Abstract
Researchers interested in the neurobiology of the acute stress response in humans require a valid and reliable acute stressor that can be used under experimental conditions. The Trier Social Stress Test (TSST) provides such a testing platform. It induces stress by requiring participants to make an interview-style presentation, followed by a surprise mental arithmetic test, in front of an interview panel who do not provide feedback or encouragement. In this review, we outline the methodology of the TSST, and discuss key findings under conditions of health and stress-related disorder. The TSST has unveiled differences in males and females, as well as different age groups, in their neurobiological response to acute stress. The TSST has also deepened our understanding of how genotype may moderate the cognitive neurobiology of acute stress, and exciting new inroads have been made in understanding epigenetic contributions to the biological regulation of the acute stress response using the TSST. A number of innovative adaptations have been developed which allow for the TSST to be used in group settings, with children, in combination with brain imaging, and with virtual committees. Future applications may incorporate the emerging links between the gut microbiome and the stress response. Future research should also maximise use of behavioural data generated by the TSST. Alternative acute stress paradigms may have utility over the TSST in certain situations, such as those that require repeat testing. Nonetheless, we expect that the TSST remains the gold standard for examining the cognitive neurobiology of acute stress in humans.
Keywords: Stress, Cognition, HPA axis, Epigenetics, Genotype
Highlights
-
•
The TSST is the human experimental gold standard for evaluating the neurobiology of acute stress.
-
•
The HPA axis response to the TSST is higher in males and lower in older adults.
-
•
Genotype and epigenetic factors moderate the neurobiological response to the TSST.
-
•
Multiple adaptations of the TSST are available for different testing contexts.
1. Introduction
Psychological stress can be defined as psychological tension or strain that is difficult to manage or endure (Colman, 2001). Although chronic (extended) stress or traumatic stress is detrimental to an organism's health, acute (short-term) stress is important for responding to threatening stimuli (McEwen, 2007), though the acute stress response can itself be dysregulated by chronic stress, such as when caring for a relative with dementia (e.g. Aschbacher et al., 2006). The impact of stress is visible across multiple psychological and physiological domains (McEwen, Gray and Nasca, 2015a), and chronic stress is considered a major contributor to neuropsychiatric diseases. Although much of this information comes from preclinical studies, the translational relevance is clear, especially when one considers the ubiquity of potential sources of stress in modern society. For example, the European Union report on “Psychological risks in Europe: Prevalence and strategies for prevention” indicated that a quarter of workers experience stress all or nearly all of their working time (Eurofund & EU-OSHA, 2014). There is also evidence that perceived stress in the United States population has increased over a number of decades (Cohen and Janicki-Deverts, 2012). In this context, a better understanding of the neurobiology of stress, as well as stress resilience (McEwen et al., 2015b, Russo et al., 2012), in human subjects is becoming increasingly relevant for individual and population health. Although some features of this field focus on chronic naturalistic stressors, such as unemployment (Dettenborn et al., 2010, Gallagher et al., 2016) or caring for a relative with dementia (Clipp and George, 1993, Kim and Schulz, 2008), valuable insights have also been derived from laboratory studies based on acute stress paradigms (c.f. Bali and Jaggi, 2015; for a review).
Researchers wishing to examine the neurobiological response to acute stress in humans are in need of a reliable and valid acute stressor-one which can robustly induce an acute stress response under experimentally controlled conditions. The Trier Social Stress Test (TSST; Kirschbaum et al., 1992, Kirschbaum et al., 1993) is an ecologically valid stressor, based on the stress induced by public speaking. It incorporates social evaluation and unpredictability, by obliging the person to speak in front of an unresponsive audience and completing a surprise mental arithmetic test. Hundreds of research studies have employed the TSST in order to examine the impact of acute stress on human neurobiology.
In the following review we will outline the methodology of the TSST, offering practical advice on employing this technique, and discussing advantages as well as potential pitfalls and challenges of the approach. We will discuss how age and gender can moderate the effects of the TSST (including modifications of the TSST for younger populations). The impact of genetic factors on acute stress response as assessed with the TSST will be outlined (including both genotype and epigenetic factors). Finally, we will discuss potential future directions for research in this area.
2. Nature of the stressors
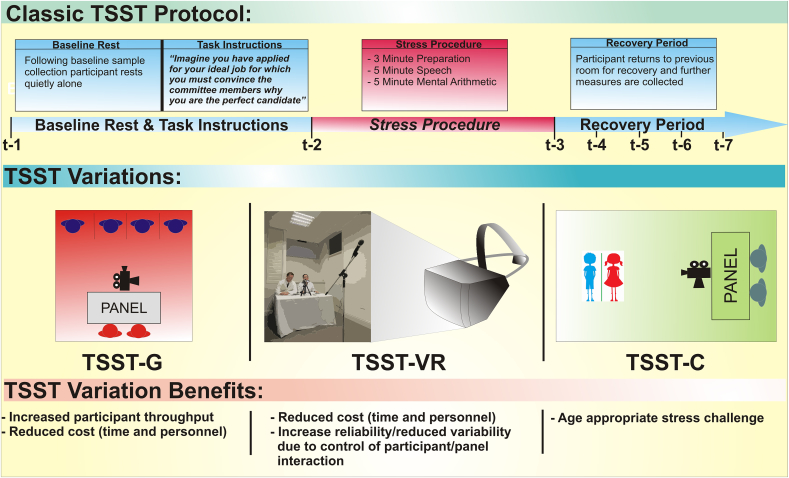
To study acute stress in humans, it is critical for researchers to have a valid and reliable method for inducing acute stress under controlled conditions. The TSST was developed over two decades ago with the primary aim to design such a task, which would produce a consistent hypothalamic-pituitary-adrenal (HPA) axis response in humans (Kirschbaum et al., 1993). Activation of the HPA axis represents the core neuroendocrine response to stress in humans and other mammals, and dysfunction of this axis is associated with a range of physical and mental health disorders (Pariante and Lightman, 2008). Other experimental stress procedures, including cognitive tasks such as the Stroop, public speaking tasks (without the other aspects of the TSST), noise exposure, emotion induction or pain induction, tend to produce variable, or no, HPA axis responses in humans. A meta-analysis of a number of laboratory-based acute psychological stress procedures, including the TSST, determined that the key psychological elements in motivated performance tasks that produce the greatest HPA axis response are a combination of social-evaluative threat and uncontrollability (Dickerson and Kemeny, 2004). The key procedural stages of the TSST incorporate each of these elements (see Fig. 1). In the standard TSST procedure, participants take part in a role-playing scenario, the most common feature of which is imagining they have applied for a job of their choice (or in some cases a pre-specified job), and after a short preparation period, must present to a panel of ‘committee members’ why they believe they are the best candidate for the particular position. Following the speech task, they are then given a surprise mental arithmetic task (c.f. Allen et al., 2014). Performing each task in front of a panel of committee members introduces the element of social-evaluative threat which is enhanced by their performance being video and voice recorded (purportedly for further analysis), the participant being informed that the committee are experts in behaviour analysis, and the committee members being trained to withhold any type of social engagement or positive feedback; they do not smile or nod, and stick closely to scripted instructions and responses. The TSST incorporates a number of elements of uncontrollability throughout the procedure; participants do not know what their task is until 3–10 min (depending on the protocol used) prior to doing it; they have a very short period of time to prepare for the task; the mental arithmetic component is completely unexpected; and the committee members are non-responsive to any attempts at social engagement.
Fig. 1.
Outline of the classic Trier Social Stress Test protocol, variations on this protocol, and the relative benefits of these variations.
In healthy humans, the TSST has been shown in a multitude of studies to activate the HPA axis as measured via salivary, plasma or serum cortisol and adrenocorticotrophic hormone (ACTH) (Kudielka et al., 2007). The TSST reliably induces a two-to-three fold increase in cortisol levels in approximately 70–80% of study participants (Dickerson and Kemeny, 2004, Frisch et al., 2015, Kudielka et al., 2007). In addition, the TSST has been employed to examine the effects of acute psychosocial stress on a range of neurobiological and other measures including immune parameters (Campisi et al., 2012, Izawa et al., 2013a, Izawa et al., 2013b, Slavich et al., 2010, Yamakawa et al., 2009), the sympathetic nervous system (El-Sheikh et al., 2008, Gold et al., 2004, Gordis et al., 2006, Graeff et al., 2003, Het et al., 2009, Jezova et al., 2004), and the cardiovascular system (Childs et al., 2006, Rimmele et al., 2007). The TSST has also proven crucial in understanding the demographic and psychological characteristics that moderate the neurobiology of the acute stress response in humans, such as age and gender (see sections 4, 5 below), personality factors such as neuroticism (Oswald et al., 2006), individual appraisals of the stress environment (Gaab et al., 2005) and social status (Gruenewald et al., 2006). See Allen et al. (2014) for an extensive review.
The TSST has also progressed our understanding of the aberrant neurobiological mechanisms which may underlie stress-related disorders. For example, individuals with a diagnosis of depression have been shown to exhibit elevated proinflammatory cytokine levels (Fagundes et al., 2013, Pace et al., 2006) in response to the TSST, as well as an altered HPA response to the TSST, evident in a higher net increase in cortisol compared to participants at risk of depression (Dienes et al., 2013), although this effect may be moderated by gender, with depression being associated with a higher cortisol response in females specifically (Chopra et al., 2009). Although findings in this area are not always consistent (Young et al., 2000), they do lend support to key neurobiological theories of depression, including those highlighting the role of immune activation (e.g. Maes, 1995, Leonard, 2000) and the HPA axis (e.g. Pariante and Lightman, 2008). Attenuated cortisol responses to the TSST have been reported in panic disorder (Petrowski et al., 2010, Petrowski et al., 2013) and individuals who have experienced early traumatic experiences and received a psychiatric diagnosis, have elevated HPA axis, cardiovascular (Heim et al., 2000) and plasma interleukin 6 (Carpenter et al., 2007) responses to the TSST. People with irritable bowel syndrome, a stress-related brain-gut axis disorder (Eisenstein, 2016, Grayson, 2016, Kennedy et al., 2012) have exhibited altered HPA axis activation in response to the TSST (Kennedy et al., 2014, Suárez-Hitz et al., 2012).
In summary, the TSST combines the key elements of social-evaluative threat and uncontrollability to produce a consistent and robust physiological and psychological stress response in humans. The reliable, robust and modifiable nature of the acute stress response produced by the TSST has led to this procedure being considered the gold standard in human experimental stress research and becoming a mainstay in stress laboratories worldwide.
3. Practical issues and adapted versions
As noted, the fact that the TSST induces a consistent and reliable physiological and psychological stress response in the majority of participants is a major advantage of this procedure. The experimental conditions during the TSST are highly standardized, and controllability is enhanced due to the availability of an extensive literature on the various environmental, psychological and physiological factors which can affect the acute stress response to the TSST. This controllability also allows an investigator to measure multiple psychological and biological outcomes prior to, during, and after the acute stress procedure. Moreover, despite being an experimental, simulated procedure, the ability to sell one's qualities in an interview scenario is a common experience for many individuals at some point in their life, which lends ecological validity to the TSST. Finally, the TSST requires little specialist equipment (only a microphone, video camera etc.) and as such, the main costs associated with the procedure will accrue depending on the biological readouts which the investigator wishes to measure.
Innovative adaptations of the original TSST have been introduced which confer relative advantages. For example, a TSST for groups (TSST-G) has been introduced which increases the throughput of study participants, and has been shown to produce a significant HPA axis and autonomic nervous system response (Boesch et al., 2014, von Dawans et al., 2011). A virtual reality version of the TSST (TSST-VR) has also been designed (Kotlyar et al., 2008) which removes any variability in data due to interactions between the study participant and committee members. However, it seems the type of VR technology utilized is important in producing a robust stress response, as an earlier version of the TSST-VR did not significantly increase cortisol levels (Kotlyar et al., 2008), whereas a more recent version, using newer technology, induced a measurable neuroendocrine response (Jordanova et al., 2007). Although the standard TSST is not well-suited to brain imaging paradigms, the Montreal Imaging Stress Task (MIST) has been developed, which can be combined with PET and fMRI (Dedovic et al., 2005). Participants must complete an arithmetic task; in the stress condition, these are manipulated to be slightly above the participant's level of mathematical skill. However, the MIST does not include a job interview task component.
The recently developed socially-evaluated cold pressor test (SECPT; Schwabe et al., 2008), is a viable alternative to the TSST. It is a shorter procedure (stress exposure lasts approximately 3 min) and requires only one committee member. In addition, the SECPT produces a significant HPA axis response, in addition to increasing other stress parameters such as blood pressure and subjective stress (Schwabe et al., 2008) and has recently been adapted for groups (Minkley et al., 2014), which further increases the throughput of study participants, and has been indicated to induce heightened HPA axis responsivity across repeated visits. However, as the SECPT involves thermal pain as a stressor, it is not possible to separate the psychologically and physically stressful aspects of the procedure, and it arguably lacks the ecological validity of the TSST. As such, the TSST is currently the best available experimental stress procedure for investigating the acute psychosocial stress response in humans.
4. Impact of age
4.1. Older and younger adults
The impact of stress on the aging brain is coming under increasing scrutiny and may inform strategies to promote healthy aging (Prenderville et al., 2015). Altered function of the immune system and HPA axis is an important biological feature of the aging process and this is reflected in the results obtained using the TSST (Kudielka et al., 2004a, Kudielka et al., 2004b). Compared to younger adults, older adults have shown lower cortisol (Hidalgo et al., 2015) and ACTH (Kudielka et al., 2004a) responses to the TSST. However, salivary alpha amylase (a marker of sympathetic nervous system activity) has increased to a greater extent in older adults (Almela et al., 2011, Strahler et al., 2010), suggesting differing neurobiological effects of age across physiological stress systems. Blunted HPA axis activity may not necessarily indicate a reduction in subjective stress; indeed, it has been argued that the psychosocial context of the TSST may be more subjectively stressful for older adults (Lupien et al., 2007). Interestingly, middle-aged men with a family history of longevity showed lower overall cortisol output and systolic blood pressure during the TSST compared to those without such a family history (Jansen, van Heemst, van der Grond, Westendorp and Oei, 2016).
The TSST may also unmask an age-specific cognitive neurobiology of acute stress, via differential effects on cognition depending on the age of participants. The TSST led to impaired verbal learning performance following interference, but only in older adults (Hidalgo et al., 2014). In contrast, only young men had picture recall impaired by the TSST (Hidalgo et al., 2015). In healthy older participants, the TSST improved working memory performance in women but not men, and for digit span forward but not digit span backward, suggesting a specific effect on memory span but not the executive component of working memory (Pulopulos et al., 2015). Of course, stress has a multifaceted impact on cognition. The impact of stress upon cognition may be more akin to an inverted U-shaped effect than a simple correlation, although even this Yerkes-Dodson law may miss complexity concerning factors such as task difficulty and whether certain changes in cognitive processing (e.g. narrow versus broad focus of attention) may be more or less suited to different cognitive demands under differing environmental conditions (c.f. Hanoch and Vitouch, 2004).
There has been a lack of longitudinal research in adults using the TSST. As research comparing younger to older adults is cross-sectional, it is possible that historical as well as developmental factors may underlie difference in acute stress response. For example, in middle-aged adults, higher levels of formal education were associated with higher overall cortisol output but a lesser increase in cortisol post-TSST (Fiocco et al., 2007); if older adults have generally received differing levels of formal education compared to younger adults, this may impact upon TSST response.
4.2. TSST for children and adolescents
There is only one recommended adaptation of the standard TSST protocol for use in children and adolescents aged 7–16 years. This adaptation, the TSST for Children (TSST-C) was first described by Buske-Kirschbaum et al. (1997), and includes a public speaking task and a mental arithmetic task. The difficulty of the mental arithmetic may be adjusted for age and ability; the speaking task is also adapted for the child or adolescent participant. The speaking task begins when the child/adolescent is given the stem of a story, and asked to complete the story in an interesting and exciting way, and the child/adolescent is told that the story ending should be better than that provided by other children their age. The participant has a 5 min preparation period and then must complete the story over the course of 5 min. If the child/adolescent finishes the story in less than 5 min, they are asked to continue, and this request is delivered in a friendly, supportive manner. The research panel generally provides the child with positive feedback; this is a distinct difference from the TSST for adults, where the panel is neutral and withholds feedback. Following the story component, the child completes a serial subtraction task, with encouragement to complete it as quickly and as accurately as possible. The number sequence is of normative ability for the child's age, and if an incorrect response is given, the child is instructed to begin again. A variation on this procedure used by some researchers is to change the task to a simpler sequence if the child repeatedly makes the same error and appears distressed by the task (e.g. Krishnaveni et al., 2014, Krämer et al., 2012, Yim et al., 2015). At the end of the protocol, the panel debriefs the participant, explaining that they were not truly judging his/her performance in comparison with other children. In one recent variation, the TSST-M, the duration of tasks was reduced, and the speech component was changed so that children were asked to imagine that they were introducing themselves to a new class (Yim et al., 2015), a task designed to more accurately map to the child/adolescent experience and ability.
The social nature, and social evaluative threat of the TSST-C may contribute to age related differences in the psychosocial stress responses, within child/adolescent groups, and in comparison to adults, and in longitudinal studies that repeat the TSST-C. A core component of the TSST-C is social evaluative threat, and this aspect deserves particular attention in child and adolescent participants, as it may explain differences in the magnitude and duration of the stress response. There is an increase in the stress response to social evaluation that occurs during childhood and adolescence due in part to typical socio-cognitive development (Somerville and Casey, 2014), with increases in emotional responding (Dahl, 2004), which may explain any differences in measurable stress responses.
The social evaluative dimension threat may be compounded, or conversely, reduced where children are being assessed by adult research panels, via differences in ego involvement (Hellhammer et al., 2009, Gruenewald et al., 2006) or a power difference in the panel and participant. In contrast to adult stress testing, participants do not usually have an age-matched panel in child stress testing, with some exceptions; Westenberg et al. (2009) developed the Leiden Public Speaking Task (Leiden-PST) which is inspired by the TSST-C but allows the child an opportunity to prepare, and uses a pre-recorded audience. These adjustments are driven by attempts to better match the tasks with child and adolescent experience, for example to mimic school presentations. The results indicate that there may be a blunting of the stress response, or a more moderate response, than when a live audience and more impromptu speech task is adopted as the protocol (Westenberg et al., 2009).
Recent variations of the TSST-C have tested the effects of replacing the adult panel with research confederates that are age-matched to the participants (Cheetham and Turner-Cobb, 2016, Cheetham et al., 2015). The precision of age matching may be especially important as typically the opinions of peers become increasingly important during adolescence (Brown, 2004). In addition, older adolescents might have more anticipatory stress in relation to the speech task (Sumter et al., 2010), as the development of cognitive abilities allows the older adolescent participant to reflect more on the upcoming task, which may contribute to increased anticipatory stress response, with subsequent difference in the time dynamics of the stress response. Finally, the social-evaluative component of the TSST-C may have differing magnitudes of effect across the course of adolescent development, related to increases in sensitivity to social stress in the adolescent, which in turn may interact with qualities of the research panel, which is more supportive and socially engaged than that of the TSST for adults. Social support from other participants, a friend or partner may moderate biological response to the TSST (e.g. Foley and Kirschbaum, 2010, Ditzen et al., 2008) and so the positive social engagement by the panel may mediate the reactivity to the tasks.
In addition to the psychosocial development that occurs during the adolescent period, pubertal development may also increase stress sensitivity (Dahl and Gunnar, 2009). However, a lack of robust longitudinal studies using multiple biological and psychological measures prevents the drawing of conclusions about unique, shared or interactive effects of pubertal and psychosocial development on stress sensitivity and stress reactivity. Sumter et al. (2010) examined the effects of development on stress reactivity to the Leiden Public Speaking Task, and their results illustrate the challenge in separating psychosocial from biological effects. The findings indicate that pubertal development contributes to age-related increases in stress responsivity, however the effect for puberty was explained by the age effect, and there are corresponding effects of age and pubertal development. Although there has been some, albeit limited, support for developmental effects on stress reactivity to the TSST-C (e.g. Gunnar et al., 2009, Stroud et al., 2000), reactivity may be captured in some neurobiological measures (e.g. cortisol) and not others (e.g. salivary alpha-amylase). Studies by Stroud et al. (Stroud et al., 2009, Stroud et al., 2002) also indicate that the stressor domain inherent in the TSST-C, in particular cognitive challenge and performance, may be less potent than more developmentally salient stressors, for example social rejection. Stroud et al. (2009) and others (Laurent, Stroud, Brush, D'Angelo and Granger, 2015) reported changes in several neurobiological stress parameters, including salivary immunoglobulin A, salivary cortisol and salivary alpha-amylase, with increasing effects of the TSST-C with age and developmental stage, however this effect was observed for some biological measures and not others. Further, in a peer-rejection stress task, stress reactivity was evident, but the biological response also differed across biological measures, and this was not consistent with the TSST effects. More research is needed to disentangle the possible effects of age, socio-emotional development, cognitive development and puberty on psychobiological responses to the TSST-C or similar stressors.
5. Studies in males and females
A number of stress-related disorders have a higher prevalence in females than in males, such as generalised anxiety disorder (e.g. Baxter et al., 2013), major depression (e.g. Ferrari et al., 2013) and irritable bowel syndrome (e.g. Lovell and Ford, 2012). There has been much interest in gender differences in acute stress response, and the TSST has frequently been used to assess such gender differences (Eisenberger et al., 2007, Uhart et al., 2006). A recent large study (N = 798) of young adults aged 18 found that males had consistently higher plasma ACTH and salivary cortisol than females, and females not on oral contraceptives had higher ACTH than those taking these contraceptives. Males were more likely than females to be reactive responders, who have increased cortisol in response to the event, and less likely than females to be non-responders, whose cortisol levels do not increase. However, a similar level of males and females were anticipatory responders, who have high baseline HPA activity levels, suggesting an anticipatory mounting of a response (Herbison et al., 2016). It should be noted that non-response may not simply be due to a lack of subjective stress appraisal, and can occur in the context of conditions associated with chronic stress (e.g. adverse early life events; Elzinga et al., 2008; panic disorder; Petrowski et al., 2010). Time series analysis indicated that plasma ACTH and salivary cortisol in males had longer peak latencies, higher post-stress peaks and more intense post-peak decline than females, which seems to suggest that although male HPA axis activity peaks at a higher level, such activity recovers to baseline levels to a similar extent as in females (Lopez-Duran et al., 2014).
In contrast to findings on ACTH and cortisol, there was not a moderating effect of gender on DHEA or DHEA-S response to the TSST, although DHEA changes were associated with changes in cortisol and ACTH (Lennartsson et al., 2012a). A lack of gender differences in salivary alpha-amylase has been demonstrated (Hidalgo et al., 2012), and there were unclear findings for a gender difference in prolactin response (Lennartsson and Jonsdottir, 2011). Although women had higher salivary immunoglobulin A than men at baseline, both genders showed similar increase and recovery in this immune marker (Birkett et al., 2016).
Sex hormones have also been examined in TSST research examining acute stress and gender. This has been examined directly, by assessing hormone levels, as well as indirectly by scheduling women to perform tests at different phases of the menstrual cycle. When women were tested in the follicular phase of their menstrual cycle (when progesterone levels are most similar to men) men still had greater ACTH and cortisol following the TSST. Testosterone negatively correlated with salivary cortisol response, and progesterone negatively with salivary ACTH and cortisol response (Stephens et al., 2016), although another study found that testosterone and estradiol were elevated by the TSST, as were ACTH and cortisol (Lennartsson et al., 2012b). Another study found that women in the follicular phase, but not the luteal phase, showed a significant increase in cortisol in response to the TSST, and cortisol levels were only associated with impaired emotional retrieval during the follicular phase (Maki et al., 2015) The changes in stress response across the menstrual cycle were not significant in another study (Herbison et al., 2016). Women with premenstrual syndrome have been found to have blunted cortisol reactivity throughout the menstrual cycle compared to control women (Huang et al., 2015). Other research has found that the higher cortisol response to the TSST in males is reduced by controlling for sex hormone levels (Juster et al., 2016). It has been argued that epinephrine may play a greater role in hemodynamic reactivity, given that epinephrine reactivity but not estradiol predicted cardiac and vascular resistance indices (Gordon and Girdler, 2014).
In a fear conditioning study, the TSST was used to assess the impact of stress on differentiation of a conditioned stimulus from a stimulus unpaired with the unconditioned stimulus in the nucleus accumbens (for early acquisition) and in the amygdala and anterior cingulate (for late acquisition); differentiation was reduced in men but increased in women taking oral contraceptives (Merz et al., 2013). Women performed better than men at a verbal learning test in a no-stress control, but men performed equally well for a TSST condition (Espin et al., 2013). Differences in emotional cognition may underlie gender differences in TSST response-females who read genuine negative news stories showed a greater cortisol response to the TSST than those who read neutral reports, but this effect was not evident in men (Marin et al., 2012). Furthermore, females displayed better recall of negative news than their male counterparts.
Given its psychosocial nature, it is plausible that gender socialisation could play a role in the neurobiological response to the TSST, with reduced differentiation in sex roles dampening difference in psychological response to the TSST (Dedovic et al., 2009). The gender of the TSST panellists is also relevant; there is evidence in young adults that a cortisol increase only occurred when the TSST panellists were of the opposite gender (Duchesne et al., 2012). During the TSST, men engage in more displacement behaviours, i.e. behaviours that do not have an apparent relevance to the situation, and so may divert attention from the stressful stimulus (Troisi, 2002). Within men, displacement was associated with reduced self-reported stress, fewer cognitive errors and a trend towards lower heart rate (Mohiyeddini, Bauer and Semple, 2013a). In contrast, high levels of public self-consciousness in healthy women were associated with displacement behaviour increasing levels of reported stress and poorer cognitive performance (Mohiyeddini, Bauer and Semple, 2013b).
There is no consistent evidence to suggest that there are sex differences in children/adolescents in their reactivity to the TSST-C. Kudielka and Kirschbaum (2005) reported no difference in cortisol reactivity to the TSST-C between male and female children, and Sumter et al. (2010) also reported no sex difference in cortisol responses to the Leiden PST, a variant of the TSST-C, although others have shown differences at some ages. For example, differences observed between 13 year old boys and girls are not evident in 11 year old boys and girls (e.g. Gunnar et al., 2009, Klimes-Dougan et al., 2001). The inconsistencies may be attributed to sex and age differences in sensitivity to social evaluation (Gunther Moor et al., 2010) and these effects of developmental stage on stress reactivity warrant further study, with particular attention to the meaning of task demands, pubertal stage and psychosocial development.
There is some evidence that sexual orientation modulates the endocrine response to acute stress, and that this effect is sex-dependent. Whereas lesbian or bisexual women had greater cortisol reactivity post-stressor, homosexual or bisexual men had lower cortisol concentrations throughout testing (Juster et al., 2015). Participants were exposed to a TSST modified to ensure maximisation of sex differences in stress response (the panel were placed behind a one-way mirror; this is associated with reduced HPA axis response compared to standard TSST in heterosexual females but not males). However, another study did not find an impact of sexual orientation on salivary cortisol in men (Jacobson et al., 2016). Sexual orientation may exert a greater influence in regions where social stigma towards homosexuality is particularly high; structural stigma (operationalised using factors such as level of exclusion of homosexual people from social institutions) was associated with a blunted response to the TSST in homosexual and bisexual young adults (Hatzenbuehler and McLaughlin, 2014).
6. Genetics and the TSST
6.1. Genotype and the TSST
Despite its reliable activation of the HPA axis, the magnitude and timing of HPA axis response to the TSST is characterised by inter-individual variation (e.g. Engert et al., 2013). As genotype may underlie such variation, the TSST has been used in research examining genetic factors implicated in HPA axis function. The FK506 binding protein (FKBP5) is an important glucocorticoid receptor regulator, implicated in negative feedback loops within the HPA axis; higher, longer-lasting cortisol reactivity has been observed in healthy subjects with TT genotype of rs1360780, a FKBP5 single nucleotide polymorphism (SNP), although such an association was not observed in a group of participants with remitted depression (Höhne et al., 2015). FKBP5 SNPs, including rs1360780, have previously been associated with a heightened TSST response (Ising et al., 2008). There was also a male-specific effect on salivary cortisol response of FKBP5 polymorphism rs3800374, with males with the TT genotype having a blunted response to the TSST (Mahon et al., 2013). Mahon et al. also observed an association between peak cortisol response and polymorphisms in the G-protein coupled type-1 corticotropin-releasing hormone (CRH) receptor (CRHR1; rs7209436, rs110402, and rs242924); they highlight that CRH is key for establishing the initial HPA axis response to a stressful stimulus, and that the polymorphisms they examined had previously been shown to interact with child abuse to predict adult depression (Bradley et al., 2008), suggesting a genotypic risk for the development of psychiatric disorder in response to severe stress.
Consistent with the neurobiological interactions between neurotransmitter systems and the HPA axis response, genotype research has implicated the serotonergic system in HPA axis TSST reactivity. The 5-HT2C receptor is the primary serotonin receptor located in the CRH neurons of the hypothalamus, and a nonsynonymous polymorphism (Cys23Ser; rs6318) in the 5-HT2C receptor gene has been associated with higher cortisol output during an emotional stress recall protocol (e.g. Brummett et al., 2012, Brummett et al., 2014). Consistent with this pattern of findings, rs6318 moderated TSST response: homozygous females and hemizygous males had greater cortisol reactivity (Way et al., 2016). Furthermore, carriers of the C-allele of rs6318 showed blunted cortisol response to the TSST, and this effect was not moderated by sex (Avery and Vrshek-Schallhorn, 2016). The serotonergic system has been implicated in other physiological responses to the TSST besides HPA axis activity; participants carrying the short-short allele for the 5HT transporter gene-linked polymorphic region (5-HTTLPR) had increased immune activation (IL-1beta) in response to the TSST (Yamakawa et al., 2015). Consistent with previous research, Yamakawa et al. also found that the short-short allele was associated with cardiovascular reactivity (Way and Taylor, 2011) as well as increased cortisol (Way and Taylor, 2010). In a sample of Caucasian males, LL-group participants had increased TSST-induced mRNA expression in the promotor region of the gene (SLC64A), compared to S-group participants (Duman and Canli, 2015). Supplementation with L-tryptophan, a precursor of serotonin, led to reduced cortisol response to the TSST, but only in S′/S′ carriers of 5-HTTLPR, not in L′/L′ carriers (Cerit et al., 2013).
Met homozygotes for the COMT (catechol-O-methyltransferase) Val158Met polymorphism had poorer performance on working memory following TSST-G exposure, compared to Val homozygotes (Buckert et al., 2012). (The substitution of Met for Val at codon 158 is associated with a reduction in activity of the COMT enzyme, which degrades dopamine; Lotta et al., 1995, Lachman et al., 1996, Chen et al., 2004, and this influence on dopamine availability is particularly pronounced in the prefrontal cortex, e.g. Sesack et al., 1998). Under conditions of prenatal stress, carriers of the seven-repeat allele of the dopamine D4 receptor (DRD4) gene had attenuated cortisol reactivity to the TSST as young adults (Buchmann et al., 2014).
In addition to monoaminergic genes, other genetic research has implicated neurotrophic factors in acute stress; the met allele of the val (66)met polymorphism in the BDNF gene was associated with increased cortisol response to the TSST in young adults and children (Armbruster et al., 2016), although this effect may be moderated by gender (Shalev et al., 2009). Carriers of the T allele of the functional neuropeptide S receptor gene polymorphism (rs324981), which increases neuropeptide S potency at its receptor, displayed larger cortisol and subjective response to the TSST for groups (Kumsta et al., 2013).
Research employing the TSST has deepened our understanding of how cognition is affected by the interaction between acute stress and the genetics underpinning neurotransmitter function. On a test of memory for neutral and emotional faces, carriers of a deletion of the ADRA2B gene (which encodes the alpha-2B adrenergic receptor) had impaired recognition and slower retrieval for neutral faces post-TSST, as well as slower retrieval of fearful faces following a low-stress control condition (Li et al., 2013). Carriers of this deletion also showed increased amygdala activation when recalling emotional faces post-TSST (compared to post-low stress control), whereas non-carriers showed TSST-induced activity changes in the right hippocampus, suggesting a central noradrenergic-glucocorticoid interaction in emotional memory retrieval (Li et al., 2015).
Many of the studies above have focused on a particular gene. Research in patients with depression examining multiple monoaminergic genes found that the effects of COMT on ACTH release in response to acute stress depended upon the presence of the MAOA variant within the same individual (Jabbi et al., 2007). This study employed a test adapted from the TSST (Uhart et al., 2006), but the findings nonetheless suggest that future research employing the TSST to study genotype effects will benefit from examination of multiple genes within the same individual. Please see Table 1 for a summary of research findings on genotype and the acute stress response to the TSST.
Table 1.
Research examining genotype and the acute stress response using the TSST.
| Paper | Sample | Genetic factors examined | Proposed functional molecular outcome | Key findings |
|---|---|---|---|---|
| Mahon et al., 2013 | N = 368, 79% female, Mean age = 23, range = 18-30 |
FKBP5 genotype (9 SNPs) CRHR1 genotype (4 SNPs) |
SNPs moderate activity of FKBP5 protein; a co-chaperone of the GR heterocomplex, and assists in terminating HPA axis response CRHR1 receptor key for establishing initial HPA axis response to stress |
TT genotype of rs4713902 had lower baseline cortisol Males with the TT genotype of rs3800374 had blunted response to the TSST TT genotype of rs242924 had lower peak cortisol response to the TSST |
| Höhne et al., 2015 | N = 116, N = 61 with remitted depression Male = 32, Female = 29, Mean age = 34.65, SD = 3.32 N = 55 healthy controls Male = 28, Female = 27, Mean age = 34.02, SD = 3.61 |
FKBP5 genotype (SNP rs1360780) | rs1360780, FKBP5 inhibits the function of GRs and the T allele results in the enhancement of FKBP5 mRNA transcription | Higher, longer-lasting cortisol reactivity in healthy subjects with TT genotype of FKBP5 polymorphism rs1360780 (but not for remitted depression group) |
| Ising et al., 2008 | N = 64 healthy volunteers, Male = 33, Female = 31, Mean age = 27.6, SD = 6.6 |
FKBP5 genotype (SNPs rs1360780, rs4713916 and rs3800737) GR polymorphisms Bcl1 and N363S MR polymorphism I180V |
SNPs moderate activity of FKBP5 protein; a co-chaperone of the GR heterocomplex, and assists in terminating HPA axis response Bcl1: Homozygous participants tend to show lower cortisol reactivity N363S: Exonic variant associated with asparagine-to-serine amino acid exchange of the GR protein and increased cortisol reactivity I180V: I180V variant associated with isoleucine-to-valine amino acid exchange of the MR protein and increased cortisol reactivity |
For all FKBP5 SNPs, subjects homozygous for minor allele had highest plasma cortisol levels corresponding to a recessive model Bcl1 carriers had lower cortisol levels in anticipation of second TSSTI180V variant associated with higher baseline ACTH levels before second TSST |
| Way et al., 2016 | N = 149, N = 81 employees 71% female Mean age = 38.34, SD = 11.47 N = 68 students 61% female, Mean age = 21.09, SD = 3.43 |
5-HT2C receptor gene polymorphism (Cys23Ser; rs6318) | Ser23 allele associated altered functionality of 5-HT (serotonin) receptor with associated changes in HPA reactivity (though previous evidence conflicting) | Homozygous females and hemizygous males had greater cortisol reactivity |
| Avery and Vrshek-Schallhorn, 2016 | N = 112, Male = 73, Female = 39, Mean age = 18.7, SD = 0.82 |
5-HT2C receptor gene polymorphism (Cys23Ser; rs6318) | (See comments above on Way et al., 2016) | Carriers of the C-allele of rs6318 showed blunted cortisol response to the TSST |
| Yamakawa et al., 2015 | N = 18, N = 9 with SL allele of 5-HTTLPR All male, Mean age = 21, SD = 0.25 N = 9 with SS allele of 5-HTTLPR All male, Mean age = 20.78, SD = 0.26 |
5-HTTLPR | 5-HTTLPR: Short (S) variant associated with lower 5-HT uptake activity | SS group: Increased IL-beta, cortisol and cardiovascular reactivity in response to TSST |
| Way and Taylor, 2010 | N = 185, Male = 39%, Female = 61%, Age range = 18–35 years |
5-HTTLPR | (See comments above on Yamakawa et al., 2015) | SS group: Increased cortisol reactivity in response to TSST |
| Way and Taylor, 2011 | N = 185, Male = 39%, Female = 61%, Age range = 18–35 years |
5-HTTLPR | (See comments above on Yamakawa et al., 2015) | SS group: Increased heart rate and blood pressure reactivity in response to TSST |
| Duman and Canli, 2015 | N = 105, All male, Men age = 28.51, SD = 13.82 |
5-HTTLPR | (See comments above on Yamakawa et al., 2015) | LL-group participants had increased TSST-induced mRNA expression in SLC64A, compared to S-group participants |
| Cerit et al., 2013 | N = 48, Male = 24, Female = 22, Age range = 18–35 years |
5-HTTLPR | (See comments above on Yamakawa et al., 2015) | Supplementation with L-tryptophan led to reduced cortisol response to the TSST, but only in S′/S′ carriers of 5-HTTLPR |
| Buckert et al., 2012 | N = 33, Male = 19, Female = 14, Mean age = 24.5, SD = 4.2 |
COMT val (158)Met | The substitution of MET for Val reduces COMT (Catechol-O-methyltransferase) enzyme activity, thus increasing synaptic dopamine availability | Met homozygotes for the COMT Val(158)Met polymorphism, had poorer performance on working memory following TSST-G exposure |
| Buchmann et al., 2014 | N = 219 (TSST sample), Male = 95, Age = 19 |
DRD4 | Seven-repeat allele of DRD4 associated with a blunted response to dopamine | Carriers of seven-repeat allele of DRD4 gene had attenuated cortisol reactivity to the TSST |
| Armbruster et al., 2016 | N = 116 (Adult sample), Male = 57, Female = 59, Mean age = 23.82, SD = 2.58 N = 123 (Child sample), Male = 71, Female = 51, Mean age = 9.33, SD = 1.02 |
BDNF val (66)met | Met allele associated with a decrease in activity dependent secretion of BDNF compared to the Val allele | Met allele of the Val(66)met polymorphism in the BDNF gene was associated with increased cortisol response to the TSST in young adults and children |
| Shalev et al., 2009 | N = 97, Male = 46, Female = 51, Mean age = 25.9, SD = 3.6 |
BDNF val (66)met | (See comments above on Armbruster et al., 2016) | In females, val/val had lowest increase (as assessed with AUCi) In males, val/val homozygotes had greater rise in cortisol than val/met |
| Kumsta et al., 2013 | N = 196, All male, Mean age = 23.7, SD = 2.9 |
Neuropeptide S receptor gene polymorphism rs324981 | T allele increases neuropeptide S receptor expression and neuropeptide S efficacy at the receptor | Carriers of the T allele of rs324981, displayed larger cortisol and subjective response to the TSST for groups |
| Li et al., 2013 | N = 42, All male, Mean age = 23.63, SD = 0.44 |
ADRA2B gene | Functional deletion variant characterised by loss of glutamic acid residues 301–303 in third intracellular loop encoding the presynaptic α2B subunit of the noradrenaline receptor increases noradrenaline availability | Carriers of the deletion of the ADRA2B gene had impaired recognition and slower retrieval for neutral faces post-TSST |
| Li et al., 2015 | N = 27, All male, Mean age = 24.25, SD = 0.75 |
ADRA2B gene | (See comments above on Li et al., 2013) | Increased amygdala activation when recalling emotional faces post-TSST, whereas non-carriers showed TSST-induced activity changes in the right hippocampus |
| Jabbi et al., 2007 | N = 70 (acute stress), Male = 30, Female = 40, Mean age = 20.14, Age range = 15–32) |
COMT & MAOA genes | Variable-number-tandem-repeat (VNTR) polymorphism in MAOA promotor region associated with high activity or low activity repeats, associated with more of less efficient transcription respectively. COMT val (158)Met (see Buckert et al., 2012) |
Effects of COMT on ACTH release depended upon the presence of the MAOA variant within the same individual |
6.2. Epigenetics and the TSST
In addition to genotype, epigenetic alterations, such as DNA methylation, are recognized as biological factors that impact on our psychological functioning. Long-term epigenetic changes may alter the biological machinery underpinning the acute stress response; such changes offer a potential mechanism whereby experience can alter the function of genes without changes in the underlying DNA sequence (Krishnan and Nestler, 2008). Epigenetic regulation of the glucocorticoid receptor gene is well documented (c.f. Dinan et al., 2010). Such regulation has been associated with stress-related psychiatric disorders (Roth, 2013), and evidence that epigenetic mechanisms may impact upon TSST response include the finding that methylation of the SKA2 gene, which plays a role in glucocorticoid receptor transactivation, was associated with reduced cortisol reactivity to the TSST (Boks et al., 2015). There was a trend within participants with the TT genotype of rs1360780 for those with remitted depression to have higher DNA methylation in intron 7 of the FKBP5 gene than healthy controls with the same genotype (Höhne et al., 2015). Furthermore, methylation of the glucocorticoid receptor (NR3C1) exon 1F predicted total cortisol output, but only in females; cortisol output in females was specifically predicted by a single CpG site in the nerve growth factor inducible protein A transcription factor (Mahon et al., 2013).
As indicated above, 5-HTTLPR genotypic differences between individuals can alter reactivity to the TSST. Epigenetic research has extended these findings; early life stressors moderated DNA methylation as a function of the 5-HTTLPR phenotype; those in the S-group with higher early life stress had lower SLC6A4 expression and increased F3 methylation (Duman and Canli, 2015), a finding that highlights the importance of gene X environment interactions. The S allele was related to increased cortisol reactivity for individuals with low methylation of SLC6A4, but not for those with high methylation, suggesting that this epigenetic factor could act as a compensatory mechanism for underlying genotypic differences (Alexander et al., 2014).
Oxytocin is a pituitary neuropeptide hormone implicated in the neurobiology of many neuropsychiatric disorders (Romano et al., 2015). In participants aged 61–67 years, oxytocin receptor gene DNA methylation increased post-TSST (Unternaehrer et al., 2012). Increased cortisol response to the TSST has been associated with reduced methylation of the oxytocin receptor in a study of social anxiety disorder (Ziegler et al., 2015).
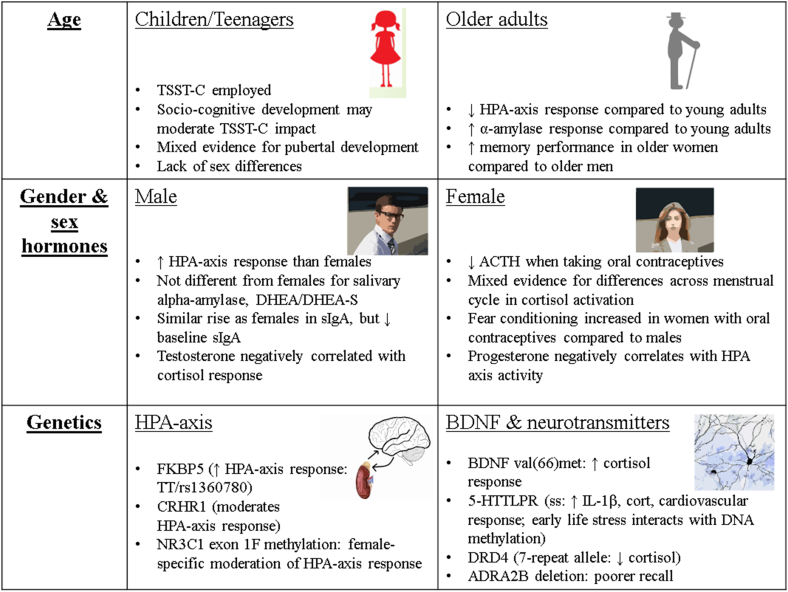
These initials findings on epigenetic mechanisms in moderating the acute stress response are highly interesting, and chime with much of the research examining the impact of genotype on TSST response, by implicating the genetics of serotonin, as well as genetic factors more directly linked with the HPA axis. The area of epigenetics and the acute stress response in humans is still in its early stages, but nonetheless provides a promising avenue for future research. For a summary of factors moderating the neurobiology response to the TSST, including age and gender as well as genetic factors, see Fig. 2.
Fig. 2.
Factors which moderate the acute stress response to the Trier Social Stress Test.
7. Limitations of the TSST
As with any experimental procedure, there are practical limitations to using the TSST. One of the major limitations of the TSST is that there is a high degree of habituation of the HPA axis response with repeated exposures (Pruessner et al., 1997, Schommer et al., 2003). As such, it has limitations when applied to intervention studies in which it is necessary to determine a pre- and post-treatment HPA axis response, as it is difficult to parse the treatment and habituation effect. Measures of autonomic nervous system show less habituation (Gerra et al., 2001, Jönsson et al., 2010, Schommer et al., 2003, von Kanel et al., 2006) and may be more suitable for intervention studies. However, it is important to note that modifications should be made to the speech and mental arithmetic task to be performed during each repeated TSST session (e.g. requiring participants to perform serial subtraction on a different set of numbers) to minimize any habituation effects (Kirschbaum et al., 1995, Petrowski et al., 2012, Schommer et al., 2003).
Although the TSST is a highly controlled procedure, and committee members will normally be well-trained prior to an experiment beginning, nuances in interactions between a specific participant and committee member cannot be controlled, as with any social interaction, and may introduce some variability to the data. In addition, dependent on the resources available to the investigator, there may be limitations on the number of participants who can be tested each day/week, as the procedure is quite labour-intensive. Depending on the requirements of a particular study, the adaptations mentioned previously can help to overcome some of these limitations; for example the TSST for groups can increase throughput and the TSST-VR can reduce variability in participant/committee interactions.
By combining a number of stressful components, the TSST induces moderate stress in a majority of participants more reliably than its constituent components (e.g. public speaking) in isolation (Kirschbaum et al., 1993). However, this leads to a difficulty in disentangling the specific role of different aspects of the stressor in inducing stress. This is a problem for a number of methods of inducing acute stress (e.g. the socially evaluated cold pressor includes both physical pain and social evaluation). Markers of stress which can be measured throughout TSST exposure, and which respond in a more time-locked manner to a stressor than cortisol (e.g. heart rate) will help in determining which aspect of the TSST is inducing a greater deal of stress for the participant.
8. Conclusion and perspectives
The TSST has allowed for a deeper understanding of the neurobiology of the acute stress response under both normal and pathological conditions, as well as how this is moderated by factors such as gender, age and genetics. Numerous adaptations of the TSST have been adapted that have allowed for acute stress to be assessed in various contexts. Nonetheless, given its extensive demonstration of acute stress across multiple laboratories, the TSST remains the gold standard of assessment of acute stress under laboratory conditions. Enhanced consistency of methodology across laboratories will ensure greater comparability of data, and increased consistency of outcomes assessed will allow for more powerful analyses to be conducted, for example through meta-analyses.
The TSST provides a flexible platform for employing technological advances in the assessment of physiology (e.g. in human epigenetics). There are already outcomes of the TSST that are under-explored; performance during the TSST (e.g. verbal performance on the interview speech, non-verbal behaviour during the interview speech) has, with some exceptions, generally not received much research interest, despite being a potential source of rich behavioural data. Nonetheless, a method such as the consensual assessment technique (Amabile, 1983) could be used to assess the interview speeches (or stories told in the TSST-C) on criteria such as creativity. Future research employing the TSST within longer-term longitudinal studies will allow for a deeper understanding of the impact of ageing upon the stress process.
Study of the neurobiology of the acute stress response may help us to better understand how chronic stress impacts upon the acute stress response. Related to this, the TSST provides an experimental platform to investigate the neurobiology of chronic stress-related disorders such as generalised anxiety disorder, major depression and irritable bowel syndrome, including maintaining factors for stress-related disorders, as well as the efficacy of pharmacological or psychological approaches to managing stress and alleviating stress-related disorder. For example, mindfulness-based interventions have been shown to lead to attenuated psychological responses to acute stress in patients with generalised anxiety disorder (Hoge et al., 2013), and partially-remitted depression (Britton et al., 2012), as well as in healthy volunteers (Creswell et al., 2014), although the latter study observed a heightened HPA axis response to TSST following meditation, particularly in those with low dispositional mindfulness.
Although the neurobiological study of acute stress in humans has developed tremendously in recent decades with advances in neuroscience and its cognate disciplines, full elucidation of processes such as acute stress responses is likely to take many decades of further work and technological advance. A controlled, effective acute stressor is invaluable in this process, and we believe that the TSST has many advantages in this regard when used in an informed way in specific contexts. Equally, it should be recognized that data from the TSST may need to be paired with information from other sources (e.g. naturalistic stressors, SECPT) for maximum insight. To better understand causal mechanisms in the neurobiology of stress, integrating the observations from TSST in human populations with preclinical data will be crucial. The neurobiological processes involved in the TSST response in health and disease depend heavily on the impact of the environment across the lifespan, and are influenced by a variety of factors. The role of the gut microbiome therein is currently underappreciated. Given evidence for changes in the composition of the gut microbiota in stress-related disorder, such as depression (Jiang et al., 2015, Kelly et al., 2016, Naseribafrouei et al., 2014) and irritable bowel syndrome (Jeffery et al., 2012), it is of interest if different microbial compositions within individuals may be predictive of altered reactivity to the TSST. The implications of improving our knowledge in this area, for both stress vulnerability and resilience, are clear. This strategy offers benefits for both the maintenance of health and for the management of stress-related neuropsychiatric disorders.
Acknowledgements
This work was supported by the Health Research Board, Ireland (HRB) through Health Research Awards (grant number HRA_POR_2014_647; GC, TGD). The APC Microbiome Institute is funded by Science Foundation Ireland (SFI; grant number SFI/12/RC/2273). This Institute has conducted studies in collaboration with several companies including GSK, Pfizer, Wyeth and Mead Johnson. The authors would like to thank Dr Pauline Luczynski for assistance with the Figures. The funding bodies supporting the authors played no role in the writing of this article. The authors declare no conflict of interest.
Contributor Information
Andrew P. Allen, Email: andrewallen@ucc.ie.
Gerard Clarke, Email: G.Clarke@ucc.ie.
References
- Alexander N., Wankerl M., Hennig J., Miller R., Zänkert S., Steudte-Schmiedgen S.…Kirschbaum C. DNA methylation profiles within the serotonin transporter gene moderate the association of 5-HTTLPR and cortisol stress reactivity. Transl. Psychiatry. 2014;4(9):e443. doi: 10.1038/tp.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Almela M., Hidalgo V., Villada C., van der Meij L., Espín L., Gómez-Amor J., Salvador A. Salivary alpha-amylase response to acute psychosocial stress: the impact of age. Biol. Psychol. 2011;87(3):421–429. doi: 10.1016/j.biopsycho.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Amabile T.M. The social psychology of creativity: a componential conceptualization. J. Personality Soc. Psychol. 1983;45(2):357–376. [Google Scholar]
- Armbruster D., Müller-Alcazar A., Strobel A., Lesch K.P., Kirschbaum C., Brocke B. BDNF val 66 met genotype shows distinct associations with the acoustic startle reflex and the cortisol stress response in young adults and children. Psychoneuroendocrinology. 2016;66:39–46. doi: 10.1016/j.psyneuen.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Aschbacher K., von Känel R., Dimsdale J.E., Patterson T.L., Mills P.J., Mausbach B.T.…Grant I. Dementia severity of the care receiver predicts procoagulant response in Alzheimer caregivers. Am. J. Geriatric Psychiatry. 2006;14(8):694–703. doi: 10.1097/01.JGP.0000227969.36850.eb. [DOI] [PubMed] [Google Scholar]
- Avery B.M., Vrshek-Schallhorn S. Nonsynonymous HTR2C polymorphism predicts cortisol response to psychosocial stress I: effects in males and females. Psychoneuroendocrinology. 2016;70:134–141. doi: 10.1016/j.psyneuen.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali A., Jaggi A.S. Clinical experimental stress studies: methods and assessment. Rev. Neurosci. 2015;26(5):555–579. doi: 10.1515/revneuro-2015-0004. [DOI] [PubMed] [Google Scholar]
- Baxter A.J., Scott K.M., Vos T., Whiteford H.A. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol. Med. 2013;43(5):897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- Birkett M., Johnson L., Gelety C. Investigation of sex differences in sIgA response to the trier social stress test. Stress Health. 2016 doi: 10.1002/smi.2680. [DOI] [PubMed] [Google Scholar]
- Boesch M., Sefidan S., Ehlert U., Annen H., Wyss T., Steptoe A., La Marca R. Mood and autonomic responses to repeated exposure to the trier social stress test for groups (TSST-G) Psychoneuroendocrinology. 2014;43:41–51. doi: 10.1016/j.psyneuen.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Boks M.P., Rutten B.P., Geuze E., Houtepen L.C., Vermetten E., Kaminsky Z., Vinkers C.H. SKA2 methylation is involved in cortisol stress reactivity and predicts the development of post-traumatic stress disorder (PTSD) after military deployment. Neuropsychopharmacology. 2015;41(5):1350–1356. doi: 10.1038/npp.2015.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.G., Binder E.B., Epstein M.P., Tang Y., Nair H.P., Liu W.…Stowe Z.N. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives General Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W.B., Shahar B., Szepsenwol O., Jacobs W.J. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: results from a randomized controlled trial. Behav. Ther. 2012;43(2):365–380. doi: 10.1016/j.beth.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.B. Adolescents' relationships with peers. Handb. Adolesc. Psychol. 2004;2:363–394. [Google Scholar]
- Brummett B.H., Babyak M.A., Kuhn C.M., Siegler I.C., Williams R.B. A functional polymorphism in the HTR2C gene associated with stress responses: a validation study. Biol. Psychol. 2014;103:317–321. doi: 10.1016/j.biopsycho.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett B.H., Kuhn C.M., Boyle S.H., Babyak M.A., Siegler I.C., Williams R.B. Cortisol responses to emotional stress in men: association with a functional polymorphism in the 5HTR2C gene. Biol. Psychol. 2012;89(1):94–98. doi: 10.1016/j.biopsycho.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A.F., Zohsel K., Blomeyer D., Hohm E., Hohmann S., Jennen-Steinmetz C.…Esser G. Interaction between prenatal stress and dopamine D4 receptor genotype in predicting aggression and cortisol levels in young adults. Psychopharmacology. 2014;231(16):3089–3097. doi: 10.1007/s00213-014-3484-7. [DOI] [PubMed] [Google Scholar]
- Buckert M., Kudielka B.M., Reuter M., Fiebach C.J. The COMT Val158Met polymorphism modulates working memory performance under acute stress. Psychoneuroendocrinology. 2012;37(11):1810–1821. doi: 10.1016/j.psyneuen.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., Jobst S., Wustmans A., Kirschbaum C., Rauh W., Hellhammer D.H. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Campisi J., Bravo Y., Cole J., Gobeil K. Acute psychosocial stress differentially influences salivary endocrine and immune measures in undergraduate students. Physiology Behav. 2012;107(3):317–321. doi: 10.1016/j.physbeh.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Carpenter L.L., Carvalho J.P., Tyrka A.R., Wier L.M., Mello A.F., Mello M.F., Anderson G.M., Wilkinson C.W., Price L.H. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerit H., Jans L.A., Van der Does W. The effect of tryptophan on the cortisol response to social stress is modulated by the 5-HTTLPR genotype. Psychoneuroendocrinology. 2013;38(2):201–208. doi: 10.1016/j.psyneuen.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Cheetham T.J., Turner-Cobb J.M. Panel manipulation in social stress testing: the bath experimental stress test for children (BEST-C) Psychoneuroendocrinology. 2016;63:78–85. doi: 10.1016/j.psyneuen.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Cheetham T., Turner-Cobb J., Family H. Using child confederates in social stress testing: impact on child cortisol reactivity. Psychosom. Med. 2015;7(33) [Google Scholar]
- Chen J., Lipska B.K., Halim N., Ma Q.D., Matsumoto M., Melhem S.…Egan M.F. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E., Vicini L.M., De Wit H. Responses to the trier social stress test (TSST) in single versus grouped participants. Psychophysiology. 2006;43(4):366–371. doi: 10.1111/j.1469-8986.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- Chopra K.K., Ravindran A., Kennedy S.H., Mackenzie B., Matthews S., Anisman H.…Levitan R.D. Sex differences in hormonal responses to a social stressor in chronic major depression. Psychoneuroendocrinology. 2009;34(8):1235–1241. doi: 10.1016/j.psyneuen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Clipp E.C., George L.K. Dementia and cancer: a comparison of spouse caregivers. Gerontologist. 1993;33(4):534–541. doi: 10.1093/geront/33.4.534. [DOI] [PubMed] [Google Scholar]
- Cohen S., Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J. Appl. Soc. Psychol. 2012;42(6):1320–1334. [Google Scholar]
- Colman A.M. Oxford University Press; Oxford, UK: 2001. Oxford dictionary of Psychology. [Google Scholar]
- Creswell J.D., Pacilio L.E., Lindsay E.K., Brown K.W. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent development and the regulation of behavior and emotion: introduction to part VIII. Ann. New York Acad. Sci. 2004;1021(1):294–295. doi: 10.1196/annals.1308.034. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Gunnar M.R. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev. Psychopathol. 2009;21(1):1. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Renwick R., Mahani N.K., Engert V. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry & Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., Wadiwalla M., Engert V., Pruessner J.C. The role of sex and gender socialization in stress reactivity. Dev. Psychol. 2009;45(1):45. doi: 10.1037/a0014433. [DOI] [PubMed] [Google Scholar]
- Dettenborn L., Tietze A., Bruckner F., Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dienes K.A., Hazel N.A., Hammen C.L. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38:927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F., Shanahan F., Napoleon Keeling P.W., Quigley E.M.M. IBS: an epigenetic perspective. Nat. Rev. Gastroenterology Hepatology. 2010;7:465–471. doi: 10.1038/nrgastro.2010.99. [DOI] [PubMed] [Google Scholar]
- Ditzen B., Schmidt S., Strauss B., Nater U.M., Ehlert U., Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. J. Psychosomatic Res. 2008;64(5):479–486. doi: 10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Duchesne A., Tessera E., Dedovic K., Engert V., Pruessner J.C. Effects of panel sex composition on the physiological stress responses to psychosocial stress in healthy young men and women. Biol. Psychol. 2012;89(1):99–106. doi: 10.1016/j.biopsycho.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Duman E.A., Canli T. Influence of life stress, 5-HTTLPR genotype, and SLC6A4 methylation on gene expression and stress response in healthy Caucasian males. Biol. Mood Anxiety Disord. 2015;5(2):1. doi: 10.1186/s13587-015-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein M. Microbiome: bacterial broadband. Nature. 2016;533(7603):S104–S106. doi: 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M., Erath S.A., Buckhalt J.A., Granger D.A., Mize J. Cortisol and children's adjustment: the moderating role of sympathetic nervous system activity. J. Abnorm. Child Psychol. 2008;36(4):601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Elzinga B.M., Roelofs K., Tollenaar M.S., Bakvis P., van Pelt J., Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology. 2008;33(2):227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Engert V., Efanov S.I., Duchesne A., Vogel S., Corbo V., Pruessner J.C. Differentiating anticipatory from reactive cortisol responses to psychosocial stress. Psychoneuroendocrinology. 2013;38(8):1328–1337. doi: 10.1016/j.psyneuen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Espin L., Almela M., Hidalgo V., Villada C., Salvador A., Gomez-Amor J. Acute pre-learning stress and declarative memory: impact of sex, cortisol response and menstrual cycle phase. Hormones Behav. 2013;63(5):759–765. doi: 10.1016/j.yhbeh.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Eurofund, EU-OSHA . Publications Office of the European Union; Luxembourg: 2014. Psychosocial Risks in Europe: Prevalence and Strategies for Prevention. [Google Scholar]
- Fagundes C.P., Glaser R., Hwang B.S., Malarkey W.B., Kiecolt-Glaser J.K. Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behav. Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J., Somerville A.J., Baxter A.J., Norman R., Patten S.B., Vos T., Whiteford H.A. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol. Med. 2013;43(3):471–481. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- Fiocco A.J., Joober R., Lupien S.J. Education modulates cortisol reactivity to the Trier Social Stress Test in middle-aged adults. Psychoneuroendocrinology. 2007;32(8):1158–1163. doi: 10.1016/j.psyneuen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Foley P., Kirschbaum C. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010;35(1):91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Frisch J.U., Häusser J.A., Mojzisch A. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front. Psychol. 2015;6:14. doi: 10.3389/fpsyg.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J., Rohleder N., Heitz V., Engert V., Schad T., Schürmeyer T.H., Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gallagher S., Sumner R.C., Muldoon O.T., Creaven A.M., Hannigan A. Unemployment is associated with lower cortisol awakening and blunted dehydroepiandrosterone responses. Psychoneuroendocrinology. 2016;69:41–49. doi: 10.1016/j.psyneuen.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Gerra G., Zaimovic A., Mascetti G., Gardini S., Zambelli U., Timpano M., Raggi M., Brambilla F. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26:91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Gold S.M., Zakowski S.G., Valdimarsdottir H.B., Bovbjerg D.H. Higher Beck depression scores predict delayed epinephrine recovery after acute psychological stress independent of baseline levels of stress and mood. Biol. Psychol. 2004;67(3):261–273. doi: 10.1016/j.biopsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gordis E.B., Granger D.A., Susman E.J., Trickett P.K. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordon J.L., Girdler S.S. Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology. 2014;51(4):309–318. doi: 10.1111/psyp.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff F.G., Parente A., Del-Ben C.M., Guimarães F.S. Pharmacology of human experimental anxiety. Braz. J. Med. Biol. Res. 2003;36(4):421–432. doi: 10.1590/s0100-879x2003000400003. [DOI] [PubMed] [Google Scholar]
- Grayson M. Irritable bowel syndrome: 4 big questions. Nature. 2016;533(7603):S118. doi: 10.1038/533S118a. S118. [DOI] [PubMed] [Google Scholar]
- Gruenewald T.L., Kemeny M.E., Aziz N. Subjective social status moderates cortisol responses to social threat. Brain, Behav. Immun. 2006;20(4):410–419. doi: 10.1016/j.bbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Wewerka S., Frenn K., Long J.D., Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev. Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B., van Leijenhorst L., Rombouts S.A., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5–6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Hanoch Y., Vitouch O. When less is more: information, emotional arousal and the ecological reframing of the Yerkes-Dodson law. Theory & Psychol. 2004;14(4):427–452. [Google Scholar]
- Hatzenbuehler M.L., McLaughlin K.A. Structural stigma and hypothalamic–pituitary–adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Ann. Behav. Med. 2014;47(1):39–47. doi: 10.1007/s12160-013-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Heit S., Graham Y.P., Wilcox M., Bonsall R., Miller A.H., Nemeroff C.B. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J. Am. Med. Assoc. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hellhammer D.H., Wüst S., Kudielka B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Herbison C.E., Henley D., Marsh J., Atkinson H., Newnham J.P., Matthews S.G.…Pennell C.E. Characterization and novel analyses of acute stress response patterns in a population-based cohort of young adults: influence of gender, smoking, and BMI. Stress. 2016;19(2):139–150. doi: 10.3109/10253890.2016.1146672. [DOI] [PubMed] [Google Scholar]
- Het S., Rohleder N., Schoofs D., Kirschbaum C., Wolf O. Neuroendocrine and psychometric evaluation of a placebo version of the 'trier social stress test'. Psychoneuroendocrinology. 2009;34:1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hidalgo V., Almela M., Villada C., Salvador A. Acute stress impairs recall after interference in older people, but not in young people. Hormones Behav. 2014;65(3):264–272. doi: 10.1016/j.yhbeh.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Hidalgo V., Pulopulos M.M., Puig-Perez S., Espin L., Gomez-Amor J., Salvador A. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behav. Brain Res. 2015;292:393–402. doi: 10.1016/j.bbr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Hidalgo V., Villada C., Almela M., Espín L., Gómez-Amor J., Salvador A. Enhancing effects of acute psychosocial stress on priming of non-declarative memory in healthy young adults. Stress. 2012;15(3):329–338. doi: 10.3109/10253890.2011.624224. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Bui E., Marques L., Metcalf C.A., Morris L.K., Robinaugh D.J.…Simon N.M. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry. 2013;74(8):1–478. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhne N., Poidinger M., Merz F., Pfister H., Brückl T., Zimmermann P.…Ising M. FKBP5 genotype-dependent DNA methylation and mRNA regulation after psychosocial stress in remitted depression and healthy controls. Int. J. Neuropsychopharmacol. 2015;18(4):pyu087. doi: 10.1093/ijnp/pyu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhou R., Wu M., Wang Q., Zhao Y. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160–168. doi: 10.3109/10253890.2014.999234. [DOI] [PubMed] [Google Scholar]
- Ising M., Depping A.M., Siebertz A., Lucae S., Unschuld P.G., Kloiber S.…Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008;28(2):389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Izawa S., Miki K., Liu X., Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain, Behav. Immun. 2013;27:38–41. doi: 10.1016/j.bbi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Izawa S., Sugaya N., Kimura K., Ogawa N., Yamada K.C., Shirotsuki K., Mikami I., Hirata K., Nagano Y., Nomura S. An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biol. Psychol. 2013;94(2):249–254. doi: 10.1016/j.biopsycho.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Korf J., Kema I.P., Hartman C., Van der Pompe G., Minderaa R.B.…Den Boer J.A. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol. psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jacobson R., Cohen H., Diamond G.M. Gender atypicality and anxiety response to social interaction stress in homosexual and heterosexual men. Archives Sex. Behav. 2016;45:713–723. doi: 10.1007/s10508-015-0528-y. [DOI] [PubMed] [Google Scholar]
- Jansen S.W., van Heemst D., van der Grond J., Westendorp R., Oei N.Y. Physiological responding to stress in middle-aged males enriched for longevity: a social stress study. Stress. 2016;19(1):28–36. doi: 10.3109/10253890.2015.1105213. [DOI] [PubMed] [Google Scholar]
- Jeffery I.B., O'Toole P.W., Öhman L., Claesson M.J., Deane J., Quigley E.M., Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- Jezova D., Makatsori A., Duncko R., Moncek F., Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2004;28(8):1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y.…Li L. Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jönsson P., Wallergård M., Österberg C., Hansen Å.M., Johansson G., Karlson B. Cardiovascular and cortisol reactivity and habituation to a virtual reality version of the Trier Social Stress Test: a pilot study. Psychoneuroendocrinology. 2010;35:1397–1403. doi: 10.1016/j.psyneuen.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Jordanova V., Stewart R., Davies E., Sherwood R., Prince M. Markers of inflammation and cognitive decline in an African-Caribbean population. Int. J. Geriatric Psychiatry. 2007;22(10):966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- Juster R.P., Hatzenbuehler M.L., Mendrek A., Pfaus J.G., Smith N.G., Johnson P.J.…Lupien S.J. Sexual orientation modulates endocrine stress reactivity. Biol. Psychiatry. 2015;77(7):668–676. doi: 10.1016/j.biopsych.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R.P., Raymond C., Desrochers A.B., Bourdon O., Durand N., Wan N.…Lupien S.J. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology. 2016;63:282–290. doi: 10.1016/j.psyneuen.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., O'Brien C., Patterson E., El Aidy S., Deane J.…Hoban A.E. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatric Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kennedy P.J., Clarke G., Quigley E.M., Groeger J.A., Dinan T.G., Cryan J.F. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012;36(1):310–340. doi: 10.1016/j.neubiorev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Kennedy P.J., Cryan J.F., Quigley E.M.M., Dinan T.G., Clarke G. A sustained hypothalamic–pituitary–adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol. Med. 2014;44(14):3123–3134. doi: 10.1017/S003329171400052X. [DOI] [PubMed] [Google Scholar]
- Kim Y., Schulz R. Family caregivers' strains: comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. J. Aging Health. 2008;20(5):483–503. doi: 10.1177/0898264308317533. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Prussner J.C., Stone A.A., Federenko I., Gaab J., Lintz D., Schommer N., Hellhammer D.H. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Wüst S., Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B., Hastings P.D., Granger D.A., Usher B.A., Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev. Psychopathol. 2001;13(03):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kotlyar M., Donahue C., Thuras P., Kushner M.G., O'Gorman N., Smith E.A., Adson D.E. Physiological response to a speech stressor presented in a virtual reality environment. Psychophysiology. 2008;45(6):1034–1037. doi: 10.1111/j.1469-8986.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- Krämer M., Seefeldt W.L., Heinrichs N., Tuschen-Caffier B., Schmitz J., Wolf O.T., Blechert J. Subjective, autonomic, and endocrine reactivity during social stress in children with social phobia. J. Abnorm. Child Psychol. 2012;40(1):95–104. doi: 10.1007/s10802-011-9548-9. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaveni G.V., Veena S.R., Jones A., Bhat D.S., Malathi M.P., Hellhammer D.…Fall C.H.D. Trier social stress test in Indian adolescents. Indian Pediatr. 2014;51(6):463–467. doi: 10.1007/s13312-014-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B., Buske-Kirschbaum A., Hellhammer D., Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Buske-Kirschbaum A., Hellhammer D.H., Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int. J. Behav. Med. 2004;11(2):116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Hellhammer D.H., Kirschbaum C. Ten years of research with the trier social stress test revisited. In: Harmon-Jones E., Winkielman P., editors. Social Neuroscience. The Guilford Press; New York: 2007. pp. 56–83. [Google Scholar]
- Kumsta R., Chen F.S., Pape H.C., Heinrichs M. Neuropeptide S receptor gene is associated with cortisol responses to social stress in humans. Biol. Psychol. 2013;93(2):304–307. doi: 10.1016/j.biopsycho.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Lachman H.M., Papolos D.F., Saito T., Yu Y.M., Szumlanski C.L., Weinshilboum R.M. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics Genomics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Laurent H.K., Stroud L.R., Brush B., D'Angelo C., Granger D.A. Secretory IgA reactivity to social threat in youth: relations with HPA, ANS, and behavior. Psychoneuroendocrinology. 2015;59:81–90. doi: 10.1016/j.psyneuen.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson A.K., Jonsdottir I.H. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology. 2011;36(10):1530–1539. doi: 10.1016/j.psyneuen.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Lennartsson A.K., Kushnir M.M., Bergquist J., Jonsdottir I.H. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012;90(2):143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lennartsson A.K., Kushnir M.M., Bergquist J., Billig H., Jonsdottir I.H. Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. Int. J. Psychophysiol. 2012;84(3):246–253. doi: 10.1016/j.ijpsycho.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Leonard B. Stress, depression and the activation of the immune system. World J. Biol. Psychiatry. 2000;1(1):17–25. doi: 10.3109/15622970009150562. [DOI] [PubMed] [Google Scholar]
- Li S., Weerda R., Guenzel F., Wolf O.T., Thiel C.M. ADRA2B genotype modulates effects of acute psychosocial stress on emotional memory retrieval in healthy young men. Neurobiol. Learn. Mem. 2013;103:11–18. doi: 10.1016/j.nlm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Li S., Weerda R., Milde C., Wolf O.T., Thiel C.M. ADRA2B genotype differentially modulates stress-induced neural activity in the amygdala and hippocampus during emotional memory retrieval. Psychopharmacology. 2015;232(4):755–764. doi: 10.1007/s00213-014-3710-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran N.L., Mayer S.E., Abelson J.L. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress. 2014;17(4):285–295. doi: 10.3109/10253890.2014.915517. [DOI] [PubMed] [Google Scholar]
- Lotta T., Vidgren J., Tilgmann C., Ulmanen I., Melen K., Julkunen I., Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lovell R.M., Ford A.C. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am. J. Gastroenterology. 2012;107(7):991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., Maheu F., Tu M., Fiocco A., Schramek T.E. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cognition. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Mahon P.B., Zandi P.P., Potash J.B., Nestadt G., Wand G.S. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227(2):231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P.M., Mordecai K.L., Rubin L.H., Sundermann E., Savarese A., Eatough E., Drogos L. Menstrual cycle effects on cortisol responsivity and emotional retrieval following a psychosocial stressor. Hormones Behav. 2015;74:201–208. doi: 10.1016/j.yhbeh.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M.F., Morin-Major J.K., Schramek T.E., Beaupré A., Perna A., Juster R.P., Lupien S.J. There is no news like bad news: women are more remembering and stress reactive after reading real negative news than men. PloS one. 2012;7(10):e47189. doi: 10.1371/journal.pone.0047189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015;226(2):T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gray J.D., Nasca C. Recognizing resilience: learning from the effects of stress on the brain. Neurobiol. Stress. 2015;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C.J., Wolf O.T., Schweckendiek J., Klucken T., Vaitl D., Stark R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013;38(11):2529–2541. doi: 10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Minkley N., Schröder T.P., Wolf O.T., Kirchner W.H. The socially evaluated cold-pressor test (SECPT) for groups: effects of repeated administration of a combined physiological and psychological stressor. Psychoneuroendocrinology. 2014;45:119–127. doi: 10.1016/j.psyneuen.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Mohiyeddini C., Bauer S., Semple S. Displacement behaviour is associated with reduced stress levels among men but not women. PloS One. 2013;8(2):e56355. doi: 10.1371/journal.pone.0056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiyeddini C., Bauer S., Semple S. Public self-consciousness moderates the link between displacement behaviour and experience of stress in women. Stress. 2013;16(4):384–392. doi: 10.3109/10253890.2012.755171. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Oswald L.M., Zandi P., Nestadt G., Potash J.B., Kalaydjian A.E., Wand G.S. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31(7):1583–1591. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- Pace T., Mletzko T., Alagbe O., Musselman D., Nemeroff C., Miller A., Heim C. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Petrowski K., Herold U., Joraschky P., Wittchen H.U., Kirschbaum C. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology. 2010;35(3):414–421. doi: 10.1016/j.psyneuen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Petrowski K., Wintermann G.B., Siepmann M. Cortisol response to repeated psychosocial stress. Appl. Psychophysiol. Biofeedback. 2012;37(2):103–107. doi: 10.1007/s10484-012-9183-4. [DOI] [PubMed] [Google Scholar]
- Petrowski K., Wintermann G., Schaarschmidt M., Bornstein S.R., Kirschbaum C. Blunted salivary and plasma cortisol response in patients with panic disorder under psychosocial stress. Int. J. Psychophysiol. 2013;88(1):35–39. doi: 10.1016/j.ijpsycho.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Prenderville J.A., Kennedy P.J., Dinan T.G., Cryan J.F. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38(1):13–25. doi: 10.1016/j.tins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Gaab J., Hellhammer D.H., Lintz D., Schommer N., Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22(8):615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pulopulos M.M., Hidalgo V., Almela M., Puig-Perez S., Villada C., Salvador A. Acute stress and working memory in older people. Stress. 2015;18(2):178–187. doi: 10.3109/10253890.2015.1004538. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Zellweger B.C., Marti B., Seiler R., Mohiyeddini C., Ehlert U., Heinrichs M. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32(6):627–635. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Romano A., Tempesta B., Di Bonaventura M.V.M., Gaetani S. From autism to eating disorders and more: the role of oxytocin in neuropsychiatric disorders. Front. Neurosci. 2015;9:497. doi: 10.3389/fnins.2015.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L. Epigenetic mechanisms in the development of behavior: advances, challenges, and promises of a new field. Dev. Psychopathol. 2013;25(4pt2):1279–1291. doi: 10.1017/S0954579413000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Murrough J.W., Han M.H., Charney D.S., Nestler E.J. Neurobiology of resilience. Nat. Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer N.C., Hellhammer D.H., Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom. Med. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Schwabe L., Haddad L., Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33(6):890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Hawrylak V.A., Matus C., Guido M.A., Levey A.I. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I., Lerer E., Israel S., Uzefovsky F., Gritsenko I., Mankuta D.…Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34(3):382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Slavich G.M., Way B.M., Eisenberger N.I., Taylor S.E. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Emotional reactivity and regulation across development. In: Gazzaniga, Mangun, editors. The Cognitive Neurosciences V. MIT Press; 2014. [Google Scholar]
- Stephens M.A.C., Mahon P.B., McCaul M.E., Wand G.S. Hypothalamic–pituitary–adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahler J., Mueller A., Rosenloecher F., Kirschbaum C., Rohleder N. Salivary α-amylase stress reactivity across different age groups. Psychophysiology. 2010;47(3):587–595. doi: 10.1111/j.1469-8986.2009.00957.x. [DOI] [PubMed] [Google Scholar]
- Stroud L.R., Foster E., Papandonatos G.D., Handwerger K., Granger D.A., Kivlighan K.T., Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev. Psychopathol. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud L.R., Salovey P., Epel E.S. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Stroud L.R., Tanofsky-Kraff M., Wilfley D.E., Salovey P. The Yale Interpersonal Stressor (YIPS): affective, physiological, and behavioral responses to a novel interpersonal rejection paradigm. Ann. Behav. Med. 2000;22(3):204–213. doi: 10.1007/BF02895115. [DOI] [PubMed] [Google Scholar]
- Suárez-Hitz K.A., Otto B., Bidlingmaier M., Schwizer W., Fried M., Ehlert U. Altered psychobiological responsiveness in women with irritable bowel syndrome. Psychosom. Med. 2012;74(2):221–231. doi: 10.1097/PSY.0b013e318244fb82. [DOI] [PubMed] [Google Scholar]
- Sumter S.R., Bokhorst C.L., Miers A.C., Van Pelt J., Westenberg P.M. Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35(10):1510–1516. doi: 10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5(1):47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Uhart M., Chong R.Y., Oswald L., Lin P.I., Wand G.S. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31(5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Unternaehrer E., Luers P., Mill J., Dempster E., Meyer A.H., Staehli S.…Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl. Psychiatry. 2012;2(8):e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dawans B., Kirschbaum C., Heinrichs M. The Trier Social Stress Test for Groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2011;36(4):514–522. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- von Kanel R., Kudielka B.M., Preckel D., Hanebuth D., Fischer J.E. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain, Behav. Immun. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Way B.M., Brown K.W., Quaglia J., McCain N., Taylor S.E. Nonsynonymous HTR2C polymorphism predicts cortisol response to psychosocial stress II: evidence from two samples. Psychoneuroendocrinology. 2016;70:142–151. doi: 10.1016/j.psyneuen.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Way B.M., Taylor S.E. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol. Psychiatry. 2010;67(5):487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way B.M., Taylor S.E. A polymorphism in the serotonin transporter gene moderates cardiovascular reactivity to psychosocial stress. Psychosom. Med. 2011;73(4):310–317. doi: 10.1097/PSY.0b013e31821195ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg P.M., Bokhorst C.L., Miers A.C., Sumter S.R., Kallen V.L., van Pelt J., Blöte A.W. A prepared speech in front of a pre-recorded audience: subjective, physiological, and neuroendocrine responses to the Leiden Public Speaking Task. Biol. Psychol. 2009;82(2):116–124. doi: 10.1016/j.biopsycho.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Matsunaga M., Isowa T., Kimura K., Kasugai K., Yoneda M., Kaneko H., Ohira H. Transient responses of inflammatory cytokines in acute stress. Biol. Psychol. 2009;82(1):25–32. doi: 10.1016/j.biopsycho.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Matsunaga M., Isowa T., Ohira H. Serotonin transporter gene polymorphism modulates inflammatory cytokine responses during acute stress. Sci. Rep. 2015;5:13852. doi: 10.1038/srep13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim I.S., Quas J.A., Rush E.B., Granger D.A., Skoluda N. Experimental manipulation of the trier social stress test-modified (TSST-M) to vary arousal across development. Psychoneuroendocrinology. 2015;57:61–71. doi: 10.1016/j.psyneuen.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Young E.A., Lopez J.F., Murphy-Weinberg V., Watson S.J., Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23(4):411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Ziegler C., Dannlowski U., Bräuer D., Stevens S., Laeger I., Wittmann H.…Lesch K.P. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology. 2015;40(6):1528–1538. doi: 10.1038/npp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]