Summary
Antibodies have a long history in antiviral therapy, but until recently they have not been actively pursued for HIV-1 due to modest potency and breadth of early human monoclonal antibodies (MAbs) and perceived insurmountable technical, financial, and logistical hurdles. Recent advances in the identification and characterization of MAbs with the ability to potently neutralize diverse HIV-1 isolates has reinvigorated discussion and testing of these products in humans, since new broadly neutralizing MAbs (bnMAbs) are more likely to be effective against worldwide strains of HIV-1. In animal models, there is abundant evidence that bnMAbs can block infection in a dose dependent manner, and the more potent bnMAbs will allow clinical testing at infusion doses that are practically achievable. Moreover, recent advances in antibody engineering are providing further improvements in MAb potency, breadth and half-life. This review summarizes the current state of the field of bnMAb protection in animal models as well as a review of variables that are critical for antiviral activity. Several bnMAbs are currently in clinical testing, and we offer perspectives on their use as pre-exposure prophylaxis (PrEP), potential benefits beyond sterilizing immunity, and a discussion of future approaches to engineer novel molecules.
Keywords: HIV-1 antibodies, pre-exposure prophylaxis, Fc-effector functions, Neutralizing antibodies, SHIV protection
Introduction
During the early part of the 20th century, scientists first began to understand the nature of antibody-based immunity. Paul Ehrlich and Ilya Mechnikov shared the 1908 Nobel Prize in Physiology and Medicine for establishing the concept that antibodies in the blood can neutralize poisons or toxins. Just prior to this, Emil von Behring had been awarded the first Nobel prize (in 1904) for serum therapy, and both Ehrlich and von Behring contributed to the concept and use of serum therapy – the transfer of blood serum to treat diseases such as diphtheria and tetanus (1, 2). After the introduction of antibiotics in the late 1930’s, the use of serum therapy declined, but subsequent improvements in immunoglobulin purification allowed antibody products to continue to play a major medical role against viral diseases – mainly to prevent infection via administration prior to exposure (e.g., hepatitis A, respiratory syncytial virus), or as early post-exposure prophylaxis (e.g., hepatitis B, rabies, varicella-zoster) (3). Importantly, in some cases, an effective vaccine has superseded the need for immunoglobulin prophylaxis (e.g. hepatitis A). Currently, there is only one licensed monoclonal antibody (MAb) product against a viral pathogen (Palivizumab) that is used to prevent respiratory syncytial virus (RSV) disease in high-risk infants. However, the ability to rapidly isolate and produce human antibodies has led to renewed interest in the development of additional MAb products against viral and bacterial pathogens, including Ebola, cytomegalovirus, Bacillus anthracis, Clostridium difficile and Staphylococcus aureus (4–11). The recent demonstration that Ebola MAbs can clear infection in primate models offers hope for this devastating disease and illustrates another example where passive immunotherapy can provide significant benefit against a lethal viral infection (5, 6, 12).
This review will focus on the potential role of HIV-1 specific neutralizing MAbs in preventing HIV-1 infection. The focus on passive immunoprophylaxis for HIV-1 arises, in part, from the difficulty in eliciting cross-reactive neutralizing antibodies (nAbs) by current vaccine immunogens, but also from research over the last 5–6 years demonstrating the isolation and characterization of highly potent and broadly reactive MAbs from B-cells of selected HIV-1 infected individuals. These neutralizing MAbs (nMAbs) provide robust protection in non-human primate challenge models at levels that can likely be readily be achieved by intermittent passive immunization of humans (13–15). In addition, engineering to improve both potency and circulating half-life of MAbs could further enhance their potential to protect (16).
Antibody development in response to HIV-1 infection
HIV-1 establishes a permanent infection by reverse transcribing and integrating its genome into that of the host as the first step toward producing new viral progeny. However, the progression to disease (in the absence of therapy) is highly variable in individual human subjects, even in subjects that are infected from the same virus source. This variation depends upon a number of key variables that will ultimately affect how much virus is produced—including but not limited to host MHC genetics (17) and the number of activated target cells available for virus spread from the site of exposure. When tested for virus in the plasma, subjects who have lower levels of virus experience a slower loss of CD4+ T cells and disease progression compared with subjects who have higher levels of virus production (18). This landmark study, and those that followed, provided the foundational knowledge that led to the use of effective antiretroviral treatment (ART) that target the virally-encoded enzymes to reduce viremia and slow or prevent disease progression.
Virus vs host dynamics
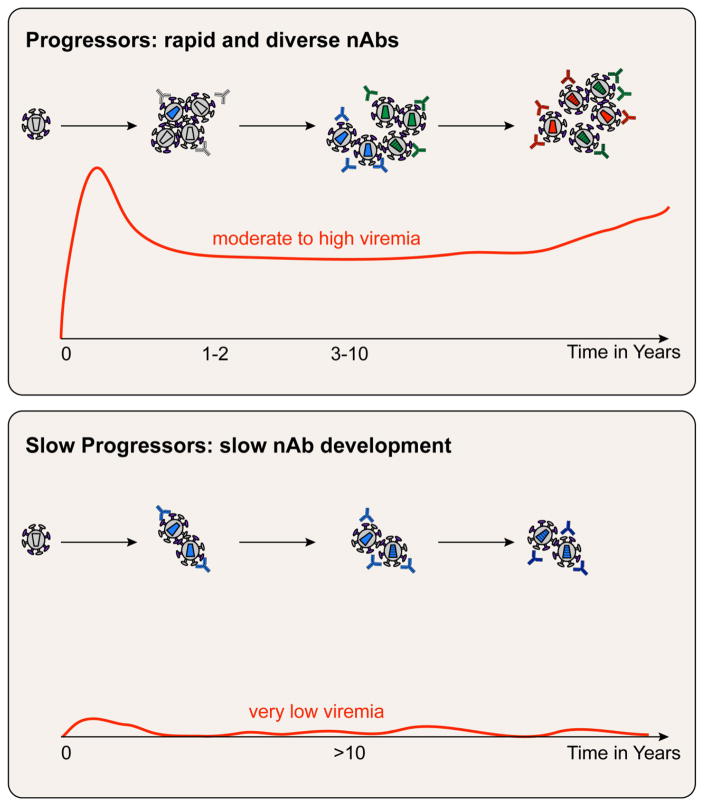
As the virus replicates, both arms of the adaptive immune response are stimulated to generate T cells and antibodies, but these fail to clear HIV-1, since the infected cell population is well established in tissues (19, 20), likely within a few days of exposure (based on primate model data), and prior to the development of immunity. HIV-1 infection is further aided by the rapid appearance of viral variants over time, termed the quasispecies (21). These quasispecies variants derive from the transmitted founder viruses (22), which are then subjected to immune selection by the developing adaptive response, including nAbs (23, 24). This complex interplay of viral variants and host immunity leads to an evolving situation in which the virus can evade responses but must remain fit enough to infect new cells. As shown in Figure 1, the timing of the development of antibodies (Abs) that can neutralize or directly block infection of HIV-1 in vitro depends upon exposure to the Envelope (Env) glycoprotein during infection. The degree of antigen exposure is proportional to the plasma virus levels, with subjects who have moderate to high levels of viremia—Progressors—developing nAbs more rapidly than in subjects who maintain very low viremia for >10 years—Slow Progressors. Individuals who tightly control viremia—Controllers—are quite rare and often have poor Ab responses. The role of viral variants in driving immunity is illustrated by the differences observed in the speed of development of nAbs, as well as how the nAbs change over time to recognize the new variants. HIV-1 evades antibodies through fixing mutations that change or obscure neutralization targets on the Env, for example by shifting or adding sites for glycosylation at or adjacent to one of these sites.
Figure 1. Timing and complexity of neutralizing antibodies in human subjects with different virus levels.
HIV-1 accumulates at least one mutation every time it replicates and thus, develops into a swarm of closely related viruses called the quasispecies that differs in each individual. The number of variants in the quasispecies depends upon the interplay between the virus and the adaptive immune response. Progressors have moderate to high viremia that is accompanied by the development of variants and antibodies that become diverse within a few years. Slow Progressors have low or undetectable viremia for 10 years or more, and they develop nAbs slowly and at low titers as a result of exposure to less antigen, and less diverse antigen.
Autologous nAbs and viral control
Despite the production of highly potent nAbs by B cells in some individuals, their virus is not cleared or fully contained, likely due to ongoing viral escape from both nAb and CD8+ T cell responses. This leads to the well-described phenomenon of autologous virus neutralization escape, i.e., an individual’s circulating plasma virus is resistant to co-circulating nAbs (23, 25). This has led researchers to suggest that antibodies are of limited importance in HIV-1 infection and control. However, both the T-cell and antibody response induce ongoing selective pressure on the viral quasispecies – and the very nature of a chronic lentiviral infection involves escape from the adaptive immune response. In addition, the virus continues to evolve, even under conditions of prolonged ART (26). Thus, not unlike CD8+ T cells, it is likely that antibodies play a role in limiting the rate of viral replication. Of note, there is at least one clinical case showing that serum nAbs contribute to viral control; Reduction in nAbs and viral rebound occurred concomitantly in vivo when CD20 cells were depleted (27). However, taken together, these data suggest that nAbs would be most effective if used as pre-exposure prophylaxis prior to the time of establishment of the viral reservoir. There were early efforts to use purified polyclonal immune globulin from HIV-positive subjects to test whether antibodies could prevent infection or affect established infection. This material, called HIV Immune Globulin or HIVIG, differed in nAb potency from batch to batch due to the source of the antibodies, and early studies in humans resulted in limited and no permanent impact upon viremia in treated adults or upon infection in mother to child transmission (28–30). It was thus incumbent upon researchers in the field to discover human MAbs that could substitute for, and improve upon, the potency of HIVIG.
Broadly neutralizing Ab development in infection
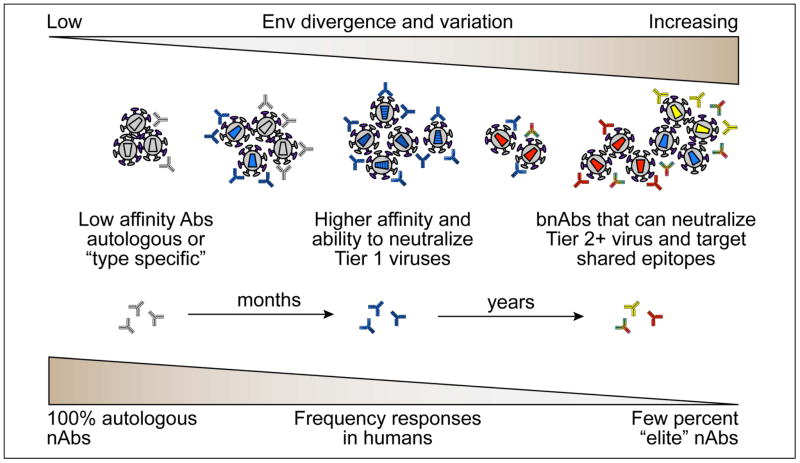
Understanding the potency and targeting of nAbs in infected subjects has been valuable for locating regions in HIV-1 Env that may be an Achilles’ heel of the virus, as described in the next section. There is good evidence that antibody responses are robust and that nearly all subjects neutralize the infecting (autologous) virus (Figure 2). Within 2 to 3 years after infection, the sera from ~50% of infected individuals can neutralize significant numbers of heterologous virus isolates (31), either due to development of an individual antibody lineage with broad reactivity or due to the cumulative effect of many individual nAbs (avidity of the polyclonal response) (32, 33). A small proportion of HIV-positive subjects eventually develop highly potent cross-reactive nAbs that target epitopes shared amongst the diverse clades of HIV-1 worldwide. The individual broadly neutralizing MAbs (bnMAbs) isolated from these subjects have been critical to enhancing our understanding of Env structure and mechanism of HIV-1 neutralization which has led directly to detailed structural information of the neutralization epitopes on the Env trimer and allowed for studies of antibody ontogeny, i.e. how such nAb lineages arise and evolve to achieve broad and potent neutralization (34). The role of bnMAbs in protection is discussed in detail in later sections of this review. Molecular examination of the Env variants in individual subjects, including the transmitted founder (T/F) Env, has provided further insights into the antigenic stimulation of specific nAb lineages (24, 35–37), and this remains an area of intense investigation (38).
Figure 2. HIV-1 Env divergence and variation during infection.
Following infection, the autologous or “type specific” response develops first, with low affinity Abs against the T/F viruses (gray) and early variants (light blue). This occurs in essentially all individuals and affinity and neutralization against the autologous virus increase over time. As the virus responds to autologous NAbs, escape variants emerge that are divergent from the T/F virus, creating more Env sequence diversity, which in turn becomes subject to the host humoral responses. As the quasispecies diverges, the nAb responses become higher affinity and broadens to accommodate resistant variants. This results in the ability of an individual sera to neutralize viruses from other individuals. After a number of years, some sera are able to cross-react with a range of heterologous strains by targeting one or more shared epitopes. This results and “broad” nAbs (bnAbs) that can neutralize many diverse Tier 2 viruses. A small percentage of individuals are “elite” neutralizers or have antibodies that can potently neutralize the large majority of virus isolates.
Animal models for HIV-1 transmission
HIV-1 and SIV models
There are several animal models that have been used as a facsimile of HIV-1 transmission in humans. Nonhuman primate (NHP) hosts exposed to members of the primate lentivirus family are widely used and are the most closely related animal models to human transmission events, summarized in a recent review (39). HIV-1 replicates in chimpanzees, but disease and pathology require 10 years or more, and use of this model for AIDS research was discontinued decades ago. Host restriction factors prevent productive infection by HIV-1 in Macaca species (40). Simian immunodeficiency virus (SIV) is endogenous in African macaques but highly pathogenic in Asian macaques. Like HIV-1, transmission of SIV isolates is by mucosal and blood-borne routes and in contrast with HIV-1, results in rapid pathogenesis despite strong antiviral immunity. Cell-mediated immunity plays an important role in the resolution of acute SIV viremia, and post-acute viral control is affected by the MHC Class I haplotype, as with HIV-1 infection. Antibody responses are similarly elicited in both HIV-1 and SIV infection, with binding Abs detected prior to any autologous nAbs and bnAb responses found only after a year or more of infection. Although HIV-1 and SIV have similar receptor and co-receptor usage, the Env proteins are sufficiently different such that nAbs specific for SIV Env are not cross neutralizing for HIV-1, and vice versa.
Introduction of SHIV models
To effectively study HIV-1 bnMAbs in NHP models, it was essential to have a virus bearing the HIV-1 Env protein, and the chimeric SIV-HIV or SHIV viruses were developed for this use (41–45). In these models, chimeric SHIV viruses that express HIV-1 envelope in the backbone of SIV have been used to challenge NHPs via various routes that include intravenous (IV), intrarectal (IR), intravaginal (IVAG) and oral routes to model natural HIV-1 transmission. The challenges are carried out using either a single dose of the challenge virus that results in close to 100% infection in all naïve animals or a series of multiple doses that results in infection of less than a third of the naive animals after each challenge. These models have been used to assess the efficacy of passive transfer of polyclonal IgG (HIVIG and SIVIG) and multiple anti-HIV-1 bnMAbs in providing protection against vaginal, rectal, and oral infection in macaques that range in age from infant to adult (Table 1). In studies of immunoprophylaxis by passive infusion, bnMAbs have been most commonly administered hours to days before challenge and have shown varying degrees of protection, likely impacted by the neutralization sensitivity of the challenge stock to the administered bnMAb as well as the epitope targeted on the HIV-1 Env (14, 15, 46–54).
Table 1.
SHIV protection studies
| HIV-1 Env epitope | Antibody | Challenge virus (route) | Rate of Protection | Conc.a (μg/ml) | Reference |
|---|---|---|---|---|---|
| CD4 binding site | b12 | SHIVSF162P4 (vaginal) | 4/4 | 705 | 53 |
| SHIVSF162P4 (vaginal) | 2/4 | 149 | 53 | ||
| SHIVSF162P4 (vaginal) | 0/4 | 16 | 53 | ||
| SHIVSF162P3 (vaginal) | 8/9 | 563 | 48 | ||
|
| |||||
| VRC01 | SHIVSF162P3 (vaginal) | 4/4 | 65 | 15 | |
| SHIVSF162P3 (rectal) | 4/4 | 79 | 15 | ||
| SHIVSF162P3 (oral) | 2/2 | 287 | 107 | ||
| SHIVSF162P3 (oral) | 4/5 | 64 | 107 | ||
| SHIVBaL.P4 (rectal) | 4/4 | 4 | Unpublished | ||
| SHIVBaL.P4 (rectal) | 4/10 | 1.3 | 15 | ||
|
| |||||
| VRC07-523LSb | SHIVSF162P3 (rectal) | 6/6 | 25 | Unpublished | |
|
| |||||
| 3BNC117 | SHIVAD8EO (rectal) | 2/2 | 91 | 54 | |
|
| |||||
| V1V2 | PG9 | SHIVBaL.P4 (rectal) | 4/6 | 32 | 15 |
|
| |||||
| V3-glycan | PGT121 | SHIVSF162P3 (vaginal) | 5/5 | 15 | 14 |
| SHIVSF162P3 (vaginal) | 3/5 | 1.8 | 14 | ||
| SHIVAD8EO (rectal) | 2/2 | 22 | 54 | ||
|
| |||||
| PGT126 | SHIVSF162P3 (vaginal) | 5/5 | 98 | 13 | |
| SHIVSF162P3 (vaginal) | 2/5 | 20 | 13 | ||
| SHIVSF162P3 (rectal) | 3/4 | 130 | 13 | ||
| SHIVSF162P3 (rectal) | 2/4 | 27 | 13 | ||
|
| |||||
| 10-1074 | SHIVAD8EO (rectal) | 2/2 | 115 | 54 | |
|
| |||||
| High mannose glycan | 2G12 | SHIVSF162P3 (vaginal) | 3/5 | 1053 | 50 |
|
| |||||
| MPER | 4E10 | SHIVBa-L (rectal) | 6/6 | 866 | 51 |
|
| |||||
| 2F5 | SHIVBa-L (rectal) | 6/6 | 742 | 51 | |
|
| |||||
| 10E8 | SHIVBaL.P4 (rectal) | 6/6 | 31 | 15 | |
| SHIVBaL.P4 (rectal) | 3/6 | 1.8 | 15 | ||
Average concentration of antibody in serum at the time of challenge is listed.
VRC07-523 contained the FcRn enhancing mutation (LS) to extend in vivo half life.
Limitations and advances in SHIV development
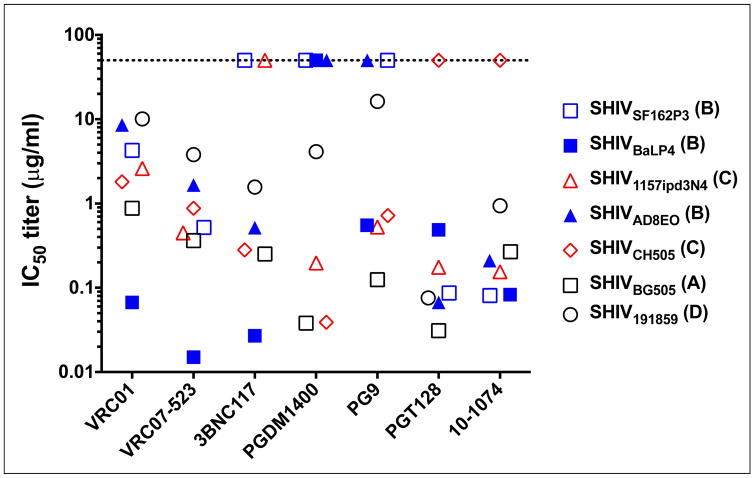
A disadvantage of the SHIV infection model has been the limited number of SHIVs available and the variable sensitivity of these SHIVs to the different bnMAbs (Figure 3). For example, the widely used SHIVSF162P3 is sensitive to most CD4 binding site (CD4bs) bnMAbs but is not sensitive to the V2-glycan reactive bnMAbs, e.g., PGDM1400 and PG9. This despite the fact that PGDM1400, targeting the trimer apex, can neutralize >75% of circulating HIV-1 strains at a very high potency. And even amongst CD4bs bnMAbs, SHIVSF162P3 is sensitive to VRC01 and VRC07-523 but resistant to 3BNC117, and yet all three bnMAbs neutralize >90% of HIV-1 strains. Also, many current SHIVs are fairly resistant to the MPER-reactive bnMAb 10E8, despite the fact that the 10E8 neutralizes ~98% of HIV-1 strains. Thus, the neutralization phenotype of commonly used SHIVs does not recapitulate the phenotype of most transmitted strains of HIV-1, making it impossible to consistently interpret the results of any given SHIV challenge study to human exposure of diverse strains of HIV-1. Recent advances in the molecular construction of SHIVs have facilitated the construction of several new SHIVs derived from founder HIV-1 strains. These new SHIVs appear to have neutralization phenotypes more similar to HIV-1 strains than to older SHIVs and are sensitive to bnMAbs targeting multiple epitopes, thereby expanding the repertoire of SHIVs and bnMAb combinations that can be tested in passive transfer studies (55–57). Most SHIVs are clonal or oligoclonal in nature, i.e., they are derived from a molecular clone and passaged briefly in vitro or in vivo. However, some SHIVs have moderate genetic complexity similar to HIV: SHIVSF162P3 that was isolated later in infection after variants had developed (58). Other new SHIV stocks may be developed to include characterized heterogeneous variants, as has been recently accomplished for SIVmac251 (59).
Figure 3. Neutralization sensitivity of SHIVs to anti-HIV-1 bnMAbs.
The IC50 titers of the bnMAbs shown were determined against a panel of replication competent SHIVs in a single-round of infection assay with TZM-bl target cells. The SHIV stocks were made in rhesus PBMCs. The clade of each SHIV is indicated in parenthesis.
Humanized mouse models
In addition to NHP, humanized mouse models are extensively used to study HIV-1 transmission. These models involve reconstitution of severe combined immunodeficiency (SCID) mice with various combinations of human hemato-lymphoid cells, and thus allow for some level of HIV-1 replication. Though there have been recent improvements in the construction of humanized mouse models, the formation of secondary lymphoid tissues is limited and these models are not likely to mimic HIV pathogenesis in humans. Nonetheless, these models appear to be useful for passive immunoprophylaxis studies where the outcome is the presence or absence of HIV-1 infection. Humanized mice were, in fact, used in some of the earliest studies to demonstrate the protective efficacy of bnMAbs (60–62) and have been used to demonstrate protection by many newer generation bnMAbs. (63–67). Complementing the findings in NHP models, experiments in humanized mice have demonstrated enhanced clearance of infected cells by bnMAbs, and this clearance was attributed to an FcγR-dependent mechanism (68).
Antibody protection studies in animal models
Polyclonal Ig studies
Early studies using polyclonal human immunoglobulin derived from HIV-1 infected donors (HIVIG) demonstrated that potency was critical for protection of chimpanzees from intravenous HIV-1 infection (69, 70). The first neutralizing antibody passive protection studies against HIV-1 primary isolates were conducted in the hu-PBL-SCID mice model (60, 62). One of the earliest studies in the SHIV challenge model used HIVIG derived from HIV-1 infected chimpanzees and a SHIV challenge stock that was based on the HIV-1 strain DH12 (71). Macaques infused with IgG from a HIV-1 DH12 infected chimpanzee, but not an HIV-1 IIIB infected animal, were protected against challenge. Importantly, DH12 IgG, but not the IIIB IgG was able to neutralize the DH12 challenge stock. These were some of the first in vivo data suggesting the importance of nAbs in protection against HIV-1.
Protection with first generation bnMAbs
Among the first anti-HIV-1 bnMAbs to show protection in SHIV models were the combination of first generation bnMAbs, 2F5 (gp41 MPER) and 2G12 (gp120 glycan-reactive), that were used together with HIVIG in adult macaques. While individual MAbs displayed partial protection, the combination of 2F5, 2G12 and HIVIG was most effective, providing complete protection against both intravenous and mucosal challenges (46, 52). A similar combination of 2F5 and 2G12 along with the CD4bs-targeting F105 MAb also provided protection against both systemic SHIV challenge as well as oral SHIV challenge in macaque adults and neonates, respectively (47). The use of a single bnMAb to protect against mucosal SHIV challenge was subsequently shown in the case of the CD4bs-targeting bnMAb, b12 (53). In fact, b12 has been used in multiple SHIV protection studies that have helped in understanding both the mechanism as well as durability of protection provided by bnMAbs (48, 49, 72). Other individual bnMAbs, including 2F5, 4E10 and 2G12, were also subsequently shown to protect in mucosal SHIV challenge models (50, 51). Although these studies provided proof of antibody-mediated protective efficacy, relatively high MAb infusion doses (>20 mg/kg) were used, with SHIV challenge performed one day after antibody infusion, when antibody concentrations in plasma were high, often substantially greater than 100 μg/ml. Thus, while these data with first generation anti-HIV-1 MAbs were encouraging proof-of-principle studies for protection with neutralizing antibodies, the amount of antibody needed and lack of data on durability of protection did not support the potential for their use in human populations.
A new generation of highly potent bnMAbs
Due to the foresight of investigators who established large cohorts of HIV-1 infected subjects, and the development of high throughput and accurate virus neutralization assays (73, 74), donors with potent serum neutralization were identified. With the advancement of technologies to screen cultured memory B cells for neutralization and to isolate antigen-specific B-cells (75, 76), many potent bnMAbs have been isolated in recent years (76–81). The bnMAbs target previously known and newly discovered sites of vulnerability on the native HIV-1 Env trimer, comprised of gp120 and gp41 subunits, and potently neutralize a majority of circulating HIV-1 strains (82). These relatively conserved sites include: (i) the V1V2-glycan site at the apex of the Env trimer targeted by bnMAbs PG9, PGT145, PGDM1400 and CAP256-VRC26.25 (83–85), (ii) the V3-glycan site centered on the glycan at Asn332 targeted by bnMAbs PGT121 and 10-1074 (86, 87), (iii) an exclusively glycan epitope on the outer domain of gp120 targeted by 2G12 (88, 89), (iv) the CD4-binding site of gp120 targeted by the VRC01-class of bnMAbs that partially mimic CD4 receptor binding and include VRC01 and 3BNC117 (75, 76, 90), and additional non-VRC01 class CD4bs bnMAbs that neutralize by other modes of recognition (24, 91, 92), (v) the membrane-proximal external region (MPER) of gp41 targeted by 2F5, 4E10 and 10E8 (81, 89), (vi) an extended region including residues from both gp120 and gp41 between the MPER and gp120 protomers targeted by 35022 and PGT151 (93, 94) and more recently, (vii) the fusion peptide of HIV-1 targeted by VRC34 and PGT151 (95, 96). Importantly however, none of these bnMAbs neutralizes 100% of viral isolates and each bnMAb has a distinct profile of potency and breadth of neutralization (84, 97). This has led to substantial interest in the use of bnMAb combinations that would be complementary (98, 99). Moreover, due to the lack of effective HIV-1 vaccines and the recent discovery of multiple highly potent bnMAbs, significant enthusiasm in the field is growing to investigate the use of these bnMAbs in both prevention and treatment of HIV-1 infection.
Protection with next generation bnMAbs
The identification of newer generation bnMAbs with potencies 10 to 100 fold greater than earlier bnMAbs has renewed interest in their potential clinical use. One of the first studies with a newer generation bnMAb was conducted by Burton and colleagues using the V3-glycan directed antibody PGT121 (14). Using the SHIVSF162P3 challenge virus, which is highly neutralization sensitive to bnMAb PGT121, sterilizing immunity was achieved in all animals administered 5 mg/kg and 1 mg/kg and three of five animals administered 0.2 mg/kg PGT121, with corresponding average antibody serum concentrations of 95 μg/mL, 15 μg/mL, and 1.8 μg/mL, respectively (Table 1). This study provided important proof of concept that newer generation potent bnMAbs could provide in vivo protection at lower serum concentrations than observed in previous studies (14). Many subsequent studies with next generation potent bnMAbs VRC01, VRC07-523, 3BNC117 (to CD4bs) and 10-1074 (V3-glycan) have also demonstrated protection (13–15, 54, 100–102).
Modifying bnMAbs for improved efficacy
Recent studies have also further delineated the parameters associated with protection including neutralization potency against the challenge virus and persistence of antibody in circulation. A potentially widely applicable approach to extend antibody half-life is to modify antibody binding to the neonatal Fc-receptor (FcRn) to substantially improve the in vivo half-life of a bnMAb and increase persistence of bnMAbs at mucosal sites of HIV-1 infection. The FcRn is related to the MHC class of molecules although it is not involved in antigen presentation (103, 104). Instead, it interacts with the Fc region of IgG and is involved in recycling and transcytosis of IgG within cells. By its capacity to bind to the Fc region with high affinity at low pH found in lysosomal compartments, it prevents degradation of the IgGs when internalized by cells and thereby regulates the serum half-life of IgGs. This interaction is also involved in actively transporting IgGs to sites of pathogen encounter and plays a role in protection against infections. In the case of VRC01, the FcRn enhancing mutation -LS (M428L/N434S (105)) lead to 2–3 fold higher in vivo half-life in macaques compared to VRC01 with the wild type Fc region. Compared to VRC01, VRC01-LS also demonstrated increased persistence in the mucosal compartments leading to improved protection against mucosal SHIV challenge (106). This improved half-life also almost doubled the median protection time in a repeated challenge study after a single infusion of the bnMAb (100).
FcRn modulation of IgG in tissues
Hence, when bnMAb VRC01 was engineered to have increased affinity for FcRn (VRC01-LS), increased and prolonged levels of the antibody were detected in mucosal tissue compared to the unmodified VRC01 (106). In fact, VRC01-LS accumulates in vaginal tissues and localizes to the basal and parabasal layers of the vaginal epithelium, similarly to FcRn, as determined by immunohistochemistry. Presumably, FcRn-mediated transport increases the monoclonal antibody levels in this tissue, where it might similarly confer protection against infection.
Since human MAbs are foreign proteins when infused in macaque monkeys, their circulating half-life is much shorter than that of human IgG in humans. This rapid clearance limits the ability to study duration of antibody effect. To overcome this, Saunders et al. modified bnMAb VRC01 to be closer to the sequence of simian antibodies (a process called simianization), without substantially impairing its antigen combining site and neutralization potency (102). After passive administration, simianized VRC01-LS maintained higher concentrations in plasma and rectal tissue than unmodified simianized VRC01. Importantly, both simianized variants of human VRC01 protected animals against SHIV challenge two months after single or multiple antibody infusions. This demonstration that passive immunization with a potent bnMAb could provide sustained protection was an important first step toward the potential clinical use of bnMabs to prevent HIV-1 infection.
Relating bnMAbs to correlates of protection
Importance of neutralization
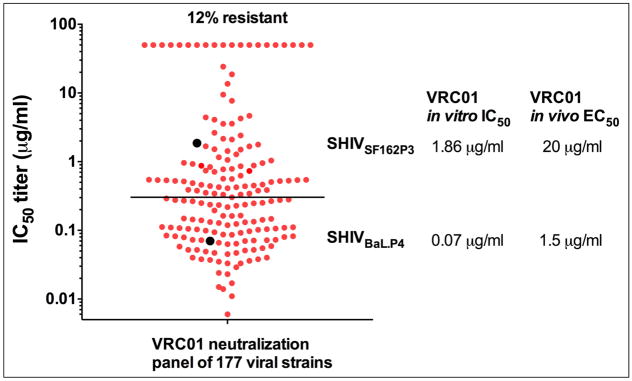
The studies noted above clearly demonstrate the ability of passively infused bnMAbs to provide complete protection against viral challenge, even when the challenge inoculum results in infecting 100% of control animals – a rate of infection known to be much greater than in the setting of human sexual transmission. A next obvious question is: what antibody function(s) can be directly associated with protection? We discussed above that more potent bnMAbs protect at lower serum concentrations than less potent first generation bnMAbs, and this suggests a key role for virus neutralization. Antibody levels have been determined by directly quantifying the concentration in the plasma just prior to SHIV challenge and these data strongly suggest that the in vitro neutralization potency of a bnMAb against a challenge SHIV is positively associated with the level of protection. For most of the earlier generation of bnMAbs like b12, 2F5, 4E10 and 2G12, plasma concentrations of several hundred micrograms or more appeared to be required for sterilizing protection against high dose mucosal SHIV challenge. The more potent bnMAbs like VRC01, PGT121 and 10E8, provide complete protection at much lower concentrations, depending on their neutralization potency to the challenge SHIV, i.e., these newer bnMAbs neutralize the challenge SHIVs at IC50s 100 to 1000-fold less than the early bnMAbs (14, 15, 48, 50, 51, 53). Thus, protection can be achieved with serum concentrations in the range of 1–10 μg/ml and sometimes lower (13, 14, 100) (Table 1). In protection studies using two SHIVs that have different sensitivities to VRC01, we demonstrated that the more resistant SHIVSF162.P3 required a higher VRC01 plasma concentration to block acquisition than the more sensitive SHIVBaL.P4 (15) (Figure 4). Hence, the ability of VRC01 to protect was associated with its neutralization potency against the specific SHIV challenge virus used. These data indicate that the neutralization sensitivity to the circulating viral strains should be a key parameter to consider when selecting a bnMAb for use in international prevention trials. The recent identification of exceptionally potent bnMAbs like PGDM1400 (83) and CAP256.VRC26.25 (84) that are 10-fold more potent than any of the previous bnMAbs, suggests that protection at concentrations lower than 1 μg/ml may be possible in these SHIV models, with the caveat that these mAbs do not neutralize all strains of HIV-1. In addition, several recent studies have used repeated low dose SHIV challenge, which may be more reflective of natural HIV-1 exposure in humans. Gautam, Martin and colleagues performed an elegant but simple study, using a single infusion of the potent mAbs VRC01, 3BNC117 and 10-1074, followed by iterative rectal challenge that resulted in infection of all controls at 6–12 weeks. The study also included the longer-half life version of VRC01 (VRC01-LS). This study confirmed the importance of bnMAb potency and half-life in protection and provided additional data demonstrating that relatively low levels of plasma antibody were associated with protection. In total, these studies confirm that relatively low serum concentration of bNAbs can protect and suggest that a single infusion of a bnMAb could provide long lasting (weeks to months) protection (49, 100).
Figure 4. VRC01 serum concentrations needed to protect against SHIV infection.
The neutralization sensitivity (IC50) of a panel of 177 HIV-1 strains to VRC01 is shown (red) along with the sensitivity of two SHIVs (SHIVSF162P3 and SHIVBaL.P4) (black). The plasma concentration of VRC01 needed to protect 50% of animals (EC50) after mucosal challenge is indicated for each SHIV.
Assessing protective in vivo titers
Regarding virus neutralization as a key component of protection, it is instructive to assess not only the plasma MAb level at the time of challenge, but also the plasma neutralization IC50 value. However, there are various formats used to measure in vitro neutralization, which may complicate cross-study comparisons. One common and highly standardized assay format involves the use of lentiviral vectors pseudotyped with the HIV-1 or SHIV Env. However, Env-pseudotyped viruses are clonal (one Env sequence) and may not represent the quasispecies of Env in the challenge SHIV stock. Thus, it is preferable to assay the actual replication competent SHIV challenge stock for neutralization to provide a direct link between in vitro neutralization assessment and in vivo protection (i.e. using the very same SHIV stock for challenge and in vitro assays). Given the variation in neutralization assay formats, including virus stock not directly linked to the challenge, and different target cells used, it is not surprising that a wide range of reciprocal serum ID50 titers ranging from <100 to >2000 has been reported to be protective. In some cases, the levels of bnMAbs at the sites of mucosal challenge were also determined (14, 50, 53). Generally much lower concentrations of bnMAbs were detected at these sites compared to the plasma, which might have implications for the mucosal titers needed to be achieved by a successful protective HIV-1 vaccine. Understanding how the levels of antibodies in plasma correlate with local concentrations necessary to prevent infection by mucosal exposure is difficult. IgG levels in mucosal samples can vary depending on the collection method used and even over time in the animal. However, recent studies using improved methods to quantify IgG in mucosal tissues have allowed a clearer understanding of the role of mucosal IgG in protection, as discussed below (106–111). Mechanisms that contribute to protection by bNmAbs are not limited to virus neutralization (48, 112), as discussed further below. Also, by engineering bnMAbs to increase affinity for FcγRs that are expressed on immune effector cells, additional details of the specific antibody-mediated effector functions that impact protection may become clear (113).
Contribution of antibody effector functions to protection
Fc receptor binding in NHPs
IgG contains two antigen combining sites (Fabs) and an Fc region that interacts with various Fc receptors (FcRs) on immune cells. The first NHP experiments to show the importance of Fc receptor binding in protection was performed with two Fc variants of IgG1 b12 in SHIV-challenged macaques. Complement activation was ablated with b12 variant KA (K322A) and both complement and Fcγ receptor binding were abolished with a double mutation variant LALA (L234A/L235A) (48). The finding was reinforced in a follow-up study regimen that more closely mimicked human transmission events using a repeated but lowered virus inoculation dose and an ongoing low-dose weekly administration of either b12 or the effector function knock-out version, LALA. Importantly, this regimen maintained a low but fairly constant and systemic presence of neutralizing antibody at the time of each mucosal challenge. When compared, b12 was more than twice as effective as LALA in reducing the infection risk at each repeated challenge (49). Hence, this bnMAb provided optimal protection when Fc-mediated effector functions were intact. Unexpectedly, protection by the KA variant, that should only be deficient in complement activation, was equal in protection to parental b12, suggesting a negligible role by complement in protection against HIV-1. Recognizing the dual functionality of all antibodies, these results were not especially surprising, but a direct confirmation of effector function involvement with protection against mucosal SHIV challenge in macaques was novel. It is important to emphasize that both of the b12 Fc variants had identical neutralization capacities as the parental b12. Thus, the findings only imply that - at least for HIV-1 protection - antibody Fc receptor binding and resulting anti-viral activities may augment neutralization and that it is most likely that full protection requires multifunctional antiviral activities of neutralizing antibodies.
Effector functions correlate with better outcomes
In HIV-infected individuals, serum titers of HIV-1 specific ADCC antibodies correlate with CD4+ T cell counts (114), suggesting a benefit of the recruitment of anti-viral immune functions. In fact, many studies have shown strong correlations between ADCC titers and improved clinical outcomes (115–118). Likewise, NHP studies have shown correlative data indicating a beneficial role for Fc-mediated effector function in controlling post-infection viremia (115, 119–121). However, no evidence has been reported in animal models that antibody effector functions alone, in the absence of neutralization, can prevent infection. For example, non-neutralizing polyclonal antibodies that showed strongly functional ADCC did not protect neonatal macaques from oral SIVmac251 challenge (122). Likewise, no protection was seen with passive transfer of non-neutralizing antibodies from HIV+ individuals that were highly enriched for potent ADCC activity (123). Thus, despite the association with reduced risk of infection by binding antibodies in RV144 vaccinees and animal models showing post-infection viral control, antibody Fc-mediated antiviral activity alone has not been shown to be adequate for blocking infection after either SIV or SHIV challenge. Furthermore, simply enhancing Fcγ receptor binding efficiency, by using a nonfucosylated variant of bnMAb b12 did not improve protection against SHIV challenge (72).
Why can’t antibody effector functions alone prevent infection?
The protection seen in animal models with neutralizing bnMAbs could be due to the theory of HIV-1 Env heterogeneity that suggests non-neutralizing antibodies do not bind forms of HIV-1 Env that can mediate viral entry into host cells. The model proposes that infectious virions also express accessible nonfunctional (i.e. not entry competent) forms of envelope, and antibody binding to these forms is sufficient to permit virion capture and even ADCC, but does not lead to neutralization (82, 124). The model also helps to reconcile the paradox of non-neutralizing mAbs with known affinity for the CD4 binding site and other epitopes associated with protection, yet do not prevent viral infectivity.
Compromised protection without neutralization
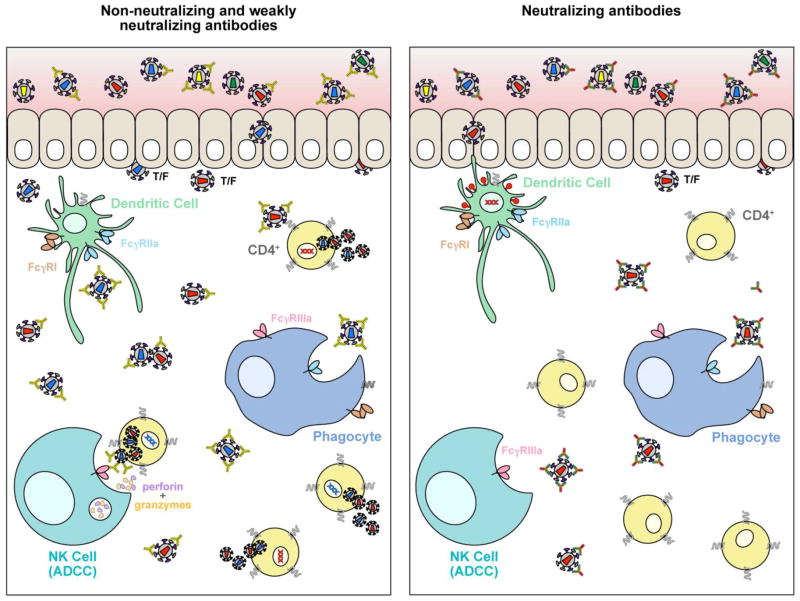
Figure 5 depicts the scenarios of how non-neutralizing or weakly neutralizing mAbs may effectively mediate FcγR anti-viral activities that result in viremia blunting and possible reduction in the risk of acquisition, but do not prevent infection. In the first panel, non-neutralizing or weakly neutralizing Abs at the mucosal surface in the presence of an HIV-1 inoculum are more likely to permit penetration of infectious virus into the epithelium through abrasions or tearing that can occur during sexual transmission or exist as a consequence of sexually-transmitted diseases (STD). Without a mechanism to neutralize the transmitted/founder (T/F) viruses, host cells within the epithelium can become infected and migrate through the mucosae and into draining lymphatics or enter the blood circulation. This occurs because Env binding by non-neutralizing mAbs leaves host cells vulnerable to infectious Env spikes not bound by mAbs that can block entry. Some non-neutralizing mAbs are capable, however, of virus capture and coating virions and signaling via FcγR binding that lead to capture and clearance of virus and killing infected cells. Based on the data to date, these processes, although important in protection, are not sufficient to completely prevent infection because infectious Env spikes are left unbound and CD4+ host cells are then vulnerable to infection. Eventually, infection spins out of control as more host cells are infected even though Ab effector functions are ongoing. In contrast, and illustrated in the second panel, is the setting of bnMAbs intercepting a virus inoculum before tissue penetration. Neutralizing-capable Abs effectively bind to Env spikes that will lead to infection, blocking viral entry while also carrying out FcγR binding and effector functions against any productively infected cells. Potentially, the right bnMAb in sufficient concentration or combinations of bnMAbs may effectively clear most or even all of the inoculum exposure. Thus, animal passive protection studies have successfully modeled sterile protection with neutralizing antibodies when a sufficient concentration of bnMAb is present at the time of challenge or shortly thereafter. These results are also in agreement with the observation that the sensitivity of the challenge virus to the bnMAb generally determines the efficacy of protection against the virus stock as discussed earlier.
Figure 5. Antiviral activities of antibodies against HIV.
The two panels depict scenarios of the protection outcome of HIV-1 exposure at mucosal sites when non-neutralizing or weakly neutralizing antibodies are present, compared to exposure in the presence of potently neutralizing antibodies. The former may be unable to block virus entry into cells, but can kill infected cells via Fc-mediated functions. These anti-viral activities are important for blunting viremia and slowing viral replication, but may not be sufficient to prevent eventual infection that overwhelms the antibody response. Conversely, neutralizing antibodies block entry into cells and also mediate Fc effector functions to kill and clear any infected cells, thus preventing establishment of infection.
Evidence in mouse models
In very early passive protection studies, bnMAb IgG1 b12, Fab b12 and the non-neutralizing Fab b13 were compared for protection against HIV-1SF2 in hu-PBL-SCID mice. Briefly, the results showed that the pattern of the in vivo protection results followed the rank order of the neutralizing potency of IgG1 b12 > Fab12 > Fab 13, i.e. IgG1 b12 gave strong protection (5/5), Fab b12 gave some protection (1/5), and Fab b13 gave no protection (61). The compromised protection with Fab b12 suggested that neutralization measured in vitro may not be the only anti-viral functions associated with protection in vivo. This finding has been reported again using humanized mice (125) and reiterates the substantial role of in vivo FcγR-mediated mechanisms in the protective activity of anti-HIV-1 bnMAbs (112).
Not all bnMAbs are equal
Recent studies suggest that not all bnMAbs are equal in the capacity to kill HIV-infected cells by Fc-mediated effector functions, and variation in this activity can occur with, e.g., lower bnMAb affinity that may cause instability of Env-bnMAb complexes on the cell surface or less activity due to poorly exposed Env epitopes on T/F viruses (126). Bruel, et al. visualized the disappearance of Gag+ target cells in the presence of NK cells in time-lapse microscopy as a readout for ADCC activity and found that even some of the most potent bnMAbs did not induce FcγRIIIa stimulation and killing of HIV-infected cells. These findings may be important for designing optimal bnMAb cocktails for immunotherapy regimens, and provides a rationale for re-engineering Fc regions of some bnMAbs to optimize therapeutics for cure research. Moreover, the differential affinity by bnMAbs for FcγRs, as well as the availability of innate immune cells, is a critical determinant of where and which type of antibody effector function could be optimized for bnMAb HIV protective therapeutics. For example, Sips, et al. found that phagocytic cells, macrophages and neutrophils, were the predominant FcγR-bearing effector cells in mucosal tissue (127). Therefore, as the authors predict, due to the landscape of effector cells available in mucosal tissue at sites of HIV-1 transmission, phagocytosis may be more frequent and relevant to HIV-1 protection and therapy than ADCC. These recent findings cause reflection on the results in macaques with the IgG1 b12 KA variant that suggested complement binding did not contribute to the protection efficacy (48). It is reasonable to hypothesize that within the specific parameters of the experimental design, including the required interactions with macaque Fcγ receptors, other effector functions such as ADCC or phagocytosis or combinations thereof were better suited for protection by b12.
An obvious challenge for bnMAb immunotherapy (i.e. treatment of those that are already infected) are escape mechanisms inherent to HIV-1 Env. Thus, continued animal modeling will be critical pre-clinical steps for determining the most likely bnMAb combinations for optimal protection efficacy and outwitting Env diversity. Regarding immunoprophylaxis, a combination of empirical data and mathematical modeling have been used to understand optimal bnMAb combinations that would cover the large majority of diverse HIV-1 isolates, as we mentioned earlier (98, 99, 128).
Implications of bnMAb biodistribution in protection
Until recently, it was assumed that passively infused IgG entered the mucosa via passive mechanisms (e.g. transudation). However, as we detailed above, the recent appreciation of the role of the FcRn in antibody tissue distribution suggests that IgG in mucosal tissues is actively modulated by interaction with FcRn, which is expressed in mucosal tissues (102, 106). Mucosal secretions collected in early passive transfer studies provided evidence that passively transferred bnMAbs are transduced systemically and through the mucosae (14, 49, 52, 53). These and other studies have documented the kinetics in blood of passively transferred polyclonal and monoclonal antibodies.
New evidence of bnMAb activities in tissues
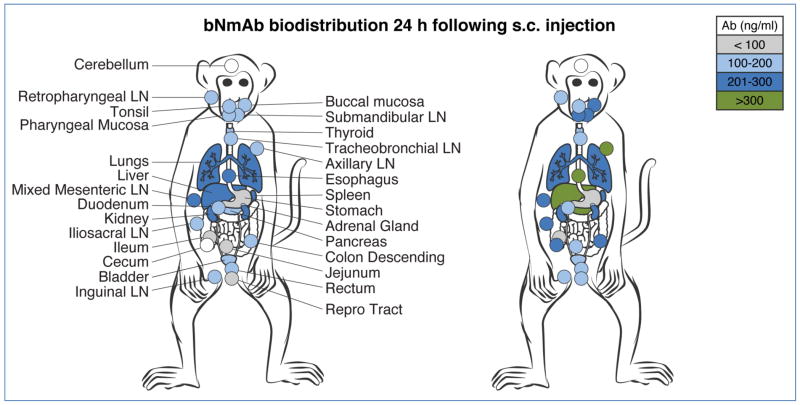
Clear evidence of IgG tissue distribution after passive infusion, although assumed to be widespread, has only recently been defined. In 2012, the concentrations and kinetics of b12 and its LALA effector function deficient variant were assessed in adult female macaques (129). The findings demonstrated that the rapid and broad distribution to lymphoid and mucosal tissues by both b12 and LALA were equivalent. Thus, the reduced protective capacity of LALA compared to b12 cannot be attributed to a differential effect of biodistribution. One prior macaque study, using polyclonal neutralizing IgG, demonstrated protection against SHIV challenge when the IgG was administered 6 hours, but not 24 hours after SHIV challenge (130). This year, we used an infant macaque oral SHIV challenge model to examine the effectiveness of early post-exposure bnMAb therapy. Short-term treatment with bnMAb cocktails started one day after virus exposure and administered three more times over 10 days prevented infection in all 10 infants, despite evidence of infection within the first few days in multiple tissues (107). These data provided direct evidence that bnMAb could impact and eradicate infection even 24 hours after viral challenge. Using serial sacrifice, the biodistribution of polyclonal SIVIG and bnMAb cocktails of PGT121 and VRC07-523 was measured in multiple lymphoid, gut and organs of infant macaques. A visualization of bnMAb spread 24 hours after subcutaneous (s.c.) delivery confirmed the rapid and extensive biodistribution of the antibodies (Figure 6). A subsequent study in adult macaques has confirmed the initial finding of bnMAb clearance of infection in diverse tissues; in this case delivered pre-exposure (131). It is important to note that (a) there was no difference in the distribution of the bnMAbs in the infants, whether or not a virus inoculum was present one day before bnMAb infusion, and (b) the kinetics and spread of virus closely matched the distribution of the bNmAbs. The targeted distribution of bnMAbs in lymphoid, gut and mucosal tissues, especially in early stages of infection and virus spread, allows for co-localization and the formation of immune complexes (ICs) that are required for a full complement of antibody-mediated anti-viral activities. For HIV-1 transmission, mucosal vulnerability in the rectum and vagina are particularly relevant for rapid delivery or vaccine induction of protective immunity, and notably, bnMAbs can be detected at both sites by one day after s.c. delivery. Thus, is seems likely that the bnMAb cocktail stopped the development of SHIV-infection by killing and clearing newly infected cells in blood and tissues (107). This study emphasizes the multi-functionality of antibodies while demonstrating that bnMAbs can be protective when administered during the earliest stages of infection. Infected cell killing requires recruitment of effector cells via antibody Fc, a reminder of the dual roles of bnMAbs in protection – neutralization and effector mechanisms – already shown to be powerful in the current cache of bnMAbs.
Figure 6. Biodistribution of bnMAb cocktail following subcutaneous (s.c.) injection.
A cocktail containing 5 mg/kg of each PGT121 and VRC07-523 was delivered s.c. in the upper back to infant (~1 month old) rhesus macaques. Multiple tissues were collected one day later, homogenized, and assayed for bnMAb cocktail concentration. Two representative animals are shown from experiments with no SHIV inoculation.
Anti-viral mechanisms of bnMAbs vs ART
An important distinction between bnMAbs and small molecule antiretroviral drugs that target protease, reverse transcriptase or integrase is that the viral reservoir includes Env variants that are selected to escape from the natural adaptive antibody response, whereas drug resistance mutations more commonly arise only after initiation of ART. This may mean that HIV-1 therapy with bnMAbs will be challenging. Initial clinical studies with single bnMAbs have shown substantial virological effects, but with evidence of fairly rapid selection of escape variants (132, 133). In animal models, combinations of bnMAbs demonstrate more sustained viral suppression than single bnMAbs (134–137). Ongoing animal model and human studies are addressing the potential role of HIV bnMAbs, in combination and with other models of ART. Our challenge may be in optimizing these weapons and designing treatment regimens, establishing a new reinforcement to curb the pandemic while vaccine efforts continue.
Effects of bnMAbs on virus control and immune modulation
Timing of bnMAb effectiveness
Early virological events are critical, and thus the timing of bnMAbs is critical to their success in preventing infection. As discussed above, widespread lymphoid, gut, and organ infection with SHIV can occur within one day of exposure (107). These results mirrored earlier studies using SIV in infants (138). A study in adult macaques infected with SHIV suggest that passive treatment beginning at ten days post-exposure is as effective as drug treatment (ART) at viral suppression, and both of these treatments result in reducing viral reservoirs (139). However, as discussed earlier, bnMAb may only be able to eradicate the very early nidus of infection, i.e., within 24 hours after exposure, and this would not be feasible for most human HIV-1 exposure events. However, in the setting of MTCT, some infants are exposed to HIV-1 during passage through the birth canal, where they are exposed to HIV-1. In this setting, it is feasible to envision passive transfer as a means to protect from intrapartum infection and additional, as immunoprophylaxis during breastfeeding.
Immune modulation by bnMAbs
In addition to blocking and blunting viral replication after passive infusion, other therapeutic effects of neutralizing mAbs independent of virus neutralization have been seen. The modulation of endogenous immune responses was reported in a murine model after a short immunotherapy using neutralizing mAbs. The mice receiving the mAbs a week following infection with FrCas(E) murine retrovirus survived healthy and mounted a long-lasting protective antiviral immunity with strong humoral and cellular immune responses (140). The enhancement of endogenous neutralizing antibody responses has been reported in three of our studies using neutralizing polyclonal IgG. The first of these was post-exposure SIVIG treatment in juvenile SIV-infected macaques (141) and the second and third were with pre-exposure prophylaxis using SHIVIG with sub-sterilizing levels of bnMAbs in newborn SHIV-infected macaques (142, 143). In these studies, an immunomodulatory effect was seen following antibody passive transfer that manifested as reduced pathogenesis and decreased progression to disease in animals receiving neutralizing antibody treatment compared to untreated animals. Importantly, this effect was not seen using SHIVIG with “mismatched” neutralizing activity specific to a different SHIV (142). One explanation for how this may work is through the process known as “antibody feedback,” a theory associated with how germinal center B cells are signaled for triggering earlier onset of somatic mutations (144). Michaud, et al. (140), posit that immune complexes formed during antibody passive immunotherapy could affect the magnitude and quality of an anti-viral humoral response. Recently, immunotherapy with 3BNC117 in HIV-1 infected individuals was reported to enhance host humoral immunity against HIV-1 providing support for this theory (145). As more evidence is gathered regarding the power of bnMAbs against HIV-1 infection, these theories will become important as a basis to understand the scope of effective mechanisms in vivo. Taken together, bnMAbs are promising therapeutics regardless of whether neutralization, effector function, or some combination of both, are the keys to their protective mechanism.
Contribution of IgA in HIV-1 protection
To date, the antibodies that have been investigated intensively have all been IgGs, in part because these are most commonly isolated by current technologies, and due to the ease of constructing and expressing antibodies in this format. Thus other isotypes have not been widely evaluated as MAbs for prophylaxis. IgA in the form of secretory IgA (SIgA) is a predominant antibody isotype in mucosal secretions and plays an essential role in mucosal immunity. For HIV-1, the role of IgA is not well defined; serum IgA levels were correlated with increased risk of infection in the setting of the RV144 vaccine trial (146). Despite its less dominant role compared to IgG, a correlation between mucosal SIgA and enhanced protection against infection has been demonstrated for vaginally transmitted viruses such as HPV and HIV-1 (147–149). HIV-1 specific IgA from highly exposed persistently seronegative (HEPS) individuals has been shown to neutralize and inhibit transcytosis of primary HIV-1 isolates across a human epithelial lining (150) and is thought to contribute to the apparent resistance of these individuals to HIV infection (151).
Evidence from SHIV/macaque in vivo studies suggest that mucosally applied IgA correlates with a higher level of protection against rectally transmitted HIV (152, 153). Nonetheless, an important experiment for evaluating the impact of neutralizing SIgA at the mucosal surface of human transmission sites will be the passive transfer of dimeric IgA (dIgA) in macaques. If shown (or engineered) to effectively interact with macaque polymeric Ig Receptor (pIgR) in plasma, a more natural deposition of SIgA at the mucosal surface could be accomplished following transcytosis through the mucosal epithelium, possibly shedding light on the impact of SIgA against a virus inoculum. More studies are warranted in this area, especially to parse the weight of neutralization vs Fcα mediated effector functions at the mucosal surface.
Passive transfer studies in humans – where are we now?
There have been a limited number of studies using anti-HIV-1 Abs in humans. Most of the earlier studies had been focused on Ab treatment of HIV-1 infected individuals with some limited success (154–157). In more recent studies, both 3BNC117 and VRC01 have been shown to have an anti-viral effect when administered to HIV-1 infected patients (132, 133, 158). In the case of prevention, no studies have been completed in humans to test the protective efficacy of bnMAbs against HIV-1 infection.
Opportunities and challenges
Recently, two large multi-center phase IIb clinical trials to test the efficacy of VRC01 in preventing HIV-1 transmission in high-risk populations have been initiated. These trials, collectively called the Antibody-Mediated Prevention (AMP) studies, are the first to test the ability of a bnMAb to prevent HIV-1 infection. Importantly, if significant protection against infection is observed, these studies are designed to also assess the level of antibody in plasma associated with protection. Thus, the results from this study could help in setting protective benchmarks for development of newer bnMAbs as well as defining the target antibody or neutralization levels needed to be induced by preventive HIV-1 vaccines.
Despite the encouraging developments in the field of HIV-1 bnMAbs and their potential use in prevention, there still lie many challenges before they can be widely used for prevention. The foremost challenge is the viral diversity observed in circulating HIV-1 strains. Although a few bnMAbs can cover more than 90% of the circulating strains, no single bnMAb is able to potently neutralize all HIV-1 strains. Therefore a combination of two or more bnMAbs would be needed to potently cover the entire range of viral strains of HIV-1 (98, 99). In this regard, use of the latest antibody technologies to make bi-specific antibodies targeting multiple HIV-1 Env epitopes to increase breadth and potency of bnMAbs shows promise (159, 160). Another challenge to development of bnMAbs for clinical use is the cost related to clinical grade production. This can be tackled in several ways. First, the use of more potent bnMAbs will lead to lower dosage at each injection. In this regard, newly discovered extremely potent bnMAbs like PGMD1400 and CAP256.VRC26.25 represent suitable candidates for development (83, 84). Second, engineering of antibodies to have longer half lives can lead to reduction in the number of doses needed to be administered every year (105, 106). Finally, ongoing improvements in efficient and cost-effective options to manufacture bnMAbs will significantly impact the overall cost of the final products and make them more feasible for global use (161).
Future advances
Alternative approaches for future clinical testing include advances in bi-specific antibodies (159, 160), engineered immunomodulatory proteins (162–164), and vector-delivered antibody molecules (165), which have the potential to mediate improved antibody effects compared to naturally-occurring antibodies. Newer visualization techniques may help to inform the mechanisms by which protection and infected cell killing occurs at mucosal sites. Although beyond the scope of this review, antibody-based treatments, including bnMAb combinations, are in testing to determine their impact on viremia and on latent viral reservoir in established infection (134, 135). Potent bnMAb treatment, combined with inducing or latency-reversing agents, may contribute to partial or functional clearance of the viral reservoir.
Concluding remarks
For more than thirty years, the HIV-1 field has cast a wide net of vaccine approaches and anti-retroviral drugs in global Herculean efforts to address the continuing threat of human transmission of this fatal virus infection. The advances in ART drugs and regimens have undoubtedly prolonged the lives of millions. Educational and humanitarian programs, especially in resource-limited areas of the world have also contributed to a slowing of the worldwide spread of HIV-1/AIDS. Vaccine designers are making progress, but at best, only 30% protection has been seen to date in the clinic and possibly 50% based on recent animal models (166, 167). The field now has a new arsenal of powerful human monoclonal antibodies with the potential to prevent HIV-1 infection, and if used early enough - to modulate or ablate viremia and potentially enhance immune responses. Antibodies are dual functioning molecules that act as operative communicators with effector immune cells. As such, HIV-1 antibodies that bring together virus neutralization and Fc-effector functions, can be effective contributors to improving the lives of infected individuals and in some demographics, preventing infection altogether.
Acknowledgments
This work was supported in part by intramural funds from the Vaccine Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health to J.R.M and by PHS grant P51-OD011092 (N.L.H.). The authors declare no conflict of interest.
References
- 1.Winau F, Winau R. Emil von Behring and serum therapy. Microbes Infect. 2002;4:185–188. doi: 10.1016/s1286-4579(01)01526-x. [DOI] [PubMed] [Google Scholar]
- 2.Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS. 2015;10:129–134. doi: 10.1097/COH.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall A, Pirofski LA. New concepts in antibody-mediated immunity. Infect Immun. 2004;72:6191–6196. doi: 10.1128/IAI.72.11.6191-6196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitlin L, Whaley KJ, Olinger GG, et al. Antibody therapeutics for Ebola virus disease. Curr Opin Virol. 2016;17:45–49. doi: 10.1016/j.coviro.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti D, Misasi J, Mulangu S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351:1339–1342. doi: 10.1126/science.aad5224. [DOI] [PubMed] [Google Scholar]
- 6.Misasi J, Gilman MS, Kanekiyo M, et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science. 2016;351:1343–1346. doi: 10.1126/science.aad6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel HD, Nikitin P, Gesner T, et al. In Vitro Characterization of Human Cytomegalovirus-Targeting Therapeutic Monoclonal Antibodies LJP538 and LJP539. Antimicrob Agents Chemother. 2016;60:4961–4971. doi: 10.1128/AAC.00382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto BJ, Shadiack AM, Carpenter S, et al. Obiltoxaximab for Inhalational Anthrax: Efficacy Projection Across a Range of Disease Severity. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.00972-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai CW, Morris S. Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration “Animal Rule”. Front Microbiol. 2015;6:1320. doi: 10.3389/fmicb.2015.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol. 2016;13:150–160. doi: 10.1038/nrgastro.2015.220. [DOI] [PubMed] [Google Scholar]
- 11.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. Antibody-Based Biologics and Their Promise to Combat Staphylococcus aureus Infections. Trends Pharmacol Sci. 2016;37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moldt B, Le KM, Carnathan DG, et al. Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques. AIDS. 2016;30:1543–1551. doi: 10.1097/QAD.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moldt B, Rakasz EG, Schultz N, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegu A, Yang ZY, Boyington JC, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sievers SA, Scharf L, West AP, Jr, Bjorkman PJ. Antibody engineering for increased potency, breadth and half-life. Curr Opin HIV AIDS. 2015;10:151–159. doi: 10.1097/COH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 19.Cohn LB, Silva IT, Oliveira TY, et al. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray AJ, Kwon KJ, Farber DL, Siliciano RF. The Latent Reservoir for HIV-1: How Immunologic Memory and Clonal Expansion Contribute to HIV-1 Persistence. J Immunol. 2016;197:407–417. doi: 10.4049/jimmunol.1600343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerhans A, Cheynier R, Albert J, et al. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989;58:901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- 22.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost SD, Wrin T, Smith DM, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore PL, Gray ES, Morris L. Specificity of the autologous neutralizing antibody response. Curr Opin HIV AIDS. 2009;4:358–363. doi: 10.1097/COH.0b013e32832ea7e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun TW, Shawn Justement J, Murray D, et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. The Journal of infectious diseases. 2013;208:1443–1447. doi: 10.1093/infdis/jit306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang KH, Bonsall D, Katzourakis A, et al. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat Commun. 2010;1:102. doi: 10.1038/ncomms1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guay LA, Musoke P, Hom DL, et al. Phase I/II trial of HIV-1 hyperimmune globulin for the prevention of HIV-1 vertical transmission in Uganda. AIDS. 2002;16:1391–1400. doi: 10.1097/00002030-200207050-00011. [DOI] [PubMed] [Google Scholar]
- 29.Onyango-Makumbi C, Omer SB, Mubiru M, et al. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY) J Acquir Immune Defic Syndr. 2011;58:399–407. doi: 10.1097/QAI.0b013e31822f8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiehm ER, Fletcher CV, Mofenson LM, et al. Use of human immunodeficiency virus (HIV) human hyperimmune immunoglobulin in HIV type 1-infected children (Pediatric AIDS clinical trials group protocol 273) The Journal of infectious diseases. 2000;181:548–554. doi: 10.1086/315224. [DOI] [PubMed] [Google Scholar]
- 31.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomaras GD, Binley JM, Gray ES, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. Journal of virology. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker LM, Simek MD, Priddy F, et al. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS pathogens. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wibmer CK, Bhiman JN, Gray ES, et al. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS pathogens. 2013;9:e1003738. doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhiman JN, Anthony C, Doria-Rose NA, et al. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nature medicine. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doria-Rose NA, Schramm CA, Gorman J, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonsignori M, Zhou T, Sheng Z, et al. Maturation Pathway from Germline to Broad HIV-1 Neutralizer of a CD4-Mimic Antibody. Cell. 2016;165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lifson JD, Haigwood NL. Lessons in nonhuman primate models for AIDS vaccine research: from minefields to milestones. Cold Spring Harb Perspect Med. 2012;2:a007310. doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirmaier A, Krupp A, Johnson WE. Understanding restriction factors and intrinsic immunity: insights and lessons from the primate lentiviruses. Future Virol. 2014;9:483–497. doi: 10.2217/FVL.14.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 42.Reimann KA, Li JT, Voss G, et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. Journal of virology. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakuragi S, Shibata R, Mukai R, et al. Infection of macaque monkeys with a chimeric human and simian immunodeficiency virus. J Gen Virol. 1992;73(Pt 11):2983–2987. doi: 10.1099/0022-1317-73-11-2983. [DOI] [PubMed] [Google Scholar]
- 44.Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shibata R, Maldarelli F, Siemon C, et al. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. The Journal of infectious diseases. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 46.Mascola JR, Lewis MG, Stiegler G, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. Journal of virology. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 48.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 49.Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nature medicine. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hessell AJ, Rakasz EG, Poignard P, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS pathogens. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hessell AJ, Rakasz EG, Tehrani DM, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. Journal of virology. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nature medicine. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 53.Parren PW, Marx PA, Hessell AJ, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. Journal of virology. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shingai M, Donau OK, Plishka RJ, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Wang S, Kong R, et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3413–3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Prete GQ, Ailers B, Moldt B, et al. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe. 2014;16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartaglia LJ, Chang HW, Lee BC, et al. Production of Mucosally Transmissible SHIV Challenge Stocks from HIV-1 Circulating Recombinant Form 01_AE env Sequences. PLoS pathogens. 2016;12:e1005431. doi: 10.1371/journal.ppat.1005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harouse JM, Gettie A, Eshetu T, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) Journal of virology. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopker MJ, Del Prete GQ, Estes JD, et al. Derivation and characterization of pathogenic transmitted/founder molecular clones from SIVsmE660 and SIVmac251 following mucosal infection. Journal of virology. 2016 doi: 10.1128/JVI.00718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nature medicine. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 61.Parren PW, Ditzel HJ, Gulizia RJ, et al. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Gauduin M-C, Safrit JT, Weir R, Fung MSC, Koup RA. Pre- and postexposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J Infect Dis. 1995;171:1203–1209. doi: 10.1093/infdis/171.5.1203. [DOI] [PubMed] [Google Scholar]
- 63.Luo XM, Lei MY, Feidi RA, et al. Dimeric 2G12 as a potent protection against HIV-1. PLoS pathogens. 2010;6:e1001225. doi: 10.1371/journal.ppat.1001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veselinovic M, Neff CP, Mulder LR, Akkina R. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology. 2012;432:505–510. doi: 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoddart CA, Galkina SA, Joshi P, et al. Efficacy of broadly neutralizing monoclonal antibody PG16 in HIV-infected humanized mice. Virology. 2014;462–463:115–125. doi: 10.1016/j.virol.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deruaz M, Moldt B, Le KM, et al. Protection of Humanized Mice From Repeated Intravaginal HIV Challenge by Passive Immunization: A Model for Studying the Efficacy of Neutralizing Antibodies In Vivo. The Journal of infectious diseases. 2016 doi: 10.1093/infdis/jiw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun M, Li Y, Yuan Z, et al. VRC01 antibody protects against vaginal and rectal transmission of human immunodeficiency virus 1 in hu-BLT mice. Arch Virol. 2016 doi: 10.1007/s00705-016-2942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prince AM, Reesink H, Pascual D, et al. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS research and human retroviruses. 1991;7:971–973. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 70.Prince AM, Horowitz B, Baker L, et al. Failure of a human immunodeficiency virus (HIV) immune globulin to protect chimpanzees against experimental challenge with HIV. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6944–6948. doi: 10.1073/pnas.85.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shibata R, Igarashi T, Haigwood N, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature medicine. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 72.Moldt B, Shibata-Koyama M, Rakasz EG, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. Journal of virology. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 74.Overbaugh J, Morris L. The Antibody Response against HIV-1. Cold Spring Harb Perspect Med. 2012;2:a007039. doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simek MD, Rida W, Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. Journal of virology. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 80.Moore PL, Gray ES, Sheward D, et al. Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. Journal of virology. 2011;85:3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang J, Ofek G, Laub L, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sok D, van Gils MJ, Pauthner M, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doria-Rose NA, Bhiman JN, Roark RS, et al. New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. Journal of virology. 2016;90:76–91. doi: 10.1128/JVI.01791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mouquet H, Scharf L, Euler Z, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trkola A, Purtscher M, Muster T, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. Journal of virology. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buchacher A, Predl R, Strutzenberger K, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS research and human retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 90.Burton DR, Pyati J, Koduri R, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 91.Zhou T, Lynch RM, Chen L, et al. Structural Repertoire of HIV-1-Neutralizing Antibodies Targeting the CD4 Supersite in 14 Donors. Cell. 2015;161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou T, Zhu J, Wu X, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang J, Kang BH, Pancera M, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Falkowska E, Le KM, Ramos A, et al. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong R, Xu K, Zhou T, et al. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science. 2016;352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JH, Ozorowski G, Ward AB. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016;351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rademeyer C, Korber B, Seaman MS, et al. Features of Recently Transmitted HIV-1 Clade C Viruses that Impact Antibody Recognition: Implications for Active and Passive Immunization. PLoS pathogens. 2016;12:e1005742. doi: 10.1371/journal.ppat.1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kong R, Louder MK, Wagh K, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. Journal of virology. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagh K, Bhattacharya T, Williamson C, et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS pathogens. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gautam R, Nishimura Y, Pegu A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rudicell RS, Kwon YD, Ko SY, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. Journal of virology. 2014;88:12669–12682. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saunders KO, Pegu A, Georgiev IS, et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. Journal of virology. 2015;89:5895–5903. doi: 10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Challa DK, Velmurugan R, Ober RJ, Sally Ward E. FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr Top Microbiol Immunol. 2014;382:249–272. doi: 10.1007/978-3-319-07911-0_12. [DOI] [PubMed] [Google Scholar]
- 104.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J Immunol. 2015;194:4595–4603. doi: 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hessell AJ, Jaworski JP, Epson E, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nature medicine. 2016;22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Voss JE, Macauley MS, Rogers KA, et al. Reproducing SIVDeltanef vaccine correlates of protection: trimeric gp41 antibody concentrated at mucosal front lines. AIDS. 2016 doi: 10.1097/QAD.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng M, Smith AJ, Shang L, et al. Mucosal Humoral Immune Response to SIVmac239nef Vaccination and Vaginal Challenge. J Immunol. 2016;196:2809–2818. doi: 10.4049/jimmunol.1500156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith AJ, Wietgrefe SW, Shang L, et al. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol. 2014;193:3126–3133. doi: 10.4049/jimmunol.1400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Q, Zeng M, Duan L, et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol. 2014;193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moldt B, Schultz N, Dunlop DC, et al. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. Journal of virology. 2011;85:10572–10581. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmad R, Sindhu ST, Toma E, et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 115.Sawyer LA, Katzenstein DA, Hendry RM, et al. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS research and human retroviruses. 1990;6:341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 116.Nag P, Kim J, Sapiega V, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. The Journal of infectious diseases. 2004;190:1970–1978. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung AW, Navis M, Isitman G, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lambotte O, Pollara J, Boufassa F, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PloS one. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moog C, Dereuddre-Bosquet N, Teillaud JL, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y, Asmal M, Lane S, et al. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. Journal of virology. 2011;85:6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asmal M, Sun Y, Lane S, et al. Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses. Journal of virology. 2011;85:5465–5475. doi: 10.1128/JVI.00313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Florese RH, Van Rompay KK, Aldrich K, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 123.Dugast AS, Chan Y, Hoffner M, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PloS one. 2014;9:e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poignard P, Moulard M, Golez E, et al. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. Journal of virology. 2003;77:353–365. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pietzsch J, Gruell H, Bournazos S, et al. A mouse model for HIV-1 entry. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bruel T, Guivel-Benhassine F, Amraoui S, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sips M, Krykbaeva M, Diefenbach TJ, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hessell AJ, Epson E, Moldt B, et al. Biodistribution of neutralizing monoclonal antibodies IgG1 b12 and LALA in mucosal and lymphatic tissues of rhesus macaques. Retrovirology. 2012:9. [Google Scholar]
- 130.Nishimura Y, Igarashi T, Haigwood NL, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu J, Ghneim K, Sok D, et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 2016 doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caskey M, Klein F, Lorenzi JC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shingai M, Nishimura Y, Klein F, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horwitz JA, Halper-Stromberg A, Mouquet H, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Milush JM, Kosub D, Marthas M, et al. Rapid dissemination of SIV following oral inoculation. AIDS. 2004;18:2371–2380. [PubMed] [Google Scholar]
- 139.Bolton DL, Pegu A, Wang K, et al. Human Immunodeficiency Virus Type 1 Monoclonal Antibodies Suppress Acute Simian-Human Immunodeficiency Virus Viremia and Limit Seeding of Cell-Associated Viral Reservoirs. Journal of virology. 2016;90:1321–1332. doi: 10.1128/JVI.02454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Michaud HA, Gomard T, Gros L, et al. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS pathogens. 2010;6:e1000948. doi: 10.1371/journal.ppat.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haigwood NL, Montefiori DC, Sutton WF, et al. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. Journal of virology. 2004;78:5983–5995. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ng CT, Jaworski JP, Jayaraman P, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nature medicine. 2010;16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jaworski JP, Kobie J, Brower Z, et al. Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. Journal of virology. 2013;87:10447–10459. doi: 10.1128/JVI.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song H, Nie X, Basu S, Cerny J. Antibody feedback and somatic mutation in B cells: regulation of mutation by immune complexes with IgG antibody. Immunol Rev. 1998;162:211–218. doi: 10.1111/j.1600-065x.1998.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 145.Schoofs T, Klein F, Braunschweig M, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 147.Belec L, Ghys PD, Hocini H, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. The Journal of infectious diseases. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 148.Bomsel M, Tudor D, Drillet AS, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 149.Rocha-Zavaleta L, Barrios T, Garcia-Carranca A, Valdespino V, Cruz-Talonia F. Cervical secretory immunoglobulin A to human papillomavirus type 16 (HPV16) from HPV16-infected women inhibit HPV16 virus-like particles-induced hemagglutination of mouse red blood cells. FEMS Immunol Med Microbiol. 2001;31:47–51. doi: 10.1111/j.1574-695X.2001.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 150.Mazzoli S, Lopalco L, Salvi A, et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. The Journal of infectious diseases. 1999;180:871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 151.Devito C, Broliden K, Kaul R, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 152.Watkins JD, Sholukh AM, Mukhtar MM, et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS. 2013;27:F13–20. doi: 10.1097/QAD.0b013e328360eac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sholukh AM, Watkins JD, Vyas HK, et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33:2086–2095. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trkola A, Kuster H, Rusert P, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 155.Mehandru S, Vcelar B, Wrin T, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. Journal of virology. 2007;81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Manrique A, Rusert P, Joos B, et al. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J Virol. 2007;81:8793–8808. doi: 10.1128/JVI.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jacobson JM, Colman N, Ostrow NA, et al. Passive immunotherapy in the treatment of advanced human immunodeficiency virus infection. The Journal of infectious diseases. 1993;168:298–305. doi: 10.1093/infdis/168.2.298. [DOI] [PubMed] [Google Scholar]
- 158.Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016 doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang Y, Yu J, Lanzi A, et al. Engineered Bispecific Antibodies with Exquisite HIV-1-Neutralizing Activity. Cell. 2016;165:1621–1631. doi: 10.1016/j.cell.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Asokan M, Rudicell RS, Louder M, et al. Bispecific Antibodies Targeting Different Epitopes on the HIV-1 Envelope Exhibit Broad and Potent Neutralization. Journal of virology. 2015;89:12501–12512. doi: 10.1128/JVI.02097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. MAbs. 2009;1:443–452. doi: 10.4161/mabs.1.5.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pegu A, Asokan M, Wu L, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sloan DD, Lam CY, Irrinki A, et al. Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells. PLoS pathogens. 2015;11:e1005233. doi: 10.1371/journal.ppat.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sung JA, Pickeral J, Liu L, et al. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gardner MR, Kattenhorn LM, Kondur HR, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barouch DH, Alter G, Broge T, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vaccari M, Gordon SN, Fourati S, et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nature medicine. 2016;22:762–770. doi: 10.1038/nm.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]