Abstract
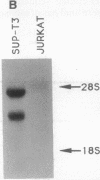
Tumor-specific alteration of the TAL1 gene occurs in almost 25% of patients with T-cell acute lymphoblastic leukemia (T-ALL). We now report the identification of TAL2, a distinct gene that was isolated on the basis of its sequence homology with TAL1. The TAL2 gene is located 33 kilobase pairs from the chromosome 9 breakpoint of t(7;9)(q34;q32), a recurring translocation specifically associated with T-ALL. As a consequence of t(7;9)(q34;q32), TAL2 is juxtaposed with sequences from the T-cell receptor beta-chain gene on chromosome 7. TAL2 sequences are actively transcribed in SUP-T3, a T-ALL cell line that harbors the t(7;9)(q34;q32). The TAL2 gene product includes a helix-loop-helix protein dimerization and DNA binding domain that is especially homologous to those encoded by the TAL1 and LYL1 protooncogenes. Hence, TAL2, TAL1, and LYL1 constitute a discrete subgroup of helix-loop-helix proteins, each of which can potentially contribute to the development of T-ALL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Guglielmi P., Jonveaux P., Cherif D., Gisselbrecht S., Mauchauffe M., Berger R., Larsen C. J., Mathieu-Mahul D. Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosomes Cancer. 1990 Jan;1(3):194–208. doi: 10.1002/gcc.2870010303. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Boehm T., Baer R., Lavenir I., Forster A., Waters J. J., Nacheva E., Rabbitts T. H. The mechanism of chromosomal translocation t(11;14) involving the T-cell receptor C delta locus on human chromosome 14q11 and a transcribed region of chromosome 11p15. EMBO J. 1988 Feb;7(2):385–394. doi: 10.1002/j.1460-2075.1988.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Foroni L., Kaneko Y., Perutz M. F., Rabbitts T. H. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4367–4371. doi: 10.1073/pnas.88.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Foroni L., Kennedy M., Rabbitts T. H. The rhombotin gene belongs to a class of transcriptional regulators with a potential novel protein dimerisation motif. Oncogene. 1990 Jul;5(7):1103–1105. [PubMed] [Google Scholar]
- Boehm T., Rabbitts T. H. The human T cell receptor genes are targets for chromosomal abnormalities in T cell tumors. FASEB J. 1989 Oct;3(12):2344–2359. doi: 10.1096/fasebj.3.12.2676678. [DOI] [PubMed] [Google Scholar]
- Brennan T. J., Chakraborty T., Olson E. N. Mutagenesis of the myogenin basic region identifies an ancient protein motif critical for activation of myogenesis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5675–5679. doi: 10.1073/pnas.88.13.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. J., Crist W. M., Link M. P., Amylon M. D., Pullen D. J., Ragab A. H., Buchanan G. R., Wimmer R. S., Vietti T. J. The t(1;14)(p34;q11) is nonrandom and restricted to T-cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1990 Sep 15;76(6):1220–1224. [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. T., Yang C. Y., Hernandez J., Embrey J., Baer R. The chromosome translocation (11;14)(p13;q11) associated with T cell acute leukemia. Asymmetric diversification of the translocational junctions. J Exp Med. 1990 Feb 1;171(2):489–501. doi: 10.1084/jem.171.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger L. R., Kagan J., Christopher G., Kurtzberg J., Hershfield M. S., Nowell P. C., Croce C. M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano M., Roberts C. W., Minden M., Crist W. M., Korsmeyer S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991 Jul 5;253(5015):79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Hsu H. L., Cheng J. T., Chen Q., Baer R. Enhancer-binding activity of the tal-1 oncoprotein in association with the E47/E12 helix-loop-helix proteins. Mol Cell Biol. 1991 Jun;11(6):3037–3042. doi: 10.1128/mcb.11.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson O. G., Kitchens R. L., Baer R. J., Buchanan G. R., Smith R. G. Rearrangements of the tal-1 locus as clonal markers for T cell acute lymphoblastic leukemia. J Clin Invest. 1991 Jun;87(6):2029–2035. doi: 10.1172/JCI115232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. A., Gonzalez-Sarmiento R., Kees U. R., Lampert F., Dear N., Boehm T., Rabbitts T. H. HOX11, a homeobox-containing T-cell oncogene on human chromosome 10q24. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8900–8904. doi: 10.1073/pnas.88.20.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Borden J., Manuelidis L., Ward D. C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988 Nov;80(3):224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Hockett R. D., Pollock K. M., Bartholdi M. F., O'Brien S. J., Korsmeyer S. J. The t(11;14)(p15;q11) in a T-cell acute lymphoblastic leukemia cell line activates multiple transcripts, including Ttg-1, a gene encoding a potential zinc finger protein. Mol Cell Biol. 1989 May;9(5):2124–2132. doi: 10.1128/mcb.9.5.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin J. D., Smith S. D., Cleary M. L. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989 Jul 14;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Reynolds T. C., Smith S. D., Sklar J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell. 1987 Jul 3;50(1):107–117. doi: 10.1016/0092-8674(87)90667-2. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shima E. A., Le Beau M. M., McKeithan T. W., Minowada J., Showe L. C., Mak T. W., Minden M. D., Rowley J. D., Diaz M. O. Gene encoding the alpha chain of the T-cell receptor is moved immediately downstream of c-myc in a chromosomal 8;14 translocation in a cell line from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1986 May;83(10):3439–3443. doi: 10.1073/pnas.83.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. D., Morgan R., Gemmell R., Amylon M. D., Link M. P., Linker C., Hecht B. K., Warnke R., Glader B. E., Hecht F. Clinical and biologic characterization of T-cell neoplasias with rearrangements of chromosome 7 band q34. Blood. 1988 Feb;71(2):395–402. [PubMed] [Google Scholar]
- Stallings R. L., Olson E., Strauss A. W., Thompson L. H., Bachinski L. L., Siciliano M. J. Human creatine kinase genes on chromosomes 15 and 19, and proximity of the gene for the muscle form to the genes for apolipoprotein C2 and excision repair. Am J Hum Genet. 1988 Aug;43(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Reynolds T. C., Smith S. D., Sklar J. Consistent breakage between consensus recombinase heptamers of chromosome 9 DNA in a recurrent chromosomal translocation of human T cell leukemia. J Exp Med. 1989 Feb 1;169(2):369–377. doi: 10.1084/jem.169.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Westbrook C. A., Rubin C. M., Le Beau M. M., Kaminer L. S., Smith S. D., Rowley J. D., Diaz M. O. Molecular analysis of TCRB and ABL in a t(7;9)-containing cell line (SUP-T3) from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1987 Jan;84(1):251–255. doi: 10.1073/pnas.84.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]