Summary
Antibiotics induce changes or dysbiosis of the intestinal microbiome. These antibiotic-induce changes may contribute to the pathogenesis of necrotizing enterocolitis (NEC) and antibiotic-associated diarrhea (AAD). Studies are beginning to unravel the contribution of specific groups of microbes to these diseases—most notably Gammaproteobacteria for NEC and bile acid- and carbohydrate-metabolizing microbes for AAD. Antibiotic-associated diarrhea occurs when antibiotic treatment induces diarrhea by altering the metabolic function of the patient’s intestinal microbiota leading to either an osmotic or infectious diarrhea, most notably Clostridium difficile infection (CDI). Antibiotic therapy impairs the host microbiota’s ability to resist colonization or expansion of pathogenic bacteria. In the case of CDI, there is growing evidence that microbiota-mediated bile acid metabolism is critical in the pathogenesis of this infection. Probiotics or other microbiota-targeted therapies may provide effective strategies to prevent and treat NEC and AAD.
Keywords: Antibiotics, microbiome, necrotizing enterocolitis, antibiotic-associated diarrhea, Clostridium difficile, probiotics
Introduction
Antibiotics are commonly prescribed medications that have saved countless lives, yet their side effects pose significant health challenges. Antibiotics are the most frequently prescribed medications in children1 and constitute a significant amount in adults2 Antibiotics function by either direct killing or inhibiting growth of bacteria. In either case, they work in conjunction with the host’s immune system to resolve infections.
Antibiotics and the microbiome
The intestinal microbiome is a complex ecosystem in which there is tremendous interdependence and cross talk between microbial species and between the microbes and their host. While antibiotics target specific types of microbes (e.g., vancomycin and Gram-positive organisms), their effects on the microbiome go beyond just those clinically targeted microbes. For example, removing certain species of bacteria opens niches for other microbes to expand which, in turn, can result in microbiome disruptions or microbial dysbiosis, such as when treatment with the Gram-positive microbe-targeted antibiotic vancomycin leads to loss of some Gram-negative taxa3. It is important to note that not all antibiotics impact intestinal microbiota to the same degree. For example, vancomycin and metronidazole both drastically change the composition of the microbiota (in different ways) but the overall bacterial density is less following metronidazole treatment yet remains the same following vancomycin treatment3. The route of exposure also matters, as parenteral antibiotic treatment can impact the intestinal microbiome via biliary excretion of antibiotic into the intestinal lumen4. Thus, while antibiotics are intended to target specific pathogenic microbes, their effects can be much more extensive, long-lasting and unpredictable5. Antibiotic-induced dysbiosis contributes in the shorter-term to antibiotic-associated diarrhea and is epidemiologically linked to a variety of longer-term health problems including obesity, asthma, allergy and inflammatory bowel disease (reviewed in5,6).
Microbiome of the neonate
Neonates face enormous challenges at parturition including developing tolerance to their new microbiota while maintaining immunity against infection. The initial colonization of the gastrointestinal (GI) tract is an intricate balance between the colonization of commensal bacteria that leads to the establishment of tolerance and the prevention of infections secondary to the selective recognition of pathogenic microbes by the host. These host-microbial interactions are critical for the development and function of both the GI tract and the immune system. For example, the microbiota of the GI tract regulates angiogenesis7, enterocyte proliferation, proper crypt formation8, along with development and function of gut-associated lymphoid tissue (GALT) and the intestinal T cell populations which prevent intestinal inflammation9,10. In a healthy neonate, this early cross talk between commensal bacteria and the host leads to pathogen recognition, epithelial barrier maturation, immune system development and development of tolerance to food antigens and commensal bacteria11.
Microbial exposures early in ontogeny are associated with a range of diseases from atopy and autoimmune disorders to obesity and cancer (reviewed in12). This process is thought to occur either through epigenetic epithelial and/or immune system changes or by providing a niche for specific microbial colonization that influences long-term health outcomes11. However, exactly how the microbiome is established, and the impacts of prenatal and postnatal exposures have on the development of the microbiome, is only starting to be elucidated but offers great promise in predicting, preventing and treating a variety of diseases.
The neonatal GI tract rapidly becomes colonized with microbiota. Newborn’s initial microbiota is acquired by vertical transmission of the maternal microbiome during delivery13–16, although there is evidence for17–19 and against20 low level microbial colonization of the placenta in utero. The mode of delivery, either via vaginal or caesarian section, influences the acquisition of the majority of the initial microbes13,14,16. As such prenatal factors which impact the maternal microbiome also influence the newborn’s microbiome (reviewed in6).
The neonatal microbiome follows a general developmental process although significant inter-individual variation is prominent13,15,16. In the first month, facultative anaerobic microbes from the Enterobacteriaceae family, a large group of Gram-negative bacteria that includes pathogens such as E. coli along with non-pathogenic bacteria, dominates the neonatal microbiome. Over the next few months, the Enterobacteriaceae are succeeded by anaerobic bacteria including the families Bifidobacteriaceae, Bacteroidaceae, Lachnospiraceae and Ruminococaccea13,15. Around the time of weaning, a varied mixture of bacterial families are present including Clostridiaceae. One way to monitor the development of the microbiome is to utilize microbial ecology concepts such as alpha diversity, which describes the number and distribution of species present in a given individual21. For example, alpha diversity is lower in infants than adults reflecting the higher number of microbial species in the adult microbiome16. The microbiome of neonates and infants is rapidly changing, but stabilizes into an adultlike microbiome by 3 years of age16,22. A multitude of factors such as the maternal microbiome, mode of delivery, diet and antibiotic exposure influence this process6.
Moreover, murine models suggest that not only is the fetus exposed to the maternal GI microbiome prior to delivery, but that colonization of mice during gestation has direct effects on the development of the offspring’s immune system23. These data raise the possibility that prenatal exposure to antibiotics or other means of altering maternal microbiomes can have profound implications for their the offspring. In support of this, prenatal exposure to antibiotics has been associated with increased risk for obesity and asthma24,25.
Mode of delivery (cesarean section versus vaginal delivery) also shapes the neonatal microbiome26. The microbiome of infants born vaginally is characterized by vaginal fecal resident microbes such as B. longum and B. catenulatum, whereas infants delivered by caesarian sections have environmental microbes as their predominant microbiota27. This difference is long lasting and can be seen even in older infants26 and children28. Moreover studies have suggested a link between the caesarian-section-associated microbiome and long-term outcomes such as asthma29, gastroenteritis, celiac disease30, and diabetes27,32.
Infant diet also contributes to the development and composition of the microbiome. The microbiomes of breast milk fed and formula fed infants are quite distinct. Breast milk is high in prebiotic compounds such as human milk oligosaccharides (HMOs) that enhance bacterial growth. It also contains live bacteria not found in formula that can influence the microbiome (reviewed in33). The microbiome of neonates fed a breast milk diet is more abundant in Bifidobacteria and Lactobacillus26,34,35. Surprisingly, several studies have found that although breastfed infants have higher bacterial counts, they have a lower species diversity than formula fed infants26,34,35.
Antibiotic effects on the microbiome
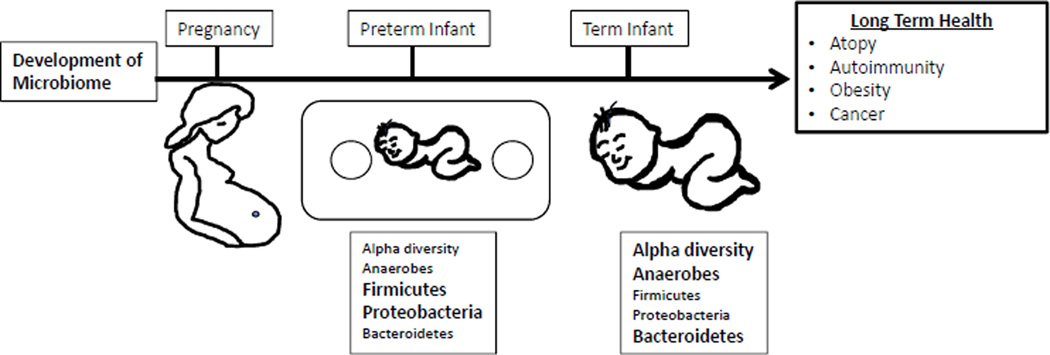
Post-natal exposure to antibiotics is another important factor that shapes the microbiome. Antibiotic treatment decreases alpha diversity of the individual’s microbiome15,36. For examples, a seminal study in three healthy adults showed that 5 days of standard dose (500mg/BID) antibiotic treatment with ciprofloxicin had a significant effect on roughly one-third of the bacterial taxa identified in the study. However, most of these disturbances only lasted approximately 4 weeks, although some taxa were still missing at 6 months after treatment36. Additional studies have corroborated these finding and demonstrated that some antibiotics have even more profound and long-lasting disruptions of the microbiome3,37. Further, antibiotic exposure in infants and young children may have significant impacts on the microbiota during critical periods of development. For example, the microbiomes of infants exposured to ampicillin and gentamicin perinatally showed a decrease in Actinobacteria species (Bifidobacterium and Lactobacillus) and an increase in Proteobacteria at 4 weeks of age and continued to have a decrease in the alpha diversity of these species even by 8 weeks of age38. In a longer study that evaluated the microbiota of 43 infants form birth to age 2, Bokulich et al., observed that early antibiotic exposure led to a decrease in the microbiome’s alpha diversity and specific deficits in the Clostridium and Ruminococcus species13. Moreover, they showed that early antibiotic exposure decreased stability and delayed the maturation of the intestinal microbiome13. Similarly, another longitudinal cohort of children from birth to age 3 also showed a decrease in the alpha diversity of the microbiota of children exposed to antibiotics15. Additionally, they showed that the species found in the microbiota of children exposed to antibiotics was dominated by a single strain rather than having multiple strains of the same species and, similarly to the previous study, had deficits in clostridium species. Finally, antibiotic-exposed microbiota had an expansion of antibiotics resistance genes15 (Figure 1).
Figure 1.
Timeline of microbiome development in premature and term infants
Microbiome of the preterm infant
Preterm infants face a more difficult challenge to maintain homeostasis with their developing microbiomes. They have immature immune and GI systems, commonly receive multiple courses of antibiotics and have abnormal feeding patterns. Not surprisingly, the colonization patterns of the GI tract differ in preterm and term infants39,40. The diversity of the premature infant’s microbiota is even more limited than in full term neonates, where the majority of the detectable species in premature infant’s microbiomes are known neonatal pathogens39,40. Additional differences include fewer anaerobes, increased abundance of Firmicutes and Proteobacteria, and decreased abundance of Bacteroides as well as a substantial delay in bifidobacterial colonization when compared to full term neonates11,41,42.
Necrotizing Enterocolitis
One of the most devastating emergencies of the premature infant is necrotizing enterocolitis (NEC), affecting up to 10% of all premature infants. Although the pathogenesis of NEC remains incompletely understood, there is growing appreciation that defects in the development of host-microbiome commensalism likely contributes. NEC is characterized by uncontrolled intestinal inflammation that can lead to tissue necrosis, perforation and sepsis. It is associated with mortality rates as high as 30% and substantial short and long term morbidity (reviewed in43). Prematurity is the predominant risk factor for NEC. Interestingly, most cases of NEC occur at 31–32 weeks of corrected gestational age, independent of gestational age at birth, suggesting that host intrinsic developmental factors may impact the pathogenesis of NEC44.
Microbiome and NEC
Murine models of NEC have suggested that bacterial colonization of the intestine is essential to the development of NEC; however, no specific bacterial species has been identified as the causative agent for NEC (reviewed in45). Human cross-sectional studies have also failed to identify a causative bacterial agent for NEC42,46,47. Longitudinal analyses of fecal microbiomes from premature infants are beginning to shed some light on the differences in microbiome composition and colonization patterns between infants that develop NEC and those who do not. A number of small, longitudinal studies have implicated a variety of bacterial species. For example, Torrazza et al., showed that Proteobacteria and Actinobacteria were more abundant in stools of infants that later develop NEC, whereas Bifidobacteria and Bacteroidetes were less abundant in those infants48. Fecal dysbiosis was also identified in a study by Morrow et al, that similarly showed an increased abundance of microbes from the Proteobacteria and Firmicutes phyla preceding the development of NEC49. Two separate small studies associated increased abundance of Clostridia species and Gammaproteobacteria with NEC50,51. In a cohort of 11 infants with NEC and 22 controls, Heida et al, showed that the meconium of infants that subsequently developed NEC was enriched for Clostridium perfringens and Bacteroides dorei species when compared to control infants52. Moreover, the abundance of staphylococci species was negatively associated with NEC development52. A recent large, multi-center study that analyzed stool samples from 166 infants of whom 46 developed NEC did not find any difference between the microbiome of the meconium in infants that developed NEC or controls. However, there were significant differences between groups by one month of age, and similar to previous smaller studies, there was a higher abundance of Gammaproteobacteria prior to the diagnosis of NEC53. Additionally, there was a reduction in strict anaerobes and alpha diversity that preceded the development of NEC in infants born less than 27 weeks of age53. Taken together, these studies suggest that there is a clear dysbiosis with shifts toward increases in Gammaproteobacteria that precedes the development of NEC.
Antibiotics and NEC: Friend or foe?
Although it remains controversial, prenatal and/or post-natal exposure to antibiotics might contribute to the dysbiosis preceeding NEC. Several older randomized controlled trials (RCTs)54 as well as animal studies have shown that prophylactic administration of enteral antibiotics can prevent this disease50. Five randomized controlled trials have been conducted to evaluate the use of prophylactic enteral antibiotics (gentamicin, vancomycin and kanamycin) for the prevention of NEC, all demonstrating significant reductions in rates of NEC54–58, with a subsequent meta-analysis showing an almost 50% reduction in the rates of NEC and a 70% reduction in NEC-related deaths59. However, these studies have limitations. The most recent study54 was conducted in 1998 (and the others more than 30 years ago), and there was limited adjusting for confounding, accounting for diet and feeding schedules, or reporting of harmful side effects of the drugs. Further, the standard of care in the NICU has changed dramatically since then, with implementation of standardized feeding protocols, earlier introduction of enteral feeds, and use of donor human milk when breast milk is not available, all of which have significantly reduced the rates of NEC60. As such, the applicability of these studies to the modern day NICU is uncertain. Additionally, there is concern that prolonged exposure to antibiotics will increase antibiotic resistance. Consistent with this, Boyle et al., found that enteral kanamycin treatment was associated with an increase in antibiotic resistant bacteria56. Interestingly, enteral vancomycin treatment was not associated with increased antibiotic resistance in this study, but was associated with significant changes in the microbiota with predominance of Gram negative bacteria and yeasts, a milieu that could be harmful to the premature host54. Thus, before recommending empiric enteral antibiotic treatment for neonates at high risk for NEC, additional data are required to ensure that the potential benefits would outweigh the risks. Yet, these older RCTs along with the new prospective characterization of the neonatal microbiome before the onset of disease provide strong evidence for the importance of intestinal microbiota in the pathogenesis of NEC and raise enthusiasm for microbiota modulating therapy.
More recent studies have addressed whether parenteral antibiotics impact the risk for NEC. Premature neonates almost universally receive broad-spectrum antibiotics during their first two days of life and many receive more prolonged antibiotic courses for treatment of culture proven or “culture-negative sepsis”. Various retrospective studies have shown that prolonged antenatal61 and post-natal antibiotic exposure is associated with an increased risk of developing NEC62–65. Specifically, a retrospective analysis of 97 matched pairs showed that prenatal exposure to ampicillin was significantly greater in infants with NEC61. Similarly, empirical (culture negative) antibiotics exposure for greater than 5 days has been associated with the development of NEC in numerous studies62–65.
This discordance in the effects of enteral vs. parenteral administration of antibiotics on the effect of NEC suggests that it is not the use of the antibiotics per se that is detrimental but alterations in the composition of the microbiome that can either predispose to, or protect against, the development of NEC. Alternatively, residual confounding by indication might explain this association, as patients who receive antibiotics may represent a sicker group with a higher risk for NEC that is independent of antibiotic exposure. Teasing this apart will require further study that explores NEC-associated pathogens such as Gammaproteobacteria in healthy and antibiotic-treated neonates.
Probiotics and NEC
Since intestinal dysbiosis is associated with the development of NEC, modulating the host’s intestinal microbiome could be a way of preventing or ameliorating the disease. Probiotics have been extensively studied and, in general, seem to reduce the incidence of NEC. A recent meta-analysis of 20 RCTs66 involving 5982 patients, showed that the relative risk of NEC was reduced by almost 50% and overall mortality by 27% with the use of probiotics. However, these studies used various probiotics (Lactobacillus, Bifidobacterium, or saccharomyces spp.) and doses. Subgroup analysis identified that Lactobacillus or a mixture of Lactobacillus and Bifidobacterium were most beneficial in reducing NEC. However, probiotics have not been used routinely to prevent NEC in the US owing to a lack of an FDA approved product for this age group and little knowledge of potential adverse outcomes and long-term data. Additionally, a recent phase III trial failed to show a benefit to using bifidobacterium breve BBG-001 in preventing NEC67, suggesting that this bifidobacterium alone is not the optimal agent to use. Initially there was concern that the use of probiotics in premature infants would increase the risk of sepsis extrapolated from case reports of probiotic-associted sepsis in immunocompromised and short bowel patients68, although the meta-analysis did not show an increase in sepsis in infants receiving probiotics66. Thus, probiotics remain a promising intervention for preventing NEC, though the most effective and safe preparation has not been clearly identified.
Antibiotic-induced diarrhea
Antibiotic-associated diarrhea (AAD), defined as diarrhea without a clear etiology that is associated with antibiotic treatment, is one of the most common medication side effects that patients and clinicians encounter69. Often AAD is mild but can also be severe and life-threatening, especially in cases of Clostridium difficile infection (CDI)69. Advances in our understanding of the intestinal microbiome has set the foundation for microbiome-targeted therapies to prevent and treat AAD.
Incidence of AAD
Diarrhea occurs in up to 35% of patients who receive antibiotics69. AAD ranges in severity from mild to life threatening, and the incidence of diarrhea varies depending on the antibiotic and the patient. Patients treated with amoxicillin-clavulanate and ampicillin have high rates of AAD (10–25%) while fluoroquinolones, macrolides, tetracyclines and cephalosporins less often induce AAD69. A recent pediatric meta-analysis reported AAD rates of 19.8%, 8.1% and 1.2% for amoxicillin-clavulanate, amoxicillin and penicillin V, respectively70. Clindamycin was historically associated with CDI in the original studies that linked CDI to pseudomembranous colitis71,72 and continues to cause a high rate of CDI73. It is important to note, however, that almost any antibiotic can increase risk for CDI73 likely reflecting both the variation in the microbiota of our patients as well as the variable responses of different microbiota to different antibiotics.
Infectious causes of AAD
Antibiotic-associated diarrhea has been recognized since the advent of antibiotics. More recently, Clostridium difficile infection has become a growing and significant problem. C. difficile is a Gram-positive, spore forming, anaerobic, toxin-producing bacteria that lives in soil and the GI tract of humans and animals. It is a significant cause of morbidity and mortality especially in hospitalized patients. In 2011, there were an estimated half-million cases of Clostridium difficile infection and almost 30,000 deaths from this infection in the United States74. CDI is the leading cause of death from gastroenteritis in the US74. Patients may become colonized but remain asymptomatic or progress to symptomatic disease or CDI. The main risk factors for CDI are antibiotic exposure along with advanced age, immune system suppression, and prolonged hospital stay75.
Other pathogens beyond Clostridium difficile have been implicated as causing AAD include Klebsiella oxytoca, Staphylococcus aureus, Clostridium perfringens, Salmonella spp. and Candida spp (reviewed in76). The majority of cases of AAD have not been linked to specific infectious agents with only 10–20% due to CDI and a much smaller contribution from the other known pathogens76 (Box 1).
Box 1. Less common infectious causes of AAD.
| Microbe |
|---|
| Klebsiella oxytoca |
| Staphylococcus aureus |
| Clostridium perfringens |
| Salmonella spp. |
| Candida spp. |
Mechanisms for antibiotic-induced diarrhea
The pathogenesis of AAD is varied. The most common mechanism is antibiotic induced microbial dysbiosis leading to altered metabolism of key intestinal nutrients, whose build-up induces an osmotic diarrhea. The second mechanism is loss of colonization resistance and subsequent infection with pathogenic bacteria e.g. Clostridium difficile. A third is direct promotility action of specific antibiotics such as erythromycin which acts as a motilin agonist69,77 (Table 1).
Table 1.
Mechanisms of antibiotic associated diarrhea
| Mechanisms Of AAD | Consequence | Example |
|---|---|---|
| Loss of microbial metabolism |
Increased metabolites lead to osmotic diarrhea |
Amoxicillin- clavulanate |
| Loss of colonization resistance |
Increased risk of infection by pathogen – C. difficile |
Clindamycin |
| Direct promotility activity |
Increased intestinal motility |
Erythromycin |
Loss of colonic metabolic function
The mechanism for non-CDI AAD is thought to be due to altered microbial metabolism (see review by Marchesi in this issue). Normally, carbohydrates that were not absorbed in the small intestines would be fermented by colonic microbes to short chain fatty acids (SCFAs) such as butyrate. It has been proposed that antibiotic induced loss of colonic bacteria such as clostridial species leads to an increase in non-absorbable carbohydrates in the large intestines. The excess carbohydrate load then induces an osmotic diarrhea78–80. Antibiotics clearly alter the intestinal metabolome81–83, yet there is little experimental evidence to directly connect these metabolic changes in the intestine to non-CDI AAD78,84. Additional study in needed to identify and test the causal relationship between specific antibiotic-induced metabolic perturbations and AAD (Table 2).
Table 2.
Features of Clostridium difficile infection
| Features | Clostridium difficile diarrhea | Non-CDI AAD |
|---|---|---|
| Commonly implicated antibiotics | Clindamycin, cephalosporins, penicillins, fluoroquinolones |
Clindamycin, cephalosporins, amoxicillin-clavulanate |
| Risk Factors | Antibiotics, PPIs, older age, hospital exposure, immune suppression, GI surgery |
Previous AAD |
| History | Fevers, cramps | No fevers |
| Diarrhea | Mild to severe, fecal leukocytes positive |
Usually mild, osmotic diarrhea |
| Mechanism | Loss of colonization resistance Altered bile acids |
Loss of metabolic function Decreased fermentation of colonic carbohydrates to SCFAs |
| Treatment | Metronidazole or oral vancomycin; FMT for recalcitrant cases; probiotics |
Supportive, probiotics, anti- motility agents |
Loss of colonization resistance
Antibiotics decrease microbial community diversity and lead to decreased colonization resistance, which is the ability of the microbiota to prevent invasion of exogenous and potentially pathogenic microbes and to limit overgrowth of endogenous potentially pathogenic organisms—most notably Clostridium difficile. The mechanisms for colonization resistance are varied and include nutrient and physical niche competition between microbes, production of bacteriocidins and induction of a host response targeting specific microbes75. While the association between antibiotic treatment and reduced colonization resistance has been observed for decades, the mechanisms have not been fully deciphered, yet recent discoveries offer some important hints. Studies involving the group of metabolites, or metabolome, of stool demonstrate that microbes regulate many metabolites, and not surprisingly, antibiotic treatment alters the metabolome with dramatic changes in bile acid, carbohydrate and amino acid composition84.
Bile acids are important regulators of the Clostridium difficile life cycle85,86. Primary bile acids induce germination of C. difficile spores, but secondary bile acids, generated by bacterial transformation of primary bile acids, inhibit C. difficile growth and prevent its domination of the intestinal microbiome85. This is an elegant example of host-microbiome interactions generating colonization resistance from a pathogen that is mutually beneficial to the host and its microbiome. This balance is disrupted by antibiotic exposure that reduces bile acid metabolizing bacteria leading to lower secondary bile acids concentrations in the colon85. The loss of secondary bile acids relieves the inhibition on C. difficile’s growth allowing it to bloom to levels high enough to induce disease87. Clostridium scindens, a bile acid metabolizer, can prevent CDI in mice and potentially in humans87. This important finding lays the foundation for microbiome-targeted therapy to prevent CDI in humans75.
Competition of host-derived resources is another mechanism by which antibiotics predispose individuals to CDI. For example, antibiotics deplete microbes that utilize host-derived sialic acids. C. difficile’s growth is enhanced in the presence of sialic acids. Therefore, antibiotic treatment predisposes the host to CDI by providing an overabundance of sialic acids that may promote CDI75,88.
AAD prevention
The cornerstone of AAD and CDI prevention is thoughtful and appropriate use of antibiotics along with proper precautions to prevent spread of Clostridium difficile89. Another more novel approach is to “protect” the intestinal microbiome from antibiotic effects by selectively inactivating antibiotics in the intestines. Concurrent administration of an intravenous beta lactam or cephalosporin antibiotic with an enteral beta lactamase that is not systemically absorbed prevents parenteral antibiotic from disrupting the intestinal microbiome90. This approach, which cleverly utilizes bacterial antibiotic resistance to our benefit, is undergoing phase II clinical trials90.
CDI infection is associated with fecal microbiome changes that precede infection including decreased alpha diversity, loss of SCFA producing microbes and elevated proportions of Proteobacteria91. The bile metabolizing microbe Clostridium scindens has been associated with protection from CDI in mice and humans87. Additional research is need to investigate whether this information could be help identify patients at high risk for developing CDI and perhaps offer novel microbiome-targeted therapies.
AAD treatment
AAD usually resolves with antibiotic cessation, but this is not clinically feasible in patients with serious bacterial infection. While anti-peristaltic agents are not recommended for patients with CDI, they can provide symptom relief for patients with non-infectious AAD. Specific treatments for CDI AAD include antibiotic treatment, such as vancomycin or metronidazole89, fecal microbiome transplantation (see review in this issue by Christina Surawicz) and probiotic therapy. We will discuss probiotics therapy for AAD.
Probiotics for AAD
Probiotics are formulations of live microorganisms intended to provide health benefits when administered into the body through alterations in the host microbiota, its metabolic function or direct effects on the host. A multitude of organisms have been utilized as probiotics for the prevention or treatment of human disease. This point is worth emphasizing when considering the use of probiotics because the effectiveness and safety of various probiotics may differ based on both the properties of the specific probiotic and the characteristics of the recipient. The most commonly used probiotics include Lactobacillus, Bifidobacterium, and Saccharomyces, Enterococcus, and Bacillus spp., delivered either by pill/capsule or through food92.
As outlined above, the pathogenesis of AAD involves antibiotic-induced dysbiosis (resulting in an altered metabolic state) or diminished colonization resistance (providing a niche for infection with pathogenic bacteria). Therefore, supplementing the gut microbiota with bacteria that are robust to these disruptions by stabilizing it against the threat of dysbiosis from offending agents (e.g. antibiotics) or creating a barrier to colonization with invading pathogens (e.g. Clostridium difficile) or altering the microbiota’s metabolic functions is an attractive preventive strategy93.
Probiotics for AAD and CDI
Several studies examining the benefits of probiotics for AAD and/or CDI have been conducted, reflecting the diversity of microbial composition, host, and delivery vehicle. A recent systematic review and meta-analysis examined the impact of probiotics (Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, and/or Bacillus) on AAD in adults and children94. Of 63 RCTs (11,811 participants) that reported enough information for meta-analysis, probiotic use was associated with a lower rate of AAD compared with patients who did not receive probiotics (RR = 0.58), an effect that remained unchanged when stratified by age category. A Cochrane review assessed the efficacy of probiotics for the prevention of AAD in children, including 23 studies with nearly 4000 subjects receiving Bacillus, Bifidobacterium, Clostridium, Lactobacilli, Lactococcus, Leuconostoc, Saccharomyces, or Streptococcus spp., either alone or in combination95. Overall, 8% of children receiving probiotics experienced AAD vs. 19% of those who did not (RR = 0.46; NNT = 10). Adverse effects were rare and not associated with probiotic use.
Two large systematic reviews and meta-analyses examining the impact of probiotics on CDI in adults and children have also been conducted. Johnston et al. analyzed data from 20 RCTs including nearly 4000 patients receiving Bifidobacterium, Lactobacillus, Saccharomyces, or Streptococcus spp, finding that probiotic use was associated with a 66% (RR= 0.34) reduction in CDI rates compared with controls96. This effect was similar for both adults and children, and probiotic use was not associated with adverse effects. A similar Cochrane review of 31 studies with nearly 4500 subjects found a similar (64%) reduction in CDI, which occurred in 2.0% of probiotic recipients and 5.5% of controls95.
Despite the consistency of findings across these systematic reviews and meta-analyses, 2 subsequently conducted multicenter RCTs highlight that some questions remain about the efficacy of probiotics for AAD and CDI. An RCT of nearly 3000 patients >65 years old found no benefit of Lactobacilli and Bifidobacterium use for the prevention of AAD (10.8% receiving probiotics vs. 10.4% receiving placebo, RR = 1.04 95% CI 0.84–1.28) or CDI (0.8% vs. 1.2%, RR (RR 0.71; 95% CI 0.34–1.47)97. Although a single RCT, the lack of an effect is notable because of its large size and multicenter, pragmatic (real-world) design. Another multicenter RCT found no benefit of Saccharomyces boulardii for prevention of AAD in 477 adults from 15 hospitals using systemic antibiotics (HR 1.02; 95% CI, .55–1.90)98. Although the balance of evidence from systematic reviews and meta-analysis supports the use if probiotics for prevention of AAD and CDI, these large, multicenter trials highlight that the observed benefits likely do not apply to all probiotic formulations or patient populations.
Safety of Probiotics
To help inform the risk/benefit ratio of probiotic use for preventing AAD and CDI, examining probiotic safety is critical. Overall, probiotic administration appears to be safe with few if any side effects92. Several case reports document infections with organisms found in probiotics, typically occurring in hosts with immune compromising conditions such as prematurity, the presence of central venous access, or neutropenia99. However, these complications seem to be rare and generally treatable with antimicrobial therapy and/or removal of the infected device. Of note, because most probiotics are sold as dietary supplements, FDA scrutiny of probiotics is limited100. Thus, hospital formulary and drug use and evaluation committees and prescribing clinicians (in the ambulatory setting) should review any products considered for patient use and to verify responsible manufacturing practices.
The data summarized above suggests that probiotics decrease the incidence of AAD and CDI in some populations of adults and children, and that they are generally safe. However, several areas require further study including the optimal microbial composition, dose, and timing of administration, as well as both efficacy and safety in hosts with compromised immunity. Better defining the structure and function of the gut microbiome as it relates to the pathogenesis of AAD and CDI has the potential to generate customized probiotics for more effective prevention of these common and sometimes devastating conditions87.
Key Points.
Antibiotics induce microbial dysbiosis
Neonatal intestinal dysbiosis may contribute to necrotizing enterocolitis
Microbiome information may help predict risk for AAD and NEC
Microbiome modulation may help prevent disease
Antibiotic induce AAD by disrupting microbiota’s metabolic functions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. PEDIATRICS. 2012;130(1):23–31. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- 2.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 3.Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1(4):279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano M, Barza M, Jacobus NV, Gorbach SL. Effect of broad-spectrum parenteral antibiotics on composition of intestinal microflora of humans. Antimicrob Agents Chemother. 1987;31(2):202–206. doi: 10.1128/aac.31.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9(4):233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 6.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 7.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99(24):15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joly F, Mayeur C, Messing B, et al. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G116–G123. doi: 10.1152/ajpgi.90657.2008. [DOI] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uronis JM, Mühlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. Bereswill S, ed. PLoS ONE. 2009;4(6):e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. 2014;2:38. doi: 10.1186/2049-2618-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert review of anti-infective therapy. 2010;8(4):435–454. doi: 10.1586/eri.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82–343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81–343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. Ruan Y, ed. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208(3):226.e1–.e7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65–237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satokari R, Grönroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009;48(1):8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- 20.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4(1):29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 24.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. International journal of obesity. 2015;39(4):665–670. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015;45(1):137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 26.Madan JC, Hoen AG, Lundgren SN, et al. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA pediatrics. 2016;170(3):212–219. doi: 10.1001/jamapediatrics.2015.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clinics in perinatology. 2011;38(2):321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388–1389. doi: 10.1136/gut.2004.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renz-Polster H, David MR, Buist AS, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2005;35(11):1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 30.Decker E, Engelmann G, Findeisen A, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. PEDIATRICS. 2010;125(6):e1433–e1440. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 31.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38(4):629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 32.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–735. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez L, Langa S, Martin V, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacological research. 2013;69(1):1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2013;185(5):385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast-and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17(6):478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pamer EG, Ubeda C, Buffie CG, et al. Profound Alterations of Intestinal Microbiota following a Single Dose of Clindamycin Results in Sustained Susceptibility to Clostridium difficile-Induced Colitis. Infection and Immunity. 2011;80(1):62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouhy F, Guinane CM, Hussey S, et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56(11):5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mai V, Young CM, Ukhanova M, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morowitz MJ, Denef VJ, Costello EK, et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc Natl Acad Sci USA. 2011;108(3):1128–1133. doi: 10.1073/pnas.1010992108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moles L, Gomez M, Heilig H, et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE. 2013;8(6):e66986. doi: 10.1371/journal.pone.0066986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claud EC, Keegan KP, Brulc JM, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):20. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker WA, Neu J. Necrotizing Enterocolitis. New England Journal of Medicine. 2011 Jan;:1–10. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. PEDIATRICS. 2012;129(2):e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 45.Afrazi A, Sodhi CP, Richardson W, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res. 2011;69(3):183–188. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raveh-Sadka T, Thomas BC, Singh A, et al. Gut bacteria are rarely shared by co-hospitalized premature infants, regardless of necrotizing enterocolitis development. eLife. 2015:4. doi: 10.7554/eLife.05477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Normann E, Fahlén A, Engstrand L, Lilja HE. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr. 2013;102(2):129–136. doi: 10.1111/apa.12059. [DOI] [PubMed] [Google Scholar]
- 48.Torrazza RM, Ukhanova M, Wang X, et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. Chakravortty D, ed. PLoS ONE. 2013;8(12):e83304. doi: 10.1371/journal.pone.0083304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow AL, Lagomarcino AJ, Schibler KR, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. Lightfoot DA, ed. PLoS ONE. 2015;10(3):e0118632. doi: 10.1371/journal.pone.0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sim K, Shaw AG, Randell P, et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis. 2015;60(3):389–397. doi: 10.1093/cid/ciu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heida FH, van Zoonen AGJF, Hulscher JBF, et al. A Necrotizing Enterocolitis-Associated Gut Microbiota Is Present in the Meconium: Results of a Prospective Study. Clin Infect Dis. 2016;62(7):863–870. doi: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 53.Warner BB, Deych E, Zhou Y, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–1936. doi: 10.1016/S0140-6736(16)00081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siu YK, Ng PC, Fung SC, et al. Double blind, randomised, placebo controlled study of oral vancomycin in prevention of necrotising enterocolitis in preterm, very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F105–F109. doi: 10.1136/fn.79.2.f105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grylack LJ, Scanlon JW. Oral gentamicin therapy in the prevention of neonatal necrotizing enterocolitis. A controlled double-blind trial. American journal of diseases of children. 1978;132(12):1192–1194. doi: 10.1001/archpedi.1978.02120370040010. [DOI] [PubMed] [Google Scholar]
- 56.Boyle R, Nelson JS, Stonestreet BS, Peter G, Oh W. Alterations in stool flora resulting from oral kanamycin prophylaxis of necrotizing enterocolitis. J Pediatr. 1978;93(5):857–861. doi: 10.1016/s0022-3476(78)81101-9. [DOI] [PubMed] [Google Scholar]
- 57.Egan EA, Mantilla G, Nelson RM, Eitzman DV. A prospective controlled trial of oral kanamycin in the prevention of neonatal necrotizing enterocolitis. J Pediatr. 1976;89(3):467–470. doi: 10.1016/s0022-3476(76)80553-7. [DOI] [PubMed] [Google Scholar]
- 58.Rowley MP, Dahlenburg GW. Gentamicin in prophylaxis of neonatal necrotising enterocolitis. The Lancet. 1978;2(8088):532. doi: 10.1016/s0140-6736(78)92265-1. [DOI] [PubMed] [Google Scholar]
- 59.Bury RG, Tudehope D. In: Enteral Antibiotics for Preventing Necrotizing Enterocolitis in Low Birthweight or Preterm Infants. Tudehope D, editor. Chichester, UK: John Wiley & Sons, Ltd; 2001. [DOI] [PubMed] [Google Scholar]
- 60.Haque K. Necrotizing enterocolitis - Some things old and some things new: A comprehensive review. J Clin Neonatol. 2016;5(2):79–12. [Google Scholar]
- 61.Weintraub AS, Ferrara L, Deluca L, et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. Journal of perinatology : official journal of the California Perinatal Association. 2012;32(9):705–709. doi: 10.1038/jp.2011.180. [DOI] [PubMed] [Google Scholar]
- 62.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. PEDIATRICS. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–526. doi: 10.5144/0256-4947.2012.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau CS, Chamberlain RS. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: A meta-analysis. Journal of pediatric surgery. 2015;50(8):1405–1412. doi: 10.1016/j.jpedsurg.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR Probiotics in Preterm Infants Study Collaborative G. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. 2016;387(10019):649–660. doi: 10.1016/S0140-6736(15)01027-2. [DOI] [PubMed] [Google Scholar]
- 68.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. PEDIATRICS. 2005;115(1):178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 69.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346(5):334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 70.Kuehn J, Ismael Z, Long PF, Barker CIS, Sharland M. Reported rates of diarrhea following oral penicillin therapy in pediatric clinical trials. J Pediatr Phar macol Ther. 2015;20(2):90–104. doi: 10.5863/1551-6776-20.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tedesco FJ, Barton RW, Alpers DH. Clindamycin-associated colitis. A prospective study. Ann Intern Med. 1974;81(4):429–433. doi: 10.7326/0003-4819-81-4-429. [DOI] [PubMed] [Google Scholar]
- 72.Bartlett JG. Clostridium difficile infection: historic review. Anaerobe. 2009;15(6):227–229. doi: 10.1016/j.anaerobe.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 73.Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1 (s1)):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 74.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincent C, Manges AR. Antimicrobial Use, Human Gut Microbiota and Clostridium difficile Colonization and Infection. Antibiotics (Basel) 2015;4(3):230–253. doi: 10.3390/antibiotics4030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larcombe S, Hutton ML, Lyras D. Involvement of Bacteria Other Than Clostridium difficile in Antibiotic-Associated Diarrhoea. Trends Microbiol. 2016;24(6):463–476. doi: 10.1016/j.tim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Peeters T, Matthijs G, Depoortere I, Cachet T, Hoogmartens J, Vantrappen G. Erythromycin is a motilin receptor agonist. Am J Physiol. 1989;257(3 Pt 1):G470–G474. doi: 10.1152/ajpgi.1989.257.3.G470. [DOI] [PubMed] [Google Scholar]
- 78.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72(1):297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 79.Clausen MR, Bonnén H, Tvede M, Mortensen PB. Colonic fermentation to short-chain fatty acids is decreased in antibiotic-associated diarrhea. Gastroenterology. 1991;101(6):1497–1504. doi: 10.1016/0016-5085(91)90384-w. [DOI] [PubMed] [Google Scholar]
- 80.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42(3):1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antunes LCM, Han J, Ferreira RBR, Lolić P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother. 2011;55(4):1494–1503. doi: 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vrieze A, Out C, Fuentes S, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 83.Mellon AF, Deshpande SA, Mathers JC, Bartlett K. Effect of oral antibiotics on intestinal production of propionic acid. Arch Dis Child. 2000;82(2):169–172. doi: 10.1136/adc.82.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theriot CM, Young VB. Interactions Between the Gastrointestinal Microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theriot CM, Bowman AA, Young VB. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. Ellermeier CD, ed. mSphere. 2016;1(1):e00045–15. doi: 10.1128/mSphere.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson KH. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol. 1983;18(4):1017–1019. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng KM, Ferreyra JA, Higginbottom SK, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen SHMD, Gerding DNMD, Johnson SMD, et al. Clinical Practice Guidelines for Clostridium difficileInfection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 90.Kaleko M, Bristol JA, Hubert S, et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe. 2016 Jun; doi: 10.1016/j.anaerobe.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Vincent C, Stephens DA, Loo VG, et al. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome. 2013;1(1):18. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agency for Healthcare Research and Quality. Safety of Probiotics to Reduce Risk and Prevent or Treat Disease. 2011 Apr;:1–645. [Google Scholar]
- 93.Parkes GC, Sanderson JD, Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. Lancet Infect Dis. 2009;9(4):237–244. doi: 10.1016/S1473-3099(09)70059-3. [DOI] [PubMed] [Google Scholar]
- 94.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 95.Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. [DOI] [PubMed] [Google Scholar]
- 96.Johnston BC, Ma SSY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157(12):878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 97.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249–1257. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 98.Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to Prevent Antibiotic-Associated Diarrhea: A Randomized, Double-Masked, Placebo-Controlled Trial. Open Forum Infect Dis. 2016;3(1):ofw011. doi: 10.1093/ofid/ofw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36(6):775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 100.Degnan FH. The US Food and Drug Administration and probiotics: regulatory categorization. Clin Infect Dis. 2008;46(Suppl 2 (s2)) doi: 10.1086/523324. S133–6–discussionS144–51. [DOI] [PubMed] [Google Scholar]