Abstract
The early postnatal period is a highly sensitive time period for the developing brain, both in humans and rodents. During this time window, exposure to adverse experiences can lastingly impact cognitive and emotional development. In this review, we briefly discuss human and rodent studies investigating how exposure to adverse early life conditions – mainly related to quality of parental care - affects brain activity, brain structure, cognition and emotional responses later in life. We discuss the evidence that early life adversity hampers later hippocampal and prefrontal cortex functions, while increasing amygdala activity, and the sensitivity to stressors and emotional behavior later in life. Exposure to early life stress may thus on the one hand promote behavioral adaptation to potentially threatening conditions later in life –at the cost of contextual memory formation in less threatening situations- but may on the other hand also increase the sensitivity to develop stress-related and anxiety disorders in vulnerable individuals.
1. Introduction
Development of the human brain starts during gestation when large numbers of neural progenitor cells undergo neuronal differentiation (Andersen, 2003, Stiles and Jernigan, 2010). Human brain development continues into adolescence, depending on gender and on the brain region studied (Mills et al., 2014, Giedd et al., 1996, Chung et al., 2002) and alterations in neuronal function and neuronal connectivity further continue throughout life (Stiles and Jernigan, 2010, Arain et al., 2013, Toga et al., 2006, Gogtay et al., 2004). During the early postnatal developmental period, the connectivity in the brain is larger than in adults (Innocenti and Price, 2005, Stiles and Jernigan, 2010, Lopez-Larson et al., 2011) which is later fine-tuned via pruning processes (Stiles and Jernigan, 2010).
The postnatal period is a particularly important and sensitive developmental window. Adverse events during this period – such as physical, sexual or emotional abuse - or being raised in an environment with a low socioeconomic status have been associated with an increased disease probability later in life (Hackman et al., 2010, Carroll et al., 2013). For example, low parental income has been associated with increases in the incidence of early-adult hypertension and arthritis (Ziol-Guest et al., 2012). Similarly, childhood maltreatment and being exposed to bullying is a risk factor for inflammatory disorders later in life (Danese et al., 2007, Copeland et al., 2014, Shirtcliff et al., 2009).
Adverse early life experiences can also have long-lasting effects on brain function, cognitive and emotional development (Teicher and Samson, 2016) and can influence the risk to develop stress-related psychopathology later in life (Kendler et al., 2000). For instance, studies on children raised in orphanages - which were lacking proper social and maternal support – report lasting adverse effects on cognitive function and increased risk for psychiatric disorders (Rutter et al., 2010, Bakermans-Kranenburg et al., 2008, Nelson et al., 2007, Zeanah et al., 2009).
Development of the brain and brain function is therefore not only determined by genetic factors but also by environmental experiences, which can interact via epigenetic programming (Bagot and Meaney, 2010). Long-term effects of these environmental factors are most pronounced when they occur during sensitive developmental periods (Meaney and Ferguson-Smith, 2010). Together, the dynamic nature of the brain's postnatal development – that is moderated by genetic and environmental factors - enables an individual to optimally adapt to environmental changes, but also renders the brain potentially sensitive to adverse environmental influences.
In this review we briefly discuss human and animal studies that investigated how adverse early life experiences determine brain development, cognition and emotional regulation. We focus on the brain regions that are critical for emotional regulation, such as the prefrontal cortex, amygdala and hippocampus (Izquierdo et al., 2016).
2. Early life adversity and human brain development
Humans grow up in a given socio-economic setting and during this early life period, they are influenced by many factors such as the extent and quality of parental care, cognitive stimulation, nutrition and social and financial status. These factors can interact and affect neurocognitive development (Hackman et al., 2010;Noble et al., 2007). Although results from various studies on how early life environment determines brain structure and brain function are sometimes variable (which may be due to the different developmental trajectories or mediating factors that were investigated; for review see Teicher and Samson, 2016), there is evidence from human studies that early life adversity can affect hippocampal, amygdala and prefrontal cortex volumes and their function (Teicher and Samson, 2016, Teicher et al., 2016).
2.1. Hippocampus
Luby et al. (2013) reported that childhood poverty is associated with smaller hippocampal volumes, which may be moderated by the extent of support from caregivers. The same group reported recently that early maternal support was positively associated with hippocampal volumes (Luby et al., 2016). Smaller hippocampal volumes have been reported in children who suffered from early life stress (Hanson et al., 2015) or after childhood maltreatment (Teicher et al., 2012, Teicher and Samson, 2016). Not only hippocampal structure is sensitive to early life adversity. Liberzon et al. (2015) reported a reduced hippocampal activation following stress exposure in children who were raised in poverty.
2.2. Prefrontal cortex
Early childhood stress and childhood emotional maltreatment have also been related to smaller volumes of the prefrontal cortex (Hanson et al., 2015) and, more specifically, the dorsal medial prefrontal cortex (van Harmelen et al., 2010). In addition, childhood emotional maltreatment is related to enhanced activity in the dorsal medial prefrontal cortex in response to social exclusion (Van Harmelen et al., 2014a). Hypo-activation of the medial prefrontal cortex has further been reported during encoding and recognition of words (Van Harmelen et al., 2014b), while less dorsolateral prefrontal activation was found during the processing of emotional faces (Fonzo et al., 2016). Early life adversity has also been correlated with reductions in executive function (Hostinar et al., 2012), and childhood poverty, but not current income, was found to negatively correlate with ventrolateral and dorsolateral prefrontal activity (Kim et al., 2013, Liberzon et al., 2015). Moreover, working memory (Evans and Schamberg, 2009) and individual differences in prefrontal cortex volumes after early life stress have been associated with decreased executive (spatial working memory) function (Hanson et al., 2015).
2.3. Amygdala
Compared to the hippocampus and prefrontal cortex, the amygdala may often respond in an opposite direction; larger amygdala volumes have e.g. been found in children who were raised by mothers who suffered from depressive symptoms (Lupien et al., 2011). By contrast, smaller amygdala volumes were recently found in children raised under conditions of early life stress (Hanson et al., 2015) and in patients suffering from posttraumatic stress-disorder with a history of early life trauma (Veer et al., 2015) and it remains unclear what explains these structural differences.
More consistent findings have been reported when activation of the amygdala is studied after early life adversity. Childhood emotional maltreatment enhances amygdala reactivity in response to emotional faces (Van Harmelen et al., 2013) and during processes of fear and anger, a greater activity of the amygdala has been associated with anxiety symptoms (Fonzo et al., 2016), whereas a failure to suppress amygdala activity has been found during negative emotions (Kim et al., 2013). Finally, enhanced amygdala activation has been found after caregiver deprivation (Maheu et al., 2010, Tottenham et al., 2011) and in conditions of family violence (McCrory et al., 2011).
2.4. Early life adversity: enhanced emotional function in humans
In addition to these structural and functional changes, childhood trauma or maltreatment alters functional connectivity (for an outstanding review see Teicher et al., 2016). Childhood trauma and maltreatment lower the connectivity between amygdala and ventromedial prefrontal cortex (Birn et al., 2014), lower resting state functional connectivity between hippocampus and subgenual nucleus, and lower amygdala - subgenual nucleus resting state connectivity in females (Herringa et al., 2013). A weakened connectivity between the amygdala and anterior cingulate cortex has also been reported after earlier maltreatment (Pagliaccio et al., 2015). Yet, Gee et al. (2013) reported that institutional rearing accelerates coupling of amygdala to prefrontal cortex, which may reflect an altered capacity to activate and control emotional responses (Meaney, 2016). These studies may emphasize the potential role of timing when examining connectivity and volumes of specific brain regions in relation to early life adversity.
Impairments in hippocampus and prefrontal cortex function on the one hand, and the enhanced amygdala function after early life adversity (Teicher and Samson, 2016) on the other, may increase emotional responses and threat detection later life (Kim et al., 2013, Loman et al., 2013, Pollak and Tolley-Schell, 2003, van Harmelen et al., 2011). These effects may reflect patterns that have been programmed during the early postnatal environment and can be adaptive under adverse conditions later in life (Champagne et al., 2009, Gee et al., 2013). Yet, these changes could in parallel increase the risk for the development of stress-related disorders in sensitive individuals (Fonzo et al., 2016, Spinhoven et al., 2010, Caspi et al., 2003). Recent reviews have summarized and conceptualized recent findings and propose that individual vulnerability and resiliency depend not only on (epi)genetic predispositions but also on maturation of stress-responsiveness by adverse (early life) experiences and impairments in the ability to cope with challenging conditions later in life if a mismatch occurs between early- and later life adverse experiences (Champagne et al., 2009, Nederhof and Schmidt, 2012, Bock et al., 2014, Daskalakis et al., 2013).
3. Early life adversity and emotional learning in rodents
Whereas human studies are important to investigate correlations between early life experiences, global brain structure/activity and neurocognitive consequences, animal studies can increase our understanding of the underlying molecular and cellular mechanisms and establish causality between early life adversity and cognitive and emotional processes later in life (Chen and Baram, 2016).
Various animal models have been developed that allow to investigate the consequences of early life adversity; some experimental results are discussed in the sections below. These models are often based on changing the amount and quality of parental care, which is one of the most important environmental influences for the offspring during the early postnatal period, both in humans and rodents (Korosi et al., 2012, Maccari et al., 2014, Krugers and Joels, 2014). Although fathers play a critical role in the development of the brain and behavior in offspring in some rodent animals (e.g. Wu et al., 2014), we focus in this part of the review on the role of maternal care, which is a critical factor in the early postnatal period of rats and mice. By examining natural variations in maternal care in rodents, the important role of parent-child relationships for later brain development, cognition, emotion and stress-sensitivity has been established as well as how lasting effects can be transmitted, i.e. via epigenetic mechanisms (Meaney and Ferguson-Smith, 2010, Liu et al., 1997, Weaver et al., 2004, Champagne et al., 2008). In these studies, natural variations in maternal care – most prominently expressed by the amount of licking and grooming by the dam – are scored during the first postnatal week. The consequences of being raised by high-licking grooming (H-LG) or low-licking grooming (L-LG) mothers can then be related to various outcome measures later in life. Other studies have used maternal separation paradigms where mothers and pups are separated for at least a few hours (sometimes repeated over days) or for 24 h (deprivation = MD) during the first week(s) of life (Oomen et al., 2010, Workel et al., 2001). More recently, a paradigm has been developed that investigates how raising dams and litters with limited nesting and bedding material (LBN) during the early postnatal period can affect the offspring later in life (Baram et al., 2012). Raising animals under these conditions, typically from postnatal days 2–9, results in patterns of unpredictable maternal care during this critical developmental period, and affects structure, cognition and emotional processes later in life (Rice et al., 2008, Brunson et al., 2005, Baram et al., 2012, Korosi et al., 2012, Naninck et al., 2015, Arp et al., 2016). It is important to mention that the developmental window of the brain – for example for whole brain growth – corresponds to a prenatal developmental time window in humans (Workman et al., 2013). Interference with parental care in rodents during the first postnatal weeks therefore may reflect alterations of gestational stress exposure in humans.
3.1. Natural variations in maternal care
When compared to adult offspring from H-LG mothers, adult offspring from L-LG mothers has less complex cells in the hippocampal CA1 area and dentate gyrus (Champagne et al., 2008, Bagot et al., 2009). The dendrites of these cells show fewer spines and the expression of hippocampal synaptic proteins is reduced (Liu et al., 2000). In addition, neurogenesis in the dentate gyrus is affected in these animals. When compared to offspring of H-LG animals, offspring of low-licking grooming animals exhibits reduced survival of newly born cells (Bredy et al., 2003). Functionally, synaptic plasticity in the dorsal part of the dentate gyrus and CA1 area is reduced in L-LG offspring when compared to H-LG offspring (Champagne et al., 2008, Bagot et al., 2009). Even within a litter there are variations in the amount of maternal care that the pups receive and this correlates positively with dendritic complexity and dorsal hippocampal synaptic plasticity later in life (van Hasselt et al., 2012a, van Hasselt et al., 2012b). Less care within a litter is related to less dendritic complexity and reduced synaptic plasticity, at least in males. In line with these structural observations, behavioral studies show that offspring of L-LG mothers displays reduced spatial memory when compared to H-LG offspring (Liu et al., 2000).
Yet, in the ventral hippocampus - the (<20%) part of the hippocampus that has been linked to emotional behavior - neuronal excitability and synaptic potentiation is enhanced in L-LG animals when compared to H-LG animals (Nguyen et al., 2015). Also, when neurons in the dorsal hippocampus of L-LG animals are exposed to stress hormones, synaptic plasticity is enhanced, indicating that these neurons do have the ability to express synaptic plasticity, in particular under conditions that mimic stressful conditions (Champagne et al., 2008, Bagot et al., 2009, Bagot et al., 2012). When compared to H-LG animals, learning under stressful conditions such as contextual fear learning (Champagne et al., 2008) is enhanced in L-LG animals and these animals display increased anxiety levels (Weaver et al., 2006, van Hasselt et al., 2012c).
Natural variations in maternal care also affect prefrontal cortex function. Van Hasselt et al. found that individual variations in maternal care correlate with decision making in the Iowa gambling task, a task that critically depends on (among other regions) the prefrontal cortex (van Hasselt et al., 2012c). In this study, H-LG correlated with more beneficial choices during the last trials of this task.
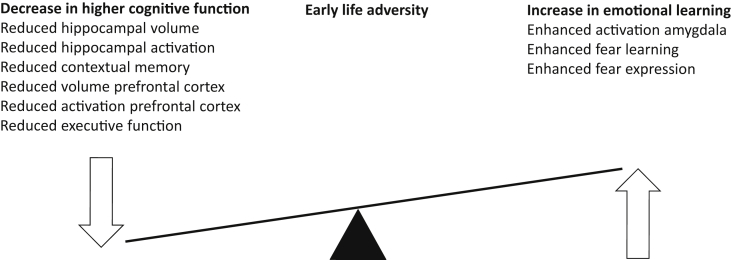
Together, these studies suggest that lower levels of maternal care received during the first postnatal week are later on accompanied by reduced spatial and executive function, but favor emotional learning processes and enhance anxiety (Fig. 1).
Fig. 1.
Early life adversity changes the balance towards enhanced cognition. Early life adversity hampers several critical measures for higher cognitive function (such as executive function) while enhancing fear learning and activation of the amygdala.
3.2. Maternal deprivation
Maternal deprivation, induced by 24 h removal of the dam from the pups increased neurogenesis in males 21 days of age (Oomen et al., 2009), but reduced proliferation of cells in 2 months' old male rats, and reduced survival and maturation of newborn cells at an adult age (Oomen et al., 2010). No effects on neurogenesis were found in female rats, while the number of granular cells in the dentate gyrus were reduced after maternal deprivation (Oomen et al., 2011). In addition, maternal deprivation slightly reduced complexity of primary dendrites in the rat dentate gyrus without affecting synaptic plasticity in this area in male rats (Oomen et al., 2010). No effects on plasticity were found in female animals (Oomen et al., 2011).
Behaviorally, Morris water maze learning was also found to be impaired after maternal deprivation (Oitzl et al., 2000, Oomen et al., 2010). As reported for animals in which natural variations in maternal care were compared, synaptic plasticity in slices of maternally deprived animals was affected by stress-hormones. Exposure to corticosterone enhanced synaptic plasticity in maternally deprived rats, suggesting a different response of hippocampal networks during exposure to stress. In line with this, contextual fear conditioning was enhanced in maternally deprived male rats (Oomen et al., 2010) while auditory fear conditioning was enhanced in both male and female rats (Oomen et al., 2011).
Also maternal separation, i.e. separating the pups from the dam repeatedly over the first postnatal days has long-lasting effects. It has been reported to enhance anxiety, reduce hippocampal synaptic plasticity and spatial memory performance (Sousa et al., 2014, Cao et al., 2014), reduce performance in the object temporal order memory task (Baudin et al., 2012, Lejeune et al., 2013) and enhance generalization of fear and fear retention (Sampath et al., 2014).
3.3. Limited bedding and nesting material
Limiting the amount of bedding and nesting material from postnatal days 2–9 (LBN) results in fragmented care and impacts cognitive and emotional function later in life (Baram et al., 2012). LBN reduced rat hippocampal CA1 dendritic complexity (Brunson et al., 2005) and also reduced the density of spines in mouse hippocampal CA3 pyramidal cells (Wang et al., 2011b, Wang et al., 2013). Moreover, neurogenesis in the mouse dentate gyrus later in life was found to be affected (Naninck et al., 2015). LBN increased neurogenesis (proliferation and differentiation of new born cells) at P9, but at the long term (P150) the survival of newly born cells as well as the volume of the dentate gyrus were reduced (Naninck et al., 2015). In line with these findings, synaptic plasticity is reduced after LBN in the hippocampal CA1 and CA3 area of middle-aged rats (Brunson et al., 2005). In slightly younger mice, LBN reduced synaptic plasticity in the adult male mouse hippocampal CA3 area (but not hippocampal CA1 area) (Wang et al., 2011b).
Behaviorally, LBN was shown to reduce spatial learning and object recognition memory (Brunson et al., 2005, Rice et al., 2008, Naninck et al., 2015). Interestingly, these effects strongly correlated with altered levels of neurogenesis (Naninck et al., 2015). While hippocampal dependent learning and memory processes were generally hampered, LBN enhanced freezing in male mice in an auditory-fear conditioning paradigm where repeated tones are presented during retrieval twenty four hours after training (Arp et al., 2016). In particular, ELS enhanced freezing between the presentation of the tones. This suggests that being raised in LBN conditions later in life hampers the ability to discriminate between potentially safe (between the tones) and non-safe (exposure to the tones) episodes.
Finally, LBN also strongly affected development of the prefrontal cortex (Yang et al., 2015). Stress exposure during the first postnatal week hampered the development of dendrites in layers II/III and V pyramidal neurons in various subregions of the prefrontal cortex and reduced performance in the temporal order memory task, which assesses prefrontal cortex function (Yang et al., 2015). In these studies, preventing apical dendritic retraction and spine loss in the prefrontal cortex after LBN also prevented impairments in prefrontal cortex-dependent cognitive tasks.
3.4. Early life adversity: towards emotional learning in rodents
Together, these rodent studies indicate that low levels and a fragmented unpredictable nature of maternal care, as well as maternal deprivation and maternal separation are accompanied in general by impaired hippocampal and prefrontal cortex functions, and by impairments in spatial and executive memory processes, whereas fear learning is enhanced (Fig. 1).
4. Early life adversity and stress-responsiveness
There is substantial evidence that early life adversity enhances activation of the hypothalamus-pituitary-adrenal (HPA)-axis and neuronal sensitivity to stress-hormones in a lasting manner (Caldji et al., 1998, Caldji et al., 2000, Francis et al., 1999, Stanton et al., 1988). Activation of the HPA-axis results in the release of corticosteroid hormones from the adrenal glands. These hormones bind in the brain to high-affinity mineralocorticoid receptors (MRs) and lower affinity glucocorticoid receptors (GRs) (de Kloet et al., 2005). Substantial evidence indicates that L-LG when compared to H-LG rats exhibit enhanced stress-responsiveness while hippocampal GR and MR levels are reduced (Liu et al., 1997, Champagne et al., 2008). The effects on GR expression may involve epigenetic (methylation) processes (Weaver et al., 2004, Weaver et al., 2006, Radtke et al., 2015).
Methylation of specific genes has also been implicated in regulating stress-responsiveness after childhood trauma in humans (Houtepen et al., 2016) and epigenetic regulation of the GR has been correlated with childhood abuse in suicide victims (McGowan et al., 2009). Early life adversity in humans has been linked to an altered sensitivity of GRs for corticosterone (Touma et al., 2011, Klengel et al., 2013). In addition, in rodents, early life experience determines the response of hippocampal synapses to corticosterone (Oomen et al., 2010, Champagne et al., 2008) since maternal deprivation and low levels of maternal care enhance hippocampal synaptic plasticity in the presence of stress-hormones.
These studies may hint to therapeutic options that could focus on targeting stress-hormones for the treatment or prevention of behavioral phenotypes after early life adversity. In line with this, Arp et al. (2016) reported that in mice that were raised under conditions of fragmented care, the increase in adult freezing behavior in a fear conditioning paradigm could be overcome by targeting GRs at adolescent age, i.e. weeks after the actual exposure to early life adversity but months before the behavioral testing (Arp et al., 2016).
Also Corticotropin Releasing Hormone (CRH) is an important mediator of early life adversity and potential target in this respect. In several studies, genetic deletion of CRH-R1 receptor (CRH-R1), or pharmacologically targeting of CRH-R1, has been reported to prevent the effects of early life adversity (Wang et al., 2011a, Wang et al., 2011b, Wang et al., 2013, Yang et al., 2015).
5. Outstanding questions
5.1. Which neurocircuitry underlies the enhanced emotional behaviors seen after early life adversity?
Early life adversity has been reported to alter functional connectivity between brain areas involved in emotional regulation (Birn et al., 2014; Herringa et al., 2013, Gee et al., 2013). Yet, exactly how this connectivity alters over time and whether it contributes to enhanced emotional behavior after early life adversity remains elusive. Optogenetic or chemogenetic tools (e.g. using DREADDs (Designer Receptors Exclusively Activated by Designer Drugs)) are now available an allow highly specific control of the activity of selective neuronal populations and their networks in brain areas. This may help to understand changes in functional connectivity between brain areas in controlling emotional regulation after early life adversity. In such studies, it will be of great interest to investigate effects of early life stress on the ability to discriminate between potentially safe and harmful contexts.
5.2. What is the cellular and molecular substrate that determines enhanced emotional behavior after early life adversity?
Activity-dependent changes in synaptic connectivity underlie learning and memory processes (Rumpel et al., 2005, Kessels and Malinow, 2009, Nabavi et al., 2014). In order to examine whether such changes underlie altered fear behavior after early life adversity, viral vectors can be used to target synaptic functions in prefrontal cortex, hippocampus and amygdala (Wang et al., 2011a).
Recent studies have demonstrated a role of so-called ‘engram’ cells in emotional memory formation (Liu et al., 2012, Redondo et al., 2014). Capturing these sparsely distributed cells during critical periods of early life adversity and fear learning (acquisition, consolidation, retrieval) will enhance our understanding of how networks and memory traces later in life are modified by early life adversity (Mayford and Reijmers, 2015, Gouty-Colomer et al., 2016). Molecular and electrophysiological tools can then be applied to identify and dissect the molecular, epigenetic and functional profile of these neurons. Using intervention studies, the causal role of these neurons and their properties (e.g. synaptic function, the (epi) genetic factors) in the effects of early life adversity on cognition can be studied.
5.3. Which factors contribute to individual variability?
An important question is why some individuals are sensitive to develop stress-related disorders, while others – which were exposed to similar adverse conditions – appear to be resilient. This requires detailed understanding of interaction between early life experiences and factors that contribute to vulnerability or resilience, such as function of GRs (Klengel et al., 2013) and MRs (Kuningas et al., 2007, Klok et al., 2015, Otte et al., 2015, Kanatsou et al., 2015), but also other genes (Caspi et al., 2003), and how they interact with the circuitry that underlies e.g. fear behavior. Preferably, these investigations are carried out in a (epi)genome-wide unbiased manner (Schraut et al., 2014, Houtepen et al., 2016).
5.4. Understanding gender differences in the effects of early life adversity
Evidence suggest gender differences in the risk to develop stress-related psychopathology. It will therefore be important to understand why – in general – females are more prone to develop pathologies. In an interesting recent paper, Loi et al. (2015) suggested that male and female mice with a history of early life adversity respond comparable in anxiety-related tasks, while hippocampal function is relatively less sensitive to early life adversity in females (Loi et al., 2015). Yet, the nature of gender differences in sensitivity to early life adversity deserves further attention.
5.5. Early life adversity and age-related cognitive alterations
Several studies indicate that stress responsiveness correlates with cognitive decline during aging and the progression of Alzheimer's pathology (Davis et al., 1986, Masugi et al., 1989). Since the activity of the HPA-axis is determined by early life adversity, it will be important to investigate whether and how early life experience determines the progression of age-related cognitive decline and in particular Alzheimer's Disease. Preliminary data indicates that this is relevant since early life adversity affects overall survival and amyloid levels in transgenic Alzheimer mice (Lesuis et al., 2016) and may modify inflammatory responses as well (Hoeijmakers et al., 2016).
5.6. Can the effects of early life adversity on cognition and emotional behavior be targeted?
-
i.
Preliminary data suggests that targeting GRs and CRH can be effective in preventing later effects of early life adversity on cognitive function (Arp et al., 2016, Wang et al., 2011a, Wang et al., 2011b, Wang et al., 2013). Future studies will be required to investigate in detail the optimal time windows for such interventions or treatments, and the role of gender.
-
ii.
Raising rodents in enriched environments generally enhances brain function and cognition. Also in humans, cognitive stimulation has beneficial effects on cognition, which may be related to increasing cognitive reserve (Barulli and Stern, 2013). It will therefore be important to investigate whether increasing cognitive abilities can prevent/overcome the effects of early life adversity on executive function and emotional behavior.
-
iii.
The notion that emotional memories become labile after retrieval, has stimulated researchers to investigate whether emotional responsiveness can be targeted by interfering with the process of reconsolidation (Nader et al., 2000, Monfils et al., 2009, Kindt et al., 2009, Schiller et al., 2010). It will be important to determine the boundary conditions that are required to reduce enhanced fear expression after early life adversity and interesting to investigate whether this window of “lability” can be used to reduce the expression of fear and
-
iv.
Finally, recent early nutrition based interventions have become of interest as a potential lead to prevent the detrimental consequences of early life adversity (Lucassen et al., 2013, Naninck et al., 2011, Naninck et al., 2016, Yam et al., 2015).
Acknowledgements
PJL is supported by NWO and ISAO/Alzheimer Nederland. MJ is supported by the Consortium on Individual Development (CID), which is funded through the Gravitation program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant number 024.001.003). MJA and HK are supported by the Netherlands Organization for Scientific Research (NWO Program Brain and Cognition: An Integrated Approach: grant # 433-09-251). SK was supported by ALW grant # 821-02-007 from the Netherlands Organization for Scientific Research NWO. SL and HK are supported by The Internationale Stichting voor Alzheimer Onderzoek (ISAO, grant #12534)
References
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arain M., Haque M., Johal L., Mathur P., Nel W., Rais A., Sandhu R., Sharma S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp J.M., Ter Horst J.P., Loi M., den Blaauwen J., Bangert E., Fernández G., Joëls M., Oitzl M.S., Krugers H.J. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol. Learn. Mem. 2016;133:30–38. doi: 10.1016/j.nlm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., van Hasselt F.N., Champagne D.L., Meaney M.J., Krugers H.J., Joëls M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., Meaney M.J. Epigenetics and the biological basis of gene x environment interactions. J. Am. Acad. Child. Adolesc. Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., Tse Y.C., Nguyen H.B., Wong A.S., Meaney M.J., Wong T.P. Maternal care influences hippocampal N-methyl-d-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol. Psychiatry. 2012;72:491–498. doi: 10.1016/j.biopsych.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M.J., van Ijzendoorn M.H., Juffer F. Earlier is better: a meta-analysis of 70 years of intervention improving cognitive development in institutionalized children. Monogr. Soc. Res. Child Dev. 2008;73:279–293. doi: 10.1111/j.1540-5834.2008.00498.x. [DOI] [PubMed] [Google Scholar]
- Baram T.Z., Davis E.P., Obenaus A., Sandman C.A., Small S.L., Solodkin A., Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D., Stern Y. Efficiency, capacity, compensation, maintenance, plasticity, emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A., Blot K., Verney C., Estevez L., Santamaria J., Gressens P., Giros B., Otani S., Daugé V., Naudon L. Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol. Learn. Mem. 2012;98:207–214. doi: 10.1016/j.nlm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J., Rether K., Gröger N., Xie L., Braun K. Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front. Neurosci. 2014;8:11. doi: 10.3389/fnins.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy T.W., Humpartzoomian R.A., Cain D.P., Meaney M.J. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Kramár E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K., Lynch G., Baram T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Diorio J., Meaney M.J. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carroll J.E., Gruenewald T.L., Taylor S.E., Janicki-Deverts D., Matthews K.A., Seeman T.E. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Huang S., Cao J., Chen T., Zhu P., Zhu R., Su P., Ruan D. The timing of maternal separation affects morris water maze performance and long-term potentiation in male rats. Dev. Psychobiol. 2014;56:1102–1109. doi: 10.1002/dev.21130. [DOI] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression, moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R., Joëls M., Krugers H. Maternal care and hippocampal plasticity, evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D.L., de Kloet E.R., Joëls M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin. Fetal Neonatal Med. 2009;14:136–142. doi: 10.1016/j.siny.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Chen Y., Baram T.Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.C., De Vries G.J., Swaab D.F. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J. Neurosci. 2002;22:1027–1033. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Wolke D., Lereya S.T., Shanahan L., Worthman C., Costello E.J. Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7570–7575. doi: 10.1073/pnas.1323641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Bagot R.C., Parker K.J., Vinkers C.H., de Kloet E.R. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.L., Davis B.M., Greenwald B.S., Mohs R.C., Mathé A.A., Johns C.A., Horvath T.B. Cortisol and Alzheimer's disease. I, Basal studies. Am. J. Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. Stress and the brain, from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Schamberg M.A. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Ramsawh H.J., Flagan T.M., Simmons A.N., Sullivan S.G., Allard C.B., Paulus M.P., Stein M.B. Early life stress and the anxious brain, evidence for a neural mechanism linking childhood emotional maltreatment to anxiety in adulthood. Psychol. Med. 2016;46:1037–1054. doi: 10.1017/S0033291715002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D., Diorio J., Liu D., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1999;286:1155–1158. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Snell J.W., Lange N., Rajapakse J.C., Casey B.J., Kozuch P.L., Vaituzis A.C., Vauss Y.C., Hamburger S.D., Kaysen D., Rapoport J.L. Quantitative magnetic resonance imaging of human brain development, ages 4–18. Cereb. Cortex. 1996;6:551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouty-Colomer L.A., Hosseini B., Marcelo I.M., Schreiber J., Slump D.E., Yamaguchi S., Houweling A.R., Jaarsma D., Elgersma Y., Kushner S.A. Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry. 2016;21:1153. doi: 10.1038/mp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain, mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral problems after early life stress, contributions of the hippocampus and amygdala. Biol. Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19119–19924. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L., Heinen Y., van Dam A.M., Lucassen P.J., Korosi A. Microglial priming and Alzheimer's disease: a possible role for (early) immune challenges and epigenetics? Front. Hum. Neurosci. 2016;10:398. doi: 10.3389/fnhum.2016.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Stellern S.A., Schaefer C., Carlson S.M., Gunnar M.R. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17208–17212. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtepen L.C., Vinkers C.H., Carrillo-Roa T., Hiemstra M., van Lier P.A., Meeus W., Branje S., Heim C.M., Nemeroff C.B., Mill J., Schalkwyk L.C., Creyghton M.P., Kahn R.S., Joëls M., Binder E.B., Boks M.P. Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nat. Commun. 2016;7:10967. doi: 10.1038/ncomms10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti G.M., Price D.J. Exuberance in the development of cortical networks. Nat. Rev. Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Furini C.R., Myskiw J.C. Fear memory. Physiol. Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Kanatsou S., Fearey B.C., Kuil L.E., Lucassen P.J., Harris A.P., Seckl J.R., Krugers H., Joels M. Overexpression of mineralocorticoid receptors partially prevents chronic stress-induced reductions in hippocampal memory and structural plasticity. PLoS One. 2015;10:e0142012. doi: 10.1371/journal.pone.0142012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Thornton L.M., Gardner C.O. Stressful life events and previous episodes in the etiology of major depression in women, an evaluation of the “kindling” hypothesis. Am. J. Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kessels H.W., Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M., Ho S.S., Sripada C.S., Swain J.E., Liberzon I., Phan K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M., Soeter M., Vervliet B. Beyond extinction, erasing human fear responses and preventing the return of fear. Nat. Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., Pace T.W., Mercer K.B., Mayberg H.S., Bradley B., Nemeroff C.B., Holsboer F., Heim C.M., Ressler K.J., Rein T., Binder E.B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok M.D., Alt S.R., Irurzun Lafitte A.J., Turner J.D., Lakke E.A., Huitinga I., Muller C.P., Zitman F.G., de Kloet E.R., Derijk R.H. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J. Psychiatry Res. 2015;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Korosi A., Naninck E.F.G., Oomen C.A., Schouten M., Krugers H., Fitzsomins C., Lucassen P.J. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 2012;227:400–409. doi: 10.1016/j.bbr.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Krugers H.J., Joels M. Long-lasting consequences of early life stress on brain structure, emotion and cognition. Curr. Top. Behav. Neurosci. 2014;18:81–93. doi: 10.1007/7854_2014_289. [DOI] [PubMed] [Google Scholar]
- Kuningas M., de Rijk R.H., Westendorp R.G., Jolles J., Slagboom P.E., van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32:1295–1301. doi: 10.1038/sj.npp.1301260. [DOI] [PubMed] [Google Scholar]
- Lejeune S., Dourmap N., Martres M.P., Giros B., Daugé V., Naudon L. The dopamine D1 receptor agonist SKF 38393 improves temporal order memory performance in maternally deprived rats. Neurobiol. Learn. Mem. 2013;106:268–273. doi: 10.1016/j.nlm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Lesuis S.L., Maurin H., Borghgraef P., Lucassen P.J., Van Leuven F., Krugers H.J. Positive and negative early life experiences differentially modulate long term survival and amyloid protein levels in a mouse model of Alzheimer's disease. Oncotarget. 2016;7:39118–39135. doi: 10.18632/oncotarget.9776. doi:10.18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Ma S.T., Okada G., Ho S.S., Swain J.E., Evans G.W. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cogn. Affect. Neurosci. 2015;10:1596–1606. doi: 10.1093/scan/nsv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky PM,Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Day J.C., Francis D.D., Meaney M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu X., Ramirez S., Pang P.T., Puryear C.B., Govindarajan A., Deisseroth K., Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M., Mossink J.C., Meerhoff G.F., Den Blaauwen J.L., Lucassen P.J., Joëls M. Effects of early-life stress on cognitive function and hippocampal structure in female rodents. Neuroscience. 2015:756–763. doi: 10.1016/j.neuroscience.2015.08.024. pii:S0306-4522(15) 00756-00763. [DOI] [PubMed] [Google Scholar]
- Loman M.M., Johnson A.E., Westerlund A., Pollak S.D., Nelson C.A., Gunnar M.R. The effect of early deprivation on executive attention in middle childhood. J. Child Psychol. Psychiatry. 2013;54:37–45. doi: 10.1111/j.1469-7610.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M.P., Anderson J.S., Ferguson M.A., Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev. Cogn. Neurosci. 2011;1:187–197. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen P.J., Naninck E.F., van Goudoever J.B., Fitzsimons C., Joels M., Korosi A. Perinatal programming of adult hippocampal structure and function, emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development, the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–11342. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A., Harms M.P., Tillman R., Barch D.M. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., Parent S., Evans A.C., Tremblay R.E., Zelazo P.D., Corbo V., Pruessner J.C., Séguin J.R. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S., Krugers H.J., Morley-Fletcher S., Szyf M., Brunton P.J. The consequences of early-life adversity, neurobiological, behavioural and epigenetic adaptations. J. Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- Maheu F.S., Dozier M., Guyer A.E., Mandell D., Peloso E., Poeth K., Jenness J., Lau J.Y., Ackerman J.P., Pine D.S., Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M., Reijmers L. Exploring memory representations with activity-based genetics. Cold Spring Harb. Perspect. Biol. 2015;8:a021832. doi: 10.1101/cshperspect.a021832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi F., Ogihara T., Sakaguchi K., Otsuka A., Tsuchiya Y., Morimoto S., Kumahara Y., Saeki S., Nishide M. High plasma levels of cortisol in patients with senile dementia of the Alzheimer's type. Methods Find. Exp. Clin. Pharmacol. 1989;11:707–710. [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., Mechelli A., Bird G., Kelly P.A., Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McGowan P.O., Sasaki A., D'Alessio A.C., Dymov S., Labonté B., Szyf M., Turecki G., Meaney M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J., Ferguson-Smith A.C. Epigenetic regulation of the neural transcriptome, the meaning of the marks. Nat. Neurosci. 2010;13:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. Mother nurture and the social definition of neurodevelopment. Proc. Natl. Acad. Sci. U. S. A. 2016;113:6094–6096. doi: 10.1073/pnas.1605859113. doi:10.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci. 2014;9:123–131. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils M.H., Cowansage K.K., Klann E., LeDoux J.E. Extinction-reconsolidation boundaries, key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S., Fox R., Proulx C.D., Lin J.Y., Tsien R.Y., Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Naninck E.F., Lucassen P.J., Bakker J. Sex differences in adolescent depression, do sex hormones determine vulnerability? J. Neuroendocrinol. 2011;23:383–392. doi: 10.1111/j.1365-2826.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- Naninck E.F., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J., Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Naninck E.F.G., Oosterink J.E., Yam K.Y., de Vries L., Schierbeek H., van Goudoever J.B., Verkaik-Schakel R.N., Plantinga J.A., Plosch T., Lucassen P.J., Korosi A. Early micronutrient supplementation protects against early stress induced cognitive impairments. FASEB J. 2016 Oct 21 doi: 10.1096/fj.201600834R. pii: fj.201600834R. [DOI] [PubMed] [Google Scholar]
- Nederhof E., Schmidt M.V. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nelson C., Zeanah C., Fox N., Marshall P., Smyke A., Guthrie D. Cognitive recovery in socially deprived young children, the Bucharest early intervention project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Nguyen H.B., Bagot R.C., Diorio J., Wong T.P., Meaney M.J. Maternal care differentially affects neuronal excitability and synaptic plasticity in the dorsal and ventral hippocampus. Neuropsychopharmacology. 2015;40:1590–1599. doi: 10.1038/npp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl M.S., Workel J.O., Fluttert M., Frosch F., De Kloet E.R. Maternal deprivation affects behaviour from youth to senescence, amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. Eur. J. Neurosci. 2000;12:3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Oomen C.A., Girardi C.E., Cahyadi R., Verbeek E.C., Krugers H., Joëls M., Lucassen P.J. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen C.A., Soeters H., Audureau N., Vermunt L., van Hasselt F.N., Manders E.M., Joëls M., Lucassen P.J., Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J. Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen C.A., Soeters H., Audureau N., Vermunt L., van Hasselt F.N., Manders E.M., Joëls M., Krugers H., Lucassen P.J. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology. 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C., Wingenfeld K., Kuehl L.K., Kaczmarczyk M., Richter S., Quante A., Regen F., Bajbouj M., Zimmermann-Viehoff F., Wiedemann K., Hinkelmann K. Mineralocorticoid receptor stimulation improves cognitive function and decreases cortisol secretion in depressed patients and healthy individuals. Neuropsychopharmacology. 2015;40:386–393. doi: 10.1038/npp.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J. Abnorm. Psychol. 2015;124:817–833. doi: 10.1037/abn0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.D., Tolley-Schell S.A. Selective attention to facial emotion in physically abused children. J. Abnorm. Psychol. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Radtke K.M., Schauer M., Gunter H.M., Ruf-Leuschner M., Sill J., Meyer A., Elbert T. Epigenetic modifications of the glucocorticoid receptor gene are associated with the vulnerability to psychopathology in childhood maltreatment. Transl. Psychiatry. 2015;5:e571. doi: 10.1038/tp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo R.L., Kim J., Arons A.L., Ramirez S., Liu X., Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S., LeDoux J., Zador A., Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Rutter M., Sonuga-Barke E.J., Castle J. I. Investigating the impact of early institutional deprivation on development, background and research strategy of the English and Romanian Adoptees (ERA) study. Monogr. Soc. Res. Child. Dev. 2010;75:1–20. doi: 10.1111/j.1540-5834.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Sampath D., Sabitha K.R., Hegde P., Jayakrishnan H.R., Kutty B.M., Chattarji S., Rangarajan G., Laxmi T.R. A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behav. Brain Res. 2014;273:144–154. doi: 10.1016/j.bbr.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Schiller D., Monfils M.H., Raio C.M., Johnson D.C., Ledoux J.E., Phelps E.A. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraut K.G., Jakob S.B., Weidner M.T., Schmitt A.G., Scholz C.J., Strekalova T., El Hajj N., Eijssen L.M., Domschke K., Reif A., Haaf T., Ortega G., Steinbusch H.W., Lesch K.P., Van den Hove D.L. Prenatal stress-induced programming of genome-wide promoter DNA methylation in 5-HTT-deficient mice. Transl. Psychiatry. 2014;4:e473. doi: 10.1038/tp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Coe C.L., Pollak S.D. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa V.C., Vital J., Costenla A.R., Batalha V.L., Sebastião A.M., Ribeiro J.A., Lopes L.V. Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol. Aging. 2014;35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Spinhoven P., Elzinga B.M., Hovens J.G., Roelofs K., Zitman F.G., van Oppen P., Penninx B.W. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J. Affect. Disord. 2010;126:103–112. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- Stanton M.E., Gutierrez Y.R., Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav. Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual Research Review, Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Büll D.R., Ionescu I.A., Heinzmann J.M., Knapman A., Siebertz A., Depping A.M., Hartmann J., Hausch F., Schmidt M.V., Holsboer F., Ising M., Cox M.B., Schmidt U., Rein T. FK506 binding protein 5 shapes stress responsiveness, modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- van Harmelen A.L., van Tol M.J., van der Wee N.J., Veltman D.J., Aleman A., Spinhoven P., van Buchem M.A., Zitman F.G., Penninx B.W., Elzinga B.M. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- van Harmelen A.L., Elzinga B.M., Kievit R.A., Spinhoven P. Intrusions of autobiographical memories in individuals reporting childhood emotional maltreatment. Eur. J. Psychotraumatol. 2011;2011:2. doi: 10.3402/ejpt.v2i0.7336. doi:10.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A.L., van Tol M.J., Demenescu L.R., van der Wee N.J., Veltman D.J., Aleman A., van Buchem M.A., Spinhoven P., Penninx B.W., Elzinga B.M. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 2013;8:362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A.L., Hauber K., Gunther Moor B., Spinhoven P., Boon A.E., Crone E.A., Elzinga B.M. Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS One. 2014;9:e85107. doi: 10.1371/journal.pone.0085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A.L., van Tol M.J., Dalgleish T., van der Wee N.J., Veltman D.J., Aleman A., Spinhoven P., Penninx B.W., Elzinga B.M. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 2014;9:2026–2033. doi: 10.1093/scan/nsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt F.N., Cornelisse S., Zhang T.Y., Meaney M.J., Velzing E.H., Krugers H.J., Joëls M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- van Hasselt F.N., Boudewijns Z.S., van der Knaap N.J., Krugers H.J., Joëls M. Maternal care received by individual pups correlates with adult CA1 dendritic morphology and synaptic plasticity in a sex-dependent manner. J. Neuroendocrinol. 2012;24:331–340. doi: 10.1111/j.1365-2826.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- van Hasselt F.N., de Visser L., Tieskens J.M., Cornelisse S., Baars A.M., Lavrijsen M., Krugers H.J., van den Bos R., Joëls M. Individual variations in maternal care early in life correlate with later life decision-making and c-fos expression in prefrontal subregions of rats. PLoS One. 2012;7:e37820. doi: 10.1371/journal.pone.0037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y., van Buchem M.A., Spinhoven P., Elzinga B.M., Rombouts S.A. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. 2015;233:436–442. doi: 10.1016/j.pscychresns.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhu J., Zhu H., Zhang Q., Lin Z., Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wang X.D., Rammes G., Kraev I., Wolf M., Liebl C., Scharf S.H., Rice C.J., Wurst W., Holsboer F., Deussing J.M., Baram T.Z., Stewart M.G., Müller M.B., Schmidt M.V. Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.D., Su Y.A., Wagner K.V., Avrabos C., Scharf S.H., Hartmann J., Wolf M., Liebl C., Kühne C., Wurst W., Holsboer F., Eder M., Deussing J.M., Müller M.B., Schmidt M.V. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat. Neurosci. 2013;16:706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Meaney M.J., Szyf M., Weaver I.C., Meaney M.J., Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workel J.O., Oitzl M.S., Fluttert M., Lesscher H., Karssen A., de Kloet E.R. Differential and age-dependent effects of maternal deprivation on the hypothalamic-pituitary-adrenal axis of brown Norway rats from youth to senescence. J. Neuroendocrinol. 2001;13:569–580. doi: 10.1046/j.1365-2826.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Workman A.D., Charvet C.J., Clancy B., Darlington R.B., Finlay B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Song Z., Wang S., Shui L., Tai F., Qiao X., He F. Early paternal deprivation alters levels of hippocampal brain-derived neurotrophic factor and glucocorticoid receptor and serum corticosterone and adrenocorticotropin in a sex-specific way in socially monogamous mandarin voles. Neuroendocrinology. 2014;100:119–128. doi: 10.1159/000366441. [DOI] [PubMed] [Google Scholar]
- Yang X.D., Liao X.M., Uribe-Mariño A., Liu R., Xie X.M., Jia J., Su Y.A., Li J.T., Schmidt M.V., Wang X.D., Si T.M. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology. 2015;40:1203–1215. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam K.Y., Naninck E.F., Schmidt M.V., Lucassen P.J., Korosi A. Early-life adversity programs emotional functions and the neuroendocrine stress system, the contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress. 2015;18:328–342. doi: 10.3109/10253890.2015.1064890. [DOI] [PubMed] [Google Scholar]
- Zeanah C.H., Egger H.L., Smyke A.T., Nelson C.A., Fox N.A., Marshall P.J., Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Ziol-Guest K.M., Duncan G.J., Kalil A., Boyce W.T. Early childhood poverty, immune-mediated disease processes, and adult productivity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17289–17293. doi: 10.1073/pnas.1203167109. [DOI] [PMC free article] [PubMed] [Google Scholar]