Abstract
Background
The investigators compared event related potential (ERP) amplitude measurements and event-related oscillations across a broad frequency range during an auditory oddball task using a comprehensive analysis approach to describe shared and unique neural auditory processing characteristics among healthy subjects (HP), schizophrenia probands (SZ) and their first-degree relatives (SZrel), and bipolar disorder I with psychosis probands (BDP) and their first-degree relatives (BDPrel).
Methods
This Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) sample consisted of clinically stable SZ (n=229) and BDP (n=188), healthy subjects (HP, n=284), SZrel (n=264) and BDPrel (n=239). They were administered an auditory oddball task in the electroencephalography (EEG) environment. Principal components analysis (PCA) derived data-driven frequency bands for measurement of evoked power. Spatial PCA reduced ERP and frequency data to component waveforms for each subject. Clusters of time-bins with significant group differences on magnitude of response were assessed for patterns of proband/relative differences from HP and familiality.
Results
Nine variables survived a linear discriminant analysis between HP, SZ, and BDP. Of those, two showed evidence (deficit in relatives and familiality) as genetic risk markers more specific to SZ (N1, P3b) while one was specific to BDP (P2) and one for psychosis in general (N2).
Conclusions
This study provides support for both shared and unique deficits in early sensory and late cognitive processing across psychotic diagnostic groups Additional ERP and time-frequency component alterations (frontal N2/P2, late high, early mid and low- frequency) may provide insight into deficits in underlying neural architecture and potential protective/compensatory mechanisms in unaffected relatives.
Keywords: ERP, auditory oddball, psychosis, time-frequency, P300, PCA
Neurophysiological methods such as event-related potentials (ERP) provide unique, state-independent, and heritable measures of psychosis risk. Abnormalities are often present before the development of clinically significant symptoms; however, the specificity of individual measures to subtypes of psychosis such as schizophrenia and bipolar disorder with psychosis is still uncertain (1). The current study investigates such measures in the auditory oddball paradigm.
The P300 ERP is a robustly replicated, multi-source brain-generated physiologic response associated with novelty/salience detection, working memory, context updating, and target recognition during visual and auditory oddball tasks (2). Reduced P3b (P300 subcomponent centered at parietal scalp locations) amplitude is frequently reported in schizophrenia (3–8) and bipolar disorder (6,7,9–11). P300 abnormalities may be useful for understanding disease risk, as reductions in this component have been reported among clinically healthy first-degree relatives of probands with schizophrenia(12–16) and bipolar disorder (17). P3b amplitude reductions among family members of affected probands suggest auditory oddball processing is associated with constitutional liability for illness. A twin study meta-analysis of P300 indicated high aggregate heritability estimates among healthy participants (50–60%) (18), consistent with the possibility that P300 may be a liability indicator for psychosis (1,19). Although P300 is the most commonly studied ERP in oddball paradigms, other ERPs to both target and standard stimuli may provide important information about psychosis as well (20). Reductions in N1 and P2 amplitude have been reported in both first episode and chronic SZ (21). N1 reductions also have been reported in first-degree relatives of SZ but not BDP (22). Similarly, SZ and BDP both show N2 abnormalities but usefulness of N2 as an endophenotype is currently unknown (22–25). In this manuscript, we evaluate multiple ERP responses during auditory oddball processing that are associated with disease effects and those that are candidates for further genetic modeling.
Inherited biomarkers (endophenotypes) should be relatively specific to persons with a disease and those at increased genetic risk for that disease (26). Most endophenotype studies of ERPs target a single diagnostic group. However, multiple lines of evidence indicate that P300 abnormalities may be liability markers for psychotic disorders generally rather than for a specific diagnostic group (11,17,27,28). Indeed, P300 abnormalities have been described for multiple neurobehavioral diagnostic categories (29).
P300 amplitude reduction may be a liability indicator for psychotic disorders generally. Alternatively, quantifying P3b amplitude, for instance, using a single sensor (e.g., Pz) at a single time point (P3b peak) may fail to capture the richness and diversity of neural activations evoked by the oddball paradigm. More complete quantification schemes may be necessary to capture neuropathological response differences between diagnostic classes. Quantifying between-groups differences on voltage over the entire ERP time-range, frequency variations in neural oscillations as a function of time, and integrating information over the whole head all may capture additional complexity of neural responding not evident in ERP peak voltage measurements (30) and may help elucidate differences between nosologically similar diagnostic groups (23,31). Quantifying event-related oscillations (EROs) has revealed early gamma band abnormalities during the auditory oddball task which are both heritable in twin studies (32) and present in first episode (33) and chronic SZ (34). Early gamma responses have been relatively understudied in BPD but have been reported as heritable but not abnormal in one study of non-psychotic bipolar patients (35) so this response may prove to be a candidate endophenotype of SZ. The current study provides direct comparison of schizophrenia (SZ), bipolar disorder with psychosis (BDP) and their first-degree relatives on ERP voltage and time-frequency measurements of the same responses, and may help illuminate the most useful approach for discriminating between psychosis groups and identifying inherited biomarkers for these disorders.
The present investigation of the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) (36) sample compared ERP amplitude measurements and EROs across a broad frequency range during an auditory oddball task using a comprehensive analysis approach to describe shared and unique neural processing characteristics among healthy subjects, SZ and their first-degree relatives (SZrel), and BDP and their first-degree relatives (BDPrel). It was predicted that (i) time-frequency measures would provide useful information beyond that of traditional ERP measures, particularly in higher frequency bands (ii) P300 response would not discriminate psychosis groups, and (iii) information from first-degree relatives would aid in identification of inherited biomarkers in components previously understudied in these groups, including the N2 ERP and mid-frequency EROs.
Methods
Participants
As part of the large, multi-site data collection project (B-SNIP), participants were recruited, interviewed, and tested at five sites: University of Illinois-Chicago, Yale University/IOL (Hartford, CT), University of Texas Southwestern (Dallas, TX), University of Maryland (Baltimore, MD), and Wayne State University (Detroit, MI)/Harvard University (Boston, MA). Participants with SZ (n=229) or BDP (all subtype I, n=188) who were clinically stable (not acutely ill and no changes in treatment for 1 month) and healthy subjects (HP, n=284) were recruited via community advertisements, linked community facilities and programs, and local NAMI-type organizations. First-degree relatives of SZ (n=264) and BDP (n=239) were recruited through initial proband participation (Table 1 for demographics). Presence of SZ or BPD-type disorder was not an exclusion criterion for relatives, as these disorders tend to run in families and a representative sample of the population was desired, however analyses removing affected relatives were also performed (Tables 2 & S2). All participants provided written informed consent. This study was approved by IRBs at each data-collection and analysis site (36).
Table 1.
Demographic data for all participants. Due to expected differences between groups on age and gender distribution, all variables were age corrected prior to analysis and gender was incorporated in all group comparisons.
| Healthy | BDP | SZ | BDP Relatives | SZ Relatives | Significance Test | |
|---|---|---|---|---|---|---|
| Subjects | 284 | 188 | 229 | 239 | 264 | |
| From each site | ||||||
| UICa | 75 | 66 | 49 | 91 | 65 | X2(16)=63.59*** |
| YU | 63 | 34 | 55 | 55 | 72 | |
| UTS | 50 | 19 | 28 | 24 | 32 | |
| UM | 26 | 45 | 55 | 39 | 48 | |
| WSU/HU | 70 | 24 | 42 | 30 | 47 | |
| Percent female | 54% | 59.6% | 31.9% | 63.2% | 66.7% | X2(4)=17.78** |
| Age | 37.2 (15–66) | 35.8 (15–64) | 34.7 (15–64) | 40.8(15–70) | 42.4(15–65) | F(4,1196)=13.42*** |
| Trials accepted | ||||||
| Standards | 524 (287–567) | 535 (315–567) | 531 (315–567) | 537 (294–567) | 534(293–567) | F(4,1119)=2.14 |
| Targets | 96 (53–100) | 96 (62–100) | 95 (52–100) | 96 (53–100) | 96 (52–100) | F(4,1119)=.33 |
| Percent Correct | 92.4% (SD=12.7) | 92.3% (SD=12.4) ES=−0.008 | 89.9% (SD=15.9) ES=−0.19 | 93.9% (SD=9.9) ES=0.12 | 92.0% (SD=14.3) ES=−0.03 | F(4,1015)=2.48* |
| Response Latency (ms) | 407.78 (SD=72.59) | 431.69 (SD=79.93) ES=0.33 | 439.60 (SD=99.81) ES=0.44 | 417.07 (SD=82.15) ES=0.13 | 416.05 (SD=82.99) ES=0.11 | F(4,1015)=4.79** |
| Clinical Scales | ||||||
| GAF | 86.1 (60–100) | 60.9 (35–92) | 48.9 (29–79) | 76.6 (40–100) | 74.6 (30–100) | F(4,1119)=352.71*** |
| PANSS-pos | - | 12.5 (7–24) | 17.0 (7–32) | - | - | t(395)=9.03*** |
| PANSS-neg | - | 12.2 (7–25) | 17.1 (7–34) | - | - | t(395)=9.71*** |
| PANSS-gen | - | 28.4 (16–52) | 33.2 (16–59) | - | - | t(395)=5.67*** |
| MADRS | - | 10.9 (0–39) | 9.0 (0–37) | - | - | t(375)=2.08* |
| YMRS | - | 5.4 (0–34) | 5.8 (0–29) | - | - | t(398)=.602 |
| Medication Class | ||||||
| Antipsychotic 1st Gen | 0% | 9.6% | 21.0% | 0.4% | 1.1% | |
| Antipsychotic 2nd Gen | 0% | 66.5% | 79.9% | 6.7% | 8.3% | |
| Mood stabilizer | 0% | 52.7% | 15.7% | 9.2% | 3.0% | |
| Lithium | 0% | 28.7% | 6.1% | 3.3% | 0.8% | |
| Antidepressant | 1.1% | 47.9% | 38.4% | 21.3% | 15.9% | |
| Sedative/anxiolytic | 1.1% | 31.4% | 20.1% | 9.6% | 8.3% | |
| Stimulant | 0.7% | 8.5% | 3.1% | 4.6% | 0.4% | |
| Anticholinergic | 0% | 7.4% | 17.5% | 0.4% | 1.5% | |
p<.05,
p<.01,
p<.001
UIC: University of Illinois, Chicago; YU: Yale University; UTS: University of Texas Southwestern Medical Center; UM: University of Maryland; WSU/HU: Wayne State University/Harvard University. ES: Effect size vs Healthy. GAF: Global Assessment of Functioning; PANSS: Positive and Negative Syndrome Scale (pos: positive, neg: negative, gen: general); MADRS: Montgomery-Asberg Depression Rating Scale; YMRS: Young Mania Rating Scale
Table 2.
Omnibus F values for group differences and proband comparisons to healthy subjects in variables surviving the linear discriminant analysis. Effect sizes represent group differences from healthy subjects (HP means shown above) and are calculated using Glass’s delta with bootstrapped two-tailed significance values. Positive effect sizes indicate a larger amplitude response in healthy subjects, except in cases where the healthy mean is negative (N1, N2, N2/P2). HP means are in μV for ERP variables and μV2/Hz for frequency variables.
| Standards | Targets | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| RANGE | N1 | P2 | Mid freq | High freq | High freq | Comp1N2 | Comp2 P2/N2 | Comp1 P3b | Comp1 Low freq |
| 50–130ms | 130–250ms | 30–110ms | 200–240ms | 460–520ms | 160–230ms | 140–240ms | 330–540ms | 20–90ms | |
| Omnibus ANOVA F value
|
12.81*** | 6.65*** | 5.79*** | 4.47** | 5.69*** | 6.79*** | 16.45*** | 13.04*** | 5.23*** |
| SZ vs HP t value | 6.32*** | 2.71** | 4.06*** | .71 | 2.35* | 4.01*** | 4.95*** | 7.83*** | 4.16*** |
| BDP vs HP t value | 4.71*** | 4.37*** | 4.11*** | 2.97** | .09 | 3.28** | 1.79 | 3.60*** | 1.82 |
|
| |||||||||
| FAMILIALITY | 0.47*** | 0.28*** | 0.72*** | 0.36*** | 0.08 | 0.13 | 0.34*** | 0.30*** | 0.71*** |
| EFFECT SIZES | |||||||||
| HP mean | −.72 | .27 | 35.65 | 6.13 | 7.54 | −.16 | −1.01 | 3.36 | 44.41 |
| BDP | −0.43** | 0.42** | 0.39** | −0.28* | −0.01 | −0.30** | −0.17 | 0.34** | 0.18 |
| SZ | −0.52** | 0.22** | 0.36** | −0.06 | −0.21* | −0.32** | −0.40** | 0.67** | 0.41** |
| BDPREL | −0.15 | 0.27** | 0.18* | −0.04 | 0.02 | −0.34** | 0.19* | 0.12 | 0.03 |
| BDPREL –psychosis | −0.11 | 0.24** | 0.16 | −0.04 | 0.03 | −0.33** | 0.22** | 0.07 | 0.01 |
| BDPREL -psych/cluster A | −0.15 | 0.24** | 0.16 | −0.06 | 0.01 | −0.28** | 0.21* | 0.05 | 0.02 |
| BDPREL - psych/cluster A/cluster B | −0.12 | 0.24** | 0.15 | −0.04 | 0.02 | −0.29** | 0.23** | 0.04 | −0.02 |
| SZREL | −0.25** | −0.12 | 0.13 | 0.06 | 0.07 | −0.34** | 0.02 | 0.22** | 0.16 |
| SZREL –psychosis | −0.24* | −0.09 | 0.13 | 0.09 | 0.09 | −0.31** | 0.04 | 0.19* | 0.16 |
| SZREL -psych/cluster A | −0.23** | −0.03 | 0.07 | 0.06 | 0.09 | −0.26** | 0.01 | 0.17 | 0.10 |
| SZREL -psych/cluster A/cluster B | −0.22** | −0.06 | 0.09 | 0.06 | 0.09 | −0.28** | 0.01 | 0.16 | 0.08 |
p<.05
p<.01
p<.001
Medical history, structured clinical interview for DSM-IV diagnosis (SCID patient or non-patient as appropriate) (37), Positive and Negative Symptom Scale (PANSS) (38), Young Mania Rating Scale (YMRS) (38), Montgomery-Asberg Depression Rating Scale (MADRS) (39), and Global Assessment of Functioning scale (GAF; Axis V of Diagnostic and Statistical Manual of Mental Disorders IV [DSM-IV]) were acquired by trained Masters or Doctoral-level clinicians. Presence of serious medical, neuro-opthalmological, or neurological illness (e.g., cancer, seizure disorders, coarse brain disease), mental retardation, head trauma with >30 minutes unconsciousness, current substance use ascertained by history plus urine drug screens on the day of testing (8 panel screen for amphetamines, barbiturates, cocaine, methadone, opiates, cannabinoids, propoxyphene and tricyclic antidepressants), abuse in the past three months, and dependence within 6 months or extensive history of drug dependence (DSM-IV) were criteria for exclusion. Healthy persons were free of any lifetime psychotic or mood disorder and family history of psychotic or BP disorders in first-degree relatives. All but 20 SZ and 14 BDP were taking psychotropic medications, while most first-degree relatives were free of psychotropic medications (Table 1).
Stimuli and Procedures
Recording conditions were equivalent and stimulus presentation and recording equipment identical across sites. Seated in a sound and electrically shielded booth (ambient sound = 61–63 dB; luminance = 0.11–0.12 foot-candles) subjects listened to tones delivered by two 8-ohm speakers located 50 cm in front of them. Stimuli were 567 standard (1000Hz) and 100 target (1500Hz) tones presented in pseudorandom order (1300ms inter-trial interval). Subjects were asked to press a button when a target was detected. Subjects refrained from smoking 1 hour prior to testing.
EEG recording
EEG was continuously recorded from 64 Ag/AgCl sensors (impedance <5 KΩ; Quik-Cap, Compumedrics Neuroscan, El Paso, TX), positioned according to the standard 10–10 EEG system plus mastoids and CP1/2 locations to provide sampling lower on the back of the head, with nose reference and forehead ground. Recordings were amplified (12,500x) and digitized (1000Hz) using Neuroscan Acquire and Synamps2 recording systems (Compumedrics Neuroscan, El Paso, TX) with a bandpass filter of DC-200Hz.
EEG processing
Raw EEG data were inspected for bad sensors and artifacts. Bad sensors were interpolated (<5% for any subject) using spherical spline interpolation (BESA 5.3; MEGIS Software, Grafelfing, Germany). Blink and cardiac artifacts were removed using Independent Components Analysis (EEGLAB 6.0) (40). Data were segmented into 1000 ms epochs from 250 ms before to 750 ms after stimulus onset and digitally band-pass filtered from 0.5 55Hz (zero-phase filter; rolloff: 6 and 48 dB/octave, respectively). The 250 ms pre-stimulus period was used for baseline adjustment. Epochs containing activity greater than 75 μV at any sensor were not included; at least 52% of trials were accepted for all subjects.
PCA data reduction
ERP
To use ERP data recorded from every sensor and, thus, to most accurately and comprehensively capture the spatial topography of evoked brain responses across time, spatial principal components analysis (PCA) on grand average data (23,31,41) was implemented using BESA (MEGIS Software, Grafelfing, Germany) and Matlab (The Mathworks, Matick, MA) to identify spatial patterns in the EEG topography (2 components for targets and 1 for standards). Target and standard conditions were averaged separately across groups. See supplemental methods, SF5 and Figures 1 and 2 for more information on PCA methods.
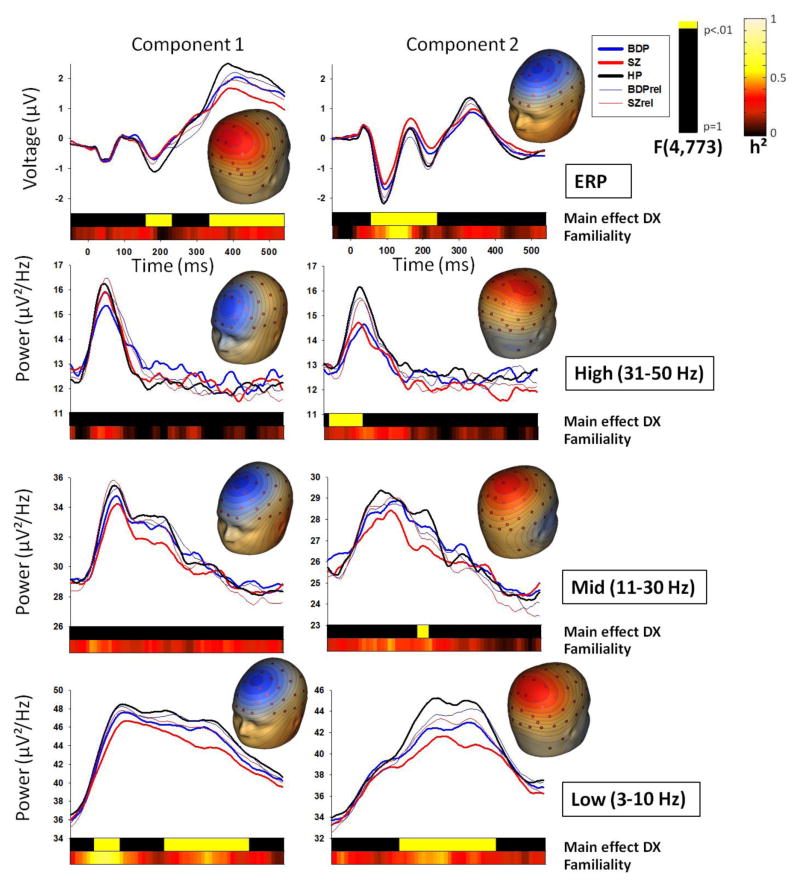
Figure 1.
Target stimuli component waveforms by group and topographies for each spatial PCA component and PCA-derived frequency band, with ERP at the top. Note that waveforms are not corrected for age, as this was done at the coarser time-bin level. Top bars in each plot indicate clusters of time-bins in which 3 consecutive time-bins showed a significant main effect of DX (group) at the .01 alpha level. Lower bars show familiality (h²) values across all families for each time-bin.
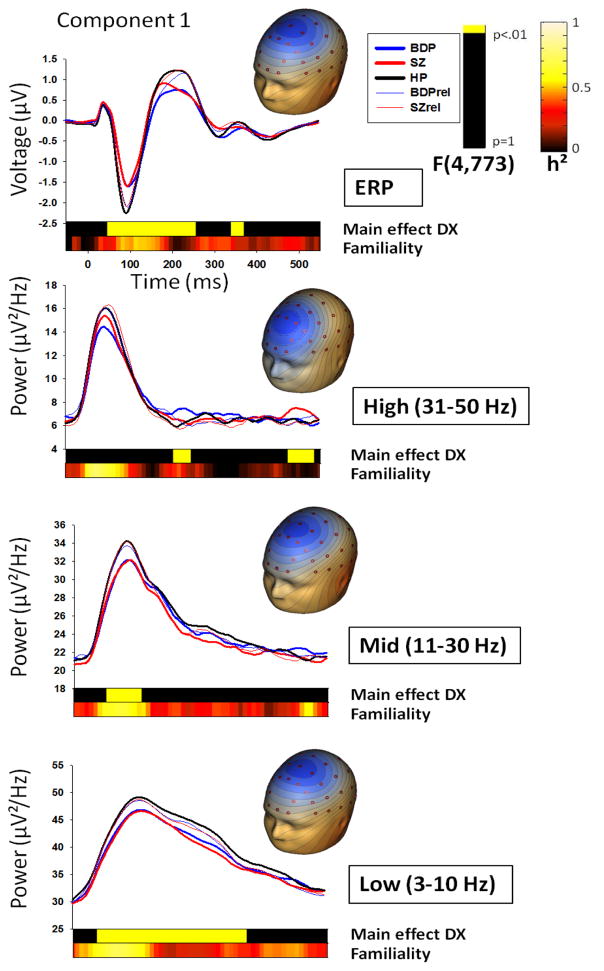
Figure 2.
Standard stimuli component waveforms by group and topographies for each spatial PCA component and PCA-derived frequency band, with ERP at the top. Note that waveforms are not corrected for age, as this was done at the coarser time-bin level. Top bars in each plot indicate clusters of time-bins in which 3 consecutive time-bins showed a significant main effect of DX (group) at the .01 alpha level. Lower bars show familiality (h²) values across all families for each time-bin.
Time-frequency
Separately from the ERP analyses, and in order to best capture data-driven frequency bands of EEG power over time, oscillatory power for frequencies from 3–52Hz was calculated with 1Hz frequency resolution using a modified Morlet wavelet transformation every 4 ms on each sensor in the averaged ERPs. To balance the tradeoff between temporal resolution at low frequencies and stability of measurement at higher frequencies (42), wavelet-length increased linearly from 1 cycle at 3Hz to 8 cycles at 52Hz. PCA was then performed on the wavelet time-frequency data, deriving data-driven frequency bands (high: 31–50 Hz, low: 3–10 Hz and midrange: 11–30 Hz) for subsequent spatial PCA (to derive frequency topographies: 2 topography components for targets and 1 for standards for each frequency band) and group analysis. See supplemental methods, SF5, Figures 1 and 2 for additional information.
ERP time-course analyses
For each condition and subject, component waveforms from −50 ms to 550 ms (matching frequency waveforms post-windowing) were grouped into sixty 10 ms bins and averaged within bin. For each bin, age effects were calculated using linear and quadratic regression analyses on healthy subjects (43). Bins with significant age effects were corrected for all subjects by subtracting the product of the linear regression coefficient and age (with the squared product of the quadratic coefficient and age for bins when significantly better-fitting quadratic effects were present) for each individual. Then, for each bin, a 2 (gender) x 5 (group: HP, SZ, BDP, SZrel, BDPrel) ANOVA was calculated to determine group differences in waveform amplitude while controlling for possible gender effects. Error degrees of freedom were adjusted based on number of families (782) to correct for lack of complete independence between groups due to genetic relatedness. To control for family-wise error due to multiple comparisons, a clustering method was implemented using Monte Carlo simulations calculated across time-bins (44). To maintain a family-wise alpha of .01, three sequential time-bins were required to be significant at alpha<.01 (Figures 1 and 2).
Time-frequency analyses
The same binning, age-correction, and 2x5 ANOVA procedure described above was utilized for time-frequency waveforms for each PCA2 component (see supplementary methods regarding PCA1 and PCA2).
Site effects
Possible site effects were examined by adding site as a variable to group comparisons; site effects were minimal for all clusters. Except for the P2 time-window to standards, where group effects remained strong with site added to comparisons, there was no overlap between significant group effects and significant group-by-site interaction so site effects were considered to be of minimal impact for variables of interest and site was removed from final analyses. (SF1 and SF2; Table 1 for subject numbers per site).
Post-hoc discriminant analyses
No variable only differentiated relatives from other groups, so to efficiently summarize variables uniquely differentiating proband groups, values from significant time-bin and time-frequency clusters were averaged within adjacent bins for each subject and submitted to linear discriminant analysis with proband group as the dependent variable (HP, SZ, BDP). Variables that minimized overall Wilks’ lambda with individual multiple F-statistics significant at p<.05 were entered in a stepwise fashion, leaving a parsimonious selection of neurophysiological measures. This analysis was included specifically to describe variables most important to group separations rather than to classify observations.
Familiality
Estimation of genetic influence on physiological responses necessitates measures of heritability, best estimated with twin/adoption studies or large family cohorts. Our sample utilized nuclear families, (where heritability cannot be disentangled from shared family environment) and families mostly consisted of one relative per proband, (underestimating heritability) so we will use the term “familiality” to describe similarities between genetically-related individuals. Overall familiality was computed for each time-bin using Sequential Oligogenic Linkage Analysis Routines (SOLAR) (45) with age and gender as covariates when significant. For cluster variables surviving linear discriminant analysis, familiality was also recomputed on each cluster (Table 2).
Results
Behavioral
For the Texas site response data was unavailable, so behavioral information was calculated on the remaining 80% of subjects. As expected, there were significant differences between groups on percent correct F(4,1015)=2.49, p=.04, although no single group differed significantly from HP. Group differences for latency of response F(4,1015)=4.79, p=.001, were driven by SZ, t(420)=3.78, p<.001 and BDP, t(392)=3.09, p=.002 showing significant slowing compared to HP. (Table 1). Accuracy was high for all groups, so all trials were included in subsequent analyses.
ERP time-bins
Group x gender ANOVAs for each condition and spatial component revealed 7 clusters that differentiated groups (Figures 1 and 2). Given a bimodal F-value distribution for the cluster spanning the N1 and P2 time-window for standards and the cluster spanning the N1/P2/N2 time-window for targets component 2, these clusters were split at points of rarity (130 ms for standards and 140 ms for targets). The target P2/N2 cluster was not split at the change in polarity due to lack of bimodal distribution in F-values and a stable pattern of group differences across this cluster.
Time-frequency
Group x gender ANOVAs for each condition, frequency band and spatial component revealed 10 clusters that differentiated groups (Figures 1 and 2). Given a bimodal F-value distribution, the low-frequency target component 2 cluster was split at the point of rarity (280 ms).
Discriminant Analyses
The 17 variables/clusters that differentiated groups were used in a linear discriminant analysis, resulting in nine variables (5 ERP time-bin and 4 time-frequency clusters) that best discriminated proband groups, (Table 2):
Standard N1 amplitude significantly discriminated probands from HP.
Standard P2 amplitude significantly discriminated probands from HP.
Standard early mid-frequency power significantly discriminated probands from HP.
Standard early high-frequency power significantly discriminated BDP, but not SZ, from HP.
Standard late high-frequency power significantly discriminated SZ, but not BDP, from HP.
Target N2 (component 1) amplitude significantly discriminated probands from HP.
Target P2/N2 complex (component 2) amplitude significantly discriminated probands from HP.
Target P3b (component 1) amplitude significantly discriminated probands from HP.
Target early frontal low-frequency power (component 1) significantly discriminated probands from HP.
Clinical Correlations
Separately for proband groups, all available clinical variables (Table 1) were submitted to Pearson correlations with the 9 discriminating EEG variables. For BDP, standard P2 was correlated with medication status for anticonvulsant/mood stabilizers (r=−.21, p<.01), lithium (r=.21, p<.01) and stimulants (r=−.21, p<.01). Medication status for SZ was correlated with standard P2 for anticonvulsant/mood stabilizers (r=−.17, p<.01), with target P2/N2 complex for first-generation antipsychotics (r=.18, p<.01), and with target frontal low-frequency for sedatives (r=.17, p<.01). Other EEG variables were not significantly correlated with any clinical assessment scores.
Relatives
As groups were ascertained by proband, relative groups could contain individuals with history of psychosis or psychosis-related personality disorders. To evaluate whether history of psychosis or Axis II characteristics influenced relative group differences, individuals with history of psychosis, then Cluster A (psychotic) personality disorder, then Cluster B (mood) personality disorder (both Cluster A and B diagnoses more liberally defined as 1 less trait count than DSM requirements to qualify (46)) were systematically removed and group comparisons were recomputed with the remaining relatives. Group differences were remarkably stable for most variables of interest, with exception of SZrel differences from HP for P3b, in which the difference was no longer significant among the healthiest relatives. Given the stability of relative group differences (SF3 and SF4), all relatives were included in omnibus group comparisons and familiality estimates; however, effect sizes are presented for complete and reduced relative groups for variables surviving linear discriminant analysis (Table 2, S2).
Familiality
Familiality calculations for each time-bin revealed higher familiality values (>0.5) for all early frequency components for standards and early low-frequency components for targets. N1 for both standards and targets also showed strong familiality. Moderate to high familiality values (0.3 to >0.5) were present across time and condition for low and mid-frequencies, as well as for P3a and P3b ERP components (Figure 1). For variables surviving linear discriminant analysis, cluster average familiality was significant for all but the standard late high-frequency and target N2 components, with particularly high (>0.70) familiality for low and mid-frequency variables (Table 2).
Discussion
Data from biological relatives of probands are important tools for assessing constitutional versus disease-related effects for etiologically complex disorders like the psychoses. The current study indicates alterations in some components of neural response to auditory oddball stimuli are biomarkers of familial risk (i) more specific to SZ (N1, P3b), (ii) more specific to BDP (P2), and (iii) for psychosis in general (N2). Use of more comprehensive ERP and time-frequency component quantifications (those mentioned above, plus frontal N2/P2, late high, early mid and low-frequency activations) may help clarify deviations in auditory sensory neural architecture associated with psychosis. This approach has been uncommon, however, especially with such large and diverse samples. It will be extremely useful to expand on this work to verify differentiation of constitution versus disease-related alterations.
Responses to standard stimuli are less studied, but data indicate they may be differentially affected in psychosis (23,47) as well as differentially heritable in psychosis and in the healthy population (35,48). For early stimulus registration, both N1 and P2 responses to standards significantly discriminated SZ and BDP from HP. Both variables were significantly familial, suggesting they are inherited biomarkers for psychosis. When examining relatives, however, only SZrel had N1 deficits and only BDPrel had P2 deficits indicating that similar deficits among probands may be constitutional in one group (N1 for SZ, P2 for BDP), and disease-related in the other. Such specific outcomes provide possible points of demarcation of variables suitable for further molecular genetic testing conditional on diagnostic group. Additionally, parietal N2 to targets was deficient in both proband groups. This variable had similar effect sizes across probands and relatives regardless of relative affectation status, suggesting this variable is an inherited biomarker for psychosis despite its marginal familiality.
The frontal P2/N2 component to targets shows an interesting effect among BDP probands, who had significantly more positive voltage than HP, while BDPrel had significantly more negative voltage than HP. Effect sizes increase slightly with removal of clinically-affected relatives, indicating this ERP may reflect a protective and/or compensatory mechanism in those at risk for psychosis who do not develop the clinical disorder. Notably, non-psychotic bipolar probands with familial risk for psychosis (first-degree relative with psychosis) show a similar pattern as BDPrel (49). Somewhat similarly, SZ and BDP share a familial predisposition but SZ and non-psychotic bipolar disorder do not (50) suggesting a specific familial risk element for psychosis that crosses clinical diagnoses.
The P2 and N2 components are associated with extended stimulus classification (35). Few auditory ERP studies have tracked high-risk individuals who either do or do not convert to psychosis, and none have reported P2/N2 amplitude effects (52,53). These studies, however, used limited sensor montages (Pz for assessing P2; Cz for assessing N2), perhaps limiting general neurophysiological utility of the information. In healthy subjects, stronger mismatch negativity (MMN), measured frontally during the 100–200 ms time-range, is highly predictive of reduced ketamine-induced psychosis (54). Those authors posited that smaller MMN indicates a NMDA receptor system more vulnerable to disruption and thus transition to psychosis. In context of our findings, stronger negativity to targets during the P2/N2 time-range is indicative of a more resilient NMDA receptor system and perhaps protection against psychosis.
The P3b showed moderate familiality, discriminated SZ and BDP from HP, but only SZrel also showed significantly reduced amplitude. This is similar to results obtained when using more traditional methodology to define P3 (Table S1). When individuals with Cluster A and/or B personality disorders were removed from SZrel, the difference was no longer significant. This pattern may indicate that disease-related effects determine SZrel differences or that removing SZrel with the sub-syndromal phenotype eliminated those with clearest genetic risk. Clinical constitution of relative groups varies across P300 reports, with none specifically eliminating psychosis-related individuals with personality disorders (12–17). Usefulness of P3b amplitude reduction as an inherited biomarker for psychosis among clinically healthy relative samples, therefore, is at present uncertain.
Using novel techniques for characterizing frequency information in evoked responses, we found multiple, largely diagnostic group-specific alterations in neural oscillatory activity. Early mid and low-frequency variables, while showing the highest familialities, did not significantly differentiate relatives from HP. Effect sizes for relatives, for both measures, were slightly less than half that of the more prominently deviant proband groups (BDP for mid; SZ for low; Table 2). This pattern, coupled with strong familiality, suggests a higher prevalence of dysfunction in family members than in the general population, with relatives falling on a continuum, perhaps based on the combination of risk alleles they carry. Particularly for the mid-frequency variable, a significant deficit in BDPrel when those with history of psychosis are included may indicate co-segregation of dysfunction with illness within BDP families. Beta frequencies are modulated by the NMDAr antagonist ketamine (55), so deficits in early sensory response along with previously discussed P2/N2 amplitude alterations may indicate stronger association of dysfunction in GABAergic NMDA networks with risk for BDP. Further exploration of this unique effect in combination with molecular genetic studies will help determine whether early stimulus processing-associated frequency alterations in relatives are related to genetic risk for psychosis.
Lastly, our previous work with a smaller proband sample suggested augmented late beta/gamma to standards as a possible biomarker for BDP (23). We observed augmented gamma to standards in BDP, but SZ also showed increased gamma activity to standards, although in a later time-range (Table 2). This gamma activity, however, was not significantly altered in relatives despite its significant familiality. This pattern indicated either these frequency deviations are not inherited biomarkers for psychosis or current clinical phenomenology does not efficiently distinguish a subgroup of families for whom this deviation may be an important clue to pathophysiology. Early gamma response has been suggested as an endophenotype for SZ but not BDP (32,35). In this study early gamma responses to targets were equally impaired in SZ and BDP and did not survive discriminant analysis, suggesting that the early gamma response may be non-specific to SZ but perhaps is an endophenotype for psychosis generally.
This study had certain limitations. First, as with all studies of chronic psychosis, medication effects may be present and cannot be easily controlled. Dosing and compliance information was based on physician choice and self-report, so firm conclusions on medication effects cannot be assessed. Many of the abnormalities in probands, however, were observed in the largely un-medicated relatives. Drug treatment and dose correlations with ERP data were also modest. Second, behavioral latency slowing in probands may be suggestive of differing evoked response latency, which was somewhat controlled for by the time-binning procedure but not directly addressed with peak latency measures. However, supplemental latency measures for P300 (Supplementary Table S1) show no group difference. Third, inferences about genetic effects on neurophysiological data were limited to familiality estimates given the nuclear family design, which confounds influences of genetic and shared family environment. Future projects will benefit from molecular genetic information to parse polygenic effects mediating psychosis risk. Nevertheless, this study provides support for shared deficits in sensory and cognitive processing across psychotic probands as well as deficits unique to single diagnostic groups, many of which were also altered in first-degree relatives and thus may serve as inherited biomarkers. Similar to many genetic studies in psychiatry, effect sizes for some of these biomarkers are modest. Still, the novel methods employed herein for quantifying neural oscillatory information based on spatial and temporal characteristics of data-driven frequency bands may provide new targets for investigating genetic underpinnings of neural alterations unique to SZ and BDP or shared across the psychosis spectrum.
Supplementary Material
Acknowledgments
We thank Dr. Gunvant Thaker for his important input and collaboration on this project. Grant support: National Institutes of Health (R01s MH077945 to GP, MH077862 to JS, MH077851 to CT, MH078113 to MK, and MH085485 to BC)
Footnotes
The remaining authors (LEE, JPH, and BAC) report no biomedical financial interests or potential conflicts of interest.
Financial Disclosures: Author JAS has served as a consultant for Takeda, Inc., Pfizer, Inc., and Eli Lilly Pharmaceuticals, BMS, Roche and has received grant funding from Janssen, Inc. Author MSK has received a grant from Sunovion Pharmaceuticals Inc. Author CAT discloses the following financial interests and associations: Intracellular Therapies (ITI, Inc.) - Advisory Board, drug development; PureTech Ventures- Ad Hoc Consultant; Eli Lilly Pharmaceuticles – Ad Hoc Consultant; Sunovion – Ad Hoc Consultant; Astellas – Ad Hoc Consultant; Merck – Ad Hoc Consultant; International Congress on Schizophrenia Research - Organizer; Unpaid volunteer; NAMI – Council Member; Unpaid Volunteer; American Psychiatric Association - Deputy Editor; Finnegan Henderson Farabow Garrett & Dunner, LLP – Expert Witness. GP- consulted for Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11(6):563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- 3.Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70(2–3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35(2):96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- 5.McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50(3):190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45(1):98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- 8.Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Front Hum Neurosci. 2010;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridberg DJ, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, Malloy FW, et al. Relationships between auditory event-related potentials and mood state, medication, and comorbid psychiatric illness in patients with bipolar disorder. Bipolar Disord. 2009;11(8):857–866. doi: 10.1111/j.1399-5618.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21(4):867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- 11.Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39(8):1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- 12.Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, et al. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41(2):325–334. doi: 10.1016/s0920-9964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 13.Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophr Res. 1991;6(1):9–13. doi: 10.1016/0920-9964(91)90015-j. [DOI] [PubMed] [Google Scholar]
- 14.Roxborough H, Muir WJ, Blackwood DH, Walker MT, Blackburn IM. Neuropsychological and P300 abnormalities in schizophrenics and their relatives. Psychol Med. 1993;23(2):305–314. doi: 10.1017/s0033291700028385. [DOI] [PubMed] [Google Scholar]
- 15.Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits In unaffected siblings of schizophrenic probands. Biol Psychiatry. 2000;47(5):380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- 16.Winterer G, Egan MF, Raedler T, Sanchez C, Jones DW, Coppola R, et al. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60(11):1158–67. doi: 10.1001/archpsyc.60.11.1158. [DOI] [PubMed] [Google Scholar]
- 17.Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, et al. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10(3):377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 18.van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31(6):533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Kiehl KA, Pearlson G, Perrone-Bizzozero NI, Eichele T, Calhoun VD. Genetic determinants of target and novelty-related event-related potentials in the auditory oddball response. Neuroimage. 2009;46(3):809–816. doi: 10.1016/j.neuroimage.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannesen JK, O'Donnell BF, Shekhar A, McGrew JH, Hetrick WP. Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder. Schizophr Bull. 2013;39(6):1219–1229. doi: 10.1093/schbul/sbs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36(5):991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Force RB, Venables NC, Sponheim SR. An auditory processing abnormality specific to liability for schizophrenia. Schizophr Res. 2008;103(1–3):298–310. doi: 10.1016/j.schres.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biol Psychiatry. 2012;72(9):766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demiralp T, Ucok A, Devrim M, Isoglu-Alkac U, Tecer A, Polich J. N2 and P3 components of event-related potential in first-episode schizophrenic patients: scalp topography, medication, and latency effects. Psychiatry Res. 2002;111(2–3):167–179. doi: 10.1016/s0165-1781(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 25.Salisbury DF, O'Donnell BF, McCarley RW, Shenton ME, Benavage A. The N2 event-related potential reflects attention deficit in schizophrenia. Biol Psychol. 1994;39(1):1–13. doi: 10.1016/0301-0511(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 26.Iacono WG, Clementz BA. A strategy for elucidating genetic influences on complex psychopathological syndromes (with special reference to ocular motor functioning and schizophrenia) Prog Exp Pers Psychopathol Res. 1993;16:11–65. [PubMed] [Google Scholar]
- 27.Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Res. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Simons CJ, Sambeth A, Krabbendam L, Pfeifer S, van Os J, Riedel WJ. Auditory P300 and N100 components as intermediate phenotypes for psychotic disorder: Familial liability and reliability. Clin Neurophys. 2011;122(10):1984–1990. doi: 10.1016/j.clinph.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 29.Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. J Abnorm Psychol. 2007;116(3):565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- 30.Privman E, Fisch L, Neufeld MY, Kramer U, Kipervasser S, Andelman F, et al. Antagonistic relationship between gamma power and visual evoked potentials revealed in human visual cortex. Cereb Cortex. 2011;21(3):616–624. doi: 10.1093/cercor/bhq128. [DOI] [PubMed] [Google Scholar]
- 31.Hamm JP, Ethridge LE, Shapiro JR, Stevens MC, Boutros NN, Summerfelt A, et al. Spatiotemporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and bipolar disorder with psychosis. Psychophys. 2012;49(4):522–530. doi: 10.1111/j.1469-8986.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2011;37(4):778–787. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor GW, McCarley RW, Salisbury DF. Early auditory gamma band response abnormalities in first hospitalized schizophrenia. Suppl Clin Neurophysiol. 2013;62:131–145. doi: 10.1016/b978-0-7020-5307-8.00009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballesteros A, Summerfelt A, Du X, Jiang P, Chiappelli J, Tagamets M, O'Donnell P, Kochunov P, Hong LE. Electrophysiological intermediate biomarkers for oxidative stress in schizophrenia. Clin Neurophysiol. 2013;124(11):2209–2215. doi: 10.1016/j.clinph.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Kane F, Murray RM, Bramon E, Sham P, Rijsdijk F. The genetic and environmental influences of event-related gamma oscillations on bipolar disorder. Bipolar Disord. 2011;13(3):260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson G, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the bipolar and schizophrenia network on intermediate phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV--clinical version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 38.Lancon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophr Res. 2000;42(3):231–239. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 40.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- 42.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. PNAS. 2010;107(37):16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dukart J, Schroeter ML, Mueller K Alzheimer's Disease Neuroimaging, Initiative. Age correction in dementia--matching to a healthy brain. PLoS One. 2011;6(7):e22193. doi: 10.1371/journal.pone.0022193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox LA., Jr Reassessing benzene risks using internal doses and Monte-Carlo uncertainty analysis. Environ Health Perspect. 1996;104(Suppl 6):1413–1429. doi: 10.1289/ehp.961041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71(10):881–9. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Donnell BF, Hokama H, McCarley RW, Smith RS, Salisbury DF, Mondrow E, Nestor PG, Shenton ME. Auditory ERPs to non-target stimuli in schizophrenia: relationship to probability, task-demands, and target ERPs. Int J Psychophysiol. 1994;17(3):219–231. doi: 10.1016/0167-8760(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 48.Ethridge LE, Malone SM, Iacono WG, Clementz BA. Genetic influences on composite neural activations supporting visual target identification. Biol Psychol. 2013;92(2):329–341. doi: 10.1016/j.biopsycho.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamm JP, Ethridge LE, Shapiro JR, Pearlson G, Tamminga CA, Sweeney JA, et al. Family history of psychosis moderates early auditory cortical response abnormalities in non-psychotic bipolar disorder. Bipolar Disord. 2013 doi: 10.1111/bdi.12110. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry. 1993;50(7):527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- 51.Gandelman-Marton R, Theitler J, Klein C, Rabey JM. The effects of immediate and short-term retest on the latencies and amplitudes of the auditory event-related potentials in healthy adults. J Neurosci Methods. 2010;186(1):77–80. doi: 10.1016/j.jneumeth.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 52.van Tricht MJ, Nieman DH, Koelman JH, Bour LJ, van der Meer JN, van Amelsvoort TA, et al. Auditory ERP components before and after transition to a first psychotic episode. Biol Psychol. 2011;87(3):350–357. doi: 10.1016/j.biopsycho.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 53.van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, et al. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 2010;68(7):642–648. doi: 10.1016/j.biopsych.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51(5):400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 55.Freye E, Partecke LB, Levy JV. Increase in delta- and beta-wave activity of the EEG during rapid opiate detoxification (ROD)-reversal by administration of the non-specific NMDA-antagonist S+ ketamine. Neurophysiol Clin. 2005;35(1):25–32. doi: 10.1016/j.neucli.2004.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.