Abstract
To investigate the neural mechanisms underlying attention deficits that are related to neoadjuvant chemotherapy in combination with cerebral perfusion. Thirty one patients with breast cancer who were scheduled to receive neoadjuvant chemotherapy and 34 healthy control subjects were included. The patients completed two assessments of the attention network tasks (ANT), neuropsychological background tests, and the arterial spin labeling scan, which were performed before neoadjuvant chemotherapy and after completing chemotherapy. After neoadjuvant chemotherapy, the patients exhibited reduced performance in the alerting and executive control attention networks but not the orienting network (p < 0.05) and showed significant increases in cerebral blood flow (CBF) in the left posterior cingulate gyrus, left middle occipital gyrus, bilateral precentral gyrus, inferior parietal gyrus, supramarginal gyrus, angular gyrus, precuneus, cuneus, superior occipital gyrus, calcarine cortex, and temporal gyrus (p < 0.01 corrected) when compared with patients before chemotherapy and healthy controls. A significant correlation was found between the decrease performance of ANT and the increase in CBF changes in some brain regions of the patients with breast cancer. The results demonstrated that neoadjuvant chemotherapy influences hemodynamic activity in different brain areas through increasing cerebral perfusion, which reduces the attention abilities in breast cancer patients.
An increasing body of evidence demonstrates that breast cancer patients suffer from many cognitive impairments related to adjuvant chemotherapy, including problems with verbal and/or visuospatial memory, attention deficits, difficulty in learning, confused thought processes1,2, and impairments in executive functions; these difficulties are commonly referred to as “chemobrain”3. Such cognitive impairment could have a significant effect on the patient’s quality of life, and thus, patients, physicians, and researchers should be more considerate of this possibility.
As a major component of the cognitive system, the attention work is involved in the centralization of brain or mental activities and the allocation of psychological resources4. Posner and Petersen proposed the attention network theory, which divides the attention systems into three distinct brain networks, that is, the alerting, orienting, and executive networks5. We recently reported findings from a cross-sectional neuropsychological study that the chemotherapy-treated breast cancer patients had significant impairment of attention networks6. Moreover, neuroimaging studies have identified the structural and functional changes that are associated with chemotherapy, including changes in gray and white matter7,8,9 and cerebral activation during performance of cognitive tasks10,11. Although previous reviews and our findings have confirmed this chemotherapy-induced cognitive impairment, the neural mechanisms underlying these deficits are still unclear.
In recent years, arterial spin labeling (ASL) has become an important noninvasive functional magnetic resonance imaging technique that uses magnetically labeled arterial blood water as an endogenous contrast agent to measure resting CBF images. This method has been widely applied to assess the cerebral perfusion in various clinical conditions, including neurodegenerative diseases12, age-related cognitive impairments13 and psychiatric disorders14. As such, ASL is a promising technique to assess whether therapy-induced cerebral perfusion changes could explain the cognitive impairments in patients with breast cancer after chemotherapy. To our knowledge, only one ASL study investigated chemotherapy-induced cerebral perfusion changes15. This study found that chemotherapy-treated patients had significantly increased post-treatment perfusion in the right precentral gyrus, which was correlated with the baseline of overall neuropsychological performance. However, in this study, some patients with breast cancer had received surgery and anti-estrogen therapy and were treated with different chemotherapeutic agents for systemic chemotherapy. Previous studies have shown that anti-estrogen therapy may impair cognitive function and may induce cerebral blood flow and metabolism alteration16,17. Different cognitive dysfunctions were also observed in patients who were treated with a variety of chemotherapy regimens18. Additionally, drug anesthesia of surgery can induce cognitive dysfunctions19,20 and cerebral perfusion change21. Therefore, it is necessary to exclude these confounding effects to determine whether the changes in cerebral perfusion and cognitive function are the result of the chemotherapy itself.
Due to the cross-sectional design used in our previous study, attention was not measured in the patients before their chemotherapy treatment. In the current study, patients with breast cancer were used to assess the potential changes in attention function before and after exposure to neoadjuvant chemotherapy in which the term “neoadjuvant” refers to the chemotherapy given before surgery, radiation, and anti-estrogen treatment, which offers advantages in terms of adding prognostic information and improving surgical options. Moreover, we used ASL to measure longitudinal differences in cerebral perfusion in these patients. Based on findings from the literature and our previous study, we hypothesize that after neoadjuvant chemotherapy, breast cancer patients have impaired performance in attention and changes in cerebral perfusion compared to themselves before neoadjuvant chemotherapy and healthy control participants. We also examined the relationships between the cerebral perfusion alterations and attention function changes that were observed in these patients.
Results
A total of 68 participants meeting the criteria were initially enrolled in the study. One patient did not complete the follow-up assessment. Two additional participants (one patient and one healthy control) were excluded from the analysis because of excessive head motion artifact during data acquisition. Therefore, the final analytical sample size was 31 patients and 34 healthy controls.
Participant demographics
Participant demographic and clinical information is shown in Table 1. All participants were premenopausal women between 28 and 52 years old. At the beginning of the chemotherapy treatment, the patients did not differ from the healthy controls with regard to age, education, depression, and fatigue score, with the exception of the anxiety score of the Hamilton Anxiety Rating Scale (HAMA). The pre-treatment patients had higher scores than the healthy controls, but these scores were below the cut-off value (HAMA scores <7). After receiving chemotherapy treatment, the patients had higher Cancer Related Fatigue (CRF) scores, but they did not have fatigue symptoms, and these scores were clearly below the cut-off value.
Table 1. Demographic characteristics and summary of neuropsychologic test.
| Healthy Controls (n = 34) | Pre-treatment (n = 31) | Post-treatment (n = 31) | P-values |
||
|---|---|---|---|---|---|
| Mean(SD) | Mean(SD) | Mean(SD) | Pre vs. Posta | HC vs. Postb | |
| Age at Baseline (years) | 46.29 (4.09) | 47.10 (4.53) | NA | 0.456 | NA |
| Education (years) | 11.21 (2.09) | 10.65 (2.36) | NA | 0.313 | NA |
| Breast cancer stage | |||||
| II | NA | 10 | NA | NA | NA |
| III | NA | 17 | NA | NA | NA |
| Inter-scan interval (days) | NA | NA | 166.61 (4.46) | NA | NA |
| Fatigue | 19.82 (3.87) | 21.19 (3.02) | 24.71 (3.12) | <0.001* | <0.001* |
| HAMA | 3.97 (1.22) | 5.19 (1.05) | 4.23 (0.96) | 0.003 | 0.354 |
| HAMD | 4.38 (1.28) | 4.94 (0.93) | 4.52 (1.24) | 0.114 | 0.670 |
| MoCA | 25.50 (1.93) | 24.74 (2.22) | 25.39 (2.33) | 0.299 | 0.832 |
| Attention/concentration | |||||
| Digit Span (forward) | 6.12 (1.27) | 5.74 (1.24) | 5.61 (1.02) | 0.670 | 0.085 |
| Digit Span (backward) | 5.24 (1.10) | 4.87 (1.09) | 4.16 (0.86) | 0.010 | <0.001* |
| Stroop Color test (sec) | 14.43 (4.17) | 15.16 (3.25) | 15.11 (3.45) | 0.955 | 0.483 |
| Trailmaking A (sec) | 52.88 (9.22) | 53.63 (9.51) | 56.64 (8.14) | 0.175 | 0.087 |
| Memory (AVLT) | |||||
| Immediate Recall | 11.44 (1.76) | 11.13 (1.73) | 10.90 (1.51) | 0.607 | 0.193 |
| Delayed Recall | 10.56 (1.93) | 10.23 (1.93) | 8.84 (1.61) | 0.013 | <0.001* |
| Recognition | 9.62 (2.16) | 9.58 (1.84) | 7.87 (1.91) | 0.005 | 0.001* |
| Executive function | |||||
| Trailmaking B (sec) | 97.02 (9.85) | 96.68 (9.15) | 102.38 (10.36) | 0.016 | 0.036 |
| Stroop Word test (sec) | 17.43 (2.47) | 18.38 (3.78) | 22.20 (6.19) | 0.010 | <0.001* |
| Stroop Interference test (sec) | 30.98 (8.38) | 31.98 (7.96) | 36.82 (9.44) | 0.022 | 0.010 |
| Verbal fluency | 13.15 (2.12) | 12.87 (1.46) | 12.26 (1.88) | 0.178 | 0.080 |
Abbreviations: SD, standard deviation; HC, Healthy Controls; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; MoCA, Montreal Cognitive Assessment Test; AVLT: Auditory Verbal Learning Test.
aPaired t-tests.
bIndependent two-sample t-tests.
*Indicates significance at FDR corrected for multiple comparisons.
Neuropsychological background tests
At baseline, there were no significant differences between the different cognitive domains of patients with breast cancer and healthy controls. Paired t-tests showed that post-treatment patients performed significantly worse in attention and concentration tests (WAIS Digit Span backward), memory (Delayed recall and Recognition test), and executive function (the Stroop Word, Stroop Interference test, Trail Making Test B, and Verbal fluency) when compared with pre-treatment patients (see Table 1). Compared with the healthy controls, the post-treatment patients also had significantly worse performance in the aforementioned cognitive domains.
The three networks of ANT
Compared with the healthy controls, the patients showed no significant differences in the three networks before treatment but had a significantly shorter mean altering network reaction time (RT) and a higher mean executive control network RT after treatment. Paired t-tests showed that the post-treatment patients had a significantly shorter mean altering network RT and a higher mean executive control network RT than that of the pre-treatment patients. Additionally, we found that the overall mean RTs of the post-treatment patients were significantly longer than that of the pre-treatment patients and healthy controls. For this task, to eliminate this influence of the overall mean RTs, ratios can be used to examine the specific effects of the three networks. For each subject, the median RT in each condition was divided by the subject’s overall RT. The network ratio scores of the breast cancer patients and healthy controls are presented in Table 2. There were significant differences in alerting and executive control effects but not in the orienting effect for the pre- and post-treatment patients. We also found a significant difference in the alerting effect between the post-treatment patients and the healthy controls. No significant differences were found in the ratio scores for the three networks between the pre-treatment patients and healthy controls.
Table 2. Attention network scores of breast cancer patients and healthy controls.
| Healthy Controls (n = 34) | Pre-treatment (n = 31) | Post-treatment (n = 31) | P-values |
||
|---|---|---|---|---|---|
| Mean RT(ms) (SD) | Mean RT(ms) (SD) | Mean RT(ms) (SD) | Pre vs. Posta | HC vs. Postb | |
| Alerting | 32.21 ± 16.97 | 31.19 ± 18.81 | 19.94 ± 18.83 | 0.038 | 0.007* |
| Ratio | 0.051 ± 0.028 | 0.049 ± 0.031 | 0.030 ± 0.029 | 0.025 | 0.005* |
| Orienting | 55.79 ± 22.30 | 62.68 ± 31.25 | 64.58 ± 27.64 | 0.701 | 0.162 |
| Ratio | 0.090 ± 0.040 | 0.097 ± 0.046 | 0.098 ± 0.041 | 0.981 | 0.441 |
| Executive | 94.18 ± 29.01 | 97.65 ± 30.08 | 110.29 ± 34.21 | 0.006 | 0.044 |
| Ratio | 0.150 ± 0.048 | 0.154 ± 0.051 | 0.168 ± 0.054 | 0.044 | 0.169 |
| Mean RT | 630.97 ± 60.31 | 643.81 ± 72.39 | 666.16 ± 72.81 | 0.002* | 0.037 |
| Accuracy (%) | 98.44 ± 1.26 | 98.58 ± 1.03 | 98.23 ± 1.23 | 0.062 | 0.489 |
Abbreviations: SD, standard deviation; HC, Healthy Controls; RT, reaction time.
aPaired t-tests.
bIndependent two-sample t-tests.
*Indicates significance at FDR corrected for multiple comparisons.
Chemotherapy effects on perfusion change
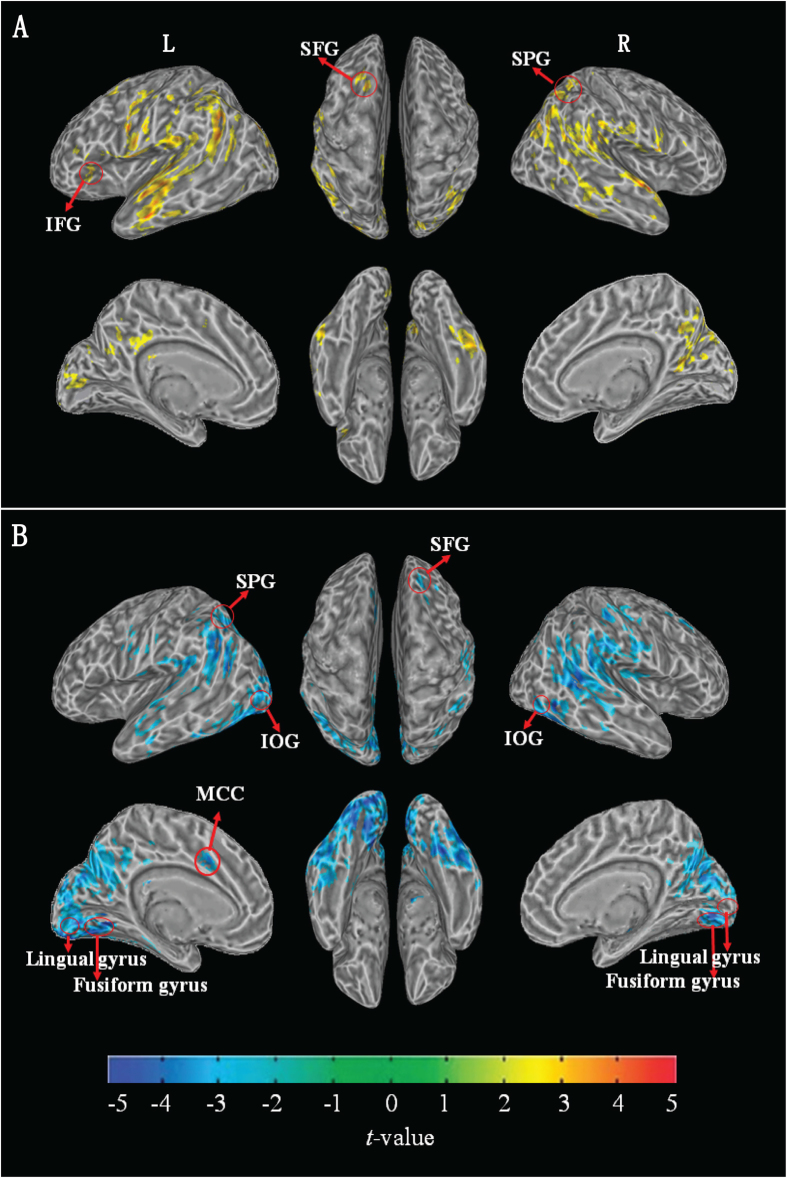
As shown in Table 3, the main effects of chemotherapy treatment were predominantly found in the left superior frontal gyrus, left posterior cingulate gyrus, left inferior frontal gyrus, right superior parietal gyrus, left middle occipital gyrus, bilateral inferior parietal gyrus, superior occipital gyrus, temporal gyrus, precentral gyrus, supramarginal gyrus, angular gyrus, precuneus, cuneus, and calcarine cortex (see Table S1 and Fig. 1A). The post-treatment patients exhibited an increase in CBF in all of the above brain regions. In the voxelwise independent two-sample t-test analysis, the post-treatment patients exhibited an increase in CBF in all of the above brain regions except for the left superior frontal gyrus, left inferior frontal gyrus, and right superior parietal gyrus. Additionally, increased CBF of the right superior frontal gyrus, left middle cingulate gyrus, left superior parietal gyrus, bilateral inferior occipital gyrus, lingual gyrus, and fusiform gyrus was observed in the post-treatment patients (see Table S2 and Fig. 1B) when compared with the healthy controls. No significant CBF differences were observed between the pre-treatment patients and healthy controls.
Table 3. Comparison of CBF values (ml/100 g / min) of corresponding parts for all participants.
| Region (CBF values) | Healthy Controls (n = 34) | Pre-treatment (n = 31) | Post-treatment (n = 31) |
P-values |
|
|---|---|---|---|---|---|
| Mean(SD) | Mean(SD) | Mean(SD) | Pre vs. Posta | HC vs. Postb | |
| Left middle cingulate gyrus | 61.90 (7.63) | NA | 54.35 (8.64) | NA | 0.015 |
| Left posterior cingulate gyrus | 65.39 (9.83) | 67.19 (9.40) | 74.79 (14.55) | 0.003 | 0.004 |
| Left superior frontal gyrus | NA | 47.70 (6.27) | 51.63 (7.73) | 0.006 | NA |
| Right superior frontal gyrus | 49.55 (6.43) | NA | 54.35 (8.64) | NA | 0.013 |
| Left inferior frontal gyrus | NA | 54.82 (7.11) | 59.47 (8.93) | 0.002* | NA |
| Left superior parietal gyrus | 45.60 (6.22) | NA | 50.25 (8.45) | NA | 0.013 |
| Right superior parietal gyrus | NA | 50.30 (7.68) | 54.20 (9.31) | 0.020 | NA |
| Inferior parietal gyrus | 51.26 (5.83) | 51.44 (7.46) | 57.33 (9.55) | 0.001* | 0.004 |
| Superior occipital gyrus | 45.05 (6.07) | 46.52 (7.71) | 51.35 (8.82) | 0.002* | 0.001* |
| Left middle occipital gyrus | 45.97 (4.73) | 47.53 (7.65) | 53.15 (9.32) | 0.003 | <0.001* |
| Inferior occipital gyrus | 49.18 (5.09) | NA | 56.02 (9.46) | NA | 0.001* |
| Superior temporal gyrus | 53.18 (5.23) | 52.76 (7.24) | 59.23 (8.08) | <0.001* | 0.001* |
| Middle temporal gyrus | 52.55 (5.47) | 52.28 (7.23) | 58.09 (8.77) | <0.001* | 0.004 |
| Inferior temporal gyrus | 50.47 (4.77) | 51.01 (7.28) | 56.57 (8.51) | 0.001* | 0.001* |
| Precentral gyrus | 50.71 (6.04) | 51.61 (6.20) | 55.93 (8.64) | 0.004 | 0.006 |
| Supramarginal gyrus | 52.45 (5.32) | 52.99 (7.67) | 58.62 (9.34) | <0.001* | 0.002* |
| Angular gyrus | 50.36 (5.36) | 50.51 (7.85) | 56.82 (9.93) | 0.002* | 0.002* |
| Precuneus | 57.24 (8.70) | 60.20 (8.88) | 64.75 (11.68) | 0.014 | 0.004 |
| Cuneus | 50.77 (7.96) | 53.49 (8.08) | 59.17 (10.25) | 0.003 | <0.001* |
| Calcarine cortex | 52.25 (7.16) | 54.37 (7.14) | 60.32 (9.91) | 0.002* | <0.001* |
| Lingual gyrus | 50.50 (6.54) | NA | 58.09 (8.38) | NA | <0.001* |
| Fusiform gyrus | 45.41 (4.66) | NA | 51.03 (7.24) | NA | 0.001* |
Abbreviations: CBF, cerebral blood flow; SD, standard deviation; HC, Healthy Controls; NA, not applicable.
aPaired t-tests.
bIndependent two-sample t-tests.
*Indicates significance at FDR corrected for multiple comparisons.
Figure 1.
(A) Demonstration of results of intragroup comparison between pre- and post-treatment patients. The results were corrected using FDR correction, with q < 0.01, p < 0.0027 and a minimum cluster size of 100. Patients with post-treatment have greater perfusion than patients with pre-treatment in yellow brain areas. Significant perfusion changes of intergroup comparison between healthy controls and post-treatment patients were not observed in brain regions with labeled red circle. (B) Demonstration of results of intergroup comparison between healthy controls and post-treatment patients. The results were corrected using FDR correction, with q < 0.01, p < 0.0046 and a minimum cluster size of 100. Healthy controls have lesser perfusion than post-treatment patients in blue brain areas. Significant perfusion changes of intergroup comparison between pre- and post-treatment patients were not observed in brain regions with labeled red circle. (IFG: inferior frontal gyrus, SFG: superior frontal gyrus, SPG: superior parietal gyrus, IOG: inferior occipital gyrus, MCC: middle cingulate gyrus, L: left, R: right).
The correlation between changes in perfusion and the attention networks
To identify the significance of the perfusion increase and the mechanism of attention deficits, we performed a correlation analysis between the perfusion change and attention performance. We calculated the change in CBF values (CBF at post-treatment minus CBF at pre-treatment) in the aforementioned significantly increased regions. Likewise, the post-treatment scores minus the pre-treatment scores of the attention networks were calculated to indicate changes in the attention networks. Significant correlations were observed between the changes in the alerting network score and the changed CBF values in the left posterior cingulate gyrus (r = −0.452; p = 0.014), bilateral inferior parietal gyrus (r = −0.550; p = 0.002), bilateral middle temporal gyrus (r = −0.381; p = 0.042), bilateral precuneus (r = −0.483; p = 0.008), and bilateral cuneus (r = −0.464; p = 0.011). Additionally, significant correlations were also found between the changes in the executive control network score and the changed CBF values in the left posterior cingulate gyrus (r = 0.680; p < 0.001), left superior frontal gyrus (r = 0.578; p = 0.001), left inferior frontal gyrus (r = 0.608; p < 0.001), bilateral inferior parietal gyrus (r = 0.507; p = 0.005), bilateral superior temporal gyrus (r = 0.548; p = 0.002), bilateral middle temporal gyrus (r = 0.601; p = 0.001), bilateral inferior temporal gyrus (r = 0.629; p < 0.001), bilateral precuneus (r = 0.654; p < 0.001), and bilateral cuneus (r = 0.567; p = 0.001).
Discussion
To the best of our knowledge, our study is the first to investigate potential neoadjuvant chemotherapy-induced changes in the attention network in combination with cerebral perfusion changes, as indicated using ASL measures. Our results demonstrate impairment of the alerting and executive control attention networks and an increase in perfusion in brain regions after chemotherapy in patients with breast cancer. We found that after chemotherapy, the patients exhibited reduced performance in the alerting and executive control attention networks, and showed a significant increase in CBF of brain regions as shown above, when compared with patients before chemotherapy and healthy controls. In contrast, these differences were not present in either the patients before chemotherapy or the healthy controls. In addition, the patients performed worse on neuropsychological background tests from baseline to post-treatment. Furthermore, we found that such a decrease in the performance of the ANT task was significantly correlated with an increase in the CBF changes in different brain regions in the patients with breast cancer. Taken together, these results demonstrate that the neoadjuvant chemotherapy-induced cerebral perfusion changes may account for the attention deficits of the patients with breast cancer.
Pre-operative systemic therapy with AC (doxorubicin/cyclophosphamide) followed by pre-operative T (docetaxel) is a commonly used regimen for the neoadjuvant chemotherapy treatment of breast cancer. The individual agents of this regimen could induce cognitive changes via several processes that influence brain structure and function, such as oxidative stress, DNA damage, altered hormone levels, and immune response deregulation22. Several previous studies have shown that docetaxel and doxorubicin have peripheral anti-angiogenic effects that are associated with vascular toxicity23,24. This anti-angiogenic effect may be due to a disruption in the balance of peripheral-released cytokines and chemicals, which also cross the blood brain barrier to cause the veins to expand, ultimately resulting in indirect cerebral effects. Therefore, it is possible that the neoadjuvant chemotherapy-induced cerebrovascular changes could transform the hemodynamic response. Previous studies showed that lower CBF was related to poorer cognitive function25,26. Cognitive function would be improved after cerebral perfusion improvement27. The increase in the CBF in brain regions would compensate for cognitive function. However, based on our aforementioned findings, it is likely that the increase in cerebral perfusion is an unsuccessful compensatory mechanism that leads to cognitive impairments, in particular, the impairment of attention networks in patients with breast cancer.
According to Posner’s dominant theory of attention network, which emphasizes the involvement of neural attention networks in alerting, orienting, and executive control networks. Clinical studies and neuroimaging evidence indicate that the alerting system is associated with the frontal and parietal cortices and thalamus and that prefrontal cortex has a central role in supporting the executive control network28,29. The results of the present study showed that after chemotherapy, the patients exhibited reduced performance in these two networks and significant increases in CBF in the left superior frontal gyrus, left inferior frontal gyrus, bilateral inferior parietal gyrus, and temporal gyrus when compared with patients before chemotherapy and/or healthy controls. This behavioral performance change in attention networks is consistent with our previous cross-sectional study findings. Functional MRI studies report that breast cancer patients prior to neoadjuvant chemotherapy treatment had increased activations during working memory tasks compared to controls in areas such as the frontal gyrus30,31, insula, thalamus31. A possible explanation for these findings is that before neoadjuvant treatment, breast cancer patients are engaging in compensatory hyperactivation of brain neural circuitry to support working memory function in response to effects of the cancer disease30. Interestingly, the similar pattern has been found in this study. As we have discussed above, breast cancer patients exhibited increases in CBF in different brain areas after chemotherapy. The patients continue to maintain this hyperperfusion, likely as a result of cognitive changes, because we observed decreased cognitive performance was associated with the increases in CBF. On the basis of the discussion above, it is plausible to presume that neoadjuvant chemotherapy affects brain functions and cognition through its over-compensatory cerebral hyperperfusion in breast cancer patients.
The frontal and parietal lobes play a more direct role in regulating the focus of attention function, as previously shown. Furthermore, the posterior cingulate gyrus, precuneus and temporal cortex play an important role in attentional processing through organizing corresponding brain networks, such as the attention network, frontoparietal network, and default mode network (DMN), to help guide attention tasks32. Hosseini et al. revealed that patients with chemotherapy showed an altered functional connectivity in the right frontoparietal and left superior frontal gyri networks33. Decreased connectivity was also observed after chemotherapy in regions of the dorsal attention network that are involved in attention tasks34. The posterior cingulate cortex (PCC) and precuneus are the main hubs of the DMN, which consists of the PCC, precuneus, medial frontal, middle temporal, and lateral parietal regions35, and a disruption in DMN connectivity has been observed in patients following chemotherapy, which was related to cognitive deficits34,36. Previous neuroimaging studies have demonstrated that breast cancer patients with chemotherapy had disturbed functional and structural networks, which reduced cognitive function. Our findings based on cerebral perfusion changes presented the existence of chemotherapy-induced attention deficits in the current study. As mentioned above, the ANT task is used to examine individual differences in the three attention networks. Thus, we hypothesized that the changes in brain networks for breast cancer patients receiving neoadjuvant chemotherapy could contribute to the attention network deficits and other cognitive impairments. Our data appear to support these hypotheses. The current study shows that after neoadjuvant chemotherapy, patients had significant increases in cerebral perfusion in the left PCC and the superior and inferior frontal, precuneus, and bilateral temporal and parietal regions compared to before chemotherapy, in addition to the right superior frontal regions when compared to healthy controls. Furthermore, the extent of regions with a segmental increase correlated with a decrease in the performance of the attention network. Interestingly, the brain regions that increased consisted of a brain network for attention function that particularly involved the DMN. Our findings suggest that neoadjuvant chemotherapy influences the hemodynamic activity in these brain networks through increasing cerebral perfusion, which reduced performance in attention networks in breast cancer patients.
Several limitations of the present study should be considered. First, the design of this study lacks the matched interval time for healthy controls to eliminate time factors that could affect brain structure and function. Previous studies show that changes in cognitive function, including those related to the ANT task, do not exist in short period intervals for healthy persons37,38. Furthermore, Nudelman et al. showed that the healthy controls showed no change in cerebral perfusion using the ASL when the inter-scan interval was approximately 160 days15. Therefore, it was appropriate to use similar controls for comparison to explore the effects of neoadjuvant chemotherapy on cerebral perfusion and attention function. Second, for the current study, we have eliminated the effects of chemotherapy-induced acute symptoms such as fatigue, vomiting, and anemia, which are involved in cognitive functioning. However, it is difficult to eliminate the potential effects of other factors, for example, the use of glucocorticoids and antiemetic drugs39,40, which could also influence cognitive functioning across the blood brain barrier. Thus, future studies should control for these factors when examining cognitive function to clarify the neural mechanisms of chemotherapy-induced cognitive impairment. Third, there is one methodological limitation that should be considered when interpreting our findings. Our results supported indirectly the existence of chemotherapy-induced attention deficits based on cerebral perfusion changes in this study. Future studies should investigate the underlying neural mechanisms of attention networks deficits more directly by combining ASL functional MRI techniques during attention tasks.
In conclusion, our study represents a significant step toward understanding potential neoadjuvant chemotherapy-induced changes in the attention network and cerebral perfusion. Our findings demonstrated that the neoadjuvant chemotherapy-induced breast cancer patients exhibited impairment of attention networks, which includes the reduced performance in the alerting and executive control attention networks and an increase in the perfusion of specific brain regions. We also reveal the reduced performance in the attention networks, which was correlated with an increase in CBF in related brain regions.
Materials and Methods
Participants
The present study was approval from the Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University. We recruited a total of 33 young women with middle-to-late-stage breast cancer who were scheduled to receive neoadjuvant chemotherapy. The patients received preoperative systemic therapy with AC (doxorubicin/cyclophosphamide) followed by preoperative docetaxel for 4 cycles after breast cancer diagnosis. The dosing schedules for AC were followed by docetaxel chemotherapy. Specifically, doxorubicin at 60 mg/m intravenously (IV) was given on day 1, and cyclophosphamide at 600 mg/m IV was given on day 1, which were cycled every 21 days for 4 cycles. Subsequently, docetaxel treatment at 100 mg/m IV was given on day 1 and cycled every 21 days for 4 cycles. Pre-treatment assessment was conducted after diagnosis but before neoadjuvant chemotherapy. Follow-up post-treatment assessment was performed within 1 month after completing eight cycles of neoadjuvant chemotherapy. Thirty-five matched healthy controls participated in the present study. There was only a single assessment for these healthy controls. The detailed information recorded from each participant is described in Table 1. For these participants, The inclusion and exclusion criteria were described in our previous study6. Following an explanation of the study objective, informed consent was obtained from all individual participants included in the study. The study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Neuropsychological background tests and ANT task
As in our previous studies, neuropsychological background tests and the ANT task were assessed for all patients and healthy controls. General cognitive functions were assessed using the Beijing version of the Montreal Cognitive Assessment (MoCA) test. The digit span test was used to measure attention. The Hamilton Depression Rating Scale (HAMD) and HAMA were used to assess participants’ possible depressive and anxiety symptoms. The Stroop test, Trail Making test and Verbal fluency test were used to measure executive function. The Chinese version of the Auditory Verbal Learning Test (AVLT) was used to assess memory. The ANT task41, as described in our previous cross-sectional study, is usually used to investigate alerting, orienting, and executive functions in the attention networks. Briefly, lower alerting and orienting network scores indicate reduced performance in the alerting and orienting networks, but higher executive control network scores indicate reduced conflict resolving capabilities. Neuropsychological evaluation and magnetic resonance imaging scans were performed on the same day.
MRI scan acquisition
All imaging data were acquired on a 3.0-T MR system (Discovery MR750 W, General Electric, Milwaukee, WI, USA) at the First Affiliated Hospital of Anhui Medical University. Multiple modalities included 3D T1-weighted, T2-weighted turbo spin echo (TSE), fluid attenuated inversion recovery (FLAIR) images, and whole brain 3D pseudo-continuous ASL (pcASL) (see details in Supplementary Materials).
Image analysis
All image data were preprocessed using customized scripts of FSL (FMRIB Software Library, the Analysis Group, FMRIB, Oxford, UK). A voxelwise two-sample t-test was performed using AFNI (Medical College of Wisconsin, Milwaukee, Wisconsin, USA) between every two of the three groups except for the comparison between after treatment and before treatment, where a paired t-test was used instead. The multiple comparison issue was addressed at the cluster level using false discovery rate (FDR) correction, with q < 0.01. A minimum voxel size of 100 was set to exclude small clusters (see details in Supplementary Materials).
Statistical analysis
SPSS 19.0 (SPSS, Chicago, IL, USA) was used to analyze the clinical and demographic data. Paired t-tests were used to assess perfusion change scores and changes in neuropsychological test performance between pre- and post-treatment within the breast cancer patient group. To compare with the healthy controls, independent two-sample t-tests were used to assess the differences in these variables. Because the breast cancer patients in the post-treatment group had lower HAMA scores and higher CRF scores compared with the pre-treatment group, we included the variable as a covariate. We also subtracted pre- from post-treatment scores of the attention networks and CBF values to obtain a change score for each chemotherapy-treated individual. A partial correlation analysis with HAMA and CRF scores as the nuisance covariates was used to assess the relationships between the changes in scores of the attention networks and the changed values of CBF in different regions pre- and post-treatment, with p < 0.05 defined as the significance level.
Additional Information
How to cite this article: Chen, X. et al. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: Evidence from an arterial spin labeling perfusion study. Sci. Rep. 7, 42684; doi: 10.1038/srep42684 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank all participants for their cooperation in the study. This work was supported by the Natural Science Foundation of China (Nos 31571149, 91432301, 81171273, 81372487, 81300944, 81301176), National Science and Technology Support Program (2011CB707802/5, 2012CB720704, 2015CB856405).
Footnotes
The authors declare no competing financial interests.
Author Contributions X.C. and X.H. were primarily responsible for the design and conduct of the study and the analysis of the data, writing the first draft of the manuscript. L.T., H.C., J.L., J.Z. and B.Q. played a role in subject recruitment and contributed to data collection. K.W. and Y.Y. was the Research Co-ordinator of the project and designed the study. All authors have revised and approved the final manuscript.
References
- Ahles T. A. et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 28, 4434–4440 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel J. S. & Schagen S. B. Chemotherapy-related cognitive dysfunction. Current neurology and neuroscience reports 12, 267–275 (2012). [DOI] [PubMed] [Google Scholar]
- Wefel J. S. et al. ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer 101, 466–475 (2004). [DOI] [PubMed] [Google Scholar]
- Anderson J. R. Cognitive Psychology and its Implications (6th edn). Worth Publishers: New York, 519 (2004). [Google Scholar]
- Posner M. I. & Petersen S. E. The attention system of the human brain. Annual review of neuroscience 13, 25–42 (1990). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Selective impairment of attention networks in breast cancer patients receiving chemotherapy treatment. Psycho-oncology 23, 1165–1171 (2014). [DOI] [PubMed] [Google Scholar]
- de Ruiter M. B. et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Human brain mapping 33, 2971–2983 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S. et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 30, 274–281 (2012). [DOI] [PubMed] [Google Scholar]
- Lepage C. et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. SpringerPlus 3, 444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S. et al. S. Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol 32, 2031–2038 (2014). [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A. & Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast cancer research and treatment 137, 33–43 (2013). [DOI] [PubMed] [Google Scholar]
- Steketee R. M. et al. Early-stage differentiation between presenile Alzheimer’s disease and frontotemporal dementia using arterial spin labeling MRI. European radiology 26, 244–253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekardaki A. et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 274, 490–499 (2015). [DOI] [PubMed] [Google Scholar]
- Zhu J. et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. Journal of psychiatric research 63, 28–35 (2015). [DOI] [PubMed] [Google Scholar]
- Nudelman K. N. et al. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PloS one 9, e96713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele F. W., Schilder C. M., de Roode M. L., Deijen J. B. & Schagen S. B. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause (New York, N.Y 22, 17–25 (2015). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Decision-making impairments in breast cancer patients treated with tamoxifen. Hormones and behavior 66, 449–456 (2014). [DOI] [PubMed] [Google Scholar]
- Kreukels B. P. et al. ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin Neurophysiol 119, 533–541 (2008). [DOI] [PubMed] [Google Scholar]
- Heinke W. & Koelsch S. The effects of anesthetics on brain activity and cognitive function. Current opinion in anaesthesiology 18, 625–631 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang B. et al. The effects of isoflurane and desflurane on cognitive function in humans. Anesthesia and analgesia 114, 410–415 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraghavan L. et al. Noninvasive Measurement of Cerebral Blood Flow Under Anesthesia Using Arterial Spin Labeling MRI: A Pilot Study. Journal of neurosurgical anesthesiology (2015). [DOI] [PubMed] [Google Scholar]
- Ahles T. A. & Saykin A. J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews 7, 192–201 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Joseph H. et al. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PloS one 6, e23492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A. et al. Docetaxel versus paclitaxel for antiangiogenesis. Journal of hematotherapy & stem cell research 11, 103–118 (2002). [DOI] [PubMed] [Google Scholar]
- Rabbitt P. et al. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology 20, 549–557 (2006). [DOI] [PubMed] [Google Scholar]
- Spilt A. et al. Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology 236, 990–995 (2005). [DOI] [PubMed] [Google Scholar]
- Calviere L. et al. Improvement in cognitive function and cerebral perfusion after bur hole surgery in an adult with moyamoya disease. Case report. J Neurosurg 115, 347–349 (2011). [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B. D., Fossella J., Flombaum J. I. & Posner M. I. The activation of attentional networks. NeuroImage 26, 471–479 (2005). [DOI] [PubMed] [Google Scholar]
- Rinne P. et al. Triple dissociation of attention networks in stroke according to lesion location. Neurology 81, 812–820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B. C., Conroy S. K., Ahles T. A., West J. D. & Saykin A. J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol 30, 2500–2508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherling C., Collins B., Mackenzie J., Bielajew C. & Smith A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front Hum Neurosci 5, 122, doi: 10.3389/fnhum.2011.00122. eCollection 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N., Sepulcre J., Turner G. R., Stevens W. D. & Schacter D. L. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of cognitive neuroscience 25, 74–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S. M. & Kesler S. R. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc 20, 391–401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J. A. et al. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain imaging and behavior 7, 524–532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J. S. et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America 103, 13848–13853 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S. R. et al. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proceedings of the National Academy of Sciences of the United States of America 110, 11600–11605 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. et al. [Cognitive function in breast cancer patients on chemotherapy: a longitudinal study]. Zhonghua yi xue za zhi 94, 27–30 (2014). [PubMed] [Google Scholar]
- Spagna A. et al. Clozapine improves the orienting of attention in schizophrenia. Schizophrenia research 168, 285–291 (2015). [DOI] [PubMed] [Google Scholar]
- Hasler W. L. Nausea, gastroparesis, and aerophagia. Journal of clinical gastroenterology 39, S223–229 (2005). [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Glucocorticoid receptors in the brain. Hospital practice (Office ed. 23, 107–111, 114, 119–121 (1988). [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B. D., Sommer T., Raz A. & Posner M. I. Testing the efficiency and independence of attentional networks. Journal of cognitive neuroscience 14, 340–347 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.