an HSIL pooled prevalence of 29.1%, and found an anal cancer rate of 45.9 per 100000 in HIV positive MSM[12].
This pattern of elevated risk for AIN in the HIV positive population has been demonstrated in several studies[14-16], and is also seen in studies evaluating anal cancer. A 2012 study by Silverberg et al[17] reported rates of anal cancer in HIV+ MSM of 135 per 100000, HIV+ non-MSM of 45 per 100000, and HIV negative non-MSM with a rate of 2 per 100000. The latter figure reflects the risk of anal cancer in the general population. The same study also found anal cancer rates of 30 per 100000 for HIV positive women; there were no cases of anal cancer in the HIV negative women.
The reason that high-risk sexual behavior and HIV infection is associated with an increased risk for AIN is likely due to the fact that both are associated with infection with HPV, inability to clear HPV infection, and for simultaneous infections with multiple strains of HPV[18].
HPV
The HPV family includes double-stranded DNA viruses that infect mucosal and cutaneous epithelia and induce cellular proliferation[19]. HPV is extraordinarily common, with most sources estimating a prevalence of 45% in the general population, with 75% of the general population acquiring an HPV infection at some point in their lifetime[20,21]. HPV has been shown to be causally associated with anogenital neoplasia, including AIN and anal cancer[22,23]. The association of HPV with anal cancer led to the realization that anal cancer shares features with genitourinary tract malignancies, such as cervical, vaginal and penile cancer, which are also SCCs closely linked to HPV infection[24,25]. It has been appreciated that the histologic transition zone in the anal canal, as in the cervix, is the most common site of the histopathologic changes associated with HPV infection[26]. The anus and cervix also share embryological origins and susceptibility to HPV infection, which might also explain the similarities between these malignancies.
Identification of a common disease pathway and causative agent prompted the adoption of the same pathological terminology across anogenital cancers as discussed above. The terms “LSIL” and “HSIL” are felt to be the most appropriate as they describe the histologic changes seen with transient vs chronic changes related to HPV infection; chronic HPV infection is the condition associated with anogenital cancer[27].
Other risk factors
A number of other risk factors for AIN have been described, though none appear to be as strongly associated with AIN as HIV and/or HPV infection and high risk sexual behavior. When considering an individual’s risk for AIN and anal SCC, a personal history of CIN and gynecological cancers should be sought by clinicians, since a history of genital neoplasia is a risk factor for anal neoplasia.
Tobacco smoking has been consistently implicated as a risk for AIN[28,29], despite a lack of clear understanding of the mechanism(s) involved. A recent study by Gautier et al[30] found that AIN regression after therapy failed to occur in any smoking patient (n = 14; 30%), while in nonsmoking patients AIN regressed in n = 29 (63%).
The presence of HPV-related dysplasia in other anatomical site in an individual is a well-established risk factor for AIN since the development of an HPV-related malignancy implies chronic infection with an oncogenic HPV strain, thus increasing the risk for other HPV-related malignancies[31]. Lastly, chronic immunosuppression has been implicated as a risk factor for the development of AIN and for the progression of AIN to cancer, presumably due to the increased HPV burden related to reduced viral clearance. This risk is best described in the post-transplant population[32], but more data is needed to clarify the risks in other populations, particularly with the rapid development and employment of new biologic and other immunomodulatory therapies[30].
PROGRESSION TO ANAL CANCER
AIN is assumed to be the precursor lesion to anal cancer, in that some cases of LSIL progress to HSIL, and then to SCC. However, the rate and risk factors associated with AIN progression, as well as the factors associated with regression, are poorly characterized. Several trials support the concept that AIN progresses to SCC, with one trial reporting rates of progression of AINII/III (HSIL) to SCC of 11% over a median period of 42 mo in a cohort of 72 patients enrolled in an AIN surveillance program. Approximately one third of this population experienced a decrease in stage or regression of disease[33]. Another trial reported progression to SCC in 3 out of 35 (8.6%) of patients with AIN III followed for 53 mo; it is noteworthy that all three who progressed were being treated with systemic steroids for extended periods of time[34].
Among high risk patients, Tong et al[35] reported a progression rate from AIN I to AIN III in 25 of 199 (12.6%) male patients in an anal cancer screening clinic, equivalent to a rate of 8.1 per 100 person-years. HIV positive patients were at the greatest risk for progression, with a hazard ratio (HR) of 2.8 for AIN I to III progression. Interestingly, CD4 count was not a significant factor affecting progression rates. This study also reported spontaneous regression rates of AIN III, with 26 of 55 (47%) regressing from AIN III, equivalent to an incidence of 68.9 per 100 person-years. Among the 26 patients who spontaneously regressed, 11 (42%) regressed to AIN II, 11 (42%) regressed to AIN I, and four (15%) regressed to no disease (negative biopsies). Similar progression rates were described by Burgos et al[36] with progression occurring at 10.5/100 person-years among 556 HIV infected men followed for 649 person-years. This trial found that being on highly-active anti-retroviral therapy or in a stable personal relationship with another individual were protective, with progression rates of 2.8/100 person-years in these sub groups, while infection with HPV strains 16 or 18 were independent risk factors for progression. An additional retrospective study of patients with anal cancer found that 19.6% of individuals (n = 27/138) had previously documented HSIL, with an average time from HSIL to cancer of 57 mo in prevalent HSIL, and 64 mo for incident HSIL[37]. Taken together, these studies suggest that while AIN can progress to SCC, the overall rates of progression are relatively low, are highest in high-risk populations, and that spontaneous regression from HSIL to LSIL and LSIL to normal will occur in some individuals (Table 3).
Table 3.
Progression rates of anal intraepithelial neoplasia to squamous cell carcinoma
| Progression | No. patients | Rate of progression | Median or average progression time | Ref. |
| AIN II/III to SCC | 72 | 11% | 42 mo | [33] |
| AIN III to SCC | 35 | 8.6% | 53 mo | [34] |
| AIN I to AIN III | 199 | 12.6% (8.1/100 person-years) | 18 mo | [35] |
| ASCUS/AIN I to AIN II/III | 556 | 24.5% (10.5/100 person-years) | 36 mo | [36] |
| HSIL to SCC | 138 | 19.6% | 57 mo. w/prevalent HSIL; 64 mo. w/incident HSIL | [37] |
ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; AIN: Anal intraepithelial neoplasia; SCC: Squamous cell carcinoma.
HPV VACCINATION
Infection with HPV is now recognized to be responsible for nearly all cervical cancers, 95% of anal cancers, 65% of vaginal cancers, 50% of vulvar cancers, and 35% of penile cancers[38,39], as well as a significant portion of head and neck cancers[40]. There are over 100 types of HPV that infect humans, with approximately 50% infecting the anogenital tract. Some of these subtypes are commonly found in anogenital cancers, whereas others never are, leading to the terminology of “high-risk”, “intermediate-risk” and “low-risk” strains of HPV[19]. HPV types 16 and 18, and to a lesser extent 6 and 11, are the primary oncogenic strains found in cervical and anal cancer. These HPV strains are thought to drive oncogenesis primarily by inducing p53 degradation and upregulation of Rb, resulting in cellular proliferation[41,42]. The discovery of these strains led to the creation of vaccines targeting them. The first vaccine was designed against HPV types 16 and 18, and was soon followed by the “quadrivalent” vaccine targeting HPV types 6, 11, 16, and 18.
The quadrivalent vaccine was initially approved for the prevention of cervical cancer, but has since been shown to be efficacious in reducing rates of AIN. A recent study demonstrated 75% reductions in LSIL and HSIL in a population of 602 HIV negative MSM, with rates of persistent HPV infection reduced by 95%[43]. Vaccination also appears to be effective in preventing recurrent high grade AIN when administered after the diagnosis and treatment of high-grade AIN in HIV negative MSM. One study reported decreased rates of recurrent HSIL and anal cancer when vaccination was administered after diagnosis of HSIL, with 12/88 (13.6%) vaccinated patients and 35/114 (30.7%) unvaccinated patients developing recurrent high grade AIN during 340.4 person-years follow up[44]. Markov modeling has demonstrated that vaccination with the quadrivalent vaccine for this indication was cost effective[45].
More recently, a “nonavalent” vaccine has been developed, adding protection against HPV types 31, 33, 45, 52, and 58 to the previous four types, with rates of cervical and vulvar disease from these additional strains reduced from 1.6 per 1000 person-years in those receiving the quadrivalent vaccine to 0.1 per 1000 person-years in those receiving the nonavalent vaccine[46]. These trials provide strong evidence that HPV vaccination is effective at preventing progression of AIN and cancer, thus clinicians should be knowledgeable about HPV vaccination and advise their patients, particularly those with AIN or at high risk for AIN/anal cancer, to be vaccinated. Given the well-established similarities between cervical and anal HPV-related diseases, the CDC has recommended HPV vaccination for children of both genders to be given at age 11 or 12, and to men and women at high risk for AIN or cervical intraepithelial neoplasia (CIN), or anyone not previously vaccinated up to 26 years of age[47].
DIAGNOSIS
As discussed, anal and cervical cancer demonstrate similarities in tumor biology, and given the success of cervical cancer screening programs, a similar anal cancer screening program would seem reasonable[48]. Anal cancer is often detected in advanced stages, with local-regional spread, a 20%-40% rate of lymph node involvement, and a 10% rate of metastatic disease frequently present at the time of diagnosis based on SEER data[49]. Additionally, changes to colorectal cancer screening (CRC) guidelines in 1997 which eliminated digital rectal examination (DRE) as an appropriate screening test for CRC, along with the de-emphasis of DRE for prostate cancer screening since 2009, has possibly contributed to delayed anal cancer diagnosis[50]. Anorectal symptoms are commonly seen in individuals presenting to gastroenterologists[51], thus an understanding of diagnosing AIN is important for gastroenterologists and other clinicians, particularly as many patients with AIN are symptomatic at presentation[33].
Anal cytology
Anal cytology is currently one mode of screening for AIN. The technique of anal cytology consists of inserting a water-moistened polyester fiber swab into the rectum until encountering the rectal wall, then removing the swab with a twisting motion while applying lateral pressure, allowing for sampling of the transitional zone and anal canal. The swab is then processed using a liquid cytology technique prior to Papanicolaou staining as with cervical specimens, and then analyzed by a pathologist (Figure 1). Sampling can be performed by either the clinician or the patient; the sensitivity has been shown to be slightly higher when the clinician performs the procedure, though compliance may be improved when the patient performs the sampling[45]. Screening the general population with anal cytology has not been studied and is not currently recommended.
Figure 1.
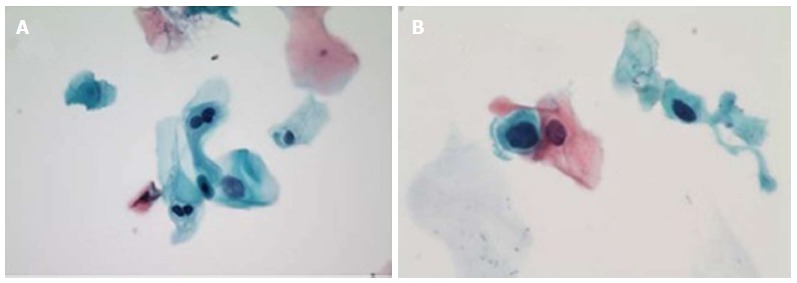
Cytology of anal intraepithelial lesions. A: LSIL, with representative binucleate hyperchromatic cells (koilocytes) and nuclear enlargement (Papanicolaou stain, original magnification × 400); B: HSIL, with representative markedly increased nuclear to cytoplasmis ratio as comparted to LSIL at left (Papanicolaou stain, oil immersion, original magnification × 1000). Reproduced with permission[85]. LSIL: Low grade squamous intraepithelial lesions; HSIL: High grade squamous intraepithelial lesions.
In studies of HIV negative MSM, the sensitivity of anal cytology reported in the literature varies, ranging from 47%-70% for the detection of AIN of any grade[52,53]. Clinicians need to be aware that while HSIL on anal cytology correlates well with high-grade AIN (i.e., AIN II or III) obtained with a biopsy, findings of ASCUS and LSIL have been shown to have near equal distribution for low grade and high grade AIN on subsequent histological examination of a tissue biopsy. For this reason, it is recommended that direct anal examination and tissue biopsy be performed following any abnormal anal cytology result[54]. Finally, given the success of HPV testing in cervical cancer screening programs using PCR of cytology specimens[55], HPV molecular testing on anal cytology specimens may provide improved diagnostic sensitivity. Initial studies comparing HPV testing to anal cytology have demonstrated equivalent sensitivity, but thus far combining cytology with HPV testing has not improved overall sensitivity, thus the roll of HPV molecular testing in the diagnosis of AIN is still under investigation[56].
High resolution anoscopy
If abnormal cytology is detected with an anal cytological exam, the next step in management of AIN is HRA to attempt to localize the source of atypical cells. High resolution anoscopy (HRA) consists of examining the squamocolumnar junction, anal canal, and perianal skin under magnification using a colposcope in a procedure that is very similar to colposcopy of the cervix. During anoscopy, an anoscope is placed into the anus with lidocaine lubrication, and then a swab soaked in 3%-5% acetic acid solution is inserted into the anal canal while the anoscope is removed for several minutes. After acetic acid application, which causes an “acetowhite change” in areas of abnormal transitional epithelium, the mucosa is carefully inspected for changes characteristic of AIN, including flat or slightly raised areas of thickened mucosa with or without vascular pattern abnormalities[57]. Lugol’s iodine is then applied in similar fashion, but in this case concerning lesions fail to stain with iodine (“Lugol’s negative”) because iodine is glycophillic and dysplastic tissues lack glycogen and appear thick mustard colored (Figure 2). Any suspicious lesions, including condylomas, atypical surface configurations, punctuations, mosaicism, or atypical vessels, are then biopsied under direct visualization[58]. Areas with color changes seen on acetic acid staining that are subsequently found to be Lugol’s negative are highly suspicious for dysplasia, and are biopsied under direct visualization during HRA. Examples of H&E stained high-grade AIN lesions obtained from biopsies are shown in Figure 3.
Figure 2.
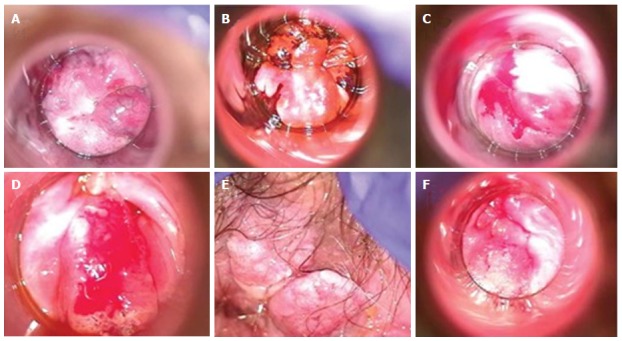
High-resolution anoscopy of representative examples of anal intraepithelial lesions. A: Low grade AIN lesion after acetic acid application with representative acetowhitening; B: Low grade AIN lesion after application of Lugol’s iodine with brown area representing normal uptake by glycogenated cells, and “mustard” colored area representing negative uptake and suggestive of dysplasia; C: High grade AIN seen after application of acetic acid and the dense acetowhite change; D: High grade AIN with concern for invasion; E: External/perianal high grade AIN after application of acetic acid; F: High grade AIN with concern for invasion. Reproduced with permission[85]. AIN: Anal intraepithelial neoplasia.
Figure 3.
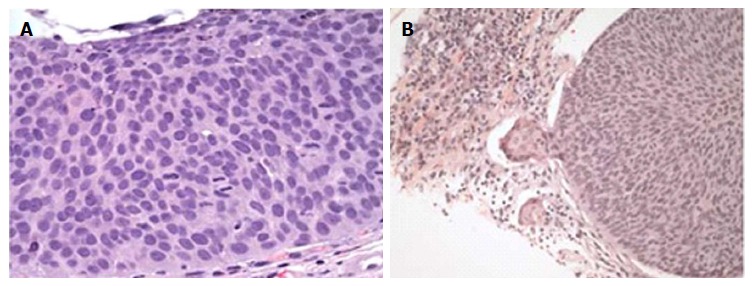
Histologic examples of high grade anal intraepithelial neoplasia, hematoxylin and eosin stain. A: High grade AIN as demonstrated by nuclear pleomorphism, numerous mitoses and no maturation of the epithelium (original magnification × 400); B: Microinvasion of a high grade AIN demonstrated by a budding off of atypical cells with paradoxical maturation and a marked inflammatory response (original magnification × 200). Reproduced with permission[85]. AIN: Anal intraepithelial neoplasia.
The appearance of lesions under HRA with acetic acid staining is similar to those seen in cervical dysplasia (Figure 2)[59]. HRA is considered superior to standard anoscopy as shown by Camus et al[60] who reported that in a population of 102 patients [68% male; 57.3% HIV positive; mean 1.6 lesions (standard deviation 0.8) per patient] only 38.7% (65/168) of all lesions seen using HRA were visible with standard anoscopy[60].
In addition to detection of AIN, HRA also facilitates the application of therapies targeting AIN, which are discussed in more detail below. Although HRA is generally considered safe for patients and not difficult for clinicians to perform, substantial training time is required in order to recognize anal lesions, which can be subtle in appearance. Due to the limited number of patients with atypical findings associated with AIN in the general population, HRA is ideally performed at centers specializing in its use rather than at clinics lacking trained experts[61].
SCREENING FOR AIN
The combination of anal cytology, possibly paired with anal HPV molecular testing, followed by HRA in individuals with positive results, represents a reasonable strategy to screen for AIN. However, at this time there are no randomized clinical trials demonstrating the value of screening at risk populations or the general population. A randomized trial evaluating the effect of screening for AIN on anal cancer incidence and/or mortality would presumably be difficult given the low incidence of anal cancer, as the number of patients and duration of follow up necessary would likely be prohibitive. The rationale for AIN screening instead relies on the cervical cancer data given the similarities between the two malignancies, as well as the identification of certain high-risk populations that might benefit from screening. As mentioned previously, these at-risk populations with elevated rates of anal cancer development include HIV-positive individuals, MSM, women with a prior history of HPV related neoplasia (cervical, vulvar, etc.), and individuals with a history of solid organ transplants (Table 2)[62,63]. Notably, the incidence of anal cancer in HIV+ MSM is higher than the rate of colorectal cancer in the general population, providing some support for screening this at-risk group for anal cancer. One possible screening algorithm for high-risk populations, e.g., HIV+ MSM, is shown in Figure 4.
Figure 4.
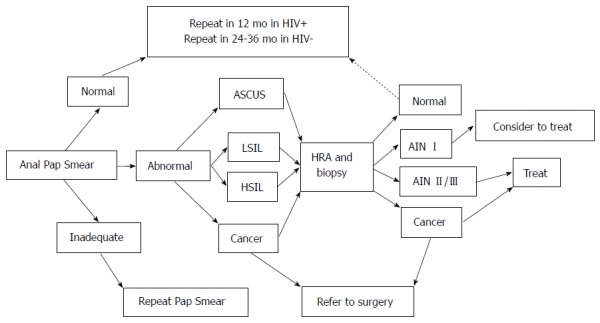
Algorithm for diagnosis, treatment and surveillance of anal intraepithelial neoplasia. Adapted from Palefsky and Rubin, 2009[86]. ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; AIN: Anal intraepithelial neoplasia.
In a study analyzing cost, Goldie et al[64] reported that screening for AIN in HIV+ MSM would be cost effective, with a trend towards cost effectiveness in post-transplant patients. Despite the elevated risk for anal cancer in these populations, the CDC, the American Society of Colon and Rectal surgeons, and most other organizations do not recommend screening high-risk groups, whereas the HIV Medicine Association of the Infectious Disease Society of America does recommend screening in the HIV+ MSM population[65].
Screening in the inflammatory bowel disease population
Another patient population that could conceivably benefit from AIN screening includes individuals diagnosed with inflammatory bowel disease (IBD), particularly those taking potent immunosuppressive medications for their disease, given the generally accepted mechanism of chronic immunosuppression as a risk factor for anal cancer[34,66]. Research describing the risk of AIN in patients with IBD is limited; most of the literature is confined to case reports and case series. In one study, Ruel et al[67] performed a single-center retrospective review to assess rates of HPV infection and AIN in their cohort of IBD patients. They reported six cases of anal SCC, nine cases of HSIL and two cases of LSIL among their population, and demonstrated concomitant infection with HPV in 50% of SCC cases and all of the HSIL/LSIL cases. Of the six cases of SCC, one patient was on immunomodulatory therapy, defined only as “azathioprine/6-MP/methotrexate”, two were not taking any immunosuppressive medications, and the immunosuppressive medications of the other three was reported as “unknown”. Although this study suggests that individuals with IBD can develop AIN, the report is limited by the fact that the size of the IBD population was not noted, thus the prevalence rates of AIN cannot be determined.
Additional studies have suggested increased rates of HPV infection in IBD patients, particularly in those individuals who were taking immunosuppressive medications[68]. In an attempt to determine the prevalence of AIN in IBD patients, Shah et al[69] performed a cross-sectional study to determine the prevalence of AIN in 270 patients, including 100 with IBD on immunosuppression, 94 with IBD not on immunosuppression, and 76 healthy controls. This group found a non-statistically significant trend towards a higher prevalence of ASCUS in individuals with IBD regardless of immunosuppressive status compared to healthy controls (P = 0.10), but no difference in IBD patients on vs off immunosuppressive medications (P = 0.90). No patients in this study were diagnosed with LSIL, HSIL or SCC, which would suggest there is no increased risk of these conditions, at least in this relatively small population of IBD patients. Based on the lack of convincing data available, experts do not advocate screening patients with IBD for AIN on a routine basis, but it would be reasonable to consider HPV vaccination in this group.
MANAGEMENT OF AIN
Because AIN and early anal cancer remain relatively rare conditions and require a level of expertise to diagnose and treat, it is recommended that individuals found to have positive anal cytology be referred to expert centers for HRA as well as any associated treatment. Additionally, because AIN can be misdiagnosed, and can progress to cancer in some yet regress in others, expert centers are best equipped to determine which individuals should be treated and what treatment modalities should be considered, particularly as randomized trials comparing treatment modalities are lacking[70].
Topical therapy
Topical therapy consists of direct application of a medication to either a specific lesion or to the entire anal canal. Medications, which can be applied in some cases by the patient, include trichloroacetic acid (TCA), 5-flurouracil, or the immune modulator imiquimod. TCA is generally well tolerated, can be applied relatively simply without requiring specialized equipment, and is efficacious, with only 1-2 treatments necessary for most patients. TCA can be reapplied during further courses of treatment if necessary. Two retrospective studies of small populations of biopsy-confirmed high-grade AIN lesions reported rates of HSIL regressing to LSIL or complete resolution in 71%-79% of cases[71,72]. In a separate prospective pilot study, 46 patients with AIN were treated with 5-flurouracil, with 39% having complete clearance, 17% experiencing a partial response, and 37% having no response. However, 50% of the complete responders had recurrence of AIN at 6 mo[73].
Imiquimod therapy was evaluated in a double-blind, randomized controlled trial of 53 HIV+ MSM, with 28 patients on active drug and 25 patients on placebo. Of the 28 individuals on active drug therapy, 43% experienced either resolution or downgrading of their lesion, and 61% of imiquimod responders achieved sustained response at 36 mo[74]. Taken together, topical therapy appears to be generally well tolerated and has reasonable efficacy, although a substantial portion of patients will not respond and others will recur. For these reasons, topical therapy may best be utilized as an adjunct to local ablative therapy.
Local ablative therapy
Local ablative therapy consists of targeted destructive therapy, most commonly radiofrequency ablation (RFA) or electrocautery, applied to anal lesions during an HRA examination. Electrocautery therapy has been studied more extensively than RFA. Chang et al[75] reported a somewhat favorable response to electrocautery therapy for high grade AIN, with 0% (n = 0/8) of HIV negative patients having recurrence of disease, although 79% (n = 23/29) of HIV positive patients experienced disease recurrence during a two-year follow up period. A more recent observational study of 83 HIV+ MSM with high grade AIN treated with electrocautery found that 32.5% (n = 27/83) experienced complete response, 33.7% (n = 28/83) partial response, and 33.7% (n = 28/83) no response. Increased success was seen in those patients treated with two to four sessions compared to those treated with only one session. Similar to prior studies, the authors noted a recurrence rate of 25% in the responders after a median of 30 mo[76].
RFA applied to the anal mucosa to treat AIN has been shown to be safe and tolerable, but evidence of treatment efficacy is limited at this time[77]. One retrospective clinical study of n = 74 biopsy-proven high-grade AIN lesions in a population of 68 HIV+ MSM treated with RFA found 64% (n = 47/74) had resolution or downgrading to low-grade AIN at 140 d follow up[78]. Similar results were demonstrated by Goldstone et al[79] who evaluated long term follow up of high grade AIN lesions treated by RFA in HIV- and HIV+ MSM. They reported 62% of HIV- MSM had recurrence during a mean time of 14 mo, while disease recurred in 91% of HIV+ MSM during a mean of follow up of 17 mo. Both electrocautery and RFA therapy are associated with minimal morbidity, particularly with a greater number of treatment sessions, but both are reasonably well tolerated[80].
Surgical treatment
With the wide availability of the local and targeted therapies discussed above, surgical therapy is largely historic in regards to AIN, although still a mainstay of therapy for anal cancer, which is beyond the scope of this review. Surgery for AIN is associated with significant morbidity, often requiring large excision of healthy tissue, occasionally necessitating rectal diversion, and yet is associated with a recurrence rate of 9%-63%[80,81]. As recurrence of AIN is presumably mediated by ongoing exposure to predisposing risk factors, notably ongoing HPV infection, and given the improvement in local therapy outcomes, surgical excision for AIN is not recommended as a routine treatment option.
Surveillance of AIN
As discussed above, the recurrence rates of AIN are significant, particularly with high grade AIN, thus post treatment surveillance is essential. However, surveillance programs vary, due to uncertainties regarding the most appropriate surveillance interval as well as the best surveillance modality (i.e., cytology alone, routine HRA, etc.). To investigate the potential role of surveillance, Crawshaw et al[82] retrospectively reviewed 424 patients with biopsy proven AIN who were treated with topical or ablative therapy and then enrolled in a surveillance program. All patients underwent annual anal cytology and DRE, while 220 also received serial HRA examinations (the remaining 204 only underwent HRA if cytology or DRE were positive.) Overall, the five-year anal cancer rate was 6% for the expectant management group and 4.5% for the group also undergoing serial HRA, which was not significantly different (P = 0.37). However, it is difficult to form conclusions based on differences in surveillance modalities because progression was rare overall: Only two patients in the expectant management group and one in the HRA group developed anal cancer, and all three of these patients were considered to be non-compliant with the recommended surveillance plan[82]. The authors of this study proposed that for highly compliant patients, active surveillance of AIN is effective regardless of the method, and that compliance with the recommended program is the most important factor in reducing progression or recurrence of AIN.
Given the limited data regarding the benefits of AIN surveillance, further research, ideally prospective randomized trials, is needed. Currently, an active clinical study called the Anal Cancer HSIL Outcomes Research (ANCHOR) trial is enrolling patients across 12 United States sites. This trial aims to determine whether screening and treatment of HSIL is effective in reducing subsequent anal cancer in HIV+ men and women compared with active monitoring via regular exams (including anal cytology combined with HRA and biopsy of any concerning lesions) vs observation. Treatments can include imiquimod, fluorouracil, electrocautery, and laser therapy, and should provide further insight into the safety of these treatments[83].
CONCLUSIONS AND FUTURE DIRECTIONS
Anal intraepithelial neoplasia is the precursor lesion to squamous cell cancer of the anus. Although it is accepted that AIN progresses to SCC in a subset of patients, the actual risk of progression remains unclear. It has been shown, however, that the progression risk is elevated in certain high-risk groups, including: (1) those with persistent infection with high-risk HPV strains; (2) HIV-positive individuals, especially those with low CD4 counts; (3) MSM; and (4) individuals with a history of HPV-mediated genital cancers (particularly cervical cancer). Individuals in these groups will likely benefit from enrollment into formal AIN screening programs.
AIN can be challenging to diagnose and manage, thus referral to expert centers with the capability of interpreting cytology and pathology, performing HRA, and treating AIN is essential. The optimal treatment modalities and intervals have not been conclusively determined at this time, and recommendations are primarily based on expert consensus and driven by local expertise. Regardless of treatment modality, the recurrence rates of high-grade AIN remain high, and ongoing surveillance is recommended in patients with history of AIN.
Novel imaging technologies may identify high risk lesions without the need for tissue biopsy. Confocal laser microscopy, which has been shown to be at least as effective as tissue biopsy for detection of superficial esophageal squamous cell cancer, might be effective for the detection and grading of AIN[84-86]. Further studies are necessary to define the role and efficacy of confocal laser microscopy in AIN management.
In summary, AIN is a clinically important lesion that is frequently underappreciated by many clinicians. Although anal cancer remains relatively uncommon, the incidence of this malignancy is increasing. With the availability of effective HPV vaccines, it is important for clinicians to be aware of AIN, particularly in high-risk groups who might benefit most from vaccination.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts of interest for this article.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 11, 2016
First decision: September 12, 2016
Article in press: November 29, 2016
P- Reviewer: Gaudet M, Nathan M, Sunesen KG S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.SEER Cancer Statistics Factsheets: Anal Cancer. National Cancer Institute. Available from: http//seer.cancer.gov/statfacts/html/anus.html.
- 2.SEER Cancer Statistics Factsheets: Colon and Rectum. National Cancer Institute. Available from: http//seer.cancer.gov/statfacts/html/colorect.html.
- 3. Available from: http//www.cancer.org/cancer/analcancer/detailedguide/anal-cancer-what-is-anal-cancer.
- 4.Abbas A, Yang G, Fakih M. Management of anal cancer in 2010. Part 1: Overview, screening, and diagnosis. Oncology (Williston Park) 2010;24:364–369. [PubMed] [Google Scholar]
- 5.Frisch M. On the etiology of anal squamous carcinoma. Dan Med Bull. 2002;49:194–209. [PubMed] [Google Scholar]
- 6.Fenger C, Nielsen VT. Intraepithelial neoplasia in the anal canal. The appearance and relation to genital neoplasia. Acta Pathol Microbiol Immunol Scand A. 1986;94:343–349. doi: 10.1111/j.1699-0463.1986.tb03003.x. [DOI] [PubMed] [Google Scholar]
- 7.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 8.Carter PS, Sheffield JP, Shepherd N, Melcher DH, Jenkins D, Ewings P, Talbot I, Northover JM. Interobserver variation in the reporting of the histopathological grading of anal intraepithelial neoplasia. J Clin Pathol. 1994;47:1032–1034. doi: 10.1136/jcp.47.11.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 10.Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, Buchbinder S, Colfax G, Da Costa M, Darragh T, Benet DJ, Judson F, et al. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study. J Natl Cancer Inst. 2005;97:896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 11.Schofield AM, Sadler L, Nelson L, Gittins M, Desai M, Sargent A, McMahon RF, Hill J, Crosbie EJ, Patnick J, et al. A prospective study of anal cancer screening in HIV-positive and negative MSM. AIDS. 2016;30:1375–1383. doi: 10.1097/QAD.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 12.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, Hillman RJ, Petoumenos K, Roberts J, Tabrizi SN, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 13.Holly EA, Ralston ML, Darragh TM, Greenblatt RM, Jay N, Palefsky JM. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–849. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 14.Gandra S, Azar A, Wessolossky M. Anal high-risk human papillomavirus infection and high-grade anal intraepithelial neoplasia detected in women and heterosexual men infected with human immunodeficiency virus. HIV AIDS (Auckl) 2015;7:29–34. doi: 10.2147/HIV.S73880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman HB, Saah AJ, Sherman ME, Busseniers AE, Blackwelder WC, Kaslow RA, Ghaffari AM, Daniel RW, Shah KV. Human papillomavirus, anal squamous intraepithelial lesions, and human immunodeficiency virus in a cohort of gay men. J Infect Dis. 1998;178:45–52. doi: 10.1086/515608. [DOI] [PubMed] [Google Scholar]
- 16.Sendagorta E, Herranz P, Guadalajara H, Bernardino JI, Viguer JM, Beato MJ, García-Olmo D, Peña JM. Prevalence of abnormal anal cytology and high-grade squamous intraepithelial lesions among a cohort of HIV-infected men who have sex with men. Dis Colon Rectum. 2014;57:475–481. doi: 10.1097/DCR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, Kirk GD, D’Souza G, Bosch RJ, Brooks JT, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor S, Bunge E, Bakker M, Castellsagué X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis. 2016;16:293. doi: 10.1186/s12879-016-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 20.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, Markowitz LE. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011;204:566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 21.Palefsky J. Human papillomavirus infection in HIV-infected persons. Top HIV Med. 2007;15:130–133. [PubMed] [Google Scholar]
- 22.Shah KV. Human papillomaviruses and anogenital cancers. N Engl J Med. 1997;337:1386–1388. doi: 10.1056/NEJM199711063371911. [DOI] [PubMed] [Google Scholar]
- 23.Abramowitz L, Jacquard AC, Jaroud F, Haesebaert J, Siproudhis L, Pradat P, Aynaud O, Leocmach Y, Soubeyrand B, Dachez R, et al. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer. 2011;129:433–439. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 24.Palefsky JM, Holly EA. Molecular virology and epidemiology of human papillomavirus and cervical cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:415–428. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. HPV-associated cancers statistics: HPV and cancer (accessed 2015 Jun 30) Available from: http// www.cdc.gov/cancer/hpv/statistics/cases.htm.
- 26.Palefsky JM. Anal human papillomavirus infection and anal cancer in HIV-positive individuals: an emerging problem. AIDS. 1994;8:283–295. doi: 10.1097/00002030-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12:495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Palefsky JM, Holly EA, Ralston ML, Arthur SP, Hogeboom CJ, Darragh TM. Anal cytological abnormalities and anal HPV infection in men with Centers for Disease Control group IV HIV disease. Genitourin Med. 1997;73:174–180. doi: 10.1136/sti.73.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slama J, Sehnal B, Dusek L, Zima T, Cibula D. Impact of risk factors on prevalence of anal HPV infection in women with simultaneous cervical lesion. Neoplasma. 2015;62:308–314. doi: 10.4149/neo_2015_037. [DOI] [PubMed] [Google Scholar]
- 30.Gautier M, Brochard C, Lion A, Henno S, Mallet AL, Bodere A, Bouguen G, Lièvre A, Siproudhis L. High-grade anal intraepithelial neoplasia: Progression to invasive cancer is not a certainty. Dig Liver Dis. 2016;48:806–811. doi: 10.1016/j.dld.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Robison K, Cronin B, Bregar A, Luis C, DiSilvestro P, Schechter S, Pisharodi L, Raker C, Clark M. Anal Cytology and Human Papillomavirus Genotyping in Women With a History of Lower Genital Tract Neoplasia Compared With Low-Risk Women. Obstet Gynecol. 2015;126:1294–1300. doi: 10.1097/AOG.0000000000001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogunbiyi OA, Scholefield JH, Raftery AT, Smith JH, Duffy S, Sharp F, Rogers K. Prevalence of anal human papillomavirus infection and intraepithelial neoplasia in renal allograft recipients. Br J Surg. 1994;81:365–367. doi: 10.1002/bjs.1800810313. [DOI] [PubMed] [Google Scholar]
- 33.Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg. 2006;76:715–717. doi: 10.1111/j.1445-2197.2006.03837.x. [DOI] [PubMed] [Google Scholar]
- 34.Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg. 2005;92:1133–1136. doi: 10.1002/bjs.4994. [DOI] [PubMed] [Google Scholar]
- 35.Tong WW, Jin F, McHugh LC, Maher T, Sinclair B, Grulich AE, Hillman RJ, Carr A. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS. 2013;27:2233–2243. doi: 10.1097/QAD.0b013e3283633111. [DOI] [PubMed] [Google Scholar]
- 36.Burgos J, Curran A, Tallada N, Guelar A, Navarro J, Landolfi S, Villar J, Crespo M, Ribera E, Falcó V. Risk of progression to high-grade anal intraepithelial neoplasia in HIV-infected MSM. AIDS. 2015;29:695–702. doi: 10.1097/QAD.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 37.Berry JM, Jay N, Cranston RD, Darragh TM, Holly EA, Welton ML, Palefsky JM. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer. 2014;134:1147–1155. doi: 10.1002/ijc.28431. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol. 2010;116:177–185. doi: 10.1097/AOG.0b013e3181e4629f. [DOI] [PubMed] [Google Scholar]
- 39.Saleem AM, Paulus JK, Shapter AP, Baxter NN, Roberts PL, Ricciardi R. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol. 2011;117:643–649. doi: 10.1097/AOG.0b013e31820bfb16. [DOI] [PubMed] [Google Scholar]
- 40.Woods R, O’Regan EM, Kennedy S, Martin C, O’Leary JJ, Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J Clin Cases. 2014;2:172–193. doi: 10.12998/wjcc.v2.i6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 42.Ruttkay-Nedecky B, Jimenez Jimenez AM, Nejdl L, Chudobova D, Gumulec J, Masarik M, Adam V, Kizek R. Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review) Int J Oncol. 2013;43:1754–1762. doi: 10.3892/ijo.2013.2105. [DOI] [PubMed] [Google Scholar]
- 43.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 44.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–898. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- 45.Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32:6941–6947. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 47.Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 48.van Oortmarssen GJ, Habbema JD. Duration of preclinical cervical cancer and reduction in incidence of invasive cancer following negative pap smears. Int J Epidemiol. 1995;24:300–307. doi: 10.1093/ije/24.2.300. [DOI] [PubMed] [Google Scholar]
- 49. Available from: http//seer.cancer. gov/csr/1975_2009_pops09.
- 50.American Cancer Society. [accessed 2016 Jun 15] Available from: http//www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations.
- 51.Schubert MC, Sridhar S, Schade RR, Wexner SD. What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol. 2009;15:3201–3209. doi: 10.3748/wjg.15.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, Darragh TM. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:415–422. doi: 10.1097/00042560-199704150-00004. [DOI] [PubMed] [Google Scholar]
- 53.Nathan M, Singh N, Garrett N, Hickey N, Prevost T, Sheaff M. Performance of anal cytology in a clinical setting when measured against histology and high-resolution anoscopy findings. AIDS. 2010;24:373–379. doi: 10.1097/QAD.0b013e328333ab8e. [DOI] [PubMed] [Google Scholar]
- 54.Panther LA, Wagner K, Proper J, Fugelso DK, Chatis PA, Weeden W, Nasser IA, Doweiko JP, Dezube BJ. High resolution anoscopy findings for men who have sex with men: inaccuracy of anal cytology as a predictor of histologic high-grade anal intraepithelial neoplasia and the impact of HIV serostatus. Clin Infect Dis. 2004;38:1490–1492. doi: 10.1086/383574. [DOI] [PubMed] [Google Scholar]
- 55.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Cuzick J. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208:184.e1–184.e11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 56.Padilla-España L, Repiso-Jiménez JB, Fernández-Sánchez F, Pereda T, Rivas-Ruiz F, Fernández-Morano T, de la Torre-Lima J, Palma F, Redondo M, de Troya-Martín M. Effectiveness of human papillomavirus genotyping for detection of high-grade anal intraepithelial neoplasia compared to anal cytology. Enferm Infecc Microbiol Clin. 2016;34:400–405. doi: 10.1016/j.eimc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Palefsky JM. Practising high-resolution anoscopy. Sex Health. 2012;9:580–586. doi: 10.1071/SH12045. [DOI] [PubMed] [Google Scholar]
- 58.Richel O, Hallensleben ND, Kreuter A, van Noesel CJ, Prins JM, de Vries HJ. High-resolution anoscopy: clinical features of anal intraepithelial neoplasia in HIV-positive men. Dis Colon Rectum. 2013;56:1237–1242. doi: 10.1097/DCR.0b013e3182a53568. [DOI] [PubMed] [Google Scholar]
- 59.Jay N, Berry JM, Hogeboom CJ, Holly EA, Darragh TM, Palefsky JM. Colposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathology. Dis Colon Rectum. 1997;40:919–928. doi: 10.1007/BF02051199. [DOI] [PubMed] [Google Scholar]
- 60.Camus M, Lesage AC, Fléjou JF, Hoyeau N, Atienza P, Etienney I. Which lesions should be biopsied during high-resolution anoscopy? Prospective descriptive study of simple morphological criteria. J Low Genit Tract Dis. 2015;19:156–160. doi: 10.1097/LGT.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 61.Albuquerque A. High-resolution anoscopy: Unchartered territory for gastroenterologists? World J Gastrointest Endosc. 2015;7:1083–1087. doi: 10.4253/wjge.v7.i13.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 63.Sunesen KG, Nørgaard M, Thorlacius-Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer. 2010;127:675–684. doi: 10.1002/ijc.25080. [DOI] [PubMed] [Google Scholar]
- 64.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 65.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg. 2016;8:41–51. doi: 10.4240/wjgs.v8.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13:3202–3209. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruel J, Ko HM, Roda G, Patil N, Zhang D, Jharap B, Harpaz N, Colombel JF. Anal Neoplasia in Inflammatory Bowel Disease Is Associated With HPV and Perianal Disease. Clin Transl Gastroenterol. 2016;7:e148. doi: 10.1038/ctg.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seksik P, Cosnes J, Sokol H, Nion-Larmurier I, Gendre JP, Beaugerie L. Incidence of benign upper respiratory tract infections, HSV and HPV cutaneous infections in inflammatory bowel disease patients treated with azathioprine. Aliment Pharmacol Ther. 2009;29:1106–1113. doi: 10.1111/j.1365-2036.2009.03973.x. [DOI] [PubMed] [Google Scholar]
- 69.Shah SB, Pickham D, Araya H, Kamal A, Pineda CE, Ghole S, Shih L, Kong C, Pai R, Welton M. Prevalence of Anal Dysplasia in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2015;13:1955–61.e1. doi: 10.1016/j.cgh.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Macaya A, Muñoz-Santos C, Balaguer A, Barberà MJ. Interventions for anal canal intraepithelial neoplasia. Cochrane Database Syst Rev. 2012;12:CD009244. doi: 10.1002/14651858.CD009244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cranston RD, Baker JR, Liu Y, Wang L, Elishaev E, Ho KS. Topical application of trichloroacetic acid is efficacious for the treatment of internal anal high-grade squamous intraepithelial lesions in HIV-positive men. Sex Transm Dis. 2014;41:420–426. doi: 10.1097/OLQ.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 72.Singh JC, Kuohung V, Palefsky JM. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. J Acquir Immune Defic Syndr. 2009;52:474–479. doi: 10.1097/QAI.0b013e3181bc0f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richel O, Wieland U, de Vries HJ, Brockmeyer NH, van Noesel C, Potthoff A, Prins JM, Kreuter A. Topical 5-fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus-positive men. Br J Dermatol. 2010;163:1301–1307. doi: 10.1111/j.1365-2133.2010.09982.x. [DOI] [PubMed] [Google Scholar]
- 74.Fox PA, Nathan M, Francis N, Singh N, Weir J, Dixon G, Barton SE, Bower M. A double-blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high-grade anal intraepithelial neoplasia in HIV-positive MSM on HAART, with long-term follow-up data including the use of open-label imiquimod. AIDS. 2010;24:2331–2335. doi: 10.1097/QAD.0b013e32833d466c. [DOI] [PubMed] [Google Scholar]
- 75.Chang GJ, Berry JM, Jay N, Palefsky JM, Welton ML. Surgical treatment of high-grade anal squamous intraepithelial lesions: a prospective study. Dis Colon Rectum. 2002;45:453–458. doi: 10.1007/s10350-004-6219-8. [DOI] [PubMed] [Google Scholar]
- 76.Burgos J, Curran A, Landolfi S, Navarro J, Tallada N, Guelar A, Crespo M, Ocaña I, Ribera E, Falcó V. The effectiveness of electrocautery ablation for the treatment of high-grade anal intraepithelial neoplasia in HIV-infected men who have sex with men. HIV Med. 2016;17:524–531. doi: 10.1111/hiv.12352. [DOI] [PubMed] [Google Scholar]
- 77.Smulian AG, Moore DM, Robertson JC, Kralovic SM. Phase I study demonstrates safety and tolerability of radiofrequency ablation (RFA) of the anal mucosa. HIV Clin Trials. 2014;15:36–44. doi: 10.1310/hct1501-36. [DOI] [PubMed] [Google Scholar]
- 78.Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with men. Int J STD AIDS. 2008;19:118–120. doi: 10.1258/ijsa.2007.005665. [DOI] [PubMed] [Google Scholar]
- 79.Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum. 2011;54:1284–1292. doi: 10.1097/DCR.0b013e318227833e. [DOI] [PubMed] [Google Scholar]
- 80.Long KC, Menon R, Bastawrous A, Billingham R. Screening, Surveillance, and Treatment of Anal Intraepithelial Neoplasia. Clin Colon Rectal Surg. 2016;29:57–64. doi: 10.1055/s-0035-1570394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marchesa P, Fazio VW, Oliart S, Goldblum JR, Lavery IC. Perianal Bowen’s disease: a clinicopathologic study of 47 patients. Dis Colon Rectum. 1997;40:1286–1293. doi: 10.1007/BF02050810. [DOI] [PubMed] [Google Scholar]
- 82.Crawshaw BP, Russ AJ, Stein SL, Reynolds HL, Marderstein EL, Delaney CP, Champagne BJ. High-resolution anoscopy or expectant management for anal intraepithelial neoplasia for the prevention of anal cancer: is there really a difference? Dis Colon Rectum. 2015;58:53–59. doi: 10.1097/DCR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 83.SEER Cancer Statistics Factsheets: Anal Cancer. National Cancer Institute. Bethesda, MD. Available from: http//anchorstudy.org/about.
- 84.Huang J, Yang YS, Lu ZS, Wang SF, Yang J, Yuan J. Detection of superficial esophageal squamous cell neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. World J Gastroenterol. 2015;21:6974–6981. doi: 10.3748/wjg.v21.i22.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siekas LL, Aboulafia DM. Establishing an anal dysplasia clinic for HIV-infected men: initial experience. AIDS Read. 2009;19:178–186. [PubMed] [Google Scholar]
- 86.Palefsky JM, Rubin M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am. 2009;36:187–200. doi: 10.1016/j.ogc.2009.02.003. [DOI] [PubMed] [Google Scholar]


