Abstract
Understanding the role of the social environment in the development of stress related diseases requires a more fundamental understanding of stress. Stress includes not only the stimulus and the response but also the individual appraisal of the situation. The social environment is not only essential for survival it is at the same time an important source of stressors. This review discusses the social stress concept, how it has been studied in rodents in the course of time and some more recent insights into the appraisal process. In addition to the factors controllability and predictability, outcome expectancy and feedback of the victim's own actions during the social stress are suggested to be important factors in the development of stress related disease. It is hypothesized that individual differences in the way in which these factors are used in the appraisal of everyday life situations may explain individual vulnerability.
Keywords: Appraisal stress, Controllability, Predictability, Expectancy, Feedback, Corticosterone, Noradrenaline, Adrenaline
1. Introduction
During the last decade there is a renewed interest in the use of the social environment in animal models of stress pathology. This is based on the fact that many animal species, including human beings spend most of their daily life in close proximity of conspecifics. It is generally assumed that a focus on social stress enhances the translational value of animal models. In some species, the social environment can be quite complex with a diversity of hierarchical relationships among group members. The general idea is that evolution has shaped these social structures for optimal survival. Living in a social community implies adaptation to the behavior and presence of other group members. In a stable social group, the social relationships are well established and there are no clear signs of stress pathology. From an evolutionary point of view, such a social structure should be optimal for health, reproduction and survival. However, social structures can be quite dynamic and have to be (re)established and maintained. This requires adaptation of the individual colony members and the degrees in which adaptation processes are activated depend of course on the stability of the social structure. Hence, the social structure and environment is not only essential for survival, it can be an important source of social stressors at the same time. In view of this dual nature and evolutionary significance of social structures, it is surprising that many studies using social stimuli as stressors interpret the data in terms of maladaptation and stress related disease. This biomedical pathophysiological interpretation bias of rodent models of social stress and the limitations of such models of depression have recently been discussed in two papers (Chaouloff, 2013, Gray et al., 2015). A similar discussion on the adaptive or maladaptive significance of stress induced behavioral changes can be found in the clinical literature (Nesse, 2000, Nesse et al., 2016). The present paper will discuss some issues that might help in the interpretation of the adaptive and/or maladaptive significance of the behavioral consequences of social defeat.
Some of the early pioneers of stress research have emphasized the view that stress should be considered as a process that includes the stimulus, the perceptual processing or appraisal of this input and the behavioral and physiological output (J. W. Hennessy and Levine, 1979, Plaut and Friedman, 1982). Still, many studies and preclinical studies in particular seem to neglect this aspect of cognitive, higher level cortical processing of information. To understand social stimuli as stressors, assessing the activation of the so called stress systems such as the Hypothalamic-Pituitary-Adrenocortical (HPA) axis and the Sympathetic nervous – Adrenomedullary (SAM) system is not sufficient. These neuroendocrine systems have an important function in the cardiovascular and metabolic support of any behavioral reaction to salient environmental challenges or opportunities. For example, the response of these systems to rewarding stimuli such as sexual behavior or social victory can be just as high as to aversive stimuli (Buwalda et al., 2012). Similarly, stress systems are highly activated during the use of drugs with a strong euphoric action (Goeders, 2002). Hence, without taking perceptual processes into account, there is a serious risk of misinterpretation. There is also a risk of circular reasoning. Because aversive stimuli and negative affective states are often associated with activation of the neuroendocrine stress systems, i.e. the activation of ‘stress’ systems and/or measurement of “stress hormone” levels are subsequently used as an indicator or even proof of the negative connotation of social stress exposure. In addition, preclinical studies often define their stimulus as aversive, usually from an anthropomorphic line of reasoning, and interpret the myriad of physiological, neuroendocrine, immune and neurochemical changes that occur in response to it as a stress response. In conclusion, there is a need for indices that allow an answer to the question whether a social stimulus is indeed perceived as a stressor in the sense that it is considered a serious threat to homeostasis and thus to physical health and psychological well-being. This paper will discuss these perceptual processes in more detail; in particular the individual differentiation in outcome expectancy and feedback from the stressful event.
2. Social defeat and the appraisal process
2.1. Controllability, predictability
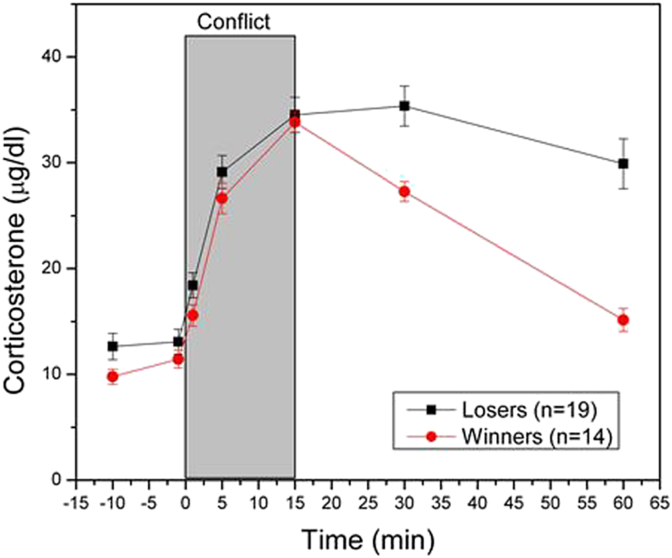
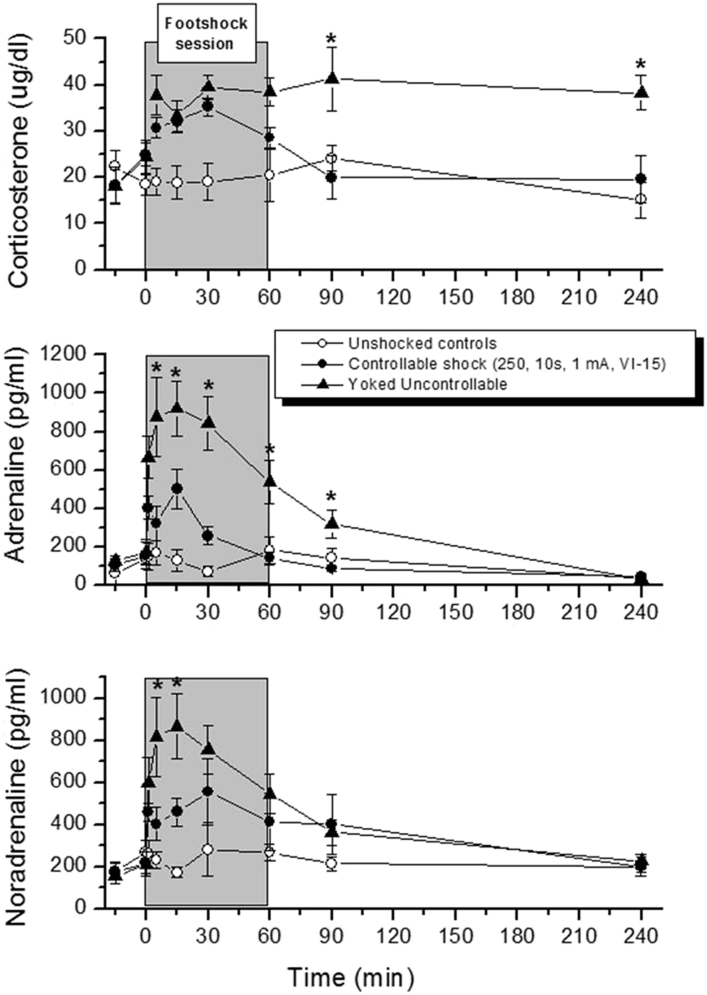
The terms controllability and predictability are central in the definition of a stressor. These terms date back to a series of elegant experiments by Martin Seligman, Steven Maier and Jay Weiss in the late sixties and early seventies of the last century (Seligman and Maier, 1967, Weiss, 1972). Using a yoked control stress paradigm, these authors concluded that it is not the physical nature of an aversive stimulus that induces somatic diseases such as stomach wall erosions or behavioral disorders such as learned helplessness, but rather the degree in which the stimulus can be controlled and/or predicted by an individual. Although the concept of controllability and predictability has strongly contributed to the present insights in stress physiology and the development of stress-related pathology, there are a few shortcomings in this concept. For example, there is evidence from the human literature that it is not the actual control that counts, but the perceived control (Salvador, 2005). This important insight necessitates a cautious interpretation of preclinical stress studies based on animal models. Stimuli that are considered as stressors from the anthropomorphic point of view may not necessarily be stressors from the animal point of view. In particular in social stress models, this is not always self-evident. This raises the question how to objectively assess whether a stimulus is perceived as a stressor in terms of predictability and controllability. In a recent paper, we argued that an uncontrollable condition can be distinguished from a controllable one by the adrenaline response and the slow recovery of the activated HPA axis and the SAM system (Koolhaas et al., 2011). This idea is illustrated for example in a comparison of the physiological response of a single social defeat with the response in the animal that wins the social interaction (Fig. 1). Although the magnitude of the acute corticosterone response is virtually identical, the recovery of the response takes almost twice as much time in the loser compared to the victor. The speed of recovery of the HPA axis response is determined by a delayed onset of negative feedback control mechanisms. This delayed onset includes a fast non-genomic action of glucocorticoids on neuronal excitability mediated by both mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) (De Kloet et al., 2008). It is suggested that the stressful nature of a stimulus acts in particular through this fast glucocorticoid action. Also the magnitude of the acute cardiovascular response to winning and losing a social interaction is identical, but the difference is in the recovery phase of this response. The defeated animal shows a delayed recovery (Koolhaas et al., 2011). Using controllable and yoked uncontrollable foot shocks in rats, Swenson and Vogel concluded already in 1983 that a delayed recovery of the corticosterone response and the release of adrenaline characterize an uncontrollable aversive situation (Swenson and Vogel, 1983). A graphic presentation of their original data is given in Fig. 2. Similar results were obtained in carefully controlled experiments using non-social stressors by de Boer and colleagues (de Boer et al., 1990). This central role of adrenaline in the acute stress response is consistent with more recent animal (Kvetnansky et al., 2013) and human research (Esler, 2010) demonstrating that the enhanced adrenaline co-transmission and/or the expression of the adrenaline synthesizing enzyme PNMT in sympathetic nerves may be an explicit biomarker of a recurrent chronic stress state and/or a pathophysiological pathway. Together, these findings suggest that a controllable stimulus can be distinguished from an uncontrollable one by the angle of the downward slope of the physiological responses and/or the presence of an adrenaline response. In experiments where repeated blood sampling is not possible, radio-telemetry techniques may be useful to assess the speed of recovery of autonomic physiological stress responses (heart rate, blood pressure) as an index of controllability.
Fig. 1.
Course of plasma corticosterone in male rats, before, during and after either winning or losing a social conflict. Animals were provided permanently implanted jugular vein cannula to allow undisturbed blood sampling during the social interaction (Koolhaas et al., 2011).
Fig. 2.
Course of plasma corticosterone, adrenaline and noradrenalin in a foot shock paradigm. Animals were either exposed to a situation in which they could switch off the shock (controllable) or a yoked control condition in which they received exactly the same amount of shocks as the controllable condition without having any control over it. The full control group did not receive any shocks (Swenson and Vogel, 1983).
Regarding predictability, it is important to notice that natural selection has sculpted physiology and behavior to meet the most likely environmental demands plus a modest safety margin. Thus, a physiological response is not only an attempt to defend a set-point, but rather a response to some prediction. McEwen has addressed this issue in his seminal work on allostasis (McEwen and Stellar, 1993, McEwen, 1998, McEwen and Wingfield, 2003). Allostasis is defined as the process of achieving stability through change in anticipation of physiological requirements (Sterling and Eyer, 1988). Indeed, in anticipation of a challenging situation, strong preparatory behavioral and physiological responses can be observed. This conditioned stress response may consist of flight or freezing behavior accompanied by a strong increase in heart rate and plasma levels of adrenaline and corticosterone (Korte et al., 1992, McCarty and Kopin, 1978). Hence, unpredictability can be assessed by the absence of such anticipatory responses.
A second problem with the classical concept of controllability and predictability is that they are generally operationally defined as binary factors; i.e. full control or complete absence of control often using strongly aversive stimuli. However, in everyday social life controllability may vary from absolute control, via threat to control to loss of control. For example, a dominant male may have full control in a stable social environment, but only partial control or may experience threat to lose control in socially unstable conditions. This condition, in which a dominant male has difficulties to control the social environment, leads to a cardiovascular type of stress pathology such as hypertension and cardiac arrhythmia's (Ely and Henry, 1978, Fokkema et al., 1995, Manuck et al., 1983, Sapolsky, 1995). Thus, while controllability/predictability generally reduces the impact of environmental challenges to the body, very effortful and demanding coping might actually be harmful. It may be argued that effortful and demanding coping has the risk of exceeding the healthy ability to cope and may represent non-coping in spite of the fact that the challenging event can be controlled. The graded degree of controllability and predictability in combination with the intensity of coping efforts in the development of stress-related pathology requires further attention. It is conceivable that individual characteristics in terms of the individual tendency to keep control will be an important factor in this (see below). An individual's appraisal of the social environment and its reaction may vary from full control to only partial to complete loss of control. Moreover, a stressor may be mild in terms of its potential consequences or it may be life-threatening. A traumatic event that is life-threatening is likely to be uncontrollable and unpredictable. Obviously, such events robustly activate the main neuroendocrine stress-responsive systems that, although initially adaptive, can become deregulated (i.e., either prolonged hyper- or hypo-(re)activity) and may increase vulnerability for stress-related psychopathologies (e.g., depression, anxiety disorders, fibromyalgia, fatigue, burn-out, PTSD).
2.2. Time domain
The chronic nature of stress is generally considered to be an important factor in the development of various forms of stress-related pathology. The first studies of chronic social stress in rats were performed by Calhoun, mainly from the perspective of population ecology (J. B. Calhoun, 1963; J. B. Calhoun, 1962). In addition to his elaborate studies of a natural population of wild rats, he created artificial rat and mice colonies in the lab. He allowed these colonies to grow to high population densities and found that with increasing densities, outbursts of extreme aggression occurred and the social structure of the colonies finally collapsed (J. B. Calhoun, 1973). After an initial explosive growth of the population, an increasing number of animals started to show clear signs of stress pathology leading to high mortality. These early studies were later criticized, mainly because of a too easy extrapolation of the results to human over-population. A second criticism was related to the rather artificial nature of these colonies with unlimited access to food and water and the absence of predators and migration. In fact, the extremely high population densities obtained in these studies would never occur in nature. However, later studies in feral mice confirmed the importance of social stress in the dynamics of natural populations (De Boer et al., 2016, Koolhaas et al., 1999, Van Oortmerssen, 1971).
Crowding is still used as a social stressor, although in a much more controlled manner. These studies are generally based on a comparison between groups of animals housed under different densities. This type of crowding has a range of cardiovascular effects including increased blood pressure and mesenteric artery reactivity and endothelial dysfunction, in particular in animals with a genetic predisposition for cardiovascular disease (Okruhlicova et al., 2008, Toot et al., 2011). A crucial factor in this crowding effect seems to be the stability of the social group. To make this social stress model more reproducible, authors have combined crowding with social instability, by regularly changing the composition of the group. This method was first introduced by Mormède (Mormede et al., 1990). His study already shows that the neuroendocrine effects of being exposed to chronic social instability depend on a complex interaction between individual characteristics and situational factors. Social instability is also one of the few effective social stress models in females (Herzog et al., 2009). More recent studies using this model mainly focus on the long-term consequences of social instability during adolescence both in males and in females (Toth and Neumann, 2013). In even more controlled studies chronic social stress is established by repeated social defeat, often combined with chronic exposure to the dominant male behind a wire mesh screen (Bartolomucci et al., 2005, Buwalda et al., 2005, Fuchs and Flugge, 2002, Veenema et al., 2005). For an extensive description of the effects of chronic (psycho)social stress in various animal species we refer to the paper by Pryce & Fuchs (this volume). Although repeated social defeat and repeated exposure to the dominant has clear neuroendocrine and behavioral effects in a wide variety of species, time may interfere in a rather complex way with the development of stress pathology. First, the procedure consists in fact of a series of intermittent exposures to stressors resulting in recurring stress responses. However, one should realize that after the first exposure to a stressor, adaptive processes such as consolidation of memory processes (see below) are activated at the same time. This implies that the response to subsequent exposures to the same stressor might be the net result of both adaptive and maladaptive processes. Second, several studies show that merely the factor time after the first exposure to a stressor is sufficient to cause changes in behavior and physiology. In preclinical social stress models, it was shown that a single or double social defeat on two consecutive days is sufficient to induce changes in behavior and neurobiology that gradually develop within three weeks and that may last for many months (Kole et al., 2004, Koolhaas et al., 1990, Koolhaas et al., 1997, Von Frijtag et al., 2000). Similarly, van Dijken observed progressive changes in behavior and HPA axis in the weeks following a single series of foot shocks (Van Dijken et al., 1992, van Dijken et al., 1993). This situation seems to be comparable to the PTSD patient, who often also experienced a short episode of stress or a single traumatic life event and may gradually develop disease symptoms months or years later. A more dynamic view of the response to a stressor implies that physiological and behavioral/cognitive processes are still changing long after the termination of the actual stressor. Evidence suggests that these dynamics are at a time scale of days, weeks and months. Consequently, the set of symptoms changes in the course of time after the stress experience.
2.3. Memory consolidation
To understand these temporal dynamics and its adaptive or maladaptive nature, we may learn from the current views on the role of stress hormones in the neurobiology of learning and memory and the role of sleep. The two main stress systems, the HPA axis and the SAM system play a major role in the consolidation of emotional memory. The action of these two systems on learning and memory strongly depends on the timing of the physiological response in relation to the stressor. Glucocorticoids from the adrenal cortex secreted during and immediately after the stressor enhance memory consolidation. They act directly on the amygdala which is the main brain area involved in emotional learning. This glucocorticoid action on memory consolidation is facilitated by circulating catecholamines from the SAM system. These catecholamines affect the processing of emotional events in the amygdala indirectly via afferent vagal nerves. In contrast to the enhancing effects on memory consolidation, memory retrieval is generally impaired by the activation of the HPA axis and adrenal medulla. For recent reviews on stress and memory, we refer to Schwabe et al., 2014 and Wolf et al., 2015 (Schwabe and Wolf, 2014, Wolf et al., 2015). The effects on memory consolidation seem to be most important for understanding the long-term consequences of stressors. A crucial factor in the consolidation of memory is sleep. It is generally thought that re-experiencing events of the past day during the sleep phase is essential for the storage of information into long-term memory (Stickgold, 2013). Sleep is essential for the strengthening and qualitative reorganization of new memories (Landmann et al., 2014). Several studies have indicated that sleep may particularly benefit the storage of emotional memories, more so than the storage of neutral information (Hu et al., 2006, Wagner et al., 2001), and such selective positive effects of sleep on emotional memory may persist for years (Wagner et al., 2006). Re-experiencing of stressful events may therefore be considered as repeated exposure to stressors and may in that way be perceived as chronic stress, despite the fact that the actual stressor has long passed by. Indeed, van Liempt and co-authors found enhanced HPA and the SAM activity related to an increased sleep fragmentation in PTSD patients (van Liempt et al., 2013). It is tempting to consider the possibility that this sleep associated re-experiencing, rumination or reappraisal of stressors and the associated activation of physiological stress systems will contribute to a further consolidation of the memory process and experience of chronic stress. At this stage, it seems relevant to mention that the development of long-term effects of stressors are prevented by social housing (Berardi et al., 2014, Fuzzo et al., 2015, Ishii et al., 2016, Ruis et al., 1999). This social buffering is not specific for social stress; it also reduces for example the effects of chronic mild stress and enhances the extinction of conditioned fear responses (Mikami et al., 2016, Westenbroek et al., 2003). Further research is required to test the hypothesis that social buffering is mediated by interfering with memory consolidation. This hypothesis may predict for example that social housing is most effective immediately after the stressor (i.e. the initial memory consolidation phase).
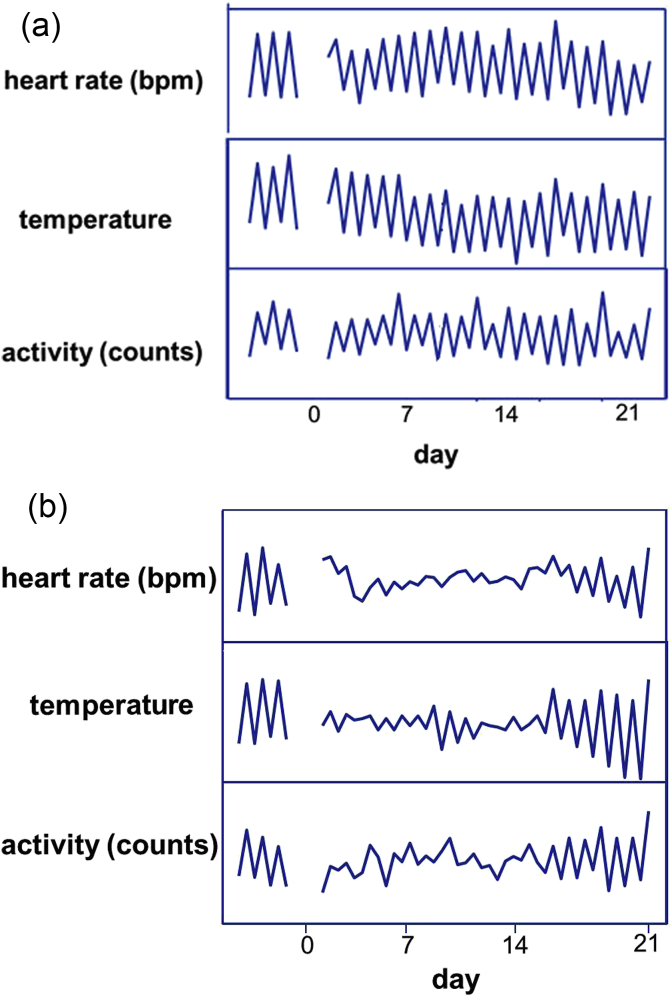
When consolidation of emotional memory is indeed the core process in both the adaptive and the maladaptive response to stressors, it becomes important to ask the question what exactly is stored and remembered. Some recent preclinical studies using social defeat in rats may shed light on this. The long-term behavioral and physiological consequences of social defeat seem to depend on the behavior of the losing animal during the actual social interaction itself. Animals that do resist the dominant for a while before true subjugation or losing the fight hardly show any long-term consequences. However, defeated animals that did not resist the dominant initially or showed clear signs of submission without any behavioral counter actions appeared to develop long-term changes in behavior and physiology (Kinn Rod et al., 2014, Meerlo et al., 1999, Walker et al., 2009, Wood et al., 2010). Fig. 3 illustrates this phenomenon using the circadian rhythm of heart rate, body temperature and activity as read out. This leads to the hypothesis that the direct feedback or evaluation of the victim's actions during the actual stressful event is crucial for its long-term consequences. Appraisal is then not only the appreciation of the challenging environment, but just as well the direct cognitive processing of the coping efforts with that challenge. Despite the overall negative outcome, which is defeat, this evaluation of its own actions might still be positive, i.e. ‘given my limited resources, I have done my best’. If the end result of this evaluation process is however negative (‘I could have done better and took the wrong decisions’), this might be the start of a downward spiral in the subsequent memory consolidation process. Although we realize that there is a serious risk of anthropomorphic reasoning based on preclinical studies in rats, we feel that understanding the cognitive processing of the victim's own behavioral response during the stressful situation itself is the key to understanding whether or not pathological consequences may occur i.e., maladaptation.
Fig. 3.
Circadian amplitude of heart rate, body temperature and activity of male rats before and after a social defeat measured by permanently implanted radio telemetry equipment. The animals were experienced winners before the exposure to social defeat. Graph A: animals that initially resisted the dominant male; Graph B: animals that submitted without resistance (Meerlo et al., 1999).
3. Individual differentiation
It is important to realize that individuals may strongly differ in the way they deal with environmental challenges. Taking post-traumatic stress disorder (PTSD) as an example, a traumatic-like event will trigger PTSD only in about 10–15% of the individuals, despite exposure to similar uncontrollable, unpredictable and potentially life-threatening situations (Creamer et al., 2006, Kessler et al., 2005). Similar percentages were obtained in a rat model of PTSD (Cohen and Zohar, 2004, Cohen et al., 2004). The notion that the majority of the population is resilient to traumatic life events has led to a paradigm shift in preclinical stress research. It appears to be essential to dissociate susceptible from resilient individuals on the basis of predefined behavioral and physiological characteristics. Considering again the recovery rate of stress-induced changes as mentioned earlier, it is tempting to speculate that the return time to baseline of stress responses may even reflect resilience level, and that those who have slow recovery may represent a risk group for developing pathologies. Relatively few studies take this approach (Russo et al., 2012, Schmidt, 2010). In addition to these experimental studies, there is accumulating evidence from a wide variety of species living in the wild that individuals may differ in their coping style (Koolhaas et al., 1999). Recent ecological evidence shows that these coping styles should be considered as individual adaptations to differential environmental conditions. Resilience and vulnerability then becomes a matter of match or mismatch between coping style and environmental demands. This individual variation in coping style within a species has fitness value and apparently protects the species against fluctuations in the natural environment (Dingemanse and Wolf, 2010, Sih et al., 2004). Understanding the individual susceptibility to stress related disease in a social environment may benefit from understanding of the biological basis and adaptive significance of this individual differentiation in coping style.
The term coping style refers to alternative response patterns in reaction to challenges that are stable over time and across various situations (Koolhaas et al., 1999). For example, animals characterized by a proactive coping style are offensive towards male conspecific rivals, are impulsive in decision-making, score high in frustration tests, take risks in the face of potential dangers and are novelty seekers (David et al., 2004, Groothuis and Carere, 2005, Steimer and Driscoll, 2005). The reactive coping style is characterized by low levels of offensive aggression and a more readily acceptance of environmental changes. Extensive studies, using a variety of learning tasks, show that behavioral flexibility is one of the main differences between proactive and reactive coping (Coppens et al., 2010). The proactive individual acts on the basis of predictions, whereas the reactive individual relies more on direct environmental input. This difference can be demonstrated for example in a reversal learning task. The proactive individual has great difficulties in changing from a once learned task into a new one. It is rigid and makes much more mistakes than a reactive coping individual (Benus et al., 1990, Bolhuis et al., 2004, Coppens et al., 2010). When the behavior of a proactive coping individual is indeed mainly based on previous experience, we have to reconsider the concepts of predictability and controllability. As discussed above, the general stress literature emphasizes the importance of unpredictability of stressors. However, notice that unpredictable is not the same as a wrong prediction. Intuitively, one may expect a large impact when an originally fully predictable and controllable situation suddenly deteriorates and becomes unpredictable and uncontrollable. It is surprising that the factor outcome expectancy has not been studied more frequently. Amat and co-workers showed that previous experience with a controllable foot shock made male rats more resilient to a subsequent uncontrollable foot shock (Amat et al., 2006). However, when the previously positive experiences suddenly change into a negative one, rats seem to be more vulnerable. This is also demonstrated for example in an experiment in which animals were socially defeated after they had already ten winning experiences (Meerlo et al., 1999). A single social defeat in part of these experienced winners completely abolished their circadian amplitude of heart rate, body temperature and physical activity for a long period of time Fig. 3b. Similarly, losing territory ownership and lowering in social rank has been demonstrated to exert greater immune suppressing effects than social subordination per se in a mouse model of chronic subordination stress (Bartolomucci, 2007). This again suggests that a violation of positive outcome expectancy might be a serious or even traumatic experience particularly in proactive individuals that strongly rely on predictions and expectancies. As a consequence of this line of reasoning, one may predict a large difference between social defeat in the victim's home territory and social defeat in someone else's territory. Unfortunately, there are to the best of our knowledge no experimental data yet to support this hypothesis.
4. Concluding remarks
The line of reasoning presented above has its consequences for both clinical and preclinical stress research. Stress research is notoriously known for its large individual variation in both the acute stress response and the development of stress related disease. A fundamental question that might explain this variation but has hardly been addressed in most studies is whether the condition to which the individual is exposed is indeed perceived as a stressor. To answer this question, research should pay attention to the speed of recovery of the physiological response and the presence or absence of an anticipatory response. In addition, the presence of a pronounced plasma adrenaline response can be considered as an indicator that a challenge is indeed perceived as uncontrollable and hence as a stressor. In line with this, the presence of adrenaline co-transmission and the progressive induction of the adrenaline synthesizing enzyme PNMT in sympathetic nerves may be an explicit biomarker of a recurrent chronic stress state and/or a pathophysiological course. By taking these aspects of the physiological response into account one might be able to distinguish susceptible individuals that have perceived the challenge as a stressor from resilient individuals that somehow managed to cope with the challenge. This focus on the individual is also important in preclinical studies using animal models. Studies of coping style and animal personality in a wide variety of species show that individuals are differentially optimized for different environmental conditions. This growing body of literature also implies that a certain challenge might be perceived as a stressor only by one type of individual and not by the other.
Although the concept of uncontrollability and unpredictability as main characteristics of a stressor are well accepted, it is important to notice that individuals are usually not naïve in everyday life. They have predictions and individuals may even differ in the degree in which they rely on these predictions. Therefore, research should pay more attention to the concept of outcome expectancies and wrong predictions by explicitly including previous experience in the experimental approach. For this, standardized social housing conditions are particularly important both preceding and during the experiment. To avoid confounding influences of social isolation or formation or social hierarchies, we recommend housing males with sterilized females.
In view of the suggestion that adaptation and maladaptation is strongly based on the processes of memory consolidation it seems important to consider in more detail what exactly is remembered from the stressful event. Recent studies of social defeat in rats suggest that feedback from the victim's own actions during the stressful event might be a crucial determinant of the course of subsequent adaptive or maladaptive processes. This requires a detailed assessment of the behavior of the victim during the stressful event and the victim's own appraisal of this event.
When we consider stress psychopathology as a disease of adaptation, it is conceivable that pathology develops in situations of a mismatch between the adaptive process and the actual environment. Such a match/mismatch hypothesis was originally developed in relation to the adult consequences of early life stress. This hypothesis suggests that early life stress prepares the individual for the environmental conditions that it is likely to meet in adulthood. When these adult conditions indeed match to the early life environment, the individual is resilient. However, in a situation where the adult environment differs from the conditions in which the individual grew up (mismatch), it may develop stress pathology (Gluckman et al., 2007, Sachser et al., 2011, Schmidt, 2010). This hypothesis may also hold for the behavioral and physiological changes induced by stressors in adulthood. In this view, the behavioral and physiological changes induced by a stressor may be adaptive and support survival in situations in which there is a high likelihood of similar environmental conditions. However, these changes are useless when similar stressors never occur again. In that situation, there is a mismatch and stress pathology can be considered as a disease of adaptation. In a social environment, this means that the more reactive, subordinate animal is resilient due to its high flexibility. The proactive individual that strongly relies on predictions is resilient under stable environmental conditions but vulnerable when outcome expectancies are suddenly violated. For example a dominant male that loses its dominant position usually ends as a social outcast with poor health.
Finally, the view presented above is mainly based on experiments in male rats. One may wonder to what extent the same perceptual processes may hold for other rodent species and for females. Unfortunately, physiological evidence supporting our view to assess the appraisal of a situation as a stressor (uncontrollable, unpredictable) is scarce. There is a general lack of data on plasma catecholamines in other species. In addition, there is usually an insufficient resolution in time of the neuroendocrine response to allow an accurate assessment of the downward slope of the response. With respect to the other aspects of the appraisal process discussed above, most of the evidence is rather anecdotal. However, the available data suggest that our line of reasoning can be generalized to other species. For example, social buffering and the underlying mechanisms has been extensively studied in male and female voles (Gobrogge and Wang, 2015) and has also been demonstrated in male mice (Adamcio et al., 2008, Liu et al., 2013) female rats (Westenbroek et al., 2003) and Guinee pigs (Hennessy et al., 2009, ). A recent study in mice confirms the temporal dynamics of the stress response in the days and weeks after the termination of the stressor (Jacobson-Pick et al., 2013). A large body of literature shows coping styles and their function in nature in a wide variety of species. It goes beyond the scope of this paper to discuss this extensively, and we may refer to some excellent reviews (Carere and Maestripieri, 2013, Dingemanse and Wolf, 2010). It shows however that individual differentiation in appraisal and coping processes is widespread in nature and seems to have a clear function in the population ecology of the species. In conclusion, we feel that social stress research in general and the validity of preclinical studies in particular may benefit from a shift in focus towards appraisal and the individual differentiation in the appraisal process.
Contributor Information
J.M. Koolhaas, Email: j.m.koolhaas@rug.nl.
S.F. de Boer, Email: s.f.de.boer@rug.nl.
B. Buwalda, Email: b.buwalda@rug.nl.
P. Meerlo, Email: p.meerlo@rug.nl.
References
- Adamcio B., Sargin D., Stradomska A., Medrihan L., Gertler C., Theis F., Ehrenreich H. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008;6 doi: 10.1186/1741-7007-6-37. 37–7007-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J., Paul E., Zarza C., Watkins L.R., Maier S.F. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. Official J. Soc. Neurosci. 2006;26(51):13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. doi:26/51/13264 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 2007;28(0091-3022; 0091-3022; 1):28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A., Palanza P., Sacerdote P., Panerai A.E., Sgoifo A., Dantzer R., Parmigiani S. Social factors and individual vulnerability to chronic stress exposure. Neurosci. Biobehav. Rev. 2005;29(1):67–81. doi: 10.1016/j.neubiorev.2004.06.009. doi:S0149-7634(04)00152-6 [pii] [DOI] [PubMed] [Google Scholar]
- Benus R.F., Den Daas S., Koolhaas J.M., van Oortmerssen G.A. Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour. 1990;112:176–193. [Google Scholar]
- Berardi A., Trezza V., Palmery M., Trabace L., Cuomo V., Campolongo P. An updated animal model capturing both the cognitive and emotional features of post-traumatic stress disorder (PTSD) Front. Behav. Neurosci. 2014;8:142. doi: 10.3389/fnbeh.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis J.E., Schouten W.G.P., Leeuw J.A.D., Schrama J.W., Wiegant V.M. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav. Brain Res. 2004;152(2):351–360. doi: 10.1016/j.bbr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Kole M.H., Veenema A.H., Huininga M., de Boer S.F., Korte S.M., Koolhaas J.M. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci. Biobehav. Rev. 2005;29(1):83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Scholte J., de Boer S.F., Coppens C.M., Koolhaas J.M. The acute glucocorticoid stress response does not differentiate between rewarding and aversive social stimuli in rats. Hormones Behav. 2012;61(2):218–226. doi: 10.1016/j.yhbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Calhoun J.B. Population density and social pathology. Sci. Am. 1962;206:139–148. doi: 10.1038/scientificamerican0262-139. [DOI] [PubMed] [Google Scholar]
- Calhoun J.B. U.S. Government Printing Office; Washington: 1963. The Ecology and Sociology of the Norway Rat. U.S. Public Health Service Publication No. 1008. [Google Scholar]
- Calhoun J.B. Death squared: the explosive growth and demise of a mouse population. Proc. R. Soc. Med. 1973;66(1 Pt 2):80–88. [PMC free article] [PubMed] [Google Scholar]
- Carere C., Maestripieri D. Animal Personalities: Behavior, Physiology and Evolution. University of Chicago Press; Chicago,London: 2013. [Google Scholar]
- Chaouloff F. Social stress models in depression research: what do they tell us? Cell Tissue Res. 2013;354(1):179–190. doi: 10.1007/s00441-013-1606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. N. Y. Acad. Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. doi:1032/1/167 [pii] [DOI] [PubMed] [Google Scholar]
- Cohen H., Zohar J., Matar M.A., Zeev K., Loewenthal U., Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29(0893–133; 0006–3223; 11):1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- Coppens C.M., de Boer S.F., Koolhaas J.M. Coping styles and behavioural flexibility: towards underlying mechanisms. Philosophical Trans. R. Soc. Lond. Ser. B, Biol. Sci. 2010;365(1560):4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M., Carboon I., Forbes A.B., McKenzie D.P., McFarlane A.C., Kelsall H.L., Sim M.R. Psychiatric disorder and separation from military service: a 10-year retrospective study. Am. J. Psychiatry. 2006;163(4):733–734. doi: 10.1176/ajp.2006.163.4.733. doi:163/4/733 [pii] [DOI] [PubMed] [Google Scholar]
- David J.T., Cervantes M.C., Trosky K.A., Salinas J.A., Delville Y. A neural network underlying individual differences in emotion and aggression in male golden hamsters. Neuroscience. 2004;126(3):567–578. doi: 10.1016/j.neuroscience.2004.04.031. [DOI] [PubMed] [Google Scholar]
- de Boer S.F., de Beun R., Slangen J.L., van der Gugten J. Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol. Behav. 1990;47(0031–9384; 4):691–698. doi: 10.1016/0031-9384(90)90079-j. [DOI] [PubMed] [Google Scholar]
- De Boer S.F., Buwal da B., Koolhaas J.M. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Van Dijken H.H., Van der Heyden J.A., Mos J., Tilders F.J. Inescapable footshocks induce progressive and long-lasting behavioural changes in male rats. Physiology Behav. 1992;51(4):787–794. doi: 10.1016/0031-9384(92)90117-k. doi:0031-9384(92)90117-K [pii] [DOI] [PubMed] [Google Scholar]
- Dingemanse N.J., Wolf M. Recent models for adaptive personality differences: a review. Philosophical Trans. R. Soc. Lond. Ser. B, Biol. Sci. 2010;365(1560):3947–3958. doi: 10.1098/rstb.2010.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely D.L., Henry J.P. Neuroendocrine response patterns in dominant and subordinate mice. Horm. Behav. 1978;10(0018–506; 0018–506; 2):156–169. doi: 10.1016/0018-506x(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Esler M. The 2009 carl ludwig lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J. Appl. Physiology (Bethesda, Md. 1985) 2010;108(2):227–237. doi: 10.1152/japplphysiol.00832.2009. [DOI] [PubMed] [Google Scholar]
- Fokkema D.S., Koolhaas J.M., van der G.J. Individual characteristics of behavior, blood pressure, and adrenal hormones in colony rats. Physiol. Behav. 1995;57(0031–9384; 0031–9384; 5):857–862. doi: 10.1016/0031-9384(94)00333-z. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Flugge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol. Biochem. Behav. 2002;73(1):247–258. doi: 10.1016/s0091-3057(02)00795-5. doi:S0091305702007955 [pii] [DOI] [PubMed] [Google Scholar]
- Fuzzo F., Matsumoto J., Kiyokawa Y., Takeuchi Y., Ono T., Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioral and neurophysiological evidence. Front. Neurosci. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P.D., Hanson M.A., Beedle A.S. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 2007;19(1042–0533; 1042–0533; 1):1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Gobrogge K., Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: lessons from voles. Hormones Behav. 2015;76:91–105. doi: 10.1016/j.yhbeh.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders N.E. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 2002;301(3):785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Gray J.M., Chaouloff F., Hill M.N. To stress or not to stress: A question of models. Current Protocols in Neuroscience / Editorial Board, Jacqueline N.Crawley [Et Al.] Vol. 70. 2015. p. 8. 33.1-22. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G., Carere C. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 2005;29(0149–7634; 0149–7634; 1):137–150. doi: 10.1016/j.neubiorev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Hennessy J.W., Levine S. Stress, arousal, and the pituitary-adrenal system: a psychoendocrine hypothesis. Progr. Psychobiol. Physiol. Psychol. 1979;8:133–174. [Google Scholar]
- Hennessy M.B., Kaiser S., Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 2009:1095–6808. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Herzog C.J., Czeh B., Corbach S., Wuttke W., Schulte-Herbruggen O., Hellweg R., Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159(3):982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Hu P., Stylos-Allan M., Walker M.P. Sleep facilitates consolidation of emotional declarative memory. Psychol. Sci. 2006;17(10):891–898. doi: 10.1111/j.1467-9280.2006.01799.x. doi:PSCI1799 [pii] [DOI] [PubMed] [Google Scholar]
- Ishii A., Kiyokawa Y., Takeuchi Y., Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Hormones Behav. 2016;81:53–58. doi: 10.1016/j.yhbeh.2016.03.003. doi:S0018-506X(15)30166-5 [pii] [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S., Audet M.C., McQuaid R.J., Kalvapalle R., Anisman H. Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PloS One. 2013;8(4):e60133. doi: 10.1371/journal.pone.0060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Merikangas K.R., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. doi:62/6/617 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E.R., Karst H., Joels M. Corticosteroid hormones in the central stress response: quick-and-slow. Front. Neuroendocrinol. 2008;29(1095–6808; 0091–3022; 2):268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kole M.H., Costoli T., Koolhaas J.M., Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125(0306–4522; 2):337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Hermann P.M., Kemperman C., Bohus B., Hoofdakker R.H., Beersma D.G. Single social interaction leading to defeat in male rats induces a gradual, but long-lasting behavioral change: a model of depression? Neurosci. Res. Commun. 1990;7:35. [Google Scholar]
- Koolhaas J.M., Meerlo P., De Boer S.F., Strubbe J.H., Bohus B. The temporal dynamics of the stress response. Neurosci. Biobehav. Rev. 1997;21(6):775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Korte S.M., De Boer S.F., Van Der Vegt B.J., Van Reenen C.G., Hopster H., Blokhuis H.J. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23(7):925–935. doi: 10.1016/s0149-7634(99)00026-3. doi:S0149-7634(99)00026-3 [pii] [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., de Boer S.F., Flugge G., Korte S.M., Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Korte S.M., Buwalda B., Bouws G.A., Koolhaas J.M., Maes F.W., Bohus B. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiology Behav. 1992;51(4):815–822. doi: 10.1016/0031-9384(92)90120-q. doi:0031-9384(92)90120-Q [pii] [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Lu X., Ziegler M.G. Stress-triggered changes in peripheral catecholaminergic systems. Adv. Pharmacol. (San Diego, Calif.) 2013;68:359–397. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
- Landmann N., Kuhn M., Piosczyk H., Feige B., Baglioni C., Spiegelhalder K., Nissen C. The reorganisation of memory during sleep. Sleep. Med. Rev. 2014;18:531. doi: 10.1016/j.smrv.2014.03.005. doi:S1087-0792(14)00026-4 [pii] [DOI] [PubMed] [Google Scholar]
- Liu X., Wu R., Tai F., Ma L., Wei B., Yang X., Jia R. Effects of group housing on stress induced emotional and neuroendocrine alterations. Brain Res. 2013;1502:71–80. doi: 10.1016/j.brainres.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Manuck S.B., Kaplan J.R., Clarkson T.B. Behaviorally induced heart rate reactivity and atherosclerosis in cynomolgous monkeys. Psychosom. Med. 1983;45:95–108. doi: 10.1097/00006842-198305000-00002. [DOI] [PubMed] [Google Scholar]
- McCarty R., Kopin I.J. Changes in plasma catecholamines and behavior of rats during the anticipation of footshock. Hormones Behav. 1978;11(2):248–257. doi: 10.1016/0018-506x(78)90052-1. doi:0018-506X(78)90052-1 [pii] [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840(0077–8923):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual. mechanisms leading to disease. Arch. Intern. Med. 1993;153(0003–9926; 18):2093–2101. [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43(0018–506; 1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Sgoifo A., de Boer S.F., Koolhaas J.M. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav. Neurosci. 1999;113(0735–7044; 6):1283–1290. doi: 10.1037//0735-7044.113.6.1283. [DOI] [PubMed] [Google Scholar]
- Mikami K., Kiyokawa Y., Takeuchi Y., Mori Y. Social buffering enhances extinction of conditioned fear responses in male rats. Physiology Behav. 2016;163:123–128. doi: 10.1016/j.physbeh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Mormede P., Lemaire V., Castanon N., Dulluc J., Laval M., Le Moal M. Multiple neuroendocrine responses to chronic social stress: interaction between individual characteristics and situational factors. Physiology Behav. 1990;47(6):1099–1105. doi: 10.1016/0031-9384(90)90358-b. [DOI] [PubMed] [Google Scholar]
- Nesse R.M. Is depression an adaptation? Archives General Psychiatry. 2000;57(1):14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nesse R.M., Bhatnagar S., Ellis B. Evolutionary origins and functions of the stress response system. In: Fink G., editor. Academic Press; Amsterdam: 2016. (Handbook of Stress). (Volume 1 ed., pp. 95–100) [Google Scholar]
- Okruhlicova L., Dlugosova K., Mitasikova M., Bernatova I. Ultrastructural characteristics of aortic endothelial cells in borderline hypertensive rats exposed to chronic social stress. Physiological Res./Acad. Sci. Bohemoslovaca. 2008;57(Suppl. l 2):S31–S37. doi: 10.33549/physiolres.931549. doi:1549 [pii] [DOI] [PubMed] [Google Scholar]
- Plaut S.M., Friedman S.B. Stress, coping behavior and resistance to disease. Psychotherapy Psychosomatics. 1982;38(1):274–283. doi: 10.1159/000287635. [DOI] [PubMed] [Google Scholar]
- Kinn Rod A.M., Murison R., Mrdalj J., Milde A.M., Jellestad F.K., Overnes L.A., Gronli J. Effects of social defeat on sleep and behaviour: importance of the confrontational behaviour. Physiology Behav. 2014;127:54–63. doi: 10.1016/j.physbeh.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Ruis M.A., te Brake J.H., Buwalda B., de Boer S.F., Meerlo P., Korte S.M., Koolhaas J.M. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24(0306–4530; 0306–4530; 3):285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachser N., Hennessy M.B., Kaiser S. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci. Biobehav. Rev. 2011;35(7):1518–1533. doi: 10.1016/j.neubiorev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Salvador A. Coping with competitive situations in humans. Neurosci. Biobehav. Rev. 2005;29(0149–7634; 0149–7634; 1):195–205. doi: 10.1016/j.neubiorev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Social subordinance as a marker of hypercortisolism. some unexpected subtleties. Ann. N. Y. Acad. Sci. 1995;771(0077–8923; 0077–8923):626–639. doi: 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.001. (1873–3360; 0306-4530) [DOI] [PubMed] [Google Scholar]
- Schwabe L., Wolf O.T. Timing matters: temporal dynamics of stress effects on memory retrieval. Cognitive, Affect. Behav. Neurosci. 2014;14(3):1041–1048. doi: 10.3758/s13415-014-0256-0. [DOI] [PubMed] [Google Scholar]
- Seligman M.E., Maier S.F. Failure to escape traumatic shock. J. Exp. Psychol. 1967;74(1):1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A., Johnson J.C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Steimer T., Driscoll P. Inter-individual vs line/strain differences in psychogenetically selected roman high-(RHA) and low-(RLA) avoidance rats: neuroendocrine and behavioural aspects. Neurosci. Biobehav. Rev. 2005;29(0149–7634; 1):99–112. doi: 10.1016/j.neubiorev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sterling P., Eyer J. Allostasis a new paradigm to explain arousal pathology. In: Fisher S., Reason J., editors. Handbook of Life Stress Cognition and Health. Oxford: Oxford University Press; 1988. pp. 629–650. [Google Scholar]
- Stickgold R. Parsing the role of sleep in memory processing. Curr. Opin. Neurobiol. 2013;23(5):847–853. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R.M., Vogel W.H. Plasma catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol. Biochem. Behav. 1983;18(5):689–693. doi: 10.1016/0091-3057(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Toot J.D., Reho J.J., Novak J., Dunphy G., Ely D.L., Ramirez R.J. Colony social stress differentially alters blood pressure and resistance-sized mesenteric artery reactivity in SHR/y and WKY male rats. Stress (Amsterdam, Neth. 2011;14(1):33–41. doi: 10.3109/10253890.2010.491876. [DOI] [PubMed] [Google Scholar]
- Toth I., Neumann I.D. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354(1):107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- van Dijken H.H., de Goeij D.C., Sutanto W., Mos J., de Kloet E.R., Tilders F.J. Short inescapable stress produces long-lasting changes in the brain-pituitary-adrenal axis of adult male rats. Neuroendocrinology. 1993;58(1):57–64. doi: 10.1159/000126512. [DOI] [PubMed] [Google Scholar]
- van Liempt S., Arends J., Cluitmans P.J., Westenberg H.G., Kahn R.S., Vermetten E. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38(1):155–165. doi: 10.1016/j.psyneuen.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Van Oortmerssen G.A. Biological significance, genetics and evolutionary origin of variability in behaviour within and between inbred strains of mice (mus musculus). A behaviour genetic study. Behaviour. 1971;38(1):1–92. doi: 10.1163/156853971x00014. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Sijtsma B., Koolhaas J.M., de Kloet E.R. The stress response to sensory contact in mice: genotype effect of the stimulus animal. Psychoneuroendocrinology. 2005;30(6):550–557. doi: 10.1016/j.psyneuen.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Von Frijtag J.C., Reijmers L.G., Van der Harst J.E., Leus I.E., Van denBos R., Spruijt B.M. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav. Brain Res. 2000;117(0166–4328; 0166–4328; 1–2):137–146. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Wagner U., Gais S., Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn. Mem. (Cold Spring Harb. N.Y.) 2001;8(2):112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U., Hallschmid M., Rasch B., Born J. Brief sleep after learning keeps emotional memories alive for years. Biol. Psychiatry. 2006;60(7):788–790. doi: 10.1016/j.biopsych.2006.03.061. doi:S0006-3223(06)00542-7 [pii] [DOI] [PubMed] [Google Scholar]
- Walker F.R., Masters L.M., Dielenberg R.A., Day T.A. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neuroscience. 2009;162(2):244–253. doi: 10.1016/j.neuroscience.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Weiss J.M. Influence of psychological variables on stress-induced pathology. In: Porter R., Knight J., editors. Physiology, Emotion and Psychosomatic Illness. CIBA Foundation Symposium () Elsevier; Amsterdam: 1972. [DOI] [PubMed] [Google Scholar]
- Westenbroek C., Ter Horst G.J., Roos M.H., Kuipers S.D., Trentani A., den Boer J.A. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2003;27(1):21–30. doi: 10.1016/s0278-5846(02)00310-x. doi:S0278-5846(02)00310-X [pii] [DOI] [PubMed] [Google Scholar]
- Wolf O.T., Atsak P., de Quervain D.J., Roozendaal B., Wingenfeld K. Stress and memory: a selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. J. Neuroendocrinol. 2015 doi: 10.1111/jne.12353. [DOI] [PubMed] [Google Scholar]
- Wood S.K., Walker H.E., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]