Abstract
BACKGROUND: The skeleton is the most common site of colonization by metastatic cancers. Zoledronic acid (ZA) has been shown to be effective for the treatment of bone metastases regardless of whether the bone lesions are osteolytic or osteoblastic. Biochemical markers of bone turnover may be useful tools to quantify the degree of bone remodeling in the presence of bone metastases. The aim of this work was to establish the correlation between tumor dispersion (bioluminescence) and biochemical markers of bone turnover in two osteolytic and osteoblastic metastasis models in mice. METHODS: The A549M1 cell line that produces osteolytic metastases and the LADOB cell line extracted from a patient with a lung carcinoma and osteoblastic metastases cells were retrovirally transduced with a luciferase reporter gene for in vivo image analysis. Forty-four-week–old mice were inoculated in the left cardiac ventricle with A549M1 or LADOB cells. Twenty mouse of each group were treated with a single dose of ZA (70 μg/kg) 5 days after i.c. Ten animals of each group were sacrificed at 21 and 28 days postinoculation in A549M1 and 60 and 75 days in the LADOB assay. Bioluminescence analysis was quantified 7, 14, 21 ,and 28 days postinoculation in A549M1 mice and 33, 45, 60, and 75 days after inoculation in LADOB mice. Osteocalcin (BGP), aminoterminal propeptide of procollagen I (PINP), carboxiterminal telopeptide of type I collagen (CTX), and 5b isoenzyme of tartrate-resistant acid phosphatase were measured by ELISA (IDS, UK). RESULTS: Bioluminescence imaging revealed a significant increase of tumor burden on time in both osteolytic and osteoblastic mice models. ZA administration resulted in a significant decrease in tumor burden at 21 and 28 days in the A549M1 animals and 60 and 70 days postinoculation in the LADOB line. Biomarkers levels were significantly increased in the untreated group at every point in the osteolytic model. In the osteoblastic model, 2 months after inoculation, all biomarkers were significantly increased. However, 2.5 months postinoculation, only PINP and CTX were significantly increased. Serum bone remodeling markers decreased in ZA-treated mice as compared with tumor groups in both models. With respect to the correlation between bone turnover markers and tumor burden, in the osteolytic model, PINP and BGP demonstrate a strong correlation with bioluminescence in both tumoral and ZA animals, and only CTX was significantly associated with bioluminescence in the group of animals that were not treated with ZA. CONCLUSIONS: We found that the best biomarkers for the diagnosis of both osteolytic and osteoblastic metastasis are formation markers, especially BGP. Moreover, these markers can be useful in the follow-up of the treatment with ZA in both types of metastasis.
Introduction
Death of cancer patients is frequently associated to its dissemination to other sites. The skeleton is the most common site of colonization by metastatic cancers, such as breast and prostate (65%-75%), thyroid (60%), kidney (25%), and lung (30%-40%). Patients with bone metastases have a high degree of morbidity, including severe pain, hypercalcemia, spinal cord compression, and pathological fractures. These skeletal-related events lead to a decrease in the quality of life and survival [1].
During the metastatic process, cancer cells from a primary tumor, beyond the organ of origin, extravasate and enter into the circulation. According to Paget's “seed and soil” theory [2], only compatible tumor cells (the seeds) with a target organ (fertile soil) nest in and proliferate, being able to develop secondary tumors. In the skeletal compartment, metastatic cells begin to release several factors deeply altering normal bone homeostasis. In the case of osteoblastic metastases, the release of ET-1, BMPs, and Wnt, among others, exacerbates the activity of osteoblasts, leading to aberrant bone deposition. In contrast, some tumors secrete factors that lead to the formation of osteolytic lesions by altering osteoclastic activity, such as PTHrP, IL-6, and IL-11, among others. This increase of bone remodeling produces the release of bone matrix-embedded factors that favor, in turn, the growth of tumor cells, thus establishing a “vicious cycle” between the tumor and the host. Of note, regulatory mechanisms try to compensate the unbalance between formation and resorption by increasing osteoclastic and osteoblastic activities, respectively (coupling remodeling phenomena) [3].
Patients diagnosed with bone metastasis benefit from the use of antiresorptive agents such as bisphosphonates that delay the onset of skeletal related events [4]. Zoledronic acid (ZA) is the most widely used bisphosphonate for the treatment of bone metastases due to its potency and administration schedule [5]. Moreover, due to the vicious cycle, ZA has been shown to be effective for the treatment of bone metastases regardless of whether the bone lesions are osteolytic or osteoblastic [6], [7].
Markers of bone metabolism are biochemical by-products that provide insight into the activity of bone cells. Most of these markers measure the collagen degradation products from osteoclastic activity (i.e., C-telopeptides of type 1 collage or CTX) [8]. Other resorption markers include 5b isoenzyme of tartrate-resistant acid phosphatase (TRAP5b) [9], secreted from activated osteoclasts. Bone formation markers are direct or indirect products of osteoblast activity, including osteocalcin (bone Gla protein or BGP) and N-terminal propeptide of procollagen type 1 (PINP) [10].
Because biochemical markers of bone turnover have proven to be sensitive and specific indicators of bone formation and resorption, they may be useful tools to quantify the degree of bone remodeling in the presence of bone metastases both in basal conditions and after their treatment with bisphosphonates such as ZA. The potential degree of association between bone turnover markers and tumor burden would be of great clinical importance for the diagnosis of patients with bone metastasis as well as for monitoring the disease treatment.
The progression of bone metastasis depends on each type of tumor, as well as the degree of osteolysis/osteosclerosis. Due to the different behavior of bone components depending on the different types of cancer and the different types of bone metastasis (osteolytic or osteoblastic), different biochemical markers should be used in case.
In this study, we analyzed two mice models of bone metastasis: one that develops osteolytic lesions and the other using cells extracted from a patient with osteoblastic lesions. We measured tumor burden over time, as well as the levels of bone formation (BGP and PINP) and bone resorption (CTX and TRAP5b) markers. The most important finding of this study was the establishment of the correlations between tumor burden and the levels of bone markers to identify the best biomarker to detect each type of metastasis and to monitor its treatment with ZA.
Materials and Methods
Cell Lines and Culture Conditions
Two human lung adenocarcinoma cell lines were used: the A549M1 cell line, which produces osteolytic metastasis, was isolated as previously described [11], and the LADOB (Lung Adenocarcinoma Osteoblastic) cell line was obtained from a patient diagnosed with lung adenocarcinoma bone metastases of osteosclerotic pattern at the Clínica Universidad de Navarra. LADOB cells were obtained from a 30-ml pleural effusion. Cells were pelleted by centrifugation and seeded in DMEM medium with 10% FCS serum. Adherent cells were subcultured, propagated (>25 passages), and frozen at different passages with no detected changes in their phenotype. Both cell lines were grown in RPMI 1640 with L-glutamine (Invitrogen, Barcelona, Spain) supplemented with 10% fetal bovine serum (Invitrogen), 1% penicillin, and streptomycin (Invitrogen). Cells were retrovirally transduced with a luciferase reporter gene for in vivo image analysis.
In Vivo Assays
Female athymic nude mice (Harlan Iberica, Spain) were maintained under specific pathogen-free conditions. Forty-four-week–old mice were inoculated in the left cardiac ventricle with 2 × 105 A549M1 cells in 100 μl of PBS and 40 with LADOB cells, as detailed elsewhere [11], [12], [13]. Twenty A549 mice and 20 LADOB mice were treated with a single dose of ZA (70 μg/kg) 5 days after i.c. Ten animals of each group were sacrificed 21 and 28 days postinoculation in the A549M1 assay and 60 or 75 days postinoculation in the LADOB assay. Blood samples were obtained under anesthesia by intracardiac puncture and collected into an EDTA-containing tube (Microvette; Sarstedt, Nümbrecht, Germany). Serum was obtained after centrifugation at 400g for 15 minutes at 4°C, and serum samples were immediately frozen at −80°C as aliquots until determination of biochemical markers of bone turnover.
Bioluminescence analysis was quantified at 7, 14, 21, and 28 days postinoculation in the A548 mice and 30, 45, 60, and 75 days after inoculation in the LADOB assay. Mice were anesthetized and injected with 100 μl of D-luciferin intraperitoneally. Imaging was completed at 2 minutes exactly for each group of mice with an IVIS 100 Imaging System (Xenogen) and analyzed with Living Image software (Xenogen Inc.). Photon flux was calculated for the hind limbs of each mouse by using a circular region of interest. Background value (from luciferin-injected mouse with no tumor cells) was subtracted in every case.
All procedures were carried out in accordance with European Community Standards on the Care and Use of Laboratory Animals and after approval of the Ethics Committee of Instituto de Investigación Sanitaria Fundación Jiménez Díaz.
Biochemical Markers of Bone Turnover
Serum BGP was measured by a specific ELISA for mouse BGP (Osteocalcin mouse; DRG, Germany). Sensitivity of this assay was 1 ng/ml. Intra- and interassay coefficients of variation of the method were <6% and <8%, respectively. Serum PINP was assayed by an ELISA specific for rat and mouse PINP (Rat/Mouse PINP EIA; IDS, UK). Sensitivity of the assay was 0.7 ng/ml. Intra- and interassay variation coefficients of the method were <5.0% and <8.2%, respectively. Serum CTX was measured by an ELISA specific for rat and mouse CTX (RatLaps ELISA; IDS, UK). Sensitivity of the assay was 2.0 ng/ml. Intra- and inter-assay variation coefficients of the method were <5.6% and <10.5%, respectively. Serum TRAP5b was measured by an ELISA specific for mouse TRAP (Mouse TRAP Assay; IDS, UK). Sensitivity of the assay was 0.1 U/L. Intra- and interassay variation coefficients of the method were <6.5% and <8%, respectively.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.01 (GraphPad Software; La Jolla, CA). The results of the experiments were expressed as the mean ± SD or median ± SEM of the different parameters. For bioluminescence, a nonparametric method, the Mann-Whitney test, was used to compare the different groups. A P value <.05 was accepted as denoting a significant difference. For biochemical markers and if the data were normally distributed, Student’s t test was used, whereas Mann-Whitney U test was employed in nonnormally distributed data. Spearman correlation was performed to study the association between bioluminescence and the levels of biochemical markers of bone turnover. Statistical significance was defined as P < .05 (*), P < .01 (**), and P < .001 (***).
Results
Model of Osteosclerosis Using LADOB Cells
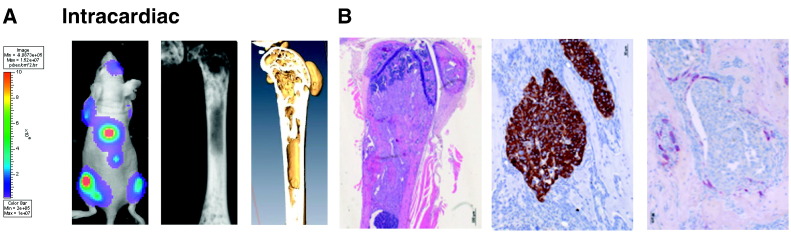
We sought to study the metastatic pattern of LADOB cells. To this end, we performed intracardiac inoculation of LADOB cells in athymic nude mice. Bioluminescence imaging showed a selective bone tropism with a time of latency of 28 to 35 days (Figure 1A). Sixty days after inoculation, osteosclerotic lesions were observed in all mice (n = 8) by X-ray imaging. Micro-CT scan revealed prominent bone formation in the metaphysis of long bones. Histological examination of these lesions confirmed the presence of tumor cell niches (Figure 1B). TRAP histochemical staining revealed the presence of some osteoclasts surrounding tumor cells with a large number of osteoblastic cells. Bone tumors were surrounded by mineralized bone matrix according to histopathological analysis. These data demonstrate that LADOB cells show metastatic ability, with the capacity to penetrate into the skeleton and to induce osteosclerotic lesions. These findings collectively suggested that LADOB cells alter bone homeostasis and induce new bone formation
Figure 1.
(A) In vivo bioluminescence imaging of a representative athymic nude mouse 7 weeks after intracardiac inoculation of LADOB cells. Note the bioluminescence emanating from different locations from the spine, long bones, and the skull compatible with the multifocal skeletal pattern of metastases. (Middle) X-ray imaging of a femur showing the radio opacity in the two metaphyses spreading toward the diaphysis. (Right) Micro-CT scan of the same bone showing osteosclerotic lesions. (B, Left) H&E staining showing the presence of tumor cells surrounding dense patches of newly formed bone at the metaphyseal-diaphyseal location. (Middle) Immunostaining for pan-cytokeratin showing tumor cells surrounded with newly formed bone. (Right) TRAP staining showing positive immunoreactivity at the tumor-bone interphase.
Tumor Burden
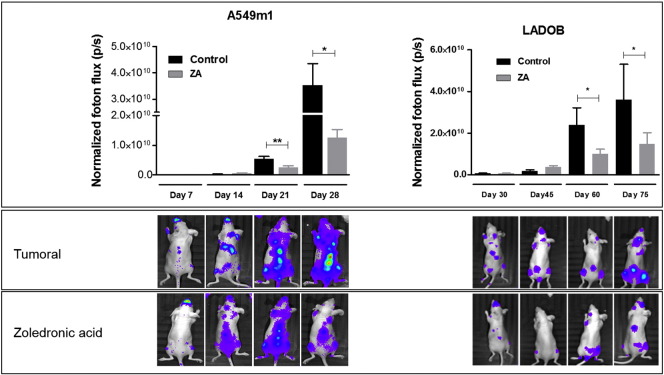
The animals were randomized into two groups (20 animals per group) after inoculation of tumor cells into the left cardiac ventricle as detailed in Figure 2. Tumor burden was monitored by in vivo bioluminescence imaging. The results of the bioluminescence imaging quantification are shown in Figure 3. As expected, bioluminescence imaging revealed a significant increase in tumor burden over time in both the osteolytic (animals inoculated with A549M1 cells) and the osteoblastic (animals inoculated with LADOB cells) mice models. Besides, compared with the nontreated group, ZA administration resulted in a significant decrease in tumor burden at 21 and 28 days postinoculation in the A549M1 animals and at 60 and 75 days postinoculation in the LADOB mice.
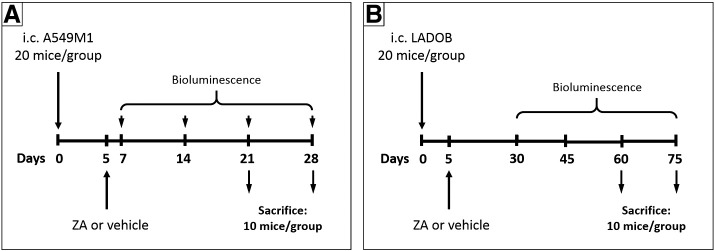
Figure 2.
Experimental design. (A) Five days after i.c. inoculation of A549M1 cells, animals were treated with a single dose of ZA (70 μg/kg) or vehicle. Ten mice for each treatment and each time point were sacrificed at 21 and 28 days. Bioluminescence was analyzed 7, 14, 21, and 28 days after A549 cells inoculation. (B) Five days after i.c. inoculation of LADOB cells, animals were treated with a single dose of ZA (70 μg/kg) or vehicle. Ten mice for each treatment and each time point were sacrificed at 60 and 75 days. Bioluminescence was analyzed 30, 45, 60, and 75 days after LADOB cells inoculation.
Figure 3.
Bioluminescence of mice inoculated with A549 or LADOB cells. (Top) Quantification of bioluminescence images was performed in the hind limbs. Data are expressed as the mean ± SEM of 10 animals/group. Mann-Whitney U test was used for comparisons between groups. Statistical significance was defined as P < .05 (*) and P < .01 (**). (Bottom) Photon flux images of the whole body of representative animals.
Biochemical Markers of Bone Turnover Levels
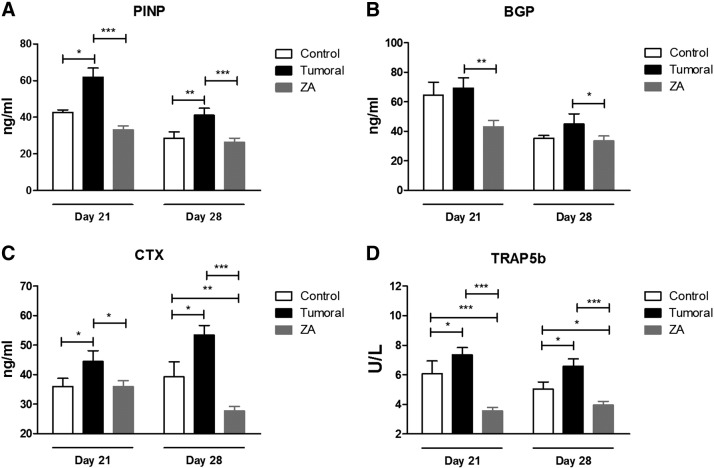
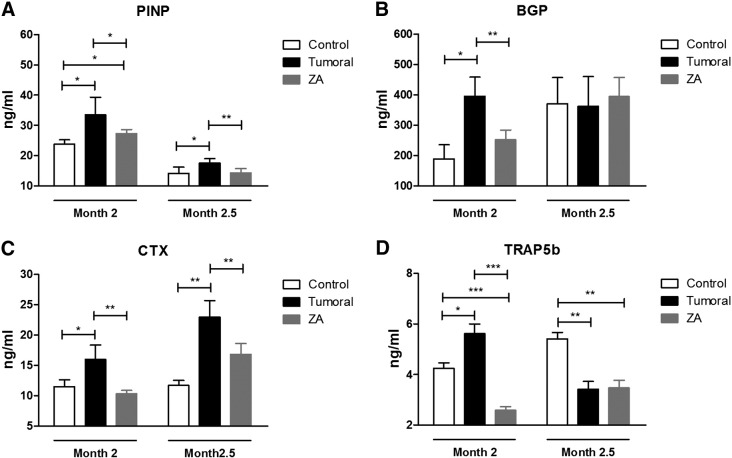
At each time point, a subset of 10 animals per condition was sacrificed, and serum biochemical markers were analyzed. Figures 4 and 5 show the levels of biochemical markers of bone turnover in mice inoculated with A549 (osteolytic metastasis) or LADOB (osteoblastic metastasis) cells, respectively. Biomarker levels were significantly increased in the untreated group at every time point in the osteolytic model. In the osteoblastic model, 2 months after inoculation, all biomarkers were significantly increased. However, 2.5 months postinoculation, only PINP and CTX were significantly increased. Consistent with the antiresorptive effects of ZA, serum bone remodeling markers significantly declined in treated mice as compared with tumor group in both models of bone metastasis.
Figure 4.
Serum levels of biochemical markers of bone turnover of the group of mice inoculated with A549 cells. (A) PINP. (B) BGP. (C) CTX. (D) TRAP5b. For comparisons, Mann-Whitney U test was used. Data are presented as mean ± SEM of 10 animals/group. Statistical significance was defined as P < .05 (*), P < .01 (**), and P < .001 (***).
Figure 5.
Serum levels of biochemical markers of bone turnover of the group of mice inoculated with LADOB cells. (A) PINP. (B) BGP. (C) CTX. (D) TRAP5b. For comparisons, Mann-Whitney U test was used. Data are presented as mean ± SEM of 10 animals/group. Statistical significance was defined as P < .05 (*), P < .01 (**), and P < .001 (***).
Correlation Between Bioluminescence and Serum Biomarkers
Bone formation marker levels exhibited a significant correlation with bioluminescence in both groups (treated and nontreated animals) of the osteolytic model. Bioluminescence in LADOB nontreated animals was significantly associated only with BGP levels. In contrast, in treated animals, a significant correlation was detected in both formation biomarkers studied with tumor burden assessed by bioluminescence imaging.
Regarding bone resorption markers, only CTX was associated with bioluminescence in the osteolytic nontreated animals. No significant correlation was found between tumor burden and bone resorption markers in the osteoblastic model (Table 1).
Table 1.
Correlation Coefficients of Biochemical Markers With Bioluminescence
| Tumoral |
ZA |
|||
|---|---|---|---|---|
| A549 | LADOB | A549 | LADOB | |
| BGP | −0.6740** | 0.6786** | −0.6739** | −0.587* |
| PINP | 0.6585** | ns | −0.4954* | 0.5123* |
| CTX | 0.6305** | ns | ns | ns |
| TRAP5b | ns | ns | ns | ns |
Pearson correlations. Statistical significance was defined as P < .05 (*), P < .01 (**), and nonsignificant (ns).
Discussion
The use of animal models provides a useful tool to assess the validity of biochemical markers, overcoming the inherent difficulties associated with human research. Xenograft models have been used to study the development of bone metastasis in human carcinomas. This strategy has previously been used by other authors in prostate [14] and breast cancer [15].
In this report, we used two xenograft models of lung cancer and bone metastasis. In these models, tumor cells (derived from patients suffering from osteolytic or osteoblastic osseous metastasis) induced selective, rapid, and reproducible bone colonization. Indeed, all inoculated animals showed an increase of tumor burden (assessed with bioluminescence imaging) on their hind limbs over time independently of the nature of the tumor cells. The fact that tumor burden increases over time in the skeleton shows that bone represents a “fertile soil” for the growth of the tumor cells.
We assessed the most common remodeling markers routinely used in the clinical setting. We selected two markers of resorption (α-CTX and TRAP5b) and two of bone formation (BGP and PINP). It is important to note that, in animal models, we can only measure the α isoform of CTX. Because the degree of β-isomerization increases with the age of collagen molecules and because rodents have a very high rate of bone remodeling, in these animals, isomerization of CTX into its β isoform does not occur.
We showed that the levels of all markers (formation and resorption) were higher in mice with bone metastasis than in the control group independently of whether the metastasis were osteolytic or osteoblastic, reflecting the increase in bone turnover produced by the presence of tumor cells in the bone tissue. In an article by Ebert et al. [16], a large set of bone remodeling markers was analyzed to determine whether bone markers can replace bone scintigraphy in the diagnosis of bone metastasis from lung cancer. They show that all markers, except for TRAP5b, were higher in patients with bone metastasis than in those without. Moreover, they did not find differences in the levels of these markers between patients suffering from osteolytic metastasis and patients with osteoblastic metastasis. The increase in the levels of bone formation or bone resorption at the onset of skeletal metastases would reflect the dominant osteoblastic or osteoclastic activity, respectively. However, due to the coupling remodeling phenomena, all markers of bone remodeling were elevated.
Several preclinical studies in vitro and in murine models of bone metastasis have shown that bisphosphonates are able to suppress osteoclastogenesis and even tumor cells growth, inhibiting the development of both osteolytic and osteoblastic bone metastasis [7]. According to our results, although ZA is able to reduce the increase of tumor burden, this treatment is not enough to avoid the seeding and the proliferation of the tumor cells in the skeleton. Furthermore, in both models of metastasis, values of all markers (formation and resorption) decreased significantly and, in most cases, reached the levels of the control group. These findings might be partially explained by the antiosteoclastic action of ZA and the high degree of bone coupling. Previous clinical studies have described similar results [17], [18], [19].
Regarding the correlation between bone turnover markers and tumor burden, in the osteolytic model, PINP and BGP demonstrated a strong association with bioluminescence in both tumoral and ZA animals, meaning that there is a significant correlation between bone matrix formation and metastasis progression, probably due to the coupling process. Interestingly, PINP and BGP, two bone formation markers, demonstrated a better correlation than bone resorption markers in a model characterized by overt osteolytic lesions. One would expect that CTX and TRAP5b would show a good association with osteolytic lesions. However, of these two resorption markers, only CTX was significantly associated with bioluminescence in the group of animals that were not treated with ZA.
Similar results were found in the osteoblastic model. Both bone formation markers correlated with tumor burden in the treated animals, whereas nontreated animals showed only a significant correlation between BGP and bioluminescence. Again, bone resorption markers did not show any significant correlation with the number of tumor cells. We found few reports in the literature studying the association of bone remodeling markers and osteoblastic metastasis treated with ZA. Most of these reports did not address osteoblastic metastasis specifically. De la Piedra et al. [20] described that PINP is a strong predictor of the appearance of skeletal related events for patients with prostate cancer and bone metastasis treated with ZA.
Interestingly, none of our mice models showed association between tumor burden and the resorption marker TRAP5b. As previously described [21], TRAP5b activity is not equal to the biological activity of osteoclasts; it reflects the total osteoclast number. Therefore, a high value may not accurately reflect the extent and degree of bone destruction.
Several clinical studies suggest that bone markers should provide useful diagnostic or prognostic information in patients suffering from bone metastasis [20], [22]. Most of these studies analyze bone turnover markers in groups of patients with bone metastasis from the same primary cancer. In a large study of patients with breast cancer and bone metastasis, Chao et al. [23] concluded that TRAP5b is a strong indicator of the presence of bone metastasis at early stages; however, it does not correlate with the extension of the disease until scintigraphy shows four or more lesions. We used the “bone resorption index” proposed by Rissanen et al. [24], in which the ratio of CTX/TRAP5b (used to estimate the amount of resorption per osteoclast) appears to be an extremely useful parameter in the postmenopausal setting. However, in this study, we did not find significant results using this parameter (data not shown).
In summary, this is the first study comparing in two mice models of osteoblastic or osteolytic metastasis the correlation between the most commonly used bone turnover markers and tumor burden. Intriguingly, we found that the best biomarkers for the diagnosis of both osteolytic and osteoblastic metastases are formation markers, especially BGP. Moreover, these markers can be useful in the follow-up of the treatment with ZA of both types of metastasis.
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of. the breast. Cancer Metastasis Rev. 1889;8:98–101. [PubMed] [Google Scholar]
- 3.Huang Q, Ouyang X. Biochemical-markers for the diagnosis of bone metastasis: a clinical review. Cancer Epidemiol. 2012;36:94–98. doi: 10.1016/j.canep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4:261–268. doi: 10.2147/tcrm.s2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung T-T, Chan J, Russell PJ, Power CA. Zoledronic acid preserves bone structure and increases survival but does not limit tumour incidence in a prostate cancer bone metastasis model. PLoS One. 2011;6:e19389. doi: 10.1371/journal.pone.0019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, Vesella RL. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. 2003;9:295–306. [PubMed] [Google Scholar]
- 8.Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Applications of an enzyme immunoassay for a new marker of bone resorption (CrossLaps): follow-up on hormone replacement therapy and osteoporosis risk assessment. J Clin Endocrinol Metab. 1995;80:864–868. doi: 10.1210/jcem.80.3.7883844. [DOI] [PubMed] [Google Scholar]
- 9.Janckila AJ, Takahashi K, Sun SZ, Yam LT. Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin Chem. 2001;47:74–80. [PubMed] [Google Scholar]
- 10.Koizumi M, Maeda H, Yoshimura K, Yamauchi T, Kawai T, Ogata E. Dissociation of bone formation markers in bone metastasis of prostate cancer. Br J Cancer. 1997;75:1601–1604. doi: 10.1038/bjc.1997.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valencia K, Martín-fernández M, Zandueta C, Ormazábal C, Martínez-canarias S, Bandrés E, de la Piedra C, Lecanda F. miR-326 associates with biochemical markers of bone turnover in lung cancer bone metastasis. Bone. 2013;52:532–539. doi: 10.1016/j.bone.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Vicent S, Luis-Ravelo D, Antón I, García-Tuñón I, Borrás-Cuesta F, Dotor J. A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res. 2008;68:2275–2285. doi: 10.1158/0008-5472.CAN-07-6493. [DOI] [PubMed] [Google Scholar]
- 13.Valencia K, Ormazábal C, Zandueta C, Luis-Ravelo D, Antón I, Pajares MJ. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18:969–980. doi: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- 14.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guisse TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 16.Ebert W, Muley T, Herb KP, Schmidt-Gayk H. Comparison of bone scintigraphy with bone markers in the diagnosis of bone metastasis in lung carcinoma patients. Anticancer Res. 2004;24:3193–3201. [PubMed] [Google Scholar]
- 17.Saad F, Lipton A. Bone-marker levels in patients with prostate cancer: potential correlations with outcomes. Curr Opin Support Palliat Care. 2010;4:127–134. doi: 10.1097/SPC.0b013e32833ac6d6. [DOI] [PubMed] [Google Scholar]
- 18.Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, Brown JE, Coleman RE. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 19.Francini F, Pascucci A, Bargagli G, Francini E, Conca R, Miano ST. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non–small cell lung cancer. Int J Clin Oncol. 2011;16:264–269. doi: 10.1007/s10147-010-0179-x. [DOI] [PubMed] [Google Scholar]
- 20.de la Piedra C, Alcaraz A, Bellmunt J, Meseguer C, Gómez-Caamano A, Ribal MJ, Vazquez F, Anido U, Smaper P, Esteban E. Usefulness of bone turnover markers as predictors of mortality risk, disease progression and skeletal-related events appearance in patients with prostate cancer with bone metastases following treatment with zoledronic acid: TUGAMO study. Br J Cancer. 2013;108:2565–2572. doi: 10.1038/bjc.2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao T-Y, Wu Y-Y, Janckila AJ. Tartrate-resistant acid phosphatase isoform 5b (TRACP 5b) as a serum maker for cancer with bone metastasis. Clin Chim Acta. 2010;411:1553–1564. doi: 10.1016/j.cca.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Barnadas A, Manso L, de la Piedra C, Meseguer C, Crespo C, Gómez P, Calvo L, Martinez P, Ruiz-Borrego P, Perello A. Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: results from a 2-year multicentre observational study (ZOMAR study) Bone. 2014;68C:32–40. doi: 10.1016/j.bone.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Chao T-Y, Yu J-C, Ku C-H, Chen MM, Lee S-H, Janckila AJ, Yam LT. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11:544–550. [PubMed] [Google Scholar]
- 24.Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–115. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]