Abstract
The anterior pituitary originates from the adenohypophyseal placode. Both the preplacode region and neural crest (NC) derive from subdivision of the neural border region, and further individualization of the placode domain is established by a reciprocal interaction between placodal precursors and NC cells (NCCs). It has long been known that NCCs are present in the adenohypophysis as interstitial cells. A recent report demonstrated that NCCs also contribute to the formation of pericytes in the developing pituitary. Here, we attempt to further clarify the role of NCCs in pituitary development using P0‐Cre/EGFP reporter mice. Spatiotemporal analyses revealed that GFP‐positive NCCs invaded the adenohypophysis in a stepwise manner. The first wave was detected on mouse embryonic day 9.5 (E9.5), when the pituitary primordium begins to be formed by adenohypophyseal placode cells; the second wave occurred on E14.5, when vasculogenesis proceeds from Atwell's recess. Finally, fate tracing of NCCs demonstrated that NC‐derived cells in the adenohypophysis terminally differentiate into all hormone‐producing cell lineages as well as pericytes. Our data suggest that NCCs contribute to pituitary organogenesis and vasculogenesis in conjunction with placode‐derived pituitary stem/progenitor cells.
Keywords: neural crest cell, pituitary development, PROP1, SOX2, stem/progenitor cell
Introduction
The pituitary gland is an important endocrine organ composed of two anatomically different entities: the neurohypophysis and the adenohypophysis. While the neurohypophysis originates from the neuroectoderm, the adenohypophysis (anterior pituitary), which is composed of the anterior lobe, intermediate lobe and pars tuberalis, originates from the adenohypophyseal placode (Kouki et al. 2001). The adenohypophyseal placode is one of the cranial placodes and originates in a common preplacode region. Placode development begins at the border between the neural and non‐neural ectoderm during early gastrulation. After the neural border region is established, it is induced to subdivide into two populations during gastrulation: the preplacode region and the neural crest (NC; reviewed in Singh & Groves, 2016). Further individualization of the placode domain is established by a reciprocal interaction between placode precursors and NC cells (NCCs).
Placode cells are unipotent and migrate to a region restricted to the head in mammals. In contrast, NCCs exhibit multipotency and are able to differentiate into many types of cells after migrating to the entire body axis. This multipotency of NCCs is sustained in various adult tissues. In the olfactory system, the olfactory epithelium and olfactory ensheathing cells have long been believed to derive solely from the olfactory placode, which is located adjacent to the adenohypophyseal placode during placode development. Recent cell lineage tracing studies by our and other groups using Wnt1‐Cre/EGFP (enhanced green fluorescent protein) and/or P0‐Cre/EGFP transgenic mice, in which migrating NCCs and their descendants permanently express GFP, revealed that GFP‐positive NCCs play a role not only in embryonic but also in postnatal olfactory development and maintenance (Katoh et al. 2011; Suzuki et al. 2013).
In the developing anterior lobe of the anterior pituitary, the presence of NCCs as interstitial cells has been analyzed using quail‐chick chimeras (Couly & Le Douarin, 1987). Most recently, ablation of NCCs using Wnt1‐Cre and P0‐Cre to delete Ctnnb1 (β‐catenin) revealed that NCCs play a role in pituitary vascularization, supplying pericytes (Davis et al. 2016). The contribution of NCCs to the brain vasculature has previously been reported in mice by our and other groups using P0‐Cre and human tissue plasminogen activator (HtPA)‐Cre reporter lines (Pietri et al. 2003; Yamanishi et al. 2012). However, aside from their role in vasculogenesis, the involvement of NCCs in pituitary development remains unclear. In this study, we further analyzed the contribution of NCCs to pituitary development during the early embryonic period using P0‐Cre/EGFP transgenic mice (Kawakami et al. 2011), in which P0‐promoter active NCCs and their descendant NC‐derived cells (NCDCs) are labeled with GFP. Our results demonstrate that NCCs play a pivotal role in pituitary development by differentiating into all types of hormone‐producing cell lineages in addition to pericytes.
Materials and methods
Animals
We used P0‐Cre/EGFP transgenic mice generated by crossing P0‐Cre mice and CAG‐CAT‐EGFP mice (Kawakami et al. 2011). The original P0‐Cre and CAG‐CAT‐EGFP transgenic mouse lines were kindly provided by K. Yamamura (Kumamoto University School of Medicine, Kumamoto, Japan) and J. Miyazaki (Osaka University School of Medicine, Osaka, Japan). Mice were housed individually in a temperature‐controlled room under a 12‐h light/12‐h dark cycle. Embryonic stages were determined by observation of vaginal plug day, which was designated as embryonic day 0.5 (E0.5). The present study was approved by the Institute Animal Care and Use Committee, Meiji University, based on NIH Guidelines for the Care and Use of Laboratory Animals.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Yoshida et al. 2009, 2015). The following primary antibodies were used: chicken IgY against jelly fish GFP (1 : 500 dilution; Aves Labs, Tigard, OR, USA); rabbit IgG against Cre recombinase (1 : 150 dilution; CST, Danvers, MA, USA); goat IgG against human SOX2 (1 : 400 dilution; Neuromics, Edina, MN, USA); guinea pig IgG against rat PROP1 [5 ng μL−1; generated in‐house (Yoshida et al. 2009)]; and rabbit IgG against rat NG2 (1 : 400 dilution; Millipore, Darmstadt, Germany). Antisera against anterior pituitary hormones included guinea pig antisera against human growth hormone (GH; 1 : 1500 dilution), which was kindly provided by Dr S. Tanaka (Shizuoka University, Shizuoka, Japan), and rabbit antisera against rat prolactin (PRL; 1 : 5000 dilution), which was kindly provided by Dr Wakabayashi (Institute for Molecular and Cellular Regulation, Gunma University, Maebashi, Japan). Guinea pig antisera against rat adrenocorticotropic hormone (ACTH; 1 : 10000 dilution), rat thyroid‐stimulating hormone (TSH)β (1 : 50000 dilution), rat luteinizing hormone (LH)β (1 : 3000 dilution) and rat follicle‐stimulating hormone (FSH)β (1 : 3000 dilution) were kindly provided by Dr A. F. Parlow (National Hormone and Pituitary Program, Torrance, CA, USA). A cocktail of antisera against six pituitary hormones was prepared by mixing each antiserum in appropriate ratios, as described above. Secondary antibodies used were Cy3‐, Cy5‐, or fluorescein isothiocyanate (FITC)‐conjugated AffiniPure donkey IgG anti‐chicken IgY or anti‐rabbit, anti‐goat and anti‐guinea pig IgG (1 : 500 dilution; Jackson ImmunoResearch, West Grove, PA, USA). Finally, the sections were enclosed in VECTASHIELD mounting medium containing 4,6‐diamidino‐2‐phenylindole (DAPI; Vector, Burlingame, CA, USA). Immunofluorescence was observed under a BZX‐700 fluorescence microscope (Keyence, Osaka, Japan).
Cell numbers positive for GFP, individual hormones, SOX2 and DAPI were counted for each section (6500–9500 cells per section) prepared from three male mice (P60).
Results
GFP‐positive cells invade the developing anterior pituitary
P0‐Cre/EGFP mice express the Cre recombinase under the P0‐promoter, resulting in permanent labeling of NCCs, NCDCs and a population of postnatal vascular precursor cells by GFP (Kubota et al. 2011). The localization of Cre allows us to identify whether GFP‐positive cells are P0‐promoter active or P0‐promoter inactive. We first performed double‐immunostaining for Cre and GFP during pituitary embryonic development, determining that a small number of GFP‐positive cells existed inside the invaginating pituitary primordium as early as E9.5 (Fig. 1A). Whereas Cre/GFP‐double positive cells were localized in the rostral region of the craniofacial mesenchyme, GFP‐positive cells inside the pituitary primordium were Cre‐negative (Fig. 1A, boxed area). On E10.5 and E11.5, the number of spindle‐shaped GFP‐positive cells increased in the pituitary primordium of Rathke's pouch (Fig. 1B,C). Cre activity inside Rathke's pouch was not detected, whereas some GFP‐positive cells outside Rathke's pouch were positive for Cre (Fig. 1C, boxed area), indicating that GFP‐positive but Cre‐negative cells invade the pituitary gland during the early stage of development. Subsequently, during the middle stage of pituitary development (E14.5), in addition to Cre‐negative/GFP‐positive cells, a large number of Cre/GFP‐double positive cells localized to Atwell's recess (Fig. 1D, arrowheads), which is an intraglandular fossa that receives several blood vessels (Daikoku et al. 1981), and to the rostral tip, which is a prospective pars tuberalis. This was in addition to Cre/GFP‐double positive cells together with Cre‐negative GFP‐positive cells located in the parenchyma of the expanding anterior lobe. This phenomenon was quite different from that on E10.5 and E11.5. On E16.5, when Atwell's recess was almost closed, Cre/GFP‐double positive cells and Cre‐negative/GFP‐positive cells were evenly distributed from the rostral to the caudal region of the parenchyma of the anterior lobe (Fig. 1E). These results demonstrate that the pituitary gland receives at least two waves of invasion by GFP‐positive cells during embryonic development. Furthermore, the populations involved in the two invading waves were different in quality and quantity, consisting of a small P0‐promoter inactive population in the first wave and a large population of P0‐promoter active and P0‐promoter inactive cells in the second wave.
Figure 1.
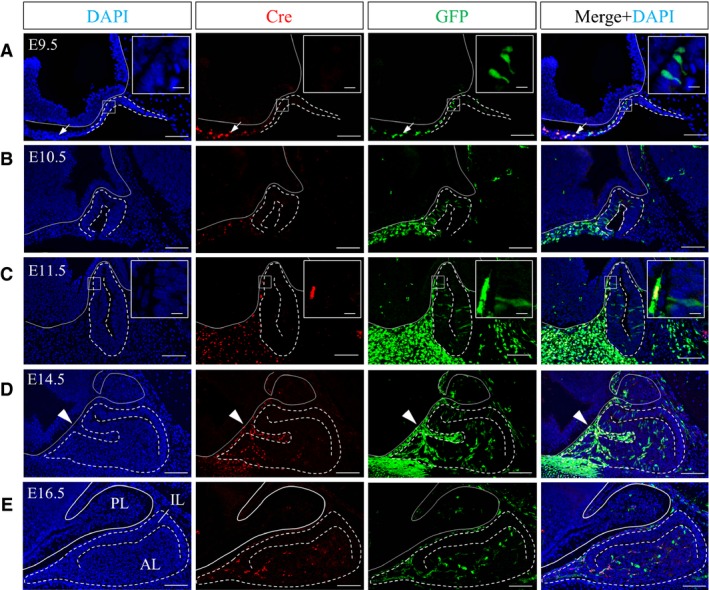
Localization of GFP‐ and/or Cre‐positive cells during embryonic pituitary development. Sagittal sections on (A) E9.5, (B) E10.5, (C) E11.5, (D) E14.5 and (E) E16.5 were reacted with anti‐Cre antibody (visualized with Cy3, red) and anti‐GFP antibody (visualized with FITC, green). Images of individual staining with DAPI, Cre or GFP, and merged images (left to right panels, respectively) are shown. Boxed areas in (A) and (C) are enlarged in the upper right of each panel. Dotted lines show the outline of the pituitary gland. Solid lines show the outline of the neuroectoderm. Arrowheads indicate Atwell's recess. AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Scale bars: 100 μm (enlarged area: 10 μm).
GFP‐positive cells acquire the characteristics of pituitary stem/progenitor cells
We previously reported that all cells in Rathke's pouch, except for the rostral tip, express Sox2, a pituitary stem/progenitor cell marker, and Prop1, a pituitary‐specific progenitor cell marker, on rat E13.5 (Yoshida et al. 2009). Therefore, we performed triple‐immunostaining for GFP, PROP1 and SOX2 using sections on mouse E11.5 and E14.5 to evaluate the relationship between GFP‐positive cells and pituitary stem/progenitor cells.
On E11.5, after the pituitary primordium received the first wave of invasion, all seven GFP‐positive cells that were observed inside Rathke's pouch were positive for PROP1 and SOX2 (Fig. 2A). GFP‐positive cells were clearly compartmentalized at the border between Rathke's pouch and the surrounding head mesenchyme, as shown by GFP‐single positive cells in the mesenchyme (Fig. 2B, arrows) and GFP/PROP1/SOX2‐triple positive cells inside Rathke's pouch (Fig. 2B,C, arrowheads). These data indicate that GFP‐positive cells inside the pouch acquire characteristics of pituitary stem/progenitor cells after the first wave of invasion and settle in the pituitary together with placode‐derived stem/progenitor cells. In contrast, on E14.5, when the embryonic pituitary receives the second wave of invasion, GFP‐single positive cells were located not only in Atwell's recess but also in the parenchyma of the anterior lobe (Fig. 2D–F′, arrows) together with GFP/PROP1/SOX2‐triple positive cells (Fig. 2D–F′, arrowheads).
Figure 2.
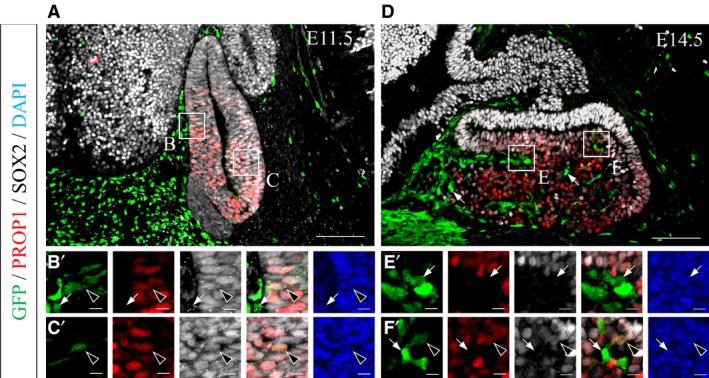
Characterization of GFP‐positive cells as pituitary stem/progenitor cells. Sagittal sections on (A) E11.5 and (D) E14.5 were reacted with anti‐GFP antibody (visualized with FITC, green), anti‐PROP1 antibody (visualized with Cy3, red) and anti‐SOX2 antibody (visualized with Cy5, gray). Triple‐merged images of the pituitary glands are shown in the upper panels. Boxed areas in A (B, C) and D (E, F) are magnified in the lower panels (B′–F′) as individual images, triple‐merged image and DAPI‐stained image. Arrowheads indicate GFP/PROP1/SOX2‐triple positive cells, and arrows indicate GFP‐single positive cells. Scale bars: 100 μm (enlarged area: 10 μm).
Taken together, our observations suggest that at least GFP‐positive cells penetrated by the first wave of invasion transform into pituitary stem/progenitor cells.
GFP‐positive cells differentiate into hormone‐producing cells and pericytes
We next focused on the GFP‐single positive cells located in the parenchyma of the developing anterior lobe. Because we assume that cells negative for SOX2 and PROP1 in the embryonic anterior lobe commit and terminally differentiate into hormone‐producing cells or cells implicated in vasculogenesis (Yoshida et al. 2009; Higuchi et al. 2015; Kanno et al. 2015), we first performed double‐immunostaining for pituitary hormones and GFP. As the five types of hormone‐producing cells do not appear simultaneously during pituitary development (Savage et al. 2003), we analyzed the pituitary gland on E16.5, when some hormone‐producing cell lineages have already appeared. Double‐immunostaining with anti‐GFP antibody and a cocktail of antibodies against six pituitary hormones clearly demonstrated that a small number of GFP/hormone‐double positive cells were present in the parenchyma of the developing anterior lobe (Fig. 3A, upper panel). The proportion of GFP/hormone‐double positive cells among hormone‐producing cells was approximately 3.3%. Additional immunohistochemistry using antibodies against each pituitary hormone revealed GFP‐immunoreactive signals in GH‐, ACTH‐, TSH‐, and LH‐producing cells (Fig. 3A, lower panels), though signals for PRL and FSH were not detected at this stage.
Figure 3.
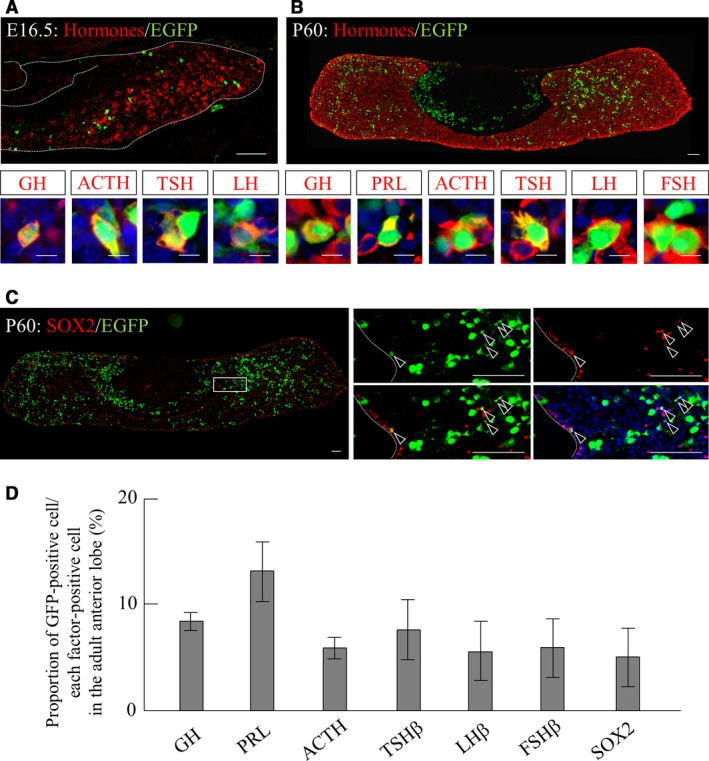
Differentiation of GFP‐positive cells into all pituitary hormone‐producing cell lineages. Immunostaining for GFP and pituitary hormones or SOX2 was performed. Merged images of pituitary glands on (A) E16.5 and (B) P60 using anti‐GFP antibody (visualized with FITC, green) and a cocktail of antibodies against six pituitary hormones (visualized with Cy3, red) are shown in the upper panels. Images of co‐localization with GFP (visualized with FITC, green) and each hormone (visualized with Cy3, red) merged with DAPI (blue) are magnified in the lower panels. (C) Images of the pituitary gland on P60 for anti‐GFP (visualized with FITC, green) and anti‐SOX2 (visualized with Cy3, red) are shown. Boxed area is magnified in individual images (GFP, SOX2, GFP/SOX2) and in the merged image with nuclear staining with DAPI (blue). (D) Proportion of GFP‐positive cells among individual hormones‐ or SOX2‐positive cells are shown. Dotted lines in (A) indicate the boundary regions and in (C) indicate the marginal cell layer (MCL). Arrowheads in (C) indicate SOX2/GFP‐double positive cells. ACTH, adrenocorticotrophic hormone; FSH, follicle‐stimulating hormone; GH, growth hormone; LH, luteinizing hormone; PRL, prolactin; TSH, thyroid‐stimulating hormone. Scale bars: 100 μm [enlarged area (A and B): 10 μm].
We next tried to confirm the existence of GFP/hormone‐double positive cells in the adult pituitary gland. In the pituitaries of postnatal day 60 (P60) mice, GFP‐positive cells were located not only in the anterior lobe but also in the intermediate lobe (Fig. 3B, upper panel). Double‐immunostaining for GFP and six pituitary hormones using the antibody cocktail demonstrated that the proportion of GFP/hormone‐double positive cells among hormone‐producing cells had increased to approximately 10%. Further analysis using antibodies against individual hormones confirmed that GFP‐positive cells differentiate into all hormone‐producing cell lineages (Fig. 3B, lower panels). The proportion of GFP‐positive cells in each hormone‐producing cell was 8.5% (GH), 13.2% (PRL), 6.0% (ACTH), 7.7% (TSH), 5.7% (LH) and 6.0% (FSH; Fig. 3D), including some melanocyte‐stimulating hormone‐producing cells in the intermediate lobe (data not shown). In addition, we further detected GFP/SOX2‐double positive pituitary stem/progenitor cells in the adult pituitary gland (Fig. 3C). These cells were located in the marginal cell layer (MCL), a pituitary stem/progenitor cell niche, and the parenchyma of the anterior lobe (Fig. 3C, boxed area). The proportion of GFP‐positive cells among SOX2‐positive cells was 5.1% (Fig. 3D).
Finally, we performed double‐immunostaining for GFP and NG2, a potent pericyte marker used to study the developing mouse telencephalon (Yamanishi et al. 2012), although no marker is absolutely specific for pericytes. Ng2, for example, is expressed not only by pericytes but also by oligodendrocyte precursor cells. However, oligodendrocytes are absent from the embryonic anterior pituitary. Therefore, NG2‐positive cells in the embryonic anterior pituitary should consist purely of pericytes. The results showed that NG2‐positive cells were located in Atwell's recess and the parenchyma of the embryonic anterior lobe on E14.5 (Fig. 4A). A portion of NG2‐positive cells in both regions were positive for GFP (Fig. 4C′,E′, arrowheads), whereas GFP‐negative/NG2‐positive cells were also located in the pituitary gland (Fig. 4B′,D′, arrows). Hence, our data demonstrate that GFP‐positive cells in the pituitary gland differentiate into two types of cells, hormone‐producing cells and vascular pericytes.
Figure 4.
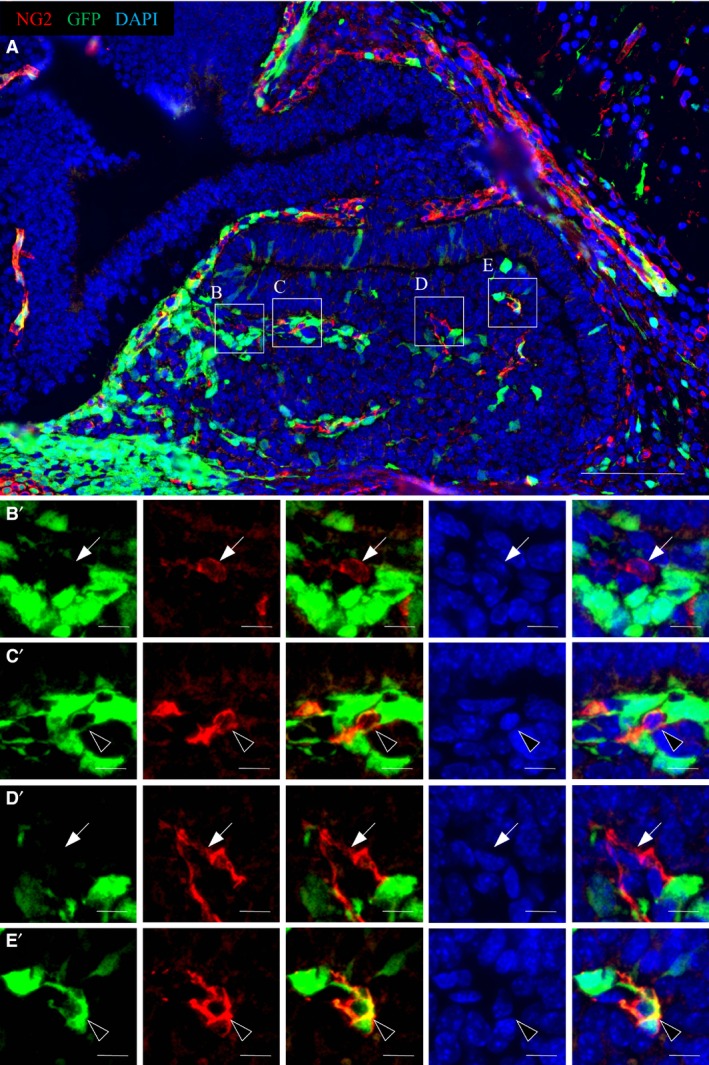
Differentiation of GFP‐positive cells into pericytes. Sagittal sections on E14.5 were reacted with anti‐GFP antibody (visualized with FITC, green) and anti‐NG2 antibody (visualized with Cy3, red). The image of the pituitary gland is shown as a merged image with DAPI in the upper panel (A). Boxed areas (B–E) are magnified in the lower panels (B′–E′) as individual images, merged image, DAPI‐stained image and triple‐merged image. Arrowheads indicate GFP/NG2‐double positive cells and arrows indicate GFP‐negative/NG2‐positive cells. Scale bars: 100 μm (enlarged area: 10 μm).
Discussion
Interactions between the pituitary gland and surrounding mesenchymal cells are indispensable for the development of the pituitary gland (Gleiberman et al. 1999), but their precise roles in pituitary development remain unclear. In the present study, we demonstrated that NCCs invade the embryonic anterior pituitary, which is believed to derive solely from the adenohypophyseal placode, in a stepwise manner and differentiate into all hormone‐producing cell lineages and vascular pericytes.
A reciprocal interaction between placode precursors and NCCs contributes to placode development and individualization, eventually resulting in segregation into the adenohypophyseal, olfactory, lens, trigeminal, epibranchial and otic placodes. Studies focusing on placode‐derived tissues other than the anterior pituitary offer a clue into the contribution of NCCs or NCDCs to the development of placode‐derived tissues. Lineage analyses using P0‐Cre transgenic mice have demonstrated that the P0 promoter is a potent tool for labeling NCCs at stages later than E9.0 (Yamauchi et al. 1999; Kanakubo et al. 2006). Indeed, using P0‐Cre/EGFP and/or Wnt1‐Cre/EGFP transgenic mice, we and another group have reported that NCCs invade the olfactory system during embryogenesis and play a lifelong role in olfactory development together with stem cells derived from the olfactory placode (Katoh et al. 2011; Suzuki et al. 2013). In particular, stem cells derived from the NC significantly contribute to reconstruction of the adult olfactory epithelium after severe injury (Suzuki et al. 2013).
In the present study, using P0‐Cre/EGFP transgenic mice, we revealed that GFP‐positive cells invade the anterior pituitary in at least a two‐step process. The first wave of invasion was detected on E9.5, when adenohypophyseal placode cells arrive at an appropriate position to initiate the formation of the pituitary primordium. In the second wave of invasion, we found that a large number of GFP‐positive cells invade the anterior lobe through Atwell's recess on E14.5, when vasculogenesis begins. Immunostaining for Cre showed that GFP‐positive cell populations in the second wave of invasion are composed of both Cre‐positive and Cre‐negative cells, whereas those in the first wave of invasion are Cre‐negative only.
In our previous study using P0‐Cre/EGFP mouse embryos in whole embryo culture from E8.5 to E9.5, GFP‐expressing cells are indeed labeled with DiI, demonstrating that GFP‐positive cells in the early developmental stage are migrating NCCs (Kanakubo et al. 2006). Furthermore, in the dental placode, Wang et al. (2011) used P0‐Cre/EGFP mice to demonstrate that Cre‐positive migrating NCCs on E9.0 are transformed into Cre‐negative NC‐derived tooth germ cells on E12.5 and contribute to neonatal dental enamel formation. Taken together, it is reasonable to assume that GFP‐positive cells from the first invasion on E9.5 in the present study are NCDCs. The transcription factor Prop1 is known to be expressed only in pituitary stem/progenitor cells, and previous reports revealed that all cells in Rathke's pouch except for those in the rostral tip are positive for PROP1 (Yoshida et al. 2009, 2011). NCDCs in the first wave of invasion were P0‐promoter inactive and expressed Prop1 and Sox2 within Rathke's pouch on E11.5, whereas surrounding mesenchymal cells were P0‐promoter active NCCs. These data suggest the possibility that NCCs convert into P0‐promoter inactive NCDCs after invasion and transform into pituitary stem/progenitor cells. Immunostaining for six hormones in E16.5 and P60 pituitaries revealed that a small number of hormone‐producing NCDCs are present in the embryonic pituitary gland and differentiate into all hormone‐producing cell lineages, accounting for 10% of all hormone‐producing cells in the adult anterior pituitary gland. These data suggest that NCCs in the first wave of invasion exhibit multipotency, converting into tissue‐specific stem/progenitor cells after invasion and thereafter coexisting with placode‐derived stem/progenitor cells throughout life.
Although the significance of stem/progenitor cells of dual origin in the pituitary gland is poorly understood, the contribution of NC‐derived olfactory stem cells to the regeneration system (Suzuki et al. 2013) suggests a similar role of NC‐derived stem/progenitor cells in the pituitary gland. Further analyses of regeneration, such as ablation of the target organs, in addition to analyses of the physiological alteration during gestation, lactation and weaning, are needed to clarify this issue. Moreover, in the pituitary gland, folliculo‐stellate cells are known to appear after birth and some of these functions as stem/progenitor cells. One of the folliculo‐stellate cell markers S100β, is expressed in various NC‐derived tissues. Indeed, we recently analyzed the rat late embryonic pituitary gland using anti‐p75, a marker for NCCs, and detected a small number of S100β/p75‐double positive cells (Horiguchi et al. 2016). Therefore, further analysis of the relationship between NCCs and folliculo‐stellate cells is necessary.
Our observations are consistent with those of a previous tracing analysis using Nestin‐Cre/GFP transgenic mice. Gleiberman et al. (2008) demonstrated that some GFP‐positive Nestin‐Cre lineage cells first settle in Rathke's pouch. These subsequently contribute to the expansion of the neonatal pituitary gland and participate in the development of the postnatal pituitary gland as adult pituitary stem cells. Nestin is known to be expressed in NC‐derived stem cells within the dorsal root ganglion (Nagoshi et al. 2009), in addition to neural stem cells in the central nervous system. Together with our present data, these observations suggest that the adult pituitary stem cells identified by analysis of Nestin‐Cre/GFP mice may include a population of cells derived from the NC.
We speculated that GFP‐positive cells involved in the second wave of invasion contribute to vasculogenesis in the developing anterior pituitary because they invade from Atwell's recess, an intraglandular fossa known to receive primitive portal vessels (Daikoku et al. 1981). An analysis using quail‐chick chimeras showed that NCDCs exist as interstitial cells in the pituitary (Couly & Le Douarin, 1985). Moreover, Davis et al. (2016) recently reported that cells labeled with X‐gal localized to Atwell's recess in the pituitaries of Wnt1‐Cre/Rosa stopLacZ mice on E14.5, and that P0‐Cre/β‐catenin fx/fx transgenic mice, which lack NCCs owing to a deletion of β‐catenin, exhibit abnormal pituitary vasculogenesis. Our findings that pericyte‐like GFP‐positive cells positive for NG2 are located in Atwell's recess and the parenchyma of the expanding anterior lobe on E14.5 support these results. We conclude that at least pericyte‐like GFP‐positive cells are NC‐lineage cells.
In addition, we should point out that P0‐Cre/EGFP mice mark not only NCCs and NCDCs but also non‐NCCs such as tissue‐resident vascular precursors that are not labeled by Wnt1‐Cre or Ht‐PA‐Cre mice (Kubota et al. 2011). These non‐NCCs are P0‐promoter active even in the postnatal period. These reports and our data suggest that plural populations of GFP‐positive cells including NCDCs and tissue‐resident vascular precursor cells invade through Atwell's recess and are involved in pituitary vasculogenesis. Considering the significant impact of NC‐derived pericytes in the formation of the avian brain (Etchevers et al. 1999), the same mechanism may be involved in the avian pituitary gland in order to construct a highly complex organ with multiple types of cells.
Conclusions
For the first time, we have observed NC‐derived stem/progenitor cells in addition to pericytes in the anterior pituitary. We previously demonstrated that NC‐derived stem cells play a pivotal role in regeneration of the olfactory epithelium after injury (Suzuki et al. 2013). A remaining question in pituitary organogenesis is whether the two distinct stem/progenitor cell populations originating from the placode and the NC in the embryonic anterior pituitary are involved in distinct functions.
Author contributions
H. Ueharu: collection and assembly of data, data analysis and interpretation, manuscript writing; S. Yoshida: collection of data, discussion; N. Kanno: maintenance of transgenic mice, collection of data; M. Higuchi: launch of the study; T. Kikkawa and N. Osumi: maintenance of transgenic mice, data analysis and interpretation, and contribution to discussion and manuscript writing; T. Kato and Y. Kato: study design, data analysis and interpretation, manuscript writing.
Conflict of interests
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgements
This work was partially supported by JSPS KAKENHI (Grant No. 21380184 to Y.K. and No. 24580435 to T.K.). Further support was provided by a MEXT‐Supported Program for the Strategic Research Foundation at Private Universities (Grant No. 2014‐2018) and by a research grant (A) to Y.K. from the Institute of Science and Technology, Meiji University. Lastly, this study was supported by the Meiji University International Institute for BioResource Research (MUIIR). The authors are grateful to Drs Kenichi Yamamura and Junichi Miyazaki for originally providing the P0‐Cre and CAG‐CAT‐EGFP mouse lines.
References
- Couly GF, Le Douarin NM (1985) Mapping of the early neural primordium in quail‐chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol 110, 422–439. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM (1987) Mapping of the early neural primordium in quail‐chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol 120, 198–214. [DOI] [PubMed] [Google Scholar]
- Daikoku S, Kawano H, Abe K, et al. (1981) Topographical appearance of adenohypophysial cells with special reference to the development of the portal system. Arch Histol Jpn 44, 103–116. [DOI] [PubMed] [Google Scholar]
- Davis SW, Mortensen AH, Keisler JL, et al. (2016) beta‐Catenin is required in the neural crest and mesencephalon for pituitary gland organogenesis. BMC Dev Biol 16, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, et al. (1999) Anterior cephalic neural crest is required for forebrain viability. Development 126, 3533–3543. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Fedtsova NG, Rosenfeld MG (1999) Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol 213, 340–353. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Michurina T, Encinas JM, et al. (2008) Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105, 6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Yoshida S, Ueharu H, et al. (2015) PRRX1‐ and PRRX2‐positive mesenchymal stem/progenitor cells are involved in vasculogenesis during rat embryonic pituitary development. Cell Tissue Res 361, 557–565. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Yako H, Yoshida S, et al. (2016) S100β‐positive cells of mesenchymal origin reside in the anterior lobe of the embryonic pituitary gland. PLoS One 11, e0163981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakubo S, Nomura T, Yamamura K, et al. (2006) Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells 11, 919–933. [DOI] [PubMed] [Google Scholar]
- Kanno N, Higuchi M, Yoshida S, et al. (2015) Expression studies of Neuronatin in the prenatal and postnatal rat pituitary. Cell Tissue Res 64, 273–288. [DOI] [PubMed] [Google Scholar]
- Katoh H, Shibata S, Fukuda K, et al. (2011) The dual origin of the peripheral olfactory system: placode and neural crest. Mol Brain 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Umeda M, Nakagata N, et al. (2011) Novel migrating mouse neural crest cell assay system utilizing P0‐Cre/EGFP fluorescent time‐lapse imaging. BMC Dev Biol 11, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouki T, Imai H, Aoto K, et al. (2001) Developmental origin of the rat adenohypophysis prior to the formation of Rathke's pouch. Development 128, 959–963. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Hirashima M, et al. (2011) Isolation and function of mouse tissue resident vascular precursors marked by myelin protein zero. J Exp Med 208, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N, Shibata S, Nakamura M, et al. (2009) Neural crest‐derived stem cells display a wide variety of characteristics. J Cell Biochem 107, 1046–1052. [DOI] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, et al. (2003) The human tissue plasminogen activator‐Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo . Dev Biol 259, 176–187. [DOI] [PubMed] [Google Scholar]
- Savage JJ, Yaden BC, Kiratipranon P, et al. (2003) Transcriptional control during mammalian anterior pituitary development. Gene 319, 1–19. [DOI] [PubMed] [Google Scholar]
- Singh S, Groves AK (2016) The molecular basis of craniofacial placode development. Wiley Interdiscip Rev Dev Biol 5, 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Yoshizaki K, Kobayashi T, et al. (2013) Neural crest‐derived horizontal basal cells as tissue stem cells in the adult olfactory epithelium. Neurosci Res 75, 112–120. [DOI] [PubMed] [Google Scholar]
- Wang SK, Komatsu Y, Mishina Y (2011) Potential contribution of neural crest cells to dental enamel formation. Biochem Biophys Res Commun 415, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi E, Takahashi M, Saga Y, et al. (2012) Penetration and differentiation of cephalic neural crest‐derived cells in the developing mouse telencephalon. Dev Growth Differ 54, 785–800. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, et al. (1999) A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol 212, 191–203. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kato T, Susa T, et al. (2009) PROP1 coexists with SOX2 and induces PIT1‐commitment cells. Biochem Biophys Res Commun 385, 11–15. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kato T, Yako H, et al. (2011) Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23, 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kato T, Chen M, et al. (2015) Localization of a juxtacrine factor ephrin‐B2 in the pituitary stem/progenitor cell niches throughout life. Cell Tissue Res 359, 755–766. [DOI] [PubMed] [Google Scholar]


