Abstract
Various fungi have the ability to colonize surfaces and to form biofilms. Fungal biofilm-associated infections are frequently refractory to targeted treatment because of resistance to antifungal drugs. One fungus that frequently colonises the respiratory tract of cystic fibrosis (CF) patients is the opportunistic black yeast–like fungus Exophiala dermatitidis. We investigated the biofilm-forming ability of E. dermatitidis and its susceptibility to various antiinfective agents and natural compounds. We tested 58 E. dermatitidis isolates with a biofilm assay based on crystal violet staining. In addition, we used three isolates to examine the antibiofilm activity of voriconazole, micafungin, colistin, farnesol, and the plant derivatives 1,2,3,4,6-penta-O-galloyl-b-D-glucopyranose (PGG) and epigallocatechin-3-gallate (EGCG) with an XTT reduction assay. We analysed the effect of the agents on cell to surface adhesion, biofilm formation, and the mature biofilm. The biofilms were also investigated by confocal laser scan microscopy. We found that E. dermatitidis builds biofilm in a strain-specific manner. Invasive E. dermatitidis isolates form most biomass in biofilm. The antiinfective agents and the natural compounds exhibited poor antibiofilm activity. The greatest impact of the compounds was detected when they were added prior cell adhesion. These findings suggest that prevention may be more effective than treatment of biofilm-associated E. dermatitidis infections.
The fungus Exophiala dermatitidis frequently colonises the respiratory tract of cystic fibrosis (CF) patients. Numerous studies have reported that the rate of occurrence of E. dermatitidis in CF patients ranges from 1% to 19%1,2. In addition, E. dermatitidis causes phaeohyphomycosis in immunosuppressed patients and in the central nervous system of immunocompetent Asian patients3,4. Outside the human body, E. dermatitidis occurs in warm and humid areas and is therefore believed to originate in tropical climates5. It is also encountered worldwide in the man-made environment, for example in dishwashers, steam baths and sauna facilities6.
Exophiala dermatitidis is metabolically active over a wide range of temperatures and is also known to be stable at extreme pH values7. Belonging to the family of black yeast-like fungi, E. dermatitidis is characterized by a melanized thick multi-layered cell wall. This darkly pigmented cell wall is linked with resistance to antifungal agents and extreme environmental conditions8. In addition, its dimorphic character is associated with pathogenicity9. The ability of this yeast to switch morphologically from the yeast state to the hyphae state is a virulence factor and an indicator of biofilm formation, because this switch is part of the biofilm formation process, as showed for Candida spp.10.
The life form “biofilm” prohibits the clearance of infections and results in chronic recurrent infections11. The embedded life mode of the microbes in the extracellular matrix of the biofilm protects the fungus against the host defence and antiinfective agents. A recent study found that E. dermatitidis can form biofilm. Sav et al., in an investigation of the biofilm behavior of 137 environmental and 7 clinical E. dermatitidis isolates, detected the ability of E. dermatitidis to form biofilm in 15% of environmental isolates and 29% of clinical isolates12.
We analyzed a set of 58 E. dermatitidis strains of various origins (CF, environmental, invasive) and compared their ability to form biofilm. Furthermore, we tested the antibiofilm activity of several antiinfective agents, the quorum-sensing molecule (QSM) farnesol, and two natural compounds with antibiofilm activity.
Results
Exophiala dermatitidis is a biofilm builder
To evaluate the biofilm formation capability of E. dermatitidis, we performed a biofilm formation assay using a total of 58 isolates of various origins. Of these isolates, 15 originated from non-CF patients, 35 originated from CF patients, and 8 originated from the environment. The crystal violet (CV)-based assay showed that E. dermatitidis was able to form biofilm. Of note, biofilm formation turned out to be strain specific, with higher amount of biomass in biofilm after 48 hours than after 24 hours (P = 0.0098). A total of 36% of the tested E. dermatitidis isolates formed biofilms with a biomass equal to or higher than the biomass of the biofilms formed by C. albicans (Figs 1, 2 and 3).
Figure 1. Relative biofilm formation of Candida albicans (ATCC 90028) and various isolates of Exophiala dermatitidis.
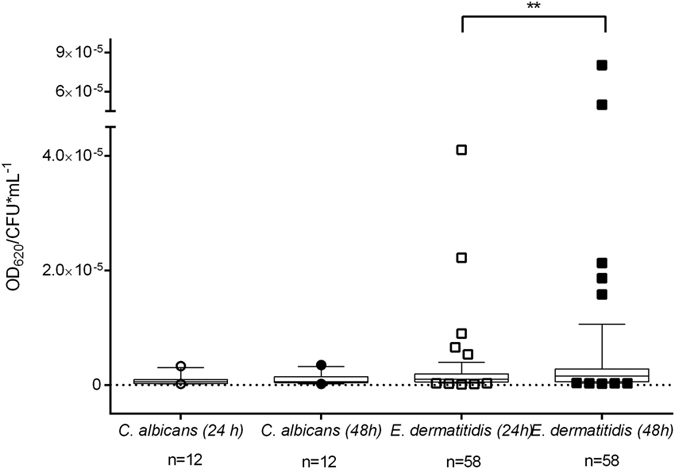
Box & whiskers with 10–90 percentile. The biofilm formation wasdetected by staining with crystal violet (0.1%) for 20 minutes. Biofilm formation at 35 °C over 24 and 48 hours. Each data point represents the mean of at least three independent experiments. *P < 0.05 as determined by Student’s t-test. OD620 = optical density at 620 nm. CFU = colony-forming units.
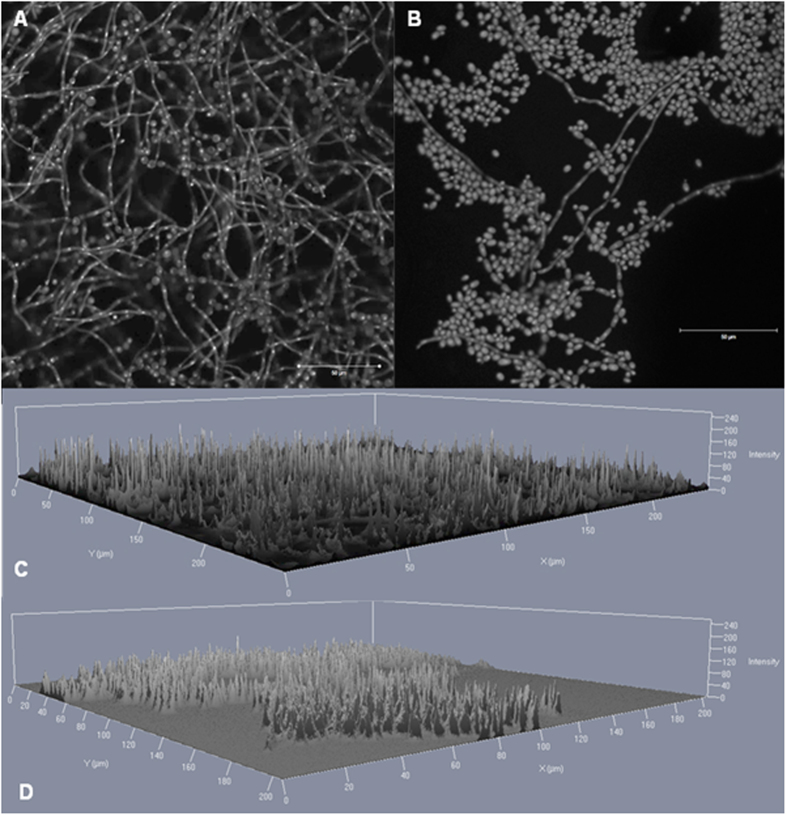
Figure 2. Confocal laser scan microscopy images of Exophiala dermatitidis biofilm grown for 48 hours at 35 °C.
The DNA of the cells was stained by 0.01% acridine orange for 2 minutes. (A) Matured biofilm of isolate P2 (CBS 116372) in a 2D image. (B) Matured biofilm of isolate CF2 (CBS 552.90) in a 2D image. (C) Matured biofilm of isolate P2 in a 2.5 D image. (D) Matured biofilm of isolate CF2 in a 2.5D image. Scale bar equals 50 μm. A laser with a wavelength of 488 nm was used.
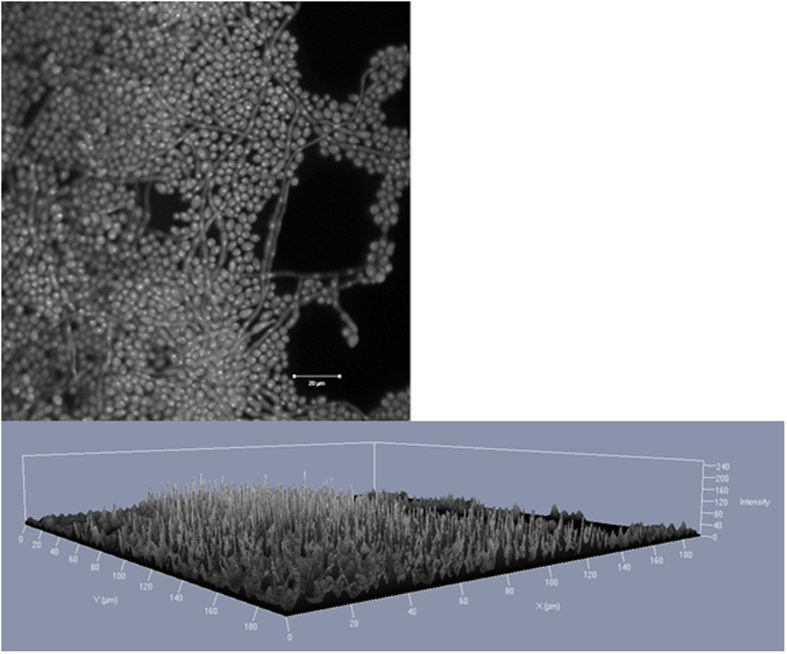
Figure 3. Confocal laser scan microscopy images of C. albicans (ATCC 90028) biofilm grown for 48 hours at 35 °C.
The DNA of the cells was stained by 0.01% acridine orange for 2 minutes. A laser with a wavelength of 488 nm was used. (A) Matured biofilm in a 2D image. Scale bar equals 50 μm. (B) Matured biofilm a 2.5 D image.
The invasive isolates from non-CF patients exhibited significantly more biomass in biofilm compared to isolates from CF patients (P = 0.0256). The biomass amount of invasive isolates tends to be higher than the biomass involved in biofilm of isolates from other origins (Fig. 4) This was also detected in the confocal laser scan microscopy (CLSM) (Fig. 2). The biofilm of the invasive isolate P2 contained more hyphal structures and was more clustered than the biofilm of the CF-patients isolate (CF2). The 2.5 D images from the invasive isolate, created in the CLSM, displayed higher signal intensity when compared to the CF-patients isolate (Fig. 2C,D).
Figure 4. Relative biofilm formation of Candida albicans (ATCC 90028) and various isolates of Exophiala dermatitidis.
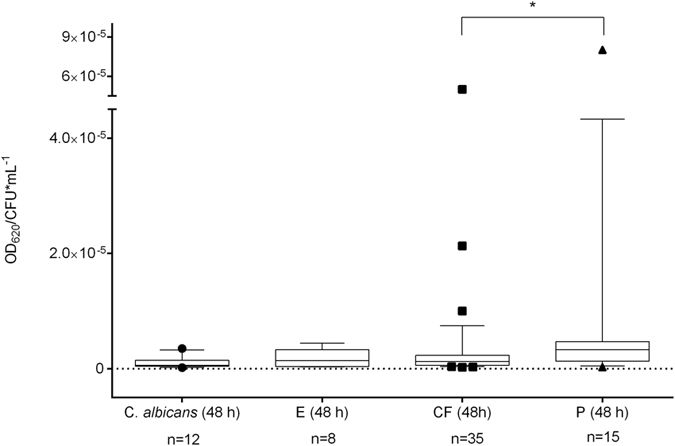
Box & whiskers with 10–90 percentile. E = environmental origin. CF = isolate from cystic fibrosis (CF) patient. P = invasive isolate from non-CF patient. Relative biofilm formation detected by staining with crystal violet (0.1%) for 20 minutes after biofilm formation at 35 °C over 48 hours. Each data point represents the mean of at least three independent experiments. *P < 0.05 as determined by Student’s t-test. CFU = colony-forming units.
XTT reduction assay is suitable for metabolically active biofilm detection
Because the CV-based biofilm formation assay can detect only the biomass in biofilm, a 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5[(phenylamino) carbonyl] – 2Htetrazolium hydroxide (XTT) reduction assay was introduced to investigate the metabolic activity of the biofilm. This assay was carried out for studying antibiofilm activity. Therefore, we investigated the suitability of the XTT assay for detecting the metabolic activity of E. dermatitidis biofilm. Metabolic activity of biofilm formation was measurable over 48 hours. Heat-inactivated cells showed no significant results upon XTT reduction.
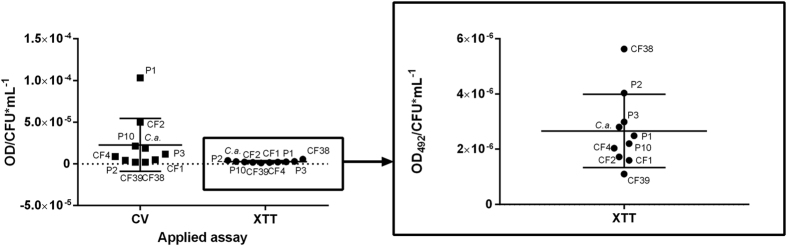
To understand the correlation, if any, between the metabolic activity and the biomass of E. dermatitidis biofilm, we compared the results of the CV assay and the XTT assay. The comparison of metabolic activity as detected by the XTT assay and of biomass, detected by CV staining, showed that the metabolic activity of E. dermatitidis biofilm is not linearly associated with the biomass involved in biofilm. Thus, the two assays are complementary procedures (Fig. 5).
Figure 5. Exophiala dermatitidis biofilm formation.
Optical density at 620 nm (OD620) as measured after staining with crystal violet (CV), and optical density at 492 nm (OD492) after XTT reduction. Each data point represents the mean of at least three independent experiments. P1, P2, P10 = invasive E. dermatitidis isolates from non-CF patients. CF1, CF2, CF4, CF39, CF38 = E. dermatitidis isolates from CF patients. C.a. = Candida albicans. CFU = colony-forming units.
Exophiala dermatitidis biofilms are mostly resistant against antibiofilm agents
Biofilm formation is a survival strategy by which fungi adapt to their environment. Thus, treating an infection caused by a biofilm-forming organism is difficult. To detect substances that may inhibit E. dermatitidis biofilm, we tested the effect of six possible antibiofilm agents on E. dermatitidis cell-adhesion, biofilm formation, and mature biofilm with the introduced XTT assay. We also tested the antibiofilm activity of these six compounds on C. albicans biofilm as a control. Before doing so, we determined the minimum inhibitory concentrations (MICs) of the substances and respectively the minimum eradication concentration (MEC) for micafungin by broth microdilution method to identify the range for antibiofilm activity testing (Table 1).
Table 1. Average minimum inhibitory concentration (MIC) values (in mg/L) for voriconazole, 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG), epigallocatechin gallate (EGCG), colistin and farnesol and the minimum effective concentration (MEC) of micafungin against three E. dermatitidis isolates. (P1, P2, CF2) and C. albicans (ATCC 90028) detected by microdilution method after 48 hours of incubation at 35 °C.
| Voriconazole mg/L | PGG mg/L | EGCG mg/L | Farnesol mg/L | Colistin mg/L | Micafungin mg/L | |
|---|---|---|---|---|---|---|
| P1 | 0.25 | 2048 | 1024 | 512 | 64 | 8 |
| P2 | >16 | >2048 | >2048 | 2048 | 128 | 8 |
| CF2 | >16 | >2048 | >2048 | >2048 | >512 | 8 |
| C. albicans | 0.023 | >2048 | >2048 | >2048 | >512 | 0.016 |
In general, E. dermatitidis biofilm exhibited a higher resistance to the tested compounds than did C. albicans. Compared to the addition of agents on cells that were already adherent, the antibiofilm activity of all tested agents was higher before cell-surface adherence and when they were added to mature biofilm. The process of E. dermatitidis biofilm formation, which usually occurs 2 to 48 hours after inoculation, showed higher resistance against treatment with antiinfective agents and natural substances.
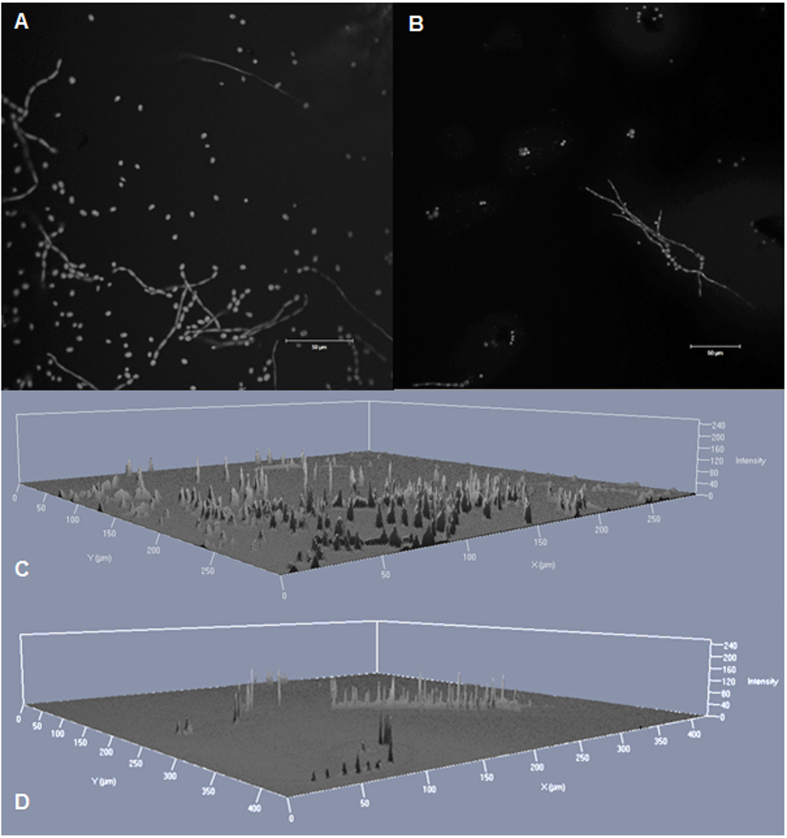
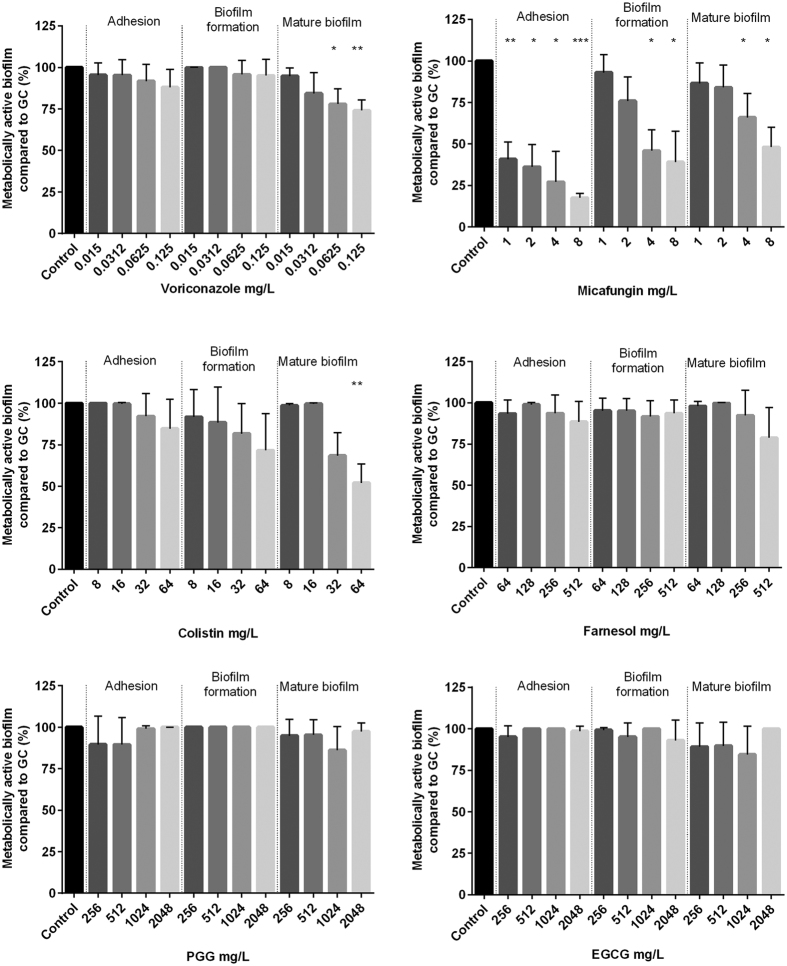
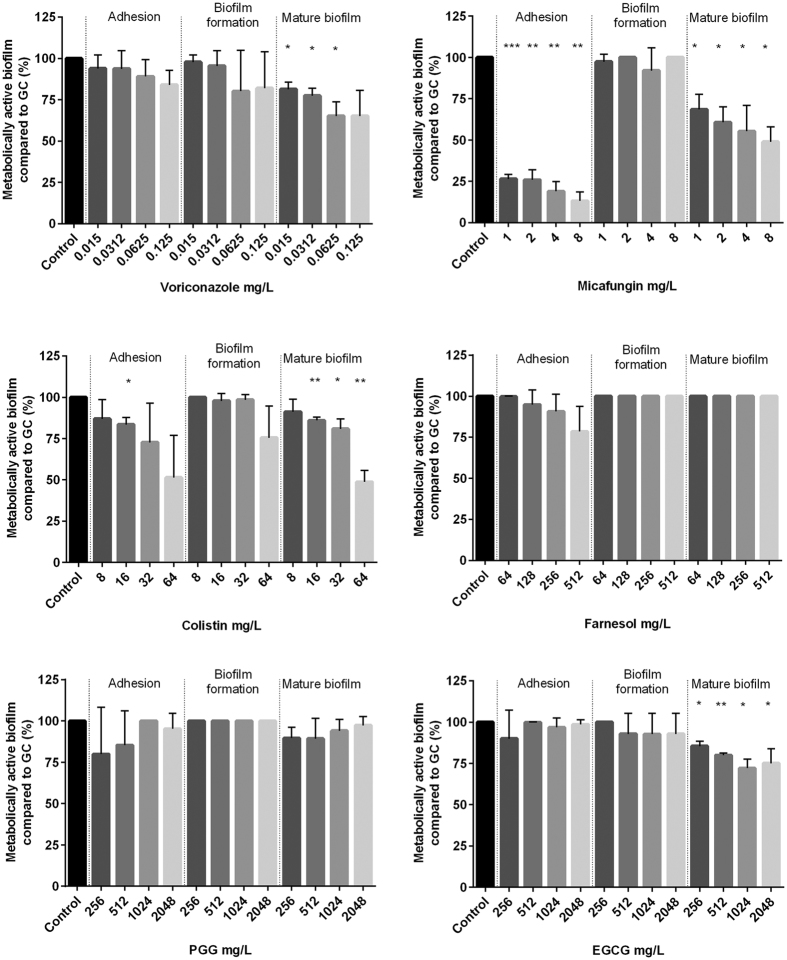
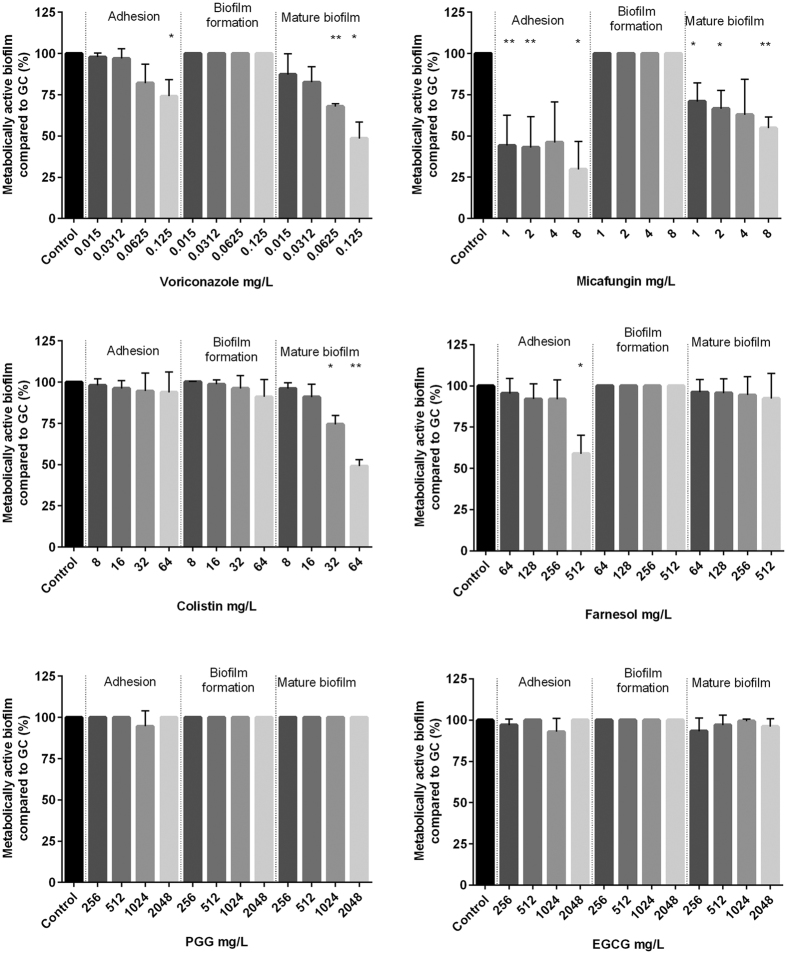
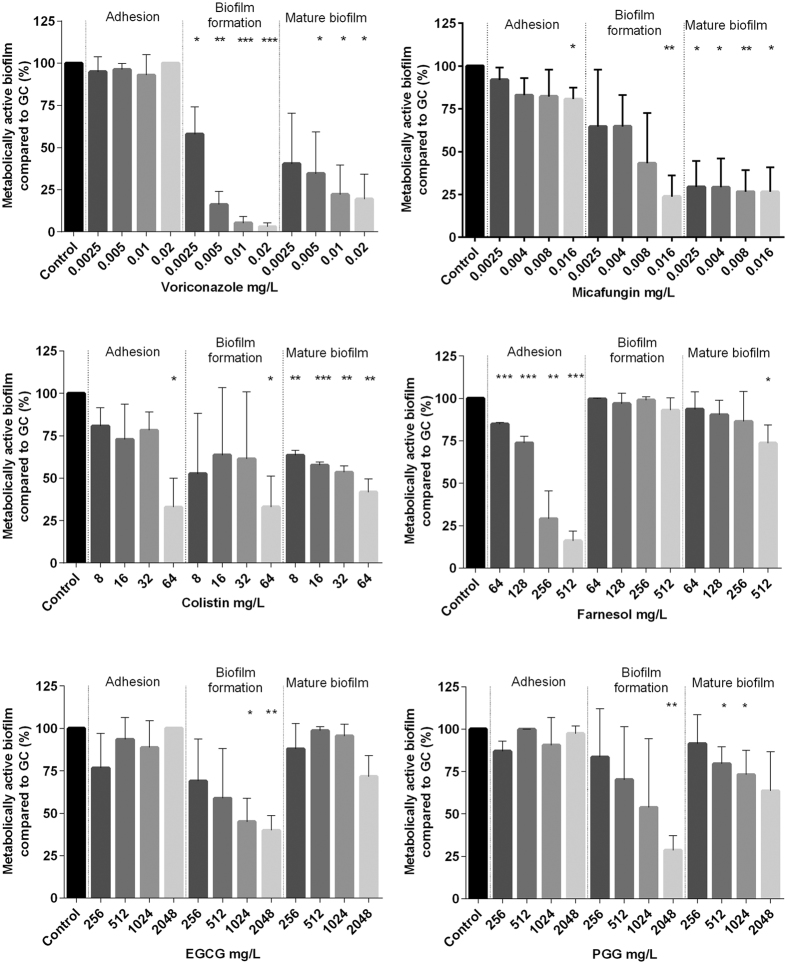
The best antibiofilm activity against E. dermatitidis was exhibited by the antifungal agent micafungin. The second highest growth reduction resulted from treatment with the antibiotic colistin. The antibiofilm effect of both agents was also documented in the CLSM (Fig. 6). An additional analysis correlates with the results of this assay. The biofilm was reduced by approximately 90% when treated with micafungin and 62% when treated with colistin as detected by gray-value measurements of the CLSM created images. In contrast, the natural substances PGG and EGCG had no visible effect on biofilm formation of E. dermatitidis at any step in the process (Figs 7, 8 and 9). On the other hand, treatment with PGG and EGCG affected the control organism C. albicans in the process of biofilm development (Fig. 10). At a concentration of 512 mg/L, the QSM farnesol decreased adhesion by approximately 25% in average. In contrast, we detected no significant difference between the non-treated growth control and either the cells treated during biofilm formation or the preformed biofilm (Figs 7, 8 and 9). However, farnesol inhibits the cell adhesion of C. albicans and exerts an antibiofilm effect on 48-hour preformed C. albicans biofilm (Fig. 10).
Figure 6. Confocal laser scan microscopy images of E. dermatitidis isolate P2 (CBS 116372) biofilm, formed in the presence of antiinfective agents.
Biofilm was grown for 48 hours at 35 °C in the presence of (A,C) 64 mg/L colistin, (B,D) 8 mg/L micafungin. 2D (A,B) and 2.5 D (C,D) images were taken. The DNA of the cells was stained by 0.01% acridine orange for 2 minutes. Scale bar equals 50 μm. A laser with a wavelength of 488 nm was used.
Figure 7.
Growth (mean with standard deviation in %) of E. dermatitidis (P1) biofilm after treatment with voriconazole (A), micafungin (B), colistin (C), farnesol (D), epigallocatechin gallate (EGCG, E) and 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG, F) in the concentrations indicated on the x-axis. Control = growth control without treatment. Adhesion = addition of drug at time point 0. Biofilm formation = drug addition after 2 hours. Mature biofilm = drug addition after 48 hours of biofilm formation. Growth was evaluated by XTT assay; optical density readings at 492 nm (OD492) were measured. *P < 0.05; **P < 0.05; ***P < 0.001. n = 4.
Figure 8.
Growth (mean with standard deviation in %) of E. dermatitidis (P2) biofilm after treatment with voriconazole (A), micafungin (B), colistin (C), farnesol (D), epigallocatechin gallate (EGCG, E) and 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG, F) in the concentrations indicated on the x-axis. Control = growth control without treatment. Adhesion = addition of drug at time point 0. Biofilm formation = drug addition after 2 hours. Mature biofilm = drug addition after 48 hours of biofilm formation. Growth was evaluated by XTT assay; optical density readings at 492 nm (OD492) were measured. *P < 0.05; **P < 0.05; ***P < 0.001. n = 4.
Figure 9.
Growth (mean with standard deviation in %) of E. dermatitidis (CF2) biofilm after treatment with voriconazole (A), micafungin (B), colistin (C), farnesol (D), epigallocatechin gallate (EGCG, E) and 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG, F) in the concentrations indicated on the x-axis. Control = growth control without treatment. Adhesion = addition of drug at time point 0. Biofilm formation = drug addition after 2 hours. Mature biofilm = drug addition after 48 hours of biofilm formation. Growth was evaluated by XTT assay; optical density readings at 492 nm (OD492) were measured. *P < 0.05; **P < 0.05; ***P < 0.001. n = 4.
Figure 10.
Growth (mean with standard deviation in %) of C. albicans (ATCC 90028) biofilm after treatment with voriconazole (A), micafungin (B), colistin (C), farnesol (D), epigallocatechin gallate (EGCG, E) and 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG, F) in the concentrations indicated on the x-axis. Control = growth control without treatment. Adhesion = addition of drug at time point 0. Biofilm formation = drug addition after 2 hours. Mature biofilm = drug addition after 48 hours of biofilm formation. Growth was evaluated by XTT assay; optical density readings at 492 nm (OD492) were measured. *P < 0.05; **P < 0.05; ***P < 0.001. n = 3.
The antifungal agent voriconazole reduced the growth of E. dermatitidis biofilm during adhesion and also reduced the growth of mature biofilm. The decrease in the number of viable cells was visible for all three strains treated with voriconazole. However, when different concentrations of voriconazole were applied to the different strains, the growth of mature biofilm was reduced at different rates. Thus, the activity of voriconazole was also strain specific (Supplementary Fig. S1). The antibiofilm activity of voriconazole was higher against the CF isolate than against the invasive isolates.
The antibiofilm activity of all agents is strain specific
Overall, strain-specific MICs/MEC of E. dermatitidis were investigated for both planktonic and biofilm cells (Supplementary Fig. S1). Treatment with 0.06 mg/L voriconazole inhibited mature biofilm by 37%. In contrast, the planktonic E. dermatitidis isolates P2 and CF2 exhibited a high MIC (>16 mg/L) against voriconazole. Voriconazole had a MIC of 0.25 mg/L against planktonic P1 cells, as detected by the microdilution method (Table 1). When the strain-specific susceptibility of biofilm was analysed, the isolate P1 exhibited the lowest biofilm reduction rate. After treatment with 0.06125 mg/L voriconazole, the biofilm of this isolate was at 78% of the growth controls. P2 (65%) and CF2 (68%) were also reduced in comparison with the growth controls. Therefore, the planktonic susceptibility and the biofilm susceptibility are not directly dependent on each other within one strain.
Combination of colistin and micafungin showed indifferent effects on E. dermatitidis biofilm
Synergistic operating agents offer the opportunity to decrease the necessary drug uptake, thus also reducing adverse effects and minimizing the risk of emerging resistance. Micafungin and colistin exhibited the highest antibiofilm activity against E. dermatitidis (Figs 7, 8 and 9). Therefore, combination treatment with micafungin and colistin was applied in the assays, in addition to single treatment. The aim was to detect a possible synergy between the two substances. The concentrations of the two drugs were the same in combination treatment as in single treatment. The checkerboard method showed that micafungin and colistin are indifferent in treatment against E. dermatitidis biofilm when applied before adhesion or when applied to mature biofilm (Supplementary Tables S1 and S2).
Discussion
In the study reported here, we systemically investigated the biofilm formation of the black yeast-like fungus E. dermatitidis. The CV assay showed that all tested E. dermatitidis isolates could form biofilm under the described conditions with a significantly higher biomass in biofilm after 48 hours of incubation. Invasive isolates produced significantly more biomass than did the isolates from CF patients. Sav et al. recently reported that only 15% of 137 environmental isolates and two of the seven (29%) clinical E. dermatitidis isolates formed biofilm over a 24-h incubation period12. When the evaluation method introduced by Sav et al.12 is applied to the results of this study, 86% of the total tested isolates, 63% of the environmental isolates, and 92% of the clinical isolates exhibited biofilm formation. In both studies, the clinical isolates showed a higher percentage of biofilm builders. However, the results vary widely; these findings may be due to differences in the period of biofilm formation and the growing conditions. In addition to the findings of Sav et al., two other publications reported biofilm capabilities of E. dermatitidis. However, they were limited by the number of included strains13 or by analyzing only isolates from non-human sources14.
The XTT assay and CV staining are complementary, because CV staining detects biomass in biofilm and the XTT assay detects metabolic activity15. The biofilm-detecting methods based on CV and XTT achieve different results, and both the quantities and the relation differ. We compared both procedures and found a 50% deviation in the results for C. albicans, a finding comparable to the results of a previous study. Marcos-Zambrano et al. tested both assays on Candida and non-Candida spp. Biofilms. They found that the overall agreement of both methods was 43.7% and that the agreement for C. albicans in particular was higher than 50%15. In our study, the difference in the quotient of optical density (OD) and colony forming units (CFUs) per mL of E. dermatitidis in both assays showed a high variance between biomass and metabolic activity.
Exophiala dermatitidis was previously identified as an exopolysaccharide producer16. A thick extracellular matrix can reduce the diffusion of oxygen and nutrients and thus can reduce metabolic activity17. A difference in the biofilm matrix structure can therefore explain the lower rates of XTT reduction15. In addition, because E. dermatitidis is a black yeast-like fungus, the thick cell wall containing melanin could influence the metabolic rate. The composition and thickness of the cell wall vary between the tested isolates, causing differences in metabolic activity. Another explanation for the weak association between biofilm mass and metabolic activity could be that some of the tested samples had already reached their maximum biomass, limiting the growth rate and the metabolic activity17.
An interstrain comparison with XTT is impossible. Various fungal species and various strains reduce XTT differently18. However, the XTT assay can measure the inhibitory effects of possible antifungal agents against E. dermatitidis biofilm.
The broth microdilution tests and the antibiofilm activity tests of the agents found an isolate-specific MIC/MEC as well as an isolate-specific minimum biofilm eradication concentration (MBEC). Determination of the MBECs of the agents against the various biofilm development stages showed that the treatment of E. dermatitidis biofilm is most effective when carried out preventive and when administered to preformed biofilms. These findings were confirmed by the study of Ramage et al. about the effect of the QSM farnesol on C. albicans biofilm formation19.
Biofilm formation processes are regulated by the secretion of QSMs. In the biofilm builder C. albicans, three QSMs have been identified, of which the most popular is farnesol. However, until now nothing was known about the existence of QSMs in the biofilm processes of E. dermatitidis. Farnesol was previously found to influence the processes of adhesions and the induction of biofilm dispersal of Candida spp.19. Here we found that farnesol had no antibiofilm activity against mature E. dermatitidis biofilm. Furthermore, the adhesion of E. dermatitidis cells was decreased by 25% when they were treated with 512 mg/L farnesol. The control C. albicans exhibited the expected reduction in biofilm growth when treated with farnesol19.
The antibiofilm activity of the analysed antifungal agents voriconazole and micafungin was, as expected, higher than that of the natural substances. Echinocandins have been shown to be effective against Candida biofilms. Micafungin exerted better antibiofilm activity against E. dermatitidis than did voriconazole. This finding was confirmed by the results of a study of Candida spp. biofilm20.
Colistin, a member of the polymyxin family, is an antibiotic used to treat infections involving gram-negative bacteria, targeting the bacterial membrane. The fungal cell wall and the cytoplasmic membrane serve as barriers against several agents. The E. dermatitidis isolates analysed in the microdilution for susceptibility testing exhibited isolate-specific MICs against colistin. With the lowest determined MIC of 64 mg/L, the in vitro activity of colistin was lower than expected. Previous reports stated that colistin exhibited a MIC50 of 12 mg/L and a MIC90 of 24 mg/L against E. dermatitidis21. Here, the antibiofilm effect of colistin against E. dermatitidis showed that colistin exerted a significant effect on cell adhesion, biofilm formation, and preformed biofilm. Schwartz et al. found that polymyxin affects the cell wall of fungi at high concentrations. At low concentrations, it increases the membrane permeability of fungi, enabling antifungal agents to more easily gain access to their site of action22.
The agents colistin and micafungin exhibited the most promising antibiofilm activity against E. dermatitidis. This was detected in the XTT assay as well as in the microscopy. However, combined treatment of micafungin and colistin exerted no synergistic effect against E. dermatitidis biofilm. In contrast, it has been shown in a previous study that the antibiotic colistin acts synergistically with antifungal agents of the echinocandin family against planktonic Candida species23. Echinocandin-mediated weakening of the cell wall may enable colistin to target the cell wall and thus reinforces the antifungal activity of echinocandins23. However, the combination of colistin and micafungin showed indifferent effects against E. dermatitidis cells during biofilm formation.
The plant derivatives EGCG and PGG were expected to exert antibiofilm activity against E. dermatitidis. Among other derivatives, polyphenols of green tea, especially EGCG, have been shown to exert anticarcinogenic, chemopreventive, antiatherogenic, antioxidant, and antimicrobial activity in vitro and in vivo24,25,26,27,28,29. Antifungal activity of EGCG has previously been demonstrated against planktonic C. albicans and against Candida spp. biofilm, especially when combined with antifungal agents28,29. PGG, a derivate of the Paeonia lactiflora root, also exerts a growth-inhibitory effect against several bacteria30,31. However, none of the natural substances, EGCG or PGG, exerted an effect on E. dermatitidis biofilm and neither planktonic growth of E. dermatitidis nor biofilm development was affected by the addition of EGCG or PGG.
Explanations for the resistance of E. dermatitidis against PGG and EGCG can be found in its thick and melanized cell wall at all life cycle stages. It has been previously reported that melanin enhances the resistance of several fungi32,33. In addition, experiments on the effect of polyphenols on the cell membrane and the cell wall of C. albicans showed that the catechins either permeabilize the cell membrane or disrupt parts of the cell wall, allowing the cells to take up substrates28. Because the cell wall of E. dermatitidis is thick and highly melanized, it is supposed to be more resistant to the effect of EGCG and PGG.
Resistance measurements of biofilm cells against all of the tested reagents at the concentrations of the detected MICs/MEC during biofilm formation demonstrated that planktonic cells are more susceptible to treatment with antiinfective agents than are cells embedded in a biofilm community. Cells embedded in the extracellular matrix of a biofilm are more likely to be resistant to host defence and antiinfective agents. Ning et al. studied the synergism of EGCG and antifungal agents against Candida spp. and found that biofilm cells exhibited significantly higher MICs (20 to 3200 times higher) than planktonic cells29.
In summary, we found that E. dermatitidis can form biofilm and that invasive isolates exhibit significantly higher biofilm-forming ability than do isolates from CF patients. The metabolic activity and the biomass involved in biofilm are strain specific.
E. dermatitidis biofilm is susceptible to the antifungal agents’ micafungin and voriconazole during adhesion and as a premature biofilm. The antibiotic colistin also reduced as well the fungal growth rate, especially during treatment of mature biofilms. In contrast, the natural substances EGCG and PGG had no significant effect on E. dermatitidis biofilm. Farnesol affects only fungal growth. Preventive treatment of the surface before biofilm formation is the most promising strategy.
Material and Methods
Strains
All clinical sputum samples were used after performing a conventional microbiological diagnosis. The study did not include patient’s details and did not result in additional constraints for the patients. All data were anonymously analysed without patients consent due to the retrospective nature of the study. All procedures and methods were carried out in accordance with approved guidelines.
We analysed 58 E. dermatitidis isolates: eight isolates (14%) from the environment, 15 (26%) invasive strains isolated from Asian patients, and 35 strains (60%) from the sputum of CF patients. As a control, we used the biofilm builder Candida albicans (American type collection (ATCC) 90028). To validate the XTT cell proliferation assay, we used the melanin-deficient mutant Mel-3 (ATCC 44504) and its corresponding wild type (ATCC 34100). For susceptibility testing, we used E. dermatitidis reference strains Centraalbureau voor Schimmelcultures (CBS) 109154, CBS 116372, and CBS 552.90. The species had been previously identified by sequence analyses of the internally transcribed spacer 1 (ITS1)34. All strains part of this study are listed in Table 2.
Table 2. List of included fungal isolates.
| Isolate abbreviation | Species | Reference Number # | Isolate origin |
|---|---|---|---|
| E1 | Exophiala dermatitidis | CBS 120550 | Steam bath, Austria |
| E2 | Exophiala dermatitidis | CBS 736.87 | Beer, Ireland |
| E3 | Exophiala dermatitidis | CBS 109142 | Berries, Netherlands |
| E4 | Exophiala dermatitidis | CBS 109143 | Sauna, Netherlands |
| E5 | Exophiala dermatitidis | CBS 120479 | Air, Germany |
| E6 | Exophiala dermatitidis | CBS 106.92 | Public pool, Japan |
| E7 | Exophiala dermatitidis | CBS 120435 | Steam bath, Thailand |
| E8 | Exophiala dermatitidis | CBS 120574 | Sauna, Thailand |
| CF 1 | Exophiala dermatitidis | CBS 154.90 | CF, Sputum, Aachen, Germany |
| CF 2* | Exophiala dermatitidis | CBS 552.90 | CF, Sputum, Aachen, Germany |
| CF 3 | Exophiala dermatitidis | CBS 149.90 | CF, Sputum, Aachen, Germany |
| CF 4 | Exophiala dermatitidis | CBS 120429 | Finland |
| CF 5 | Exophiala dermatitidis | Lfd. Nr.1951 | CF, Sputum, Essen, Germany |
| CF 6 | Exophiala dermatitidis | Lfd. Nr. 1946 | CF, Sputum, Essen, Germany |
| CF 7 | Exophiala dermatitidis | Lfd. Nr. 1952 | CF, Sputum, Essen, Germany |
| CF 8 | Exophiala dermatitidis | CBS 120155 | CF, Belgium |
| CF 9 | Exophiala dermatitidis | CBS 100337 | CF, Sweden |
| CF 10 | Exophiala dermatitidis | CBS 551.90 | CF, Germany |
| CF 11 | Exophiala dermatitidis | CBS 550.90 | CF, Germany |
| CF 12 | Exophiala dermatitidis | CBS 549.90 | CF, Germany |
| CF 13 | Exophiala dermatitidis | CBS 748.88 | CF, Norway |
| CF 14 | Exophiala dermatitidis | CBS 148.90 | CF, Germany |
| CF 15 | Exophiala dermatitidis | CBS 213.90 | CF, Germany |
| CF 16 | Exophiala dermatitidis | CBS 156.90 | CF, Germany |
| CF 17 | Exophiala dermatitidis | CBS 153.90 | CF, Germany |
| CF 18 | Exophiala dermatitidis | Lfd Nr. 1741 | CF |
| CF 19 | Exophiala dermatitidis | Lfd Nr. 1742 | CF |
| CF 20 | Exophiala dermatitidis | Lfd Nr. 1802 | CF, Sputum |
| CF 23 | Exophiala dermatitidis | Lfd Nr. 1873 | CF, Sputum |
| CF 24 | Exophiala dermatitidis | Lfd Nr. 1874 | CF, Sputum |
| CF 25 | Exophiala dermatitidis | Lfd Nr. 1875 | CF, Sputum |
| CF 26 | Exophiala dermatitidis | Lfd Nr. 1883 | CF, Sputum |
| CF 28 | Exophiala dermatitidis | Lfd Nr. 1889 | CF, Sputum |
| CF 31 | Exophiala dermatitidis | Lfd Nr. 1909 | CF |
| CF 32 | Exophiala dermatitidis | Lfd Nr. 1910 | CF |
| CF 33 | Exophiala dermatitidis | Lfd Nr. 1911 | CF |
| CF 34 | Exophiala dermatitidis | Lfd Nr. 1912 | CF |
| CF 35 | Exophiala dermatitidis | Lfd Nr. 1913 | CF |
| CF 36 | Exophiala dermatitidis | Lfd Nr. 1914 | CF |
| CF 37 | Exophiala dermatitidis | Lfd Nr. 1915 | CF |
| CF 38 | Exophiala dermatitidis | Lfd Nr. 1916 | CF |
| CF 39 | Exophiala dermatitidis | Lfd Nr. 1917 | CF |
| CF 41 | Exophiala dermatitidis | Lfd Nr. 1919 | CF |
| CF 43 | Exophiala dermatitidis | Lfd Nr. 1921 | CF |
| P1 | Exophiala dermatitidis | CBS 109154 | Brain, Japan |
| P2 | Exophiala dermatitidis | CBS 116372 | Brain, Japan |
| P4 | Exophiala dermatitidis | CBS 578.76 | Chromomykose, Taiwan |
| P5 | Exophiala dermatitidis | CBS 577.76 | Cervical lymphnodes, Taiwan |
| P6 | Exophiala dermatitidis | CBS 579.76 | Brain, Japan |
| P7 | Exophiala dermatitidis | CBS 207.35 | Chromomykose, Japan |
| P8 | Exophiala dermatitidis | NCPF* 2542 | Human lung |
| P9 | Exophiala dermatitidis | CBS 120546 | Skin graft, Greece |
| P10 | Exophiala dermatitidis | CBS 109148 | Human faeces, Netherlands |
| P11 | Exophiala dermatitidis | CBS 120473 | Brain, USA |
| P12 | Exophiala dermatitidis | CBS 100341 | Blood, Germany |
| P13 | Exophiala dermatitidis | CBS 109153 | External ear, Finland |
| P14 | Exophiala dermatitidis | CBS 120542 | Human faeces, Slovenia |
| P15 | Exophiala dermatitidis | CBS 424.67 | Chromomycosis, Germany |
| P16 | Exophiala dermatitidis | CBS 109153 | External ear, Finland |
| WT | Exophiala dermatitidis | ATCC 34100 | Wild type of mutants, unknown origin |
| Mu2 | Exophiala dermatitidis | ATCC44504 | Mel-3, derived from ATCC34100 |
| C. albicans | C. albicans | ATCC 90028 | Blood, Iowa, USA |
E = environmental isolate. CF = isolate from cystic fibrosis (CF) patients. P = isolate from non-CF patients.
*This isolate was excluded in the CV assay.
Biofilm formation assay
The biofilm-forming ability of E. dermatitidis isolates was analysed using a CV assay. We grew 58 E. dermatitidis isolates and C. albicans over 48 hours in Sabouraud bouillon containing 2% glucose at 35 °C and high shaking conditions (200 rpm), ensuring culturing of yeast-formed cells. Cells were washed with phosphate-buffered saline (PBS). A suspension with a cell density of 1 × 106 cells/mL in RPMI (pH 7.0) was set for each isolate. A 200-μL aliquot of the suspension was added to each well of a polystyrene, sterile, flat-bottomed 96-well microtiter plate. Sterile RPMI was used as a control for a blank correction.
After incubation over a period of 24 to 48 h, the plate was rinsed three times with PBS, and 125 μL of a 0.1% CV solution was added to each well. The staining was carried out for a minimum of 20 min at room temperature. Three additional rinsing steps with PBS were followed by air drying overnight. Next, 200 μL of 30% acetic acid was added to each well of the plate and incubated over 30 minutes, after which 150 μL of the solution was transferred from each well to a fresh microtiter plate. The plate was read at an OD620. The OD620 value of each well was calculated by subtraction of the blank reading. An inoculum size was estimated by CFU counting as follows: 100 μL of suspension at various dilutions was plated on malt extract agar, and the grown colonies were counted after incubation for 48 hours at 35 °C.
Viability assay
The metabolic activity of E. dermatitidis biofilm was measured by using an XTT assay. Seven isolates of the eight tested exhibited a high biomass amount in biofilm whereas one exhibited a low biomass amount. Strain cultivation and biofilm formation were carried out as described above. After incubation, the plates were rinsed three times with PBS, and 100 μL of 1 μM menadione in XTT solution was added to each well. The XTT/menadione solution was prepared fresh: 0.5 mg/mL XTT (Sigma, Steinheim, Germany) in sterile NaCl (0.9%), and 10 mM menadione in ethanol (100%). The final concentration of 1 μM was achieved by the addition of 1 μL menadione stock to 10 mL XTT solution. After the addition of the XTT/menadione solution, the plates were incubated in the dark at 36 °C for 3 h, and 75 μL of solution from each well was transferred to a new, sterile, U-bottom-shaped microtiter plate for measurement of the OD492.
To exclude inaccuracy with regard to pigments, such as melanin deposits in the cell wall, we used the XTT assay to analyze planktonic living, heat-killed cells, and Mel-3 cells. We used 0.5 mL of a cell culture that had been incubated for 24 h. Cells were heated at 70 °C for 5, 10, 15, or 20 min and were then centrifuged at 3,000 × g for five min. The supernatant was then transferred to a new reagent tube, and 100 μL of the XTT/menadione solution was added to both the cells and the supernatant, followed by incubation in the dark for 2 h at 36 °C and repeated centrifugation. Subsequently, 75 μL of supernatant was transferred to a 96-well microtiter plate, and the OD492 was measured. The cell concentration and the relative XTT reduction were determined.
Antibiofilm activity of antiinfective agents and natural substances
This assay was amplified to investigate the susceptibility of the cells of E. dermatitidis biofilm against the antiinfective agents colistin, voriconazole, and micafungin and against the QSM farnesol and the natural compounds 1,2,3,4,6-pentagallolyl glucose (PGG) and epigallocatechin-3-gallate (EGCG). Three E. dermatitidis strains with high ability to form biofilm were used. The MIC of voriconazole and the MEC of micafungin against C. albicans were determined by E-test. All other MICs against C. albicans and E. dermatitidis as well as the MEC of micafungin against E. dermatitidis were investigated by microdilution method according to EUCAST document for moulds35. The test ranges of antifungal agents and natural compounds used in the microdilution assay were as follows: Voriconazole (Sigma, Steinheim, Germany) and micafungin (MedChem Express, New Jersey, USA) 0.0312–16 mg/L, colistin (Sigma, Steinheim, Germany) 1–512 mg/L and farnesol, EGCG and PGG (all from Sigma, Steinheim, Germany) 4–2048 mg/L. Exophiala dermatitidis cells were treated with the total, one-half, one-quarter and one-eighth of the concentration of the MIC/MEC. We tested the activity of the six agents in three assay variations against E. dermatitidis adhesion to surface, against biofilm development, and against mature biofilm.
The antiinfective and natural agents were diluted in 1% solvent (dimethyl sulfoxide; DMSO) and RPMI medium for the preparation of a stock solution. The working solutions were made by dilution with sterile RPMI and the stock and working solutions were stored at −18 °C until use, with one exception, the solutions containing micafungin were stored at −80 °C. The compounds to be tested were added to the wells of a polystyrene, sterile, flat-bottomed 96-well microtiter plate at amounts of 100 μL.
We investigated the anti-adhesion effect by treating the fungus with drugs from the beginning of biofilm formation. We prepared the microtiter plates with the substances in advance. For the assay, the prepared plates were inoculated with 100 μL of cell suspension prepared fresh as described above, and cell counts in form of CFUs were carried out. After 2 h of incubation at 35 °C, the plates were incubated with XTT and evaluated as described above.
We evaluated the activity of the substances against the development of biofilm, as described above for the anti-adhesion effect, except for a difference in the time point of treatment with the substances. We placed 200 μL cell suspensions in each well and incubated it for 2 h at 35 °C. Subsequently, the plate was rinsed thrice with sterile PBS, and 100 μL of drug/substance solution was added to each well. The plates were incubated for 48 h until the XTT assay was performed as described above.
We tested the activity of the substances against mature biofilms over 48 h at 35 °C. The procedures were the same as those for the biofilm inhibiting effect, but in this case the drugs were added after two days of biofilm formation.
To investigate the in vitro synergy of micafungin and colistin, we applied the checkerboard technique to the assay to obtain the fractional biofilm eradication concentration (FBEC)36. We serially diluted micafungin along the ordinate while serially diluting colistin along the abscissa. Final drug concentrations were applied as described above. For data analysis, we determined the MBEC at which 50% of the biofilm was reduced (MBEC50) for both agents when used alone and in combination. The FBEC was determined using the following formula:
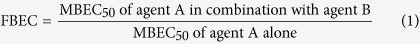 |
The FBEC index (ΣFBEC) is the sum of the FBEC of agent A and the FBEC of agent B. A ΣFBEC index smaller than or equal to 0.5 indicates synergism; a ΣFBEC index between 0.51 and 0.6 indicates partial synergism; a ΣFBEC index between 0.61 and 4.0 indicates indifference; and a ΣFBEC index higher than 4 indicates antagonism36.
Microscopy images
CLSM was used to visualize the biofilm of E. dermatitidis. Biofilms of E. dermatitidis were formed over 48 hours at 35 °C in sterile, 1 μ-Slide 4 Well Glass Bottom dishes (ibidi GmbH®, Martinsried, Germany) with an inoculum of 1 × 106 cells/mL in RPMI. The supernatant was discarded and the cells were fixed with 100% methanol for 2 min. The methanol was discarded and the cells were air-dried before the biofilm was stained with 0.01% acridine orange (Becton Dickenson, New Jersey, USA) for 3 min. Subsequently, the biofilm was rinsed three times with PBS and air-dried again. The cells were observed in the ELYRA LSM 710 (Zeiss, Oberkochen, Germany) with a laser at 488 nm and a 20 × objective. The images were processed using the Zen black software (Zeiss, Oberkochen, Germany). Two dimensional and two and a half dimensional (2.5D = pseudo-3D) images were created.
Statistical analysis
Values are presented as the mean obtained from three separate observations. Values were compared with Student’s t-test; statistical significance was set at the level of P < 0.05. Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
Additional Information
How to cite this article: Kirchhoff, L. et al. Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to antiinfective agents. Sci. Rep. 7, 42886; doi: 10.1038/srep42886 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank the Imaging Center Essen (IMCES), Faculty of Medicine, University Hospital Essen, University of Duisburg-Essen, for their assistance and the appropriation of imaging facilities.
Footnotes
The authors declare no competing financial interests.
Author Contributions L.K., M.O. and J.S. conceived and designed the experiments. K.Z. established the crystal violet assay. M.O., J.S. and P.-M.R. supervised the work. S.D. and L.K. established and validated the XTT assay. E.S., G.H., L.S. and J.B. contributed reagents/materials/analysis tools. L.K. and J.S. wrote the main manuscript text and L.K. prepared all figures. All authors reviewed the manuscript.
References
- Bakare N., Rickerts V., Bargon J. & Just-Nübling G. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses 46, 19–23 (2003). [DOI] [PubMed] [Google Scholar]
- Kondori N., Lindblad A., Welinder-Olssen C., Wennerǻs C. & Gilljam M. Development of IgG antibodies to Exophiala dermatitidis is associated with inflammatory responses in patients with cystic fibrosis. J. Cyst. Fibros. 13, 391–399 (2014). [DOI] [PubMed] [Google Scholar]
- Kantarcioğlu A. S. & de Hoog G. S. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47, 4–13 (2004). [DOI] [PubMed] [Google Scholar]
- Li D. M., Li R. Y., de Hoog G. S., Sudhadham M. & Wang D. L. Fatal Exophiala infections in China, with a report of seven cases. Mycoses 54, e136–142 (2011). [DOI] [PubMed] [Google Scholar]
- Sudhadham M. et al. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 61, 145–155 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos T., de Hoog G. S., de Boer A. G., de Crom I. & Haase G. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 45, 373–377 (2002). [DOI] [PubMed] [Google Scholar]
- Blasi B., Tafer H., Tesei D. & Sterflinger K. From glacier to sauna: RNA-Seq of the human pathogen black fungus Exophiala dermatitidis under varying temperature conditions exhibits common and novel fungal response. PLoS One 10, e0127103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo W. F. Jr. et al. Effects of disrupting the polyketide synthase gene WdPKSI in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol. 6, doi: 10.1186/1471-2180-6-55 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog G. S. & Guarro J. Exophiala dermatitidis, in: Atlas of Clinical Fungi (ed. de Hoog G. S.). Centraalbureau Voor Schimmelcultures: Utrecht, 1, p. 161 (1995). [Google Scholar]
- Blankenship J. R. & Mitchell A. P. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9, 588–594 (2006). [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells. Annu. Rev. Microbiol. 64, 357–372 (2010). [DOI] [PubMed] [Google Scholar]
- Sav H. et al. Virulence markers of opportunistic black yeast in Exophiala. Mycoses 59, 343–350 (2016). [DOI] [PubMed] [Google Scholar]
- Seneviratne C. J., Fong P. H. L. Wong S. S. W. & Lee V. H. F. Antifungal Susceptibility and phenotypic characterization of oral isolates of a black fungus from a nasopharyngal carcinoma patient under radiotherapy. BMC Oral Health 39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupannčič J., Novak Babič M., Zalar P. & Gunde-Cimerman N. The Black Yeast Exophiala dermatitidis and Other Selected Opportunistic Human Fungal Pathogens Spread from Dishwashers to Kitchens. PLoS One 11, e0148166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos-Zambrano L. J., Escribano P., Bouza E. & Guinea J. Production of biofilm by Candida and non-Candida ssp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 304, 1192–1198 (2014). [DOI] [PubMed] [Google Scholar]
- Yurlova N. A. & De Hoog G. S. Exopolysaccharides and capsules in human pathogenic Exophiala species. Mycoses 45, 443–448 (2002). [DOI] [PubMed] [Google Scholar]
- Alnuaimi A. D., O’Brien-Simpson N. M., Reynolds E. C. & McCullough M. J. Clinical isolates and laboratory reference Candida species and strains have varying abilities to form biofilm. FEMS Yeast Res. 13, 689–699 (2013). [DOI] [PubMed] [Google Scholar]
- Pierce C. G. et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3, 1494–1500 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G., Saville S. P., Wickes B. L. & López-Ribot J. L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68, 5459–5463 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katragkou A. et al. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother 52, 357–360 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemuth H. et al. In vitro activity of colistin as single agent and in combination with antifungals against filamentous fungi occurring in patients with cystic fibrosis. Mycoses 56, 297–303 (2013). [DOI] [PubMed] [Google Scholar]
- Schwartz S. N., Medoff G., Kobayashi G. S., Kwan C. N. & Schlessinger D. Antifungal Properties of polymyxin B and its potentiation of tetracycline as an antifungal agent. Antimicrob. Agents Chemother 2, 36–40 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler U. et al. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J. Antimicrob. Chemother 68, 1285–96 (2013). [DOI] [PubMed] [Google Scholar]
- Sakanaka S., Kim M., Taniguchi M. & Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric. Biol. Chem. 53, 2307–2311 (1989). [Google Scholar]
- Fang M. Z. et al. Tea polyphenol (−)-epigallotechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 63, 7563–7570 (2003). [PubMed] [Google Scholar]
- Si W. et al. Bioassay-guided purification and identification of antimicrobial components in Chinese green tea extract. J Chromatogr. A 1125, 204–210 (2006). [DOI] [PubMed] [Google Scholar]
- Steinmann J., Buer J., Pietschmann T. & Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 168, 1059–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evensen N. A. & Braun P. C. The effect of tea polyphenols on Candida albicans: inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 55, 1033–1039 (2009). [DOI] [PubMed] [Google Scholar]
- Ning. Y., Ling J. & Wu C. D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Arch. Oral. Biol. 60, 1565–1570 (2015). [DOI] [PubMed] [Google Scholar]
- Lin M. H., Chang F. R., Hua M. Y., Wu Y. C. & Liu S. T. Inhibitory effect of 1,2,3,4,6-penta-O-galloly-beta-D-glucopyranose on biofilm formation by Staphylococcus aureus. Antimicrob. Agents Chemother 55, 1021–1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan L. T. M., Moon J. K., Shibamoto T. & Ahn Y. J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J. Agric. Food Chem. 60, 9062–9073 (2012). [DOI] [PubMed] [Google Scholar]
- Dixon D. M., Polak A. & Szaniszlo P. J. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25, 97–106 (1987). [DOI] [PubMed] [Google Scholar]
- Wang Y., Aisen P. & Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63, 3131–3136 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J., Schmidt D., Buer J. & Rath P.-M. Discrimination of Scedosporium prolificans against Pseudoallescheria boydii and Scedosporium apiospermum by semiautomated repetitive sequence-based PCR. Med. Mycol. 49, 475–483 (2011). [DOI] [PubMed] [Google Scholar]
- Arendrup M. C., Guinea J., Cuenca-Estrella M., Meletiadis J., Mouton J. W., Lagrou K. & Howard S. J. & The Subcommittee On Antifungal Susceptibility Testing (Afst) Of The Escmid European Committee For Antimicrobial Susceptibility Testing (Eucast). Method for the determination of broth minimum inhibitory concentration of antifungal agents on conidia forming moulds. Eucast Definitive Document E.DEF 9.3 (2015).
- Odds F. C. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemother 52, 1 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.